Abstract
The storage and mobilization of lipid energy are central functions of adipocytes. Lipid energy is stored as triglyceride in lipid droplet structures that are now recognized as bona fide organelles and whose functions are greatly influenced by members of the perilipin family of lipid droplet scaffolds. Recent work indicates that the signaling events underlying fatty acid mobilization involve protein trafficking to a specialized subset of lipid droplets. Furthermore, the core lipolytic machinery is composed of evolutionarily conserved proteins whose functions are conserved in avian and mammalian production species. Lipolysis affects many aspects of animal nutrition and physiology, which can have an important influence on growth efficiency, lactation, and meat quality. This review focuses on recent research that addresses the organization and trafficking of key players in hormone-stimulated lipolysis, and the central role of perilipin1A in adipocyte lipolysis. The review emphasizes recent work from the laboratories of the authors that utilizes imaging techniques to explore the organization and interactions among lipolytic effectors in live cells during lipolytic activation. A mechanistic understanding of lipolysis may lead to new strategies for promoting human and animal health.
Keywords: adipose triglyceride lipase, alpha/beta hydrolase domain-containing 5, fluorescence resonance energy transfer, hormone sensitive lipase, perilipin, protein complementation
INTRODUCTION
The storage and mobilization of lipid are fundamental cellular processes, and multicellular organisms, from insects to mammals, have evolved specialized cells that store excess lipid energy for mobilization in times of need. In mammals, adipose tissue functions as a highly specialized lipid energy buffer that stores excess energy as triglycerides (TG) for systemic mobilization in the form of FFA. Adipocytes are a key source of fatty acids under various physiological conditions, such as fasting, exercise, and lactation (Sumner and McNamara, 2007; Elkins and Spurlock, 2009; Frayn, 2010), and in disease states like diabetes mellitus (Unger, 1995). Thus, alterations in adipose tissue lipolysis can have an important influence on growth, energy partitioning, and insulin sensitivity. Energy flow into and out of adipocytes also has important implications in animal agriculture because body fat content and distribution are key factors in determining meat quality and production efficiency (Hausman et al., 2009). Several key players regulating adipocyte lipolysis are highly conserved evolutionarily, including in the chicken, quail (Lee et al., 2009; Serr et al., 2009), and pig (Deiuliis et al., 2008). Therefore, a better understanding of energy storage and mobilization in rodent adipocytes should shed light on new production strategies that would allow for selective lipid deposition into desirable fat depots, such as marbling adipose tissue, without sacrificing overall production efficiency.
Although it is well established that TG stores are dynamically regulated, the cellular and molecular bases of this dynamic regulation are just now being revealed. Recent work indicates that storage and mobilization of intracellular lipid involves the assembly of specialized subcellular structures (i.e., lipid droplets), the targeting of unique protein scaffolds and enzymes, and the dynamic trafficking of lipases and regulatory proteins (Granneman and Moore, 2008).
KEY PLAYERS IN ADIPOCYTE LIPOLYSIS
Adipocyte lipolysis is a multifaceted phenomenon that is subject to distinct temporal controls, many of which are poorly understood. This review addresses protein trafficking during rapid, hormone-stimulated lipolysis (for recent reviews on the general regulation of lipolysis see Langin and Arner, 2006; Duncan et al., 2007), and focuses on work from our laboratory that examines the organization and trafficking of lipolytic effector proteins. Interest in lipolytic protein trafficking originates from the seminal work of Londos et al. (1999, 2005). These investigators demonstrated that hormone-stimulated lipolysis is directly related to the amount of protein kinase A (PKA) activation (Honnor et al., 1985a,b). Thus, major signals that acutely inhibit (e.g., insulin and adenosine) or stimulate (e.g., catecholamines) lipolysis converge at the level of PKA (Figure 1). In addition, Londos and colleagues discovered perilipin (PLIN1) as a major lipid coat protein in adipocytes, and demonstrated that this protein is the dominant target of PKA activation (Greenberg et al., 1991). Lastly, Londos and colleagues showed that hormone-sensitive lipase (HSL), itself a target of PKA, translocates to lipid droplet surfaces during PKA activation (Egan et al., 1992). Together, these observations provided a conceptual framework whereby lipolysis is regulated by the PKA-dependent trafficking of proteins to lipid droplet surfaces (Figure 1).
Figure 1.
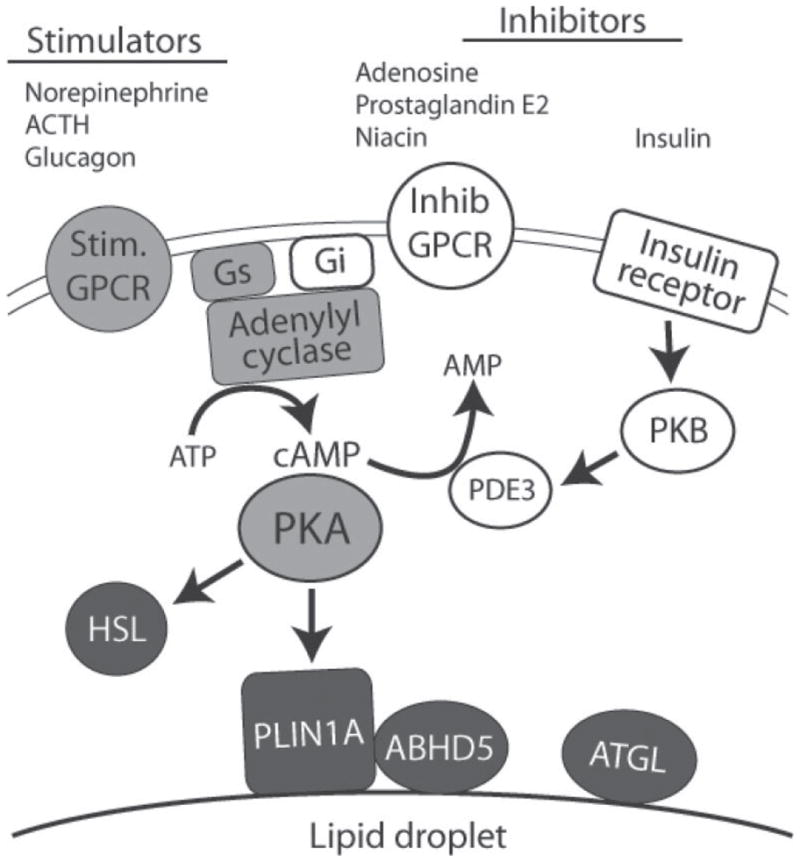
Rapid regulation of adipocyte lipolysis of protein kinase A (PKA). Adipocyte lipolysis is regulated by diverse stimulatory (Stim.) and inhibitory (Inhib) ligands acting through G-protein coupled receptors (GPCR) that transverse the plasma membrane bilayer. G-protein coupled receptor-generated signals are integrated by adenylyl cyclase, which generates cAMP. In addition, insulin suppresses lipolysis through protein kinase B (PKB)-dependent activation of phosphodiesterase 3 (PDE3), which degrades cyclic adenosine monophosphate (cAMP) to adenosine monophosphate (AMP). Signals controlling lipolysis converge at PKA, which directly phosphorylates hormone-sensitive lipase (HSL) and perilipin1A (PLIN1A), the major PKA substrate in fat cells. Perilipin1A is targeted to the surface of lipid droplets that are covered by a phospholipid monolayer. Adipose triglyceride lipase (ATGL) and its co-activator, α/β hydrolase domain-containing 5 (ABHD5), are key elements PKA-regulated lipolysis, yet neither appears to be a direct target of PKA. Gs and Gi are the stimulatory and inhibitory G-proteins, respectively, of adenylyl cyclase.
Lipid Droplets and Droplet Scaffold Proteins
Virtually all cells can accumulate small amounts of neutral lipid in structures termed lipid droplets (LD) or lipid bodies. Until relatively recently, these structures were considered to be inert repositories of lipid; however, recent proteomic and lipidomic experiments have clearly established that LD are heterogeneous, dynamic organelles involved in the synthesis, movement, and degradation of lipid (Brasaemle et al., 2004; Liu et al., 2004; Bartz et al., 2007b; Zehmer et al., 2009).
Lipid droplets of adipocytes are thought to form in a specialized subdomain of the endoplasmic reticulum (Martin and Parton, 2006) and are sequentially bound by several proteins containing conserved PAT [perilipin, adipose differentiation-related protein, tail-interacting protein-47 (TIP-47)] domains as they migrate and enlarge (Wolins et al., 2003, 2006; Nagayama et al., 2007). The biology of PAT proteins is an active area of research, and accumulating evidence indicates that specific PAT proteins play specialized roles in LD biology (Londos et al., 2005; Wolins et al., 2006; Brasaemle et al., 2009). Members of the perilipin protein family have been functionally classified into those that translocate between the cytoplasm and LD surface and those that are constitutively bound to the LD surface (Wolins et al., 2006). Exchangeable PAT appear to be involved in the assembly and enlargement of LD and include PLIN4 (S3–12), PLIN3 (also known as TIP-47), and to some extent PLIN5 (also known as muscle lipid droplet protein and oxidative-PAT). Perilipin homologs that are constitutively bound to LD (i.e., cPAT) include PLIN1 (i.e., perilipin) and PLIN2 (also known as adipose differentiation-related protein and adipophilin). Perilipin2 expression is upregulated during LD formation and is induced by conditions that expose adipocytes to increase quantity of fatty acid (Gao et al., 2000; Granneman et al., 2005); however, mature white adipocytes normally express little if any PLIN2 in vivo. Both PLIN1 and PLIN2 are degraded when cytosolic and are stabilized when targeted to LD (Brasaemle et al., 1997a,b).
Of the PAT proteins expressed in adipocytes, there is compelling evidence that perilipin1A (PLIN1A), a PLIN1 splice variant, plays a central role in orchestrating hormone-stimulated lipolysis in adipocytes (as discussed subsequently in detail). The major LD PAT protein of fully differentiated adipocytes is PLIN1 (Greenberg et al., 1991, 1993). Perilipin1A can be phosphorylated at up to 6 sites by PKA and is the major target of PKA phosphorylation in adipocytes (Greenberg et al., 1991). Recent evidence indicates that PLIN1 is a multifunctional protein, capable of reducing basal lipolysis, promoting lipolysis after PKA activation, and controlling LD fragmentation through mechanisms that are lipase dependent and lipase independent (Brasaemle et al., 2009).
Genetic deletion of Plin in mice largely abrogates hormone-stimulated lipolysis (Tansey et al., 2001; Miyoshi et al., 2006). Furthermore, expression of wild type PLIN1A, but not phosphorylation-defective mutants, restores PKA-dependent lipolysis in adipocytes differentiated from Plin1 null mice (Miyoshi et al., 2006, 2007). Reconstitution experiments in heterologous (non-fat cell) systems demonstrate that PLIN1A confers the ability of PKA to increase HSL-dependent and HSL-independent lipolysis (Souza et al., 2002; Sztalryd et al., 2003; Tansey et al., 2003). It is important to note that PLIN1 regulation of lipolysis is complex and involves phosphorylation-dependent and phosphorylation-independent interactions among different functional domains of the protein (Zhang et al., 2003).
In summary, adipocytes express multiple PAT proteins depending on developmental and nutritional status, and these proteins play distinct roles in the generation and maintenance of LD. Of perilipin (PLIN) family members, PLIN1A is the most abundant in mature unilocular fat cells, where it is centrally involved in the organization and regulation of lipolytic effector interactions in both basal and stimulated states.
Lipases
HSL
Until recently, HSL (UniGene name: LIPE) was considered to be the major, if not exclusive, lipase mediating hormone-stimulated lipolysis (reviewed in Holm, 2003; Yeaman, 2004). Hormone-sensitive lipase exhibits strong diglyceride hydrolase activity that is 10- and 5-fold greater than its activity against TG and monoglyceride substrates, respectively (Belfrage et al., 1978). Interestingly, phosphorylation of HSL by PKA increases its activity toward TG, but not aqueous esterase substrates (Yeaman, 1990). Hormone-stimulated lipolysis (measured by cellular FFA release) is significantly impaired, but not eliminated, in HSL knockout mice. Adipocytes of HSL null mice are largely incapable of releasing glycerol and have massive accumulation of cellular diacylglycerol, clearly demonstrating the importance of HSL as a diglyceride lipase (Haemmerle et al., 2002).
Adipose Triglyceride Lipase
The fact that loss of HSL does not abolish hormone-stimulated lipolysis indicated the existence of another TG lipase in adipose tissue. The identification of a second major lipase, adipose triglyceride lipase [ATGL; also known as desnutrin (TTS-2.1); UniGene name: PNPLA2; Jenkins et al. (2004); Villena et al. (2004); Zimmermann et al. (2004)] and the discovery that CGI-58 (UniGene name: ABHD5), a PLIN1-interacting protein (Subramanian et al., 2004; Yamaguchi et al., 2004), is a key co-activator of ATGL (Lass et al., 2006) greatly advanced our understanding of the biochemical basis of adipose tissue lipolysis. Adipose triglyceride lipase is a member of the patatin-domain-containing family of proteins (Kienesberger et al., 2009). Although most abundant in fat, ATGL is found in numerous tissues (Villena et al., 2004; Zimmermann et al., 2004). Adipose triglyceride lipase has strong TG hydrolase activity, but no activity against diglyceride or monoglyceride substrates (Zimmermann et al., 2004). Interestingly, ATGL has reduced, but detectable, transacylase and phospholipase activities (Jenkins et al., 2004; Notari et al., 2006); however, it is presently unclear how these activities of ATGL are regulated in adipocytes or if they are involved in hormone-stimulated lipolysis. Adipose triglyceride lipase is not a direct PKA target (Zimmermann et al., 2004); thus, its activation by hormones must involve indirect mechanisms.
Genetic disruption of Atgl in mice results in massive lipid accumulation in muscle (cardiac and skeletal), liver, kidney, and testes (Haemmerle et al., 2006). Adipose triglyceride lipase deletion in mice reduces adrenergic activation of lipolysis by more than 70% in white fat explants, corresponding to the loss in total TG hydrolase activity. Similar results have been observed using shRNA knock-down in cultured cells (Kershaw et al., 2006; Miyoshi et al., 2007). Adipose triglyceride lipase null mice are largely incapable of lipid mobilization during fasting and thus rely heavily on glucose as an energy source. Human mutations of ATGL have been discovered that result in ectopic lipid accumulation and myopathy, demonstrating the importance of this lipase in humans (Akiyama et al., 2007; Fischer et al., 2007; Kobayashi et al., 2008). Interestingly, a C-terminal truncation mutation of ATGL that abrogates activity in vivo does not inhibit activity in vitro; rather, the mutation results in mistargeting of the protein away from LD in vivo (Schweiger et al., 2008). These observations further reinforce the importance of lipase targeting and trafficking in the regulation of lipolysis.
ABHD5
Alpha/beta hydrolase domain-containing 5, also known as CGI-58 and 1-acylglycerol-3-phosphate O-acyltransferase, belongs to the esterase/lipase subfamily of proteins containing α/β hydrolase folds. However, unlike bona fide lipases, the predicted catalytic serine within the consensus GXSXG motif contains asparagine (Lefèvre et al., 2001), and thus ABHD5 exhibits no lipase activity (Lass et al., 2006; Yamaguchi et al., 2007). Rare homozygous mutations of ABHD5/CGI-58 result in Chanarin-Dorfman syndrome (MIM 27630) that is characterized by ectopic lipid accumulation in numerous tissues (Lefèvre et al., 2001; Akiyama et al., 2003). A major advance in understanding the function of ABHD5 came with the discovery that ABHD5 dramatically increases the TG hydrolase activity of ATGL owing to a direct interaction between these proteins (Lass et al., 2006). A second key feature of ABHD5, discussed below, is its ability to associate with PAT proteins, in particular PLIN1 and PLIN5 (Subramanian et al., 2004; Yamaguchi et al., 2004; Granneman et al., 2009b). Recently, ABHD5 was found to mediate the acylation of lysophosphatidic acid (Ghosh et al., 2008; Montero-Moran et al., 2010). The significance of this activity is presently unclear; however, mutations of ABHD5 that result in Chanarin-Dorfman syndrome do not affect this acyltransferase activity. It is important to note that although null mutations of ABHD5 and ATGL both result in ectopic lipid accumulation, the phenotypes differ considerably and point to unique functions of these interacting proteins (Radner et al., 2010).
Other Lipases and the Biological Significance of HSL vs. ATGL
A proteomic survey using activity-based probes concluded that ATGL, HSL, and monoglyceride lipase are the major lipases in mouse adipose tissue (Birner-Gruenberger et al., 2005), whereas combined pharmacological, immunological and genetic approaches strongly indicate that ATGL and HSL constitute greater than 90% of TG hydrolase activity in fat cell extracts (Claus et al., 2005; Schweiger et al., 2006). It is important to remember, however, that TG hydrolase activities against artificial TG emulsions may not reflect activity against biological substrates within the cell (Schweiger et al., 2008). Adipose triglyceride lipase and HSL appear to work in concert during PKA activation because of their complementary enzymatic activities. Moreover, the relative contribution of each of these lipases is unlikely to be fixed, but rather will depend on nutritional and hormonal factors (Villena et al., 2004) and the relative abundance of ATGL activators, such as ABHD5, and inhibitors, such as G0S2 (Yang et al., 2010).
Lastly, it is important to note that the neutral lipid core of adipocyte LD is surrounded by a monolayer of phospholipid (Blanchette-Mackie et al., 1995), and it has been proposed that the monolayer phospholipid composition plays a role in the activity of lipolytic effectors (Okuda et al., 1994). In this regard, a functional genetic screen of genes involved in LD formation in Drosophila S2 cells demonstrated the significance of phospholipid metabolism on LD number and size (Guo et al., 2008). It is worth noting that both ABHD5 and ATGL can directly affect phospholipid metabolism, and it is conceivable that their roles as lipolytic effectors may involve alterations in the LD phospholipid monolayer.
ORGANIZATION OF LIPOLYTIC PROTEINS: IMMUNOHISTOCHEMCIAL AND BIOCHEMICAL ANALYSES
PLIN1
As mentioned previously, there is strong evidence that lipolysis occurs at LD that have a unique protein composition, and that PLIN1 is indispensable in regulating the access and activity of lipases at these LD surfaces. Thin-section immunoelectron microscopic analysis of adipocytes demonstrated that PLIN1 is highly targeted to the lipid droplet surface (Blanchette-Mackie et al., 1995). Given its location and effect on basal lipolysis, PLIN1 was initially hypothesized to form a protective barrier that shielded TG from cellular lipases, and it was also hypothesized that PKA phosphorylation led to the dissociation of PLIN1, loss of the protective barrier, and subsequent attack by intracellular lipases. However, recent results indicate that PLIN1 does not form a continuous barrier at the LD surface, but rather may provide a scaffold for the targeting and trafficking of lipolytic effectors. First, the abundance of PLIN1 protein is largely unrelated to the adipocyte LD surface area (D’Eon et al., 2005; Nikonova et al., 2008), and confocal immunofluorescence analysis of PLIN1 indicates a discontinuous pattern on the LD surface (Blanchette-Mackie et al., 1995; Moore et al., 2005). In cultured fat cells, PLIN1 is heavily targeted to small LD, whereas large LD, which contain the bulk of cellular TG, have relatively low concentrations (Moore et al., 2005). We have recently used Fluoronanogold immunocytochemistry to directly correlate fluorescence confocal images with high resolution transmission electron micrographs of the LD surface of primary mouse fat cells. The results indicate that although PLIN1 associates with a variety of intracellular structures and is heavily targeted to microdroplets, its density is low on vast regions of the LD surface (V. A. Kimler and J. G. Granneman, unpublished results). To the extent that PLIN1 defines where lipolysis occurs, these observations indicate there is heterogeneity with respect to amount and location of droplet structures that are competent for hormone-stimulated lipolysis (Moore et al., 2005).
ABHD5
Alpha/beta hydrolase domain-containing 5 was independently discovered by 2 groups as a PLIN1-interacting protein (Subramanian et al., 2004; Yamaguchi et al., 2004). Alpha/beta hydrolase domain-containing 5 is cytosolic in preadipocytes, which do not express PLIN. Endogenous expression in adipocytes or ectopic expression of PLIN in preadipocytes results in targeting of ABHD5 to LD (Subramanian et al., 2004). Double-label immunofluorescence analysis indicates that ABHD5 and PLIN are highly co-localized in un-stimulated adipocytes (Subramanian et al., 2004; Granneman et al., 2007). Phosphorylation of PLIN by PKA rapidly decreases its proximity to ABHD5, although almost all ABHD5 remains localized to PLIN1-containing LD during the onset of lipolysis. However, ABHD5 becomes progressively cytosolic, during sustained stimulation, whereas PLIN1 remains on LD surfaces (Granneman et al., 2007; Yamaguchi et al., 2007). Experiments performed in vitro demonstrated that ABHD5 binds directly to PLIN1, but not phosphorylated PLIN1 (Yamaguchi et al., 2004).
ATGL
In the basal state, ATGL is found in the cytoplasm (Villena et al., 2004; Notari et al., 2006; Granneman et al., 2007) and to a lesser degree on LD (Zimmermann et al., 2004; Bartz et al., 2007b), including those containing PLIN (Granneman et al., 2007). Stimulation elicits minor translocation of ATGL to LD (Granneman et al., 2007), but does not improve the co-localization of ATGL with PLIN. Unlike HSL, ATGL does not interact with PLIN1, as judged by fluorescence resonance energy transfer (FRET) and bimolecular fluorescence complementation assays (Granneman et al., 2007). Interestingly, immunofluorescence studies of cultured adipocytes indicate that forskolin increases the co-localization of ABHD5 and ATGL. Because stimulation does not alter the overall co-localization of ATGL and PLIN, the stimulation-induced increase in co-localization of ABHD5 and ATGL may involve subcellular structures that lack PLIN.
HSL
Biochemical experiments have shown that HSL is almost exclusively found in the cytoplasm in the basal state and translocates to neutral lipid fractions after PKA activation (Egan et al., 1992; Clifford et al., 2000; Martin et al., 2009). Detailed immunofluorescence analysis has shown that phosphorylation of HSL on serine 659 and 660 occurs within 5 s of cell stimulation and is required for the rapid accumulation of the lipase on LD surfaces (Su et al., 2003; Martin et al., 2009). Protein kinase A-induced HSL translocation to LD is impaired in cells lacking PLIN and is enhanced by ectopic PLIN expression (Sztalryd et al., 2003; Miyoshi et al., 2006). Interestingly, although PKA-induced HSL translocation in adipocytes requires PLIN, it does not require PLIN phosphorylation (Miyoshi et al., 2006).
DYNAMIC IMAGING OF LIPOLYTIC EFFECTORS IN LIVE CELLS
Immunochemical and biochemical experiments indicate a model in which PLIN1 acts as a dynamic scaffold that regulates the trafficking and activity of lipolytic effectors at the LD surface. We recently proposed a model of hormone-stimulated lipolysis in which PLIN1 regulates lipolysis by direct and indirect mechanisms (Figure 2), and we are testing the model using dynamic imaging techniques in live cells. First, we propose that PLIN1 indirectly regulates ATGL activity by controlling the availability of its protein activator, ABHD5, in a PKA-dependent fashion. Second, we hypothesize that PLIN1 facilitates HSL-mediated lipolysis by providing a docking site on the LD where HSL gains access to TG and diglyceride substrates.
Figure 2.
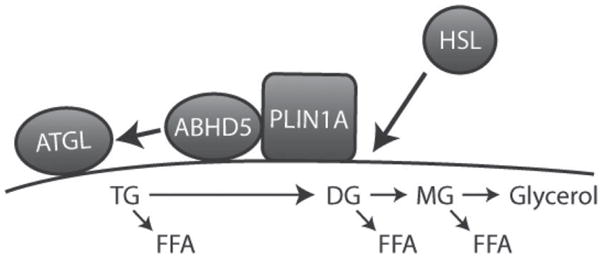
Protein trafficking during initiation of lipolysis. Under basal conditions, perilipin1A (PLIN1A) and α/β hydrolase domain-containing 5 (ABHD5) form a complex on the surface of lipid droplets (LD). Hormone-sensitive lipase (HSL) is mainly cytosolic, whereas adipose triglyceride lipase (ATGL) is localized to LD, including those containing perilipin (PLIN). Stimulation by protein kinase A (PKA) activation leads to trafficking of HSL and ABHD5 (arrows). Phosphorylation of PLIN1A induces a conformational change that decreases its proximity to ABHD5, which allows ABHD5 to activate ATGL. Because ATGL acts exclusively on triglyceride (TG), it is likely that ATGL initiates generation of FFA. Phosphorylation of HSL promotes its translocation and tight association with PLIN1. A major component of HSL activity likely depends on generation of diglyceride (DG) substrate from the action of ATGL, whereas monoglyceride lipase (MG) acts to liberate glycerol and the final FFA. Not illustrated are potential effects of PLIN phosphorylation on the biophysical properties of the LD surface and the effects of sustained activity on LD fragmentation and movement.
According to the model, an important function of PLIN1 is to sequester ABHD5 from ATGL in the un-stimulated state and thereby suppress basal lipolysis. Protein complementation assays were used to assess the protein-protein interactions in live cells and demonstrated that the interaction of ABHD5 with PLIN1 in the basal state is far stronger that its interaction with ATGL (Granneman et al., 2009a). Importantly, ABHD5 can interact with PLIN1 or ATGL, but not with both simultaneously. Thus, expression of PLIN1 dramatically reduces the interaction of ABHD5 with its target lipase and provides a mechanism whereby PLIN1 can suppress lipolysis without acting as a barrier. This mechanism was confirmed by siRNA experiments showing that disinhibition of lipolysis after knockdown of PLIN1 requires both ATGL and ABHD5 (Granneman et al., 2009a).
We have used FRET to image the dynamic interaction of fluorescently tagged PLIN1 and ABHD5 live cells (Granneman et al., 2007). The FRET signals are generated when proteins are in extremely close proximity (<8 nm), as occurs during direct interactions. Figure 3 illustrates the results of a typical experiment. In the basal state, PLIN1 and ABHD5 are nearly perfectly co-localized, and the interaction of PLIN1 and ABHD5 supports strong energy transfer. Brief stimulation of PKA with forskolin rapidly (i.e., within 1 min) decreases the proximity between these proteins as evidenced by the pronounced decrease in the FRET signal. Interestingly, over the time period in which lipolysis is maximally activated (i.e., <10 min) no loss of ABHD5 or PLIN1 from the LD surface is observed and ABHD5 may remain as a complex with PLIN1A during the initiation of lipolysis. As mentioned previously, PLIN1A has 6 potential sites for PKA phosphorylation and mutational analysis indicates that phosphorylation of either PKA site 5 or 6 is sufficient to reduce the proximity between ABDH5 and PLIN1 (Granneman et al., 2009a).
Figure 3.
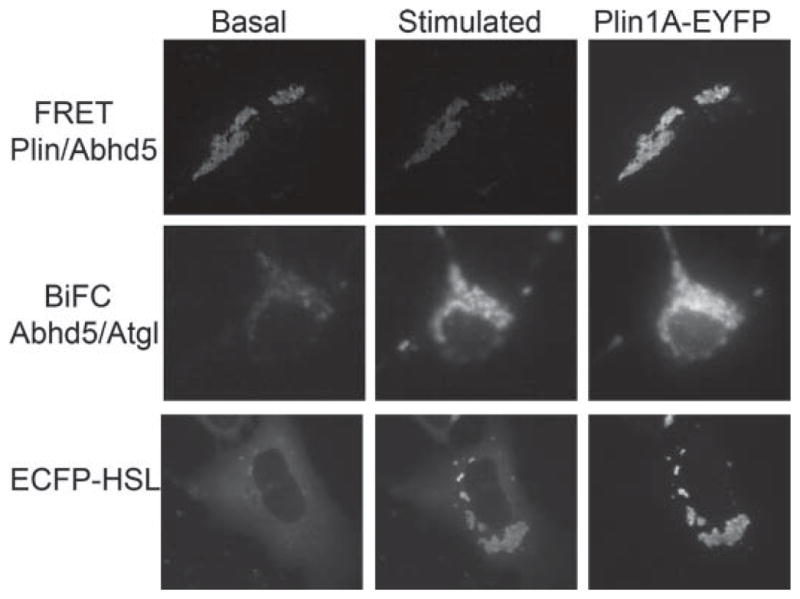
Imaging protein trafficking in live cells by confocal fluorescence microscopy. Top row: Imaging the interaction of α/β hydrolase domain-containing 5 (ABHD5) and perilipin1A (PLIN1A) by fluorescence resonance energy transfer (FRET). In the basal state, ABHD5 and PLIN1A are in close molecular proximity and exhibit strong fluorescence energy transfer between enhanced cyan fluorescent protein (ECFP) and enhanced yellow fluorescent protein (EYFP) tags. Stimulation with forskolin rapidly decreases the association and is indicated by diminished FRET signal. Perilipin1A fused to EYFP shows the intracellular location of lipid droplets (LD). Middle row: Imaging the interaction of ABHD5 and adipose triglyceride lipase (ATGL) using bimolecular fluorescence complementation (BiFC). In the basal state, there is low interaction between ABHD5 and ATGL as indicated by weak fluorescence. Stimulation greatly increases the interaction, resulting in stronger BiFC signal, which co-localizes precisely to LD marked with PLIN1A-EYFP. Lower panel: Stimulationinduced accumulation of HSL on LD containing PLIN1A. In the basal state, ECFP-hormone sensitive lipase (HSL) is largely cytosolic, and stimulation leads to rapid and precise accumulation on LD-containing PLIN1A-EYFP.
Biochemical analyses have demonstrated that ATGL is critical for triggering hormone stimulated lipolysis and that PLIN1 is involved in this process (Miyoshi et al., 2007). Unlike HSL, ATGL is not a direct target of PKA, nor does ATGL bind to PLIN1. However, release of ABHD5 provides a mechanism whereby PLIN1 can indirectly regulate ATGL in a PKA-dependent fashion. Tests of this model using protein complementation demonstrated that PKA regulates the interaction of ABHD5 with its target lipase in a manner that requires phosphorylation of PLIN1 on the same sites that promote release of ABHD5. Previous immunocytochemical analyses, which assess static subcellular co-localization, suggested that ABHD5 and ATGL interact on structures lacking PLIN1. However, bimolecular fluorescence complementation (BiFC) experiments, which monitor dynamic protein-protein interactions directly, demonstrated that the interaction of ABHD5 and ATGL occurs mainly on LD that contain PLIN1 (Granneman et al., 2009a). This is illustrated in Figure 3 (middle row), in which the BiFC signal is reduced in the basal state and is rapidly increased upon stimulation. Furthermore, the increased BiFC signals co-localize precisely with that of fluorescently tagged PLIN1A.
There are substantial biochemical and immunochemical data demonstrating that phosphorylation of HSL provokes its translocation to lipid droplets and that PLIN1 is involved in this process. An example of dynamic imaging of fluorescently tagged HSL is shown in the Figure 3 (bottom row). Tagged HSL is largely cytosolic in the basal state and stimulation leads to its rapid accumulation on PLIN1A-containing LD (Granneman et al., 2007). Translocation of HSL begins within 1 min after forskolin stimulation and is essentially complete by 5 min. Interestingly, accumulation of HSL on individual LD occurs in direct proportion to PLIN1 concentration. Experiments using FRET, protein complementation, and chemical cross-linking indicate that PKA activation generates a complex containing PLIN1 and HSL (Miyoshi et al., 2006; Granneman et al., 2007; Shen et al., 2009). However, analysis of phosphorylation-defective mutants of PLIN1 in adipocytes strongly indicates that HSL translocation is not sufficient to initiate lipolysis in the absence of PLIN phosphorylation (Miyoshi et al., 2006). Thus, PLIN phosphorylation affects HSL activity beyond its ability to promote translocation, but it is not known whether this effect involves direct interactions with phosphorylated PLIN or is brought about indirectly by such factors as ATGL activation (and generation of diglyceride substrate) or droplet remodeling.
SUMMARY AND CONCLUSIONS
A growing body of evidence indicates that PLIN1 plays a critical role in organizing LD proteins and regulating trafficking at the LD surface. Perilipin1 clearly regulates the activity and accessibility of cellular lipases during PKA activation; however, the biochemical and biophysical bases are uncertain. In this regard, it is unclear what roles the conserved acyltransferase activity of ABHD5 or phospholipase activity of ATGL might play in lipolysis and lipid droplet dynamics. Additionally, PLIN1 mediates lipase-independent LD fragmentation and dispersion, suggesting additional functions of the protein (Marcinkiewicz et al., 2006).
The PLIN1 is expressed almost exclusively in adipocytes, yet most mammalian cells store and mobilize small amounts of TG. How the interactions of lipolysis are regulated in tissues lacking PLIN1, such as muscle and liver, is an important area of future research. Available evidence indicates that specific PLIN homologs contribute to cell-specific regulation of stored TG. In this regard, we have recently shown that PLIN5, which is enriched in muscle and liver, binds ABHD5; however, PLIN5 facilitates the interaction of ABHD5 and ATGL in the basal state, whereas Plin1 suppresses it (Granneman et al., 2009a,b). This interaction between ABHD5 and PLIN5 appears to promote basal lipolysis, but may not be involved in stimulated lipolysis because forskolin does not affect FRET between ABHD5 and PLIN5 (J. G. Granneman, unpublished results).
Much of the basic research into the molecular components of lipolysis has been performed on rodents or in cell culture models. Nonetheless, the basic elements of the lipolytic machinery are evolutionarily conserved, and research in production animals is beginning to emerge. Adipose triglyceride lipase is present in poultry, pigs, and cattle (Deiuliis et al., 2008; Nie et al., 2009) and is upregulated by fasting in chickens and pigs, as it is in rodents (Villena et al., 2004; Serr et al., 2009; Zhao et al., 2009). Furthermore, SNP in the ATGL gene have been associated with growth and fat content in chickens (Nie et al., 2010), and variations in ATGL mRNA expression have been correlated with leanness in pigs (Zhao et al., 2009). These observations suggest that a greater understanding of LD biology and lipolytic protein interactions may lead to new strategies for improving growth efficiency and meat quality of production animals.
Footnotes
Presented at the Cell Biology Symposium titled “Receptors and Signaling” at the Joint Annual Meeting, July 11 to 15, 2010, Denver, Colorado. The symposium was sponsored, in part, by American Society of Animal Science (ASAS), the American Dairy Science Association (ADSA), and the National Institute of Food and Agriculture (NIFA) with publication sponsored by the Journal of Animal Science, ASAS, ADSA, and NIFA.
Supported by National Institutes of Health grants DK062292, DK 076229 and the Department of Veterans Affairs.
LITERATURE CITED
- Akiyama M, Sakai K, Ogawa M, McMillan JR, Sawamura D, Shimizu H. Novel duplication mutation in the patatin domain of adipose triglyceride lipase (PNPLA2) in neutral lipid storage disease with severe myopathy. Muscle Nerve. 2007;36:856–859. doi: 10.1002/mus.20869. [DOI] [PubMed] [Google Scholar]
- Akiyama M, Sawamura D, Nomura Y, Sugawara M, Shimizu H. Truncation of CGI-58 protein causes malformation of lamellar granules resulting in ichthyosis in Dorfman-Chanarin syndrome. J Invest Dermatol. 2003;121:1029–1034. doi: 10.1046/j.1523-1747.2003.12520.x. [DOI] [PubMed] [Google Scholar]
- Bartz R, Zehmer JK, Zhu M, Chen Y, Serrero G, Zhao Y, Liu P. Dynamic activity of lipid droplets: Protein phosphorylation and GTP-mediated protein translocation. J Proteome Res. 2007b;6:3256–3265. doi: 10.1021/pr070158j. [DOI] [PubMed] [Google Scholar]
- Belfrage P, Jergil B, Stralfors P, Tornqvist H. Identification and some characteristics of the enzyme protein of the hormone-sensitive lipase from rat adipose tissue. Adv Exp Med Biol. 1978;101:113–126. doi: 10.1007/978-1-4615-9071-2_11. [DOI] [PubMed] [Google Scholar]
- Birner-Gruenberger R, Susani-Etzerodt H, Waldhuber M, Riesenhuber G, Schmidinger H, Rechberger G, Kollroser M, Strauss JG, Lass A, Zimmermann R, Haemmerle G, Zechner R, Hermetter A. The lipolytic proteome of mouse adipose tissue. Mol Cell Proteomics. 2005;4:1710–1717. doi: 10.1074/mcp.M500062-MCP200. [DOI] [PubMed] [Google Scholar]
- Blanchette-Mackie EJ, Dwyer NK, Barber T, Coxey RA, Takeda T, Rondinone CM, Theodorakis JL, Greenberg AS, Londos C. Perilipin is located on the surface layer of intracellular lipid droplets in adipocytes. J Lipid Res. 1995;36:1211–1226. [PubMed] [Google Scholar]
- Brasaemle DL, Barber T, Kimmel AR, Londos C. Post-translational regulation of perilipin expression. Stabilization by stored intracellular neutral lipids. J Biol Chem. 1997a;272:9378–9387. doi: 10.1074/jbc.272.14.9378. [DOI] [PubMed] [Google Scholar]
- Brasaemle DL, Barber T, Wolins NE, Serrero G, Blanchette-Mackie EJ, Londos C. Adipose differentiation-related protein is an ubiquitously expressed lipid storage droplet-associated protein. J Lipid Res. 1997b;38:2249–2263. [PubMed] [Google Scholar]
- Brasaemle DL, Dolios G, Shapiro L, Wang R. Proteomic analysis of proteins associated with lipid droplets of basal and lipolytically stimulated 3T3–L1 adipocytes. J Biol Chem. 2004;279:46835–46842. doi: 10.1074/jbc.M409340200. [DOI] [PubMed] [Google Scholar]
- Brasaemle DL, Subramanian V, Garcia A, Marcinkiewicz A, Rothenberg A. Perilipin A and the control of triacyl-glycerol metabolism. Mol Cell Biochem. 2009;326:15–21. doi: 10.1007/s11010-008-9998-8. [DOI] [PubMed] [Google Scholar]
- Claus TH, Lowe DB, Liang Y, Salhanick AI, Lubeski CK, Yang L, Lemoine L, Zhu J, Clairmont KB. Specific inhibition of hormone-sensitive lipase improves lipid profile while reducing plasma glucose. J Pharmacol Exp Ther. 2005;315:1396–1402. doi: 10.1124/jpet.105.086926. [DOI] [PubMed] [Google Scholar]
- Clifford GM, Londos C, Kraemer FB, Vernon RG, Yeaman SJ. Translocation of hormone-sensitive lipase and perilipin upon lipolytic stimulation of rat adipocytes. J Biol Chem. 2000;275:5011–5015. doi: 10.1074/jbc.275.7.5011. [DOI] [PubMed] [Google Scholar]
- Deiuliis JA, Shin J, Bae D, Azain MJ, Barb R, Lee K. Developmental, hormonal, and nutritional regulation of porcine adipose triglyceride lipase (ATGL) Lipids. 2008;43:215–225. doi: 10.1007/s11745-007-3146-1. [DOI] [PubMed] [Google Scholar]
- D’Eon TM, Souza SC, Aronovitz M, Obin MS, Fried SK, Greenberg AS. Estrogen regulation of adiposity and fuel partitioning. Evidence of genomic and non-genomic regulation of lipogenic and oxidative pathways. J Biol Chem. 2005;280:35983–35991. doi: 10.1074/jbc.M507339200. [DOI] [PubMed] [Google Scholar]
- Duncan RE, Ahmadian M, Jaworski K, Sarkadi-Nagy E, Sul HS. Regulation of lipolysis in adipocytes. Annu Rev Nutr. 2007;27:79–101. doi: 10.1146/annurev.nutr.27.061406.093734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egan JJ, Greenberg AS, Chang MK, Wek SA, Moos MC, Jr, Londos C. Mechanism of hormone-stimulated lipolysis in adipocytes: Translocation of hormone-sensitive lipase to the lipid storage droplet. Proc Natl Acad Sci USA. 1992;89:8537–8541. doi: 10.1073/pnas.89.18.8537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elkins DA, Spurlock DM. Phosphorylation of perilipin is associated with indicators of lipolysis in Holstein cows. Horm Metab Res. 2009;41:736–740. doi: 10.1055/s-0029-1225359. [DOI] [PubMed] [Google Scholar]
- Fischer J, Lefèvre C, Morava E, Mussini JM, Laforet P, Negre-Salvayre A, Lathrop M, Salvayre R. The gene encoding adipose triglyceride lipase (PNPLA2) is mutated in neutral lipid storage disease with myopathy. Nat Genet. 2007;39:28–30. doi: 10.1038/ng1951. [DOI] [PubMed] [Google Scholar]
- Frayn KN. Fat as a fuel: Emerging understanding of the adipose tissue-skeletal muscle axis. Acta Physiol (Oxf) 2010;199:509–518. doi: 10.1111/j.1748-1716.2010.02128.x. [DOI] [PubMed] [Google Scholar]
- Gao J, Ye H, Serrero G. Stimulation of adipose differentiation related protein (ADRP) expression in adipocyte precursors by long-chain fatty acids. J Cell Physiol. 2000;182:297–302. doi: 10.1002/(SICI)1097-4652(200002)182:2<297::AID-JCP19>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- Ghosh AK, Ramakrishnan G, Chandramohan C, Rajasekharan R. CGI-58, the causative gene for Chanarin-Dorfman syndrome, mediates acylation of lysophosphatidic acid. J Biol Chem. 2008;283:24525–24533. doi: 10.1074/jbc.M801783200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granneman JG, Li P, Zhu Z, Lu Y. Metabolic and cellular plasticity in white adipose tissue I: Effects of beta3-adrenergic receptor activation. Am J Physiol Endocrinol Metab. 2005;289:E608–E616. doi: 10.1152/ajpendo.00009.2005. [DOI] [PubMed] [Google Scholar]
- Granneman JG, Moore HP. Location, location: Protein trafficking and lipolysis in adipocytes. Trends Endocrinol Metab. 2008;19:3–9. doi: 10.1016/j.tem.2007.10.006. [DOI] [PubMed] [Google Scholar]
- Granneman JG, Moore HP, Granneman RL, Greenberg AS, Obin MS, Zhu Z. Analysis of lipolytic protein trafficking and interactions in adipocytes. J Biol Chem. 2007;282:5726–5735. doi: 10.1074/jbc.M610580200. [DOI] [PubMed] [Google Scholar]
- Granneman JG, Moore HP, Krishnamoorthy R, Rathod M. Perilipin controls lipolysis by regulating the interactions of abhydrolase containing 5 (ABHD5) and adipose triglyceride lipase (ATGL) J Biol Chem. 2009a;284:34538–34544. doi: 10.1074/jbc.M109.068478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granneman JG, Moore HP, Mottillo EP, Zhu Z. Functional interactions between MLDP (LSDP5) and ABHD5 in the control of intracellular lipid accumulation. J Biol Chem. 2009b;284:3049–3057. doi: 10.1074/jbc.M808251200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg AS, Egan JJ, Wek SA, Garty NB, Blanchette-Mackie EJ, Londos C. Perilipin, a major hormonally regulated adipocyte-specific phosphoprotein associated with the periphery of lipid storage droplets. J Biol Chem. 1991;266:11341–11346. [PubMed] [Google Scholar]
- Greenberg AS, Egan JJ, Wek SA, Moos MC, Jr, Londos C, Kimmel AR. Isolation of cDNAs for perilipins A and B: Sequence and expression of lipid droplet-associated proteins of adipocytes. Proc Natl Acad Sci USA. 1993;90:12035–12039. doi: 10.1073/pnas.90.24.12035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Y, Walther TC, Rao M, Stuurman N, Goshima G, Terayama K, Wong JS, Vale RD, Walter P, Farese RV. Functional genomic screen reveals genes involved in lipid-droplet formation and utilization. Nature. 2008;453:657–661. doi: 10.1038/nature06928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haemmerle G, Lass A, Zimmermann R, Gorkiewicz G, Meyer C, Rozman J, Heldmaier G, Maier R, Theussl C, Eder S, Kratky D, Wagner EF, Klingenspor M, Hoefler G, Zechner R. Defective lipolysis and altered energy metabolism in mice lacking adipose triglyceride lipase. Science. 2006;312:734–737. doi: 10.1126/science.1123965. [DOI] [PubMed] [Google Scholar]
- Haemmerle G, Zimmermann R, Hayn M, Theussl C, Waeg G, Wagner E, Sattler W, Magin TM, Wagner EF, Zechner R. Hormone-sensitive lipase deficiency in mice causes diglyceride accumulation in adipose tissue, muscle, and testis. J Biol Chem. 2002;277:4806–4815. doi: 10.1074/jbc.M110355200. [DOI] [PubMed] [Google Scholar]
- Hausman GJ, Dodson MV, Ajuwon K, Azain M, Barnes KM, Guan LL, Jiang Z, Poulos SP, Sainz RD, Smith S, Spurlock M, Novakofski J, Fernyhough ME, Bergen WG. Board-invited review: The biology and regulation of preadipocytes and adipocytes in meat animals. J Anim Sci. 2009;87:1218–1246. doi: 10.2527/jas.2008-1427. [DOI] [PubMed] [Google Scholar]
- Holm C. Molecular mechanisms regulating hormone-sensitive lipase and lipolysis. Biochem Soc Trans. 2003;31:1120–1124. doi: 10.1042/bst0311120. [DOI] [PubMed] [Google Scholar]
- Honnor RC, Dhillon GS, Londos C. cAMP-dependent protein kinase and lipolysis in rat adipocytes. I. Cell preparation, manipulation, and predictability in behavior. J Biol Chem. 1985a;260:15122–15129. [PubMed] [Google Scholar]
- Honnor RC, Dhillon GS, Londos C. cAMP-dependent protein kinase and lipolysis in rat adipocytes. II. Definition of steady-state relationship with lipolytic and antilipolytic modulators. J Biol Chem. 1985b;260:15130–15138. [PubMed] [Google Scholar]
- Jenkins CM, Mancuso DJ, Yan W, Sims HF, Gibson B, Gross RW. Identification, cloning, expression, and purification of three novel human calcium-independent phospholipase A2 family members possessing triacylglycerol lipase and acylglycerol transacylase activities. J Biol Chem. 2004;279:48968–48975. doi: 10.1074/jbc.M407841200. [DOI] [PubMed] [Google Scholar]
- Kershaw EE, Hamm JK, Verhagen LA, Peroni O, Katic M, Flier JS. Adipose triglyceride lipase: Function, regulation by insulin, and comparison with adiponutrin. Diabetes. 2006;55:148–157. [PMC free article] [PubMed] [Google Scholar]
- Kienesberger PC, Oberer M, Lass A, Zechner R. Mammalian patatin domain containing proteins: A family with diverse lipolytic activities involved in multiple biological functions. J Lipid Res. 2009;50(Suppl):S63–S68. doi: 10.1194/jlr.R800082-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi K, Inoguchi T, Maeda Y, Nakashima N, Kuwano A, Eto E, Ueno N, Sasaki S, Sawada F, Fujii M, Matoba Y, Sumiyoshi S, Kawate H, Takayanagi R. The lack of the C-terminal domain of adipose triglyceride lipase causes neutral lipid storage disease through impaired interactions with lipid droplets. J Clin Endocrinol Metab. 2008;93:2877–2884. doi: 10.1210/jc.2007-2247. [DOI] [PubMed] [Google Scholar]
- Langin D, Arner P. Importance of TNFalpha and neutral lipases in human adipose tissue lipolysis. Trends Endocrinol Metab. 2006;17:314–320. doi: 10.1016/j.tem.2006.08.003. [DOI] [PubMed] [Google Scholar]
- Lass A, Zimmermann R, Haemmerle G, Riederer M, Schoiswohl G, Schweiger M, Kienesberger P, Strauss JG, Gorkiewicz G, Zechner R. Adipose triglyceride lipase-mediated lipolysis of cellular fat stores is activated by CGI-58 and defective in Chanarin-Dorfman syndrome. Cell Metab. 2006;3:309–319. doi: 10.1016/j.cmet.2006.03.005. [DOI] [PubMed] [Google Scholar]
- Lee K, Shin J, Latshaw JD, Suh Y, Serr J. Cloning of adipose triglyceride lipase complementary deoxyribonucleic acid in poultry and expression of adipose triglyceride lipase during development of adipose in chickens. Poult Sci. 2009;88:620–630. doi: 10.3382/ps.2008-00265. [DOI] [PubMed] [Google Scholar]
- Lefèvre C, Jobard F, Caux F, Bouadjar B, Karaduman A, Heilig R, Lakhdar H, Wollenberg A, Verret JL, Weissenbach J, Ozguc M, Lathrop M, Prud’homme JF, Fischer J. Mutations in CGI-58, the gene encoding a new protein of the esterase/lipase/thioesterase subfamily, in Chanarin-Dorfman syndrome. Am J Hum Genet. 2001;69:1002–1012. doi: 10.1086/324121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu P, Ying Y, Zhao Y, Mundy DI, Zhu M, Anderson RG. Chinese hamster ovary K2 cell lipid droplets appear to be metabolic organelles involved in membrane traffic. J Biol Chem. 2004;279:3787–3792. doi: 10.1074/jbc.M311945200. [DOI] [PubMed] [Google Scholar]
- Londos C, Brasaemle DL, Schultz CJ, Adler-Wailes DC, Levin DM, Kimmel AR, Rondinone CM. On the control of lipolysis in adipocytes. Ann N Y Acad Sci. 1999;892:155–168. doi: 10.1111/j.1749-6632.1999.tb07794.x. [DOI] [PubMed] [Google Scholar]
- Londos C, Sztalryd C, Tansey JT, Kimmel AR. Role of PAT proteins in lipid metabolism. Biochimie. 2005;87:45–49. doi: 10.1016/j.biochi.2004.12.010. [DOI] [PubMed] [Google Scholar]
- Marcinkiewicz A, Gauthier D, Garcia A, Brasaemle DL. The phosphorylation of serine 492 of perilipin A directs lipid droplet fragmentation and dispersion. J Biol Chem. 2006;281:11901–11909. doi: 10.1074/jbc.M600171200. [DOI] [PubMed] [Google Scholar]
- Martin S, Okano S, Kistler C, Fernandez-Rojo MA, Hill MM, Parton RG. Spatiotemporal regulation of early lipolytic signaling in adipocytes. J Biol Chem. 2009;284:32097–32107. doi: 10.1074/jbc.M109.002675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin S, Parton RG. Lipid droplets: A unified view of a dynamic organelle. Nat Rev Mol Cell Biol. 2006;7:373–378. doi: 10.1038/nrm1912. [DOI] [PubMed] [Google Scholar]
- Miyoshi H, Perfield JW, 2nd, Souza SC, Shen WJ, Zhang HH, Stancheva ZS, Kraemer FB, Obin MS, Greenberg AS. Control of adipose triglyceride lipase action by serine 517 of perilipin A globally regulates protein kinase A-stimulated lipolysis in adipocytes. J Biol Chem. 2007;282:996–1002. doi: 10.1074/jbc.M605770200. [DOI] [PubMed] [Google Scholar]
- Miyoshi H, Souza SC, Zhang HH, Strissel KJ, Christoffolete MA, Kovsan J, Rudich A, Kraemer FB, Bianco AC, Obin MS, Greenberg AS. Perilipin promotes hormone-sensitive lipase-mediated adipocyte lipolysis via phosphorylation-dependent and -independent mechanisms. J Biol Chem. 2006;281:15837–15844. doi: 10.1074/jbc.M601097200. [DOI] [PubMed] [Google Scholar]
- Montero-Moran G, Caviglia JM, McMahon D, Rothenberg A, Subramanian V, Xu Z, Lara-Gonzalez S, Storch J, Carman GM, Brasaemle DL. Cgi-58/abhd5 is a coenzyme A-dependent lysophosphatidic acid acyltransferase. J Lipid Res. 2010;51:709–719. doi: 10.1194/jlr.M001917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore HP, Silver RB, Mottillo EP, Bernlohr DA, Granneman JG. Perilipin targets a novel pool of lipid droplets for lipolytic attack by hormone-sensitive lipase. J Biol Chem. 2005;280:43109–43120. doi: 10.1074/jbc.M506336200. [DOI] [PubMed] [Google Scholar]
- Nagayama M, Uchida T, Gohara K. Temporal and spatial variations of lipid droplets during adipocyte division and differentiation. J Lipid Res. 2007;48:9–18. doi: 10.1194/jlr.M600155-JLR200. [DOI] [PubMed] [Google Scholar]
- Nie Q, Hu Y, Xie L, Zhang C, Shen X, Zhang X. Identification and characterization of adipose triglyceride lipase (ATGL) gene in birds. Mol Biol Rep. 2009;37:3487–3493. doi: 10.1007/s11033-009-9941-4. [DOI] [PubMed] [Google Scholar]
- Nie QH, Fang MX, Xie L, Shen X, Liu J, Luo ZP, Shi JJ, Zhang XQ. Associations of ATGL gene polymorphisms with chicken growth and fat traits. J Appl Genet. 2010;51:185–191. doi: 10.1007/BF03195726. [DOI] [PubMed] [Google Scholar]
- Nikonova L, Koza RA, Mendoza T, Chao PM, Curley JP, Kozak LP. Mesoderm-specific transcript is associated with fat mass expansion in response to a positive energy balance. FASEB J. 2008;22:3925–3937. doi: 10.1096/fj.08-108266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Notari L, Baladron V, Aroca-Aguilar JD, Balko N, Heredia R, Meyer C, Notario PM, Saravanamuthu S, Nueda ML, Sanchez-Sanchez F, Escribano J, Laborda J, Becerra SP. Identification of a lipase-linked cell membrane receptor for pigment epithelium-derived factor. J Biol Chem. 2006;281:38022–38037. doi: 10.1074/jbc.M600353200. [DOI] [PubMed] [Google Scholar]
- Okuda H, Morimoto C, Tsujita T. Role of endogenous lipid droplets in lipolysis in rat adipocytes. J Lipid Res. 1994;35:36–44. [PubMed] [Google Scholar]
- Radner FP, I, Streith E, Schoiswohl G, Schweiger M, Kumari M, Eichmann TO, Rechberger G, Koefeler HC, Eder S, Schauer S, Theussl HC, Preiss-Landl K, Lass A, Zimmermann R, Hoefler G, Zechner R, Haemmerle G. Growth retardation, impaired triacylglycerol catabolism, hepatic steatosis, and lethal skin barrier defect in mice lacking comparative gene identification-58 (CGI-58) J Biol Chem. 2010;285:7300–7311. doi: 10.1074/jbc.M109.081877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweiger M, Schoiswohl G, Lass A, Radner FP, Haemmerle G, Malli R, Graier W, Cornaciu I, Oberer M, Salvayre R, Fischer J, Zechner R, Zimmermann R. The C-terminal region of human adipose triglyceride lipase affects enzyme activity and lipid droplet binding. J Biol Chem. 2008;283:17211–17220. doi: 10.1074/jbc.M710566200. [DOI] [PubMed] [Google Scholar]
- Schweiger M, Schreiber R, Haemmerle G, Lass A, Fledelius C, Jacobsen P, Tornqvist H, Zechner R, Zimmermann R. Adipose triglyceride lipase and hormone-sensitive lipase are the major enzymes in adipose tissue triacylglycerol catabolism. J Biol Chem. 2006;281:40236–40241. doi: 10.1074/jbc.M608048200. [DOI] [PubMed] [Google Scholar]
- Serr J, Suh Y, Lee K. Regulation of adipose triglyceride lipase by fasting and refeeding in avian species. Poult Sci. 2009;88:2585–2591. doi: 10.3382/ps.2009-00265. [DOI] [PubMed] [Google Scholar]
- Shen WJ, Patel S, Miyoshi H, Greenberg AS, Kraemer FB. Functional interaction of hormone-sensitive lipase and perilipin in lipolysis. J Lipid Res. 2009;50:2306–2313. doi: 10.1194/jlr.M900176-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Souza SC, Muliro KV, Liscum L, Lien P, Yamamoto MT, Schaffer JE, Dallal GE, Wang X, Kraemer FB, Obin M, Greenberg AS. Modulation of hormone-sensitive lipase and protein kinase A-mediated lipolysis by perilipin A in an adenoviral reconstituted system. J Biol Chem. 2002;277:8267–8272. doi: 10.1074/jbc.M108329200. [DOI] [PubMed] [Google Scholar]
- Su CL, Sztalryd C, Contreras JA, Holm C, Kimmel AR, Londos C. Mutational analysis of the hormone-sensitive lipase translocation reaction in adipocytes. J Biol Chem. 2003;278:43615–43619. doi: 10.1074/jbc.M301809200. [DOI] [PubMed] [Google Scholar]
- Subramanian V, Rothenberg A, Gomez C, Cohen AW, Garcia A, Bhattacharyya S, Shapiro L, Dolios G, Wang R, Lisanti MP, Brasaemle DL. Perilipin A mediates the reversible binding of CGI-58 to lipid droplets in 3T3–L1 adipocytes. J Biol Chem. 2004;279:42062–42071. doi: 10.1074/jbc.M407462200. [DOI] [PubMed] [Google Scholar]
- Sumner JM, McNamara JP. Expression of lipolytic genes in the adipose tissue of pregnant and lactating Holstein dairy cattle. J Dairy Sci. 2007;90:5237–5246. doi: 10.3168/jds.2007-0307. [DOI] [PubMed] [Google Scholar]
- Sztalryd C, Xu G, Dorward H, Tansey JT, Contreras JA, Kimmel AR, Londos C. Perilipin A is essential for the translocation of hormone-sensitive lipase during lipolytic activation. J Cell Biol. 2003;161:1093–1103. doi: 10.1083/jcb.200210169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tansey JT, Huml AM, Vogt R, Davis KE, Jones JM, Fraser KA, Brasaemle DL, Kimmel AR, Londos C. Functional studies on native and mutated forms of perilipins. A role in protein kinase A-mediated lipolysis of triacylglycerols. J Biol Chem. 2003;278:8401–8406. doi: 10.1074/jbc.M211005200. [DOI] [PubMed] [Google Scholar]
- Tansey JT, Sztalryd C, Gruia-Gray J, Roush DL, Zee JV, Gavrilova O, Reitman ML, Deng CX, Li C, Kimmel AR, Londos C. Perilipin ablation results in a lean mouse with aberrant adipocyte lipolysis, enhanced leptin production, and resistance to diet-induced obesity. Proc Natl Acad Sci USA. 2001;98:6494–6499. doi: 10.1073/pnas.101042998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unger RH. Lipotoxicity in the pathogenesis of obesity-dependent NIDDM. Genetic and clinical implications. Diabetes. 1995;44:863–870. doi: 10.2337/diab.44.8.863. [DOI] [PubMed] [Google Scholar]
- Villena JA, Roy S, Sarkadi-Nagy E, Kim KH, Sul HS. Desnutrin, an adipocyte gene encoding a novel patatin domain-containing protein, is induced by fasting and glucocorticoids: Ectopic expression of desnutrin increases triglyceride hydrolysis. J Biol Chem. 2004;279:47066–47075. doi: 10.1074/jbc.M403855200. [DOI] [PubMed] [Google Scholar]
- Wolins NE, Brasaemle DL, Bickel PE. A proposed model of fat packaging by exchangeable lipid droplet proteins. FEBS Lett. 2006;580:5484–5491. doi: 10.1016/j.febslet.2006.08.040. [DOI] [PubMed] [Google Scholar]
- Wolins NE, Skinner JR, Schoenfish MJ, Tzekov A, Bensch KG, Bickel PE. Adipocyte protein S3–12 coats nascent lipid droplets. J Biol Chem. 2003;278:37713–37721. doi: 10.1074/jbc.M304025200. [DOI] [PubMed] [Google Scholar]
- Yamaguchi T, Omatsu N, Matsushita S, Osumi T. CGI-58 interacts with perilipin and is localized to lipid droplets. Possible involvement of CGI-58 mislocalization in Chanarin-Dorfman syndrome. J Biol Chem. 2004;279:30490–30497. doi: 10.1074/jbc.M403920200. [DOI] [PubMed] [Google Scholar]
- Yamaguchi T, Omatsu N, Morimoto E, Nakashima H, Ueno K, Tanaka T, Satouchi K, Hirose F, Osumi T. CGI-58 facilitates lipolysis on lipid droplets but is not involved in the vesiculation of lipid droplets caused by hormonal stimulation. J Lipid Res. 2007;48:1078–1089. doi: 10.1194/jlr.M600493-JLR200. [DOI] [PubMed] [Google Scholar]
- Yang X, Lu X, Lombes M, Rha GB, Chi YI, Guerin TM, Smart EJ, Liu J. The G(0)/G(1) switch gene 2 regulates adipose lipolysis through association with adipose triglyceride lipase. Cell Metab. 2010;11:194–205. doi: 10.1016/j.cmet.2010.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeaman SJ. Hormone-sensitive lipase—A multipurpose enzyme in lipid metabolism. Biochim Biophys Acta. 1990;1052:128–132. doi: 10.1016/0167-4889(90)90067-n. [DOI] [PubMed] [Google Scholar]
- Yeaman SJ. Hormone-sensitive lipase—New roles for an old enzyme. Biochem J. 2004;379:11–22. doi: 10.1042/BJ20031811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zehmer JK, Huang Y, Peng G, Pu J, Anderson RG, Liu P. A role for lipid droplets in inter-membrane lipid traffic. Proteomics. 2009;9:914–921. doi: 10.1002/pmic.200800584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang HH, Souza SC, Muliro KV, Kraemer FB, Obin MS, Greenberg AS. Lipase-selective functional domains of perilipin A differentially regulate constitutive and protein kinase A-stimulated lipolysis. J Biol Chem. 2003;278:51535–51542. doi: 10.1074/jbc.M309591200. [DOI] [PubMed] [Google Scholar]
- Zhao SM, Ren LJ, Chen L, Zhang X, Cheng ML, Li WZ, Zhang YY, Gao SZ. Differential expression of lipid metabolism related genes in porcine muscle tissue leading to different intramuscular fat deposition. Lipids. 2009;44:1029–1037. doi: 10.1007/s11745-009-3356-9. [DOI] [PubMed] [Google Scholar]
- Zimmermann R, Strauss JG, Haemmerle G, Schoiswohl G, Birner-Gruenberger R, Riederer M, Lass A, Neuberger G, Eisenhaber F, Hermetter A, Zechner R. Fat mobilization in adipose tissue is promoted by adipose triglyceride lipase. Science. 2004;306:1383–1386. doi: 10.1126/science.1100747. [DOI] [PubMed] [Google Scholar]


