Abstract
Objective
Elevated serum aldolase A levels occur in the absence of elevated creatine kinase M (CK) levels in a subset of myositis patients. This study was undertaken to investigate the cell biology of this unexplained clinical observation.
Methods
Cultured human myoblasts were differentiated in vitro. RNA and protein lysates were prepared and used to determine aldolase and CK gene and protein expression by QPCR and immunoblotting. Cardiotoxin was used to induce muscle injury and repair in an experimental mouse model, and aldolase A and CK were immunoblotted in the muscle lysates. Immunohistochemical staining was performed on myositis patient muscle paraffin sections to assess aldolase A and CK staining in vivo.
Results
Aldolase A mRNA and protein expression is highest in differentiating myoblasts, and remains robust throughout differentiation. In contrast, CK mRNA and protein levels are low in undifferentiated myoblasts and become strikingly upregulated as differentiation progresses. Aldolase A protein expression is high in regenerating muscle in the mouse model of injury/repair, while CK expression was low. Immunohistochemical staining of human myositis biopsies showed that muscle cells with the highest levels of aldolase and no CK staining have features of regeneration.
Conclusion
In undifferentiated muscle cells, and those early in the differentiation process, aldolase A is expressed in the absence of CK. Thereafter, both are expressed. We propose that isolated serum aldolase A elevation in myositis patients (i) reflects preferential immune-mediated damage of early regenerative cells, and (ii) is a biomarker of damaged early regenerating muscle cells.
Keywords: myositis, aldolase, creatine kinase, muscle regeneration
Introduction
Serum levels of muscle enzymes are frequently elevated in myopathic processes, including dermatomyositis (DM) and polymyositis (PM). Indeed, the most well-established diagnostic criteria for DM (1) and PM include elevation of muscle enzymes as a key clinical feature (2, 3). Furthermore, in clinical practice, many physicians rely on muscle enzyme levels as a marker of disease activity.
The muscle enzymes which can be detected in circulation following muscle damage include creatine kinase (CK), aldolase, aspartate aminotransferase, alanine aminotransferase and lactate dehydrogenase. Although serum levels of each muscle enzyme may be increased in the inflammatory myopathies, none of these proteins are found exclusively within skeletal muscle. For example, CK (especially the CK-MB isoform) is found in the heart, the transaminases and lactate dehydrogenase are found in the heart and liver, and aldolase is widely expressed with particularly high levels found in the brain and liver.
Serum CK and aldolase levels are the most sensitive measures for detecting muscle damage (3) and often parallel one another in patients with myopathic disease (4). However, multiple individual case reports have described patients with fasciitis (5, 6) or DM-like disorders (7, 8) who had normal CK levels and high serum aldolase levels. Moreover, Nozaki and Pestronk recently reviewed the clinical features of twelve patients with selectively elevated aldolase levels (9) and found that these patients had a constellation of features including myalgias, muscle weakness, and muscle biopsies showing perimysial and perifascicular pathology. These patients often had arthritis and/or pulmonary involvement. Although Nozaki and Pestronk emphasized the diagnostic utility of checking the aldolase in patients with muscle complaints and a normal CK, how aldolase might be released from muscle without a concurrent CK elevation remains unexplained.
In this study, we analyzed the gene and protein expression levels of CK and aldolase in vitro during muscle cell differentiation. Our findings show that aldolase is expressed at high levels prior to CK during in vitro muscle regeneration, identifying a phase in muscle cell differentiation in which cells express exclusively aldolase. Our findings were validated in a mouse model of muscle regeneration where we found that early regenerating myofibers expressed high levels in the absence of CK. Since accumulating data suggests that immature muscle cells express the highest concentrations of myositis autoantigens (10), we propose that isolated elevations of aldolase levels in myositis may reflect preferential damage of such immature precursors.
Methods
Cell culture and differentiation
Normal human skeletal muscle cells (Clonetics) were cultured in Ham’s F10 growth medium supplemented with 20% fetal bovine serum, 2% chick embryo extract (Accurate Chemical), and 2mM L-glutamine at low density to prevent contact differentiation. Differentiation into myotubes was induced in these cultures by replacing the growth medium with medium containing DMEM, 2% horse serum and 2mM L-glutamine, and growing the cells for 2 weeks without further subculturing.
Immunoblotting
Biochemical levels of proteins expressed in myoblasts and myotubes were assessed in lysates made from myoblasts harvested immediately prior to transfer into differentiation medium (day 0), and on days 1, 4, 10 and 13 of culture in this medium. Lysates were prepared by placing the cultures on ice, washing the cells 3 times with PBS and then scraping the cells into buffer A (20 mM Tris pH 7.4, 150 mM NaCl, 0.1 mM EDTA, 1% Nonidet P40, and a protease inhibitor cocktail). Equal protein amounts of the lysates were electrophoresed and immunoblotted with antibodies recognizing myogenic factor-5 (Myf-5;Santa Cruz), aldolase A (Santa Cruz), CK (Abcam), monoclonal antibodies against embryonic myosin heavy chain (MYH-3, Developmental Studies Hybridoma Bank) and vinculin (Sigma) and a patient serum monospecific for Mi-2. Blotted proteins were visualized using enhanced chemiluminesence (Pierce). The data shown is representative of that obtained in 2-5 separate experiments. Immunoblots were quantified by densitometry; for each protein, the scanned band of maximum optical density was assigned the value 100%, and all others in that time course were expressed relative to that.
QPCR Analysis
Total RNA was isolated from differentiating human myoblasts using the TRIzol reagent method (Invitrogen). RNA was further purified using the RNeasy miniprep kit (Qiagen). cDNA synthesis was performed using the AffinityScript QPCR cDNA synthesis kit (Agilent), and QPCR reactions were performed in triplicate with Brilliant Ii QRT-PCR Master Mix (Agilent) using the Agilent Mx3000p QPCR system. Primers specific for ALDOA, CKM, MYF5 and MYH3, and an endogenous control gene (RPLP0) were synthesized by Applied Biosystems. Expression values for ALDOA, CKM, MYF5 and MYH3 were normalized to the endogenous control gene and are represented as arbitrary units relative to RPLP0 expression at each time point.
Mouse muscle injury model
All experiments involving mice were approved by the Johns Hopkins Animal Care and Use Committee. The right anterior tibilias muscles of six-week-old C57BL/6 mice were injected with 100 microliters of 10 μM cardiotoxin (CTX), exactly as described (11). On days 1, 2, 3 and 4 after muscle injury, mice were sacrificed and the anterior tibialis muscles removed and frozen at −80°C (11). The frozen tissue pieces were used in two ways: (i) 10 micron frozen sections were cut, then processed for hematoxylin and eosin staining, and (ii) pieces of the frozen muscle were homogenized in Buffer A, and equal protein amounts of the resulting lysates were electrophoresed, immunoblotted with antibodies against aldolase A and CK and quantitated by densitometry as described above.
Human muscle tissue
Human muscle biopsies were obtained from patients seen at the Johns Hopkins Myositis Center. Surgical procedures were performed for patient management, and the research tissue samples were in excess of that required for routine diagnostic purposes. Informed consent was obtained from each study subject, and all samples were obtained under the auspices of human subject internal review board-approved protocols. All patient samples were de-identified, with clinical and laboratory features linked only to the patient code. Muscle biopsies were obtained from patients with definite DM by Bohan and Peter criteria (2, 3) with elevated levels of aldolase at the time of biopsy.
Immunohistochemistry
Muscle paraffin sections were rehydrated, soaked in target retrieval solution (Dako) for 30 mins at 95°C and blocked with PBS containing 5% BSA. This and the subsequent antibody incubations were performed at room temperature for 1 hour in a humidified chamber. Primary antibody incubations consisted of mixtures of goat anti-aldolase (Santa Cruz) and mouse anti-CK (Abcam) antibodies; both antibodies were diluted 1:50 in PBS containing 1% BSA. Following extensive washes, the sections were incubated with donkey anti-goat Alexa Fluor 488 and donkey anti-mouse Alexa Fluor 594 secondary antibodies (Invitrogen) diluted 1:200 in PBS containing 1% BSA. The sections were subsequently washed with PBS, and mounted using Prolong Gold antifade reagent containing DAPI.
Results
Aldolase A protein is robustly expressed in undifferentiated myoblasts, whereas CK is absent
In previous studies, we showed that myositis autoantigens are expressed at low levels in normal muscle, but are strikingly upregulated in myositis muscle, especially in regenerating cells (10). These findings were recapitulated in vitro: we demonstrated that autoantigens were expressed at high levels in myoblasts, and low levels after differentiation into myotubes. The observation that some patients with proven inflammatory myopathies have isolated elevation of aldolase prompted us to examine aldolase expression as a function of muscle differentiation. We therefore initially studied the patterns of aldolase A and CK expression in the cultured human myoblast differentiation model.
Equal protein amounts of lysates made from cultured differentiating myoblasts were immunoblotted (Fig 1A). Aldolase A expression was highest in myoblasts, and although it decreased during differentiation, it remained prominent. In contrast, CK expression was absent in myoblasts and during the early stages of differentiation, and was only detected after day 4. Vinculin levels (loading control) were unchanged. Myf-5 and MYH-3 were expressed exclusively in undifferentiated or differentiating/differentiated muscle cells, respectively (Fig 1A), confirming the progression of differentiation in the in vitro system. For comparison, we immunoblotted Mi-2, a DM-specific autoantigen, whose expression pattern is representative of autoantigens targeted in the rheumatic autoimmune diseases – prominently expressed in myoblasts, with levels decreasing rapidly during differentiation.
Figure 1. Aldolase A protein is expressed in the absence of creatine kinase in early undifferentiated cultured myoblasts.

(A) Cultured human myoblasts were induced to differentiate in vitro to myotubes. Equal protein amounts of lysates made from these cultures at various stages of differentiation were immunoblotted for the indicated proteins, as described in the Methods section. (B) The CK and aldolase immunoblots were quantified by densitometry. For each, the scanned band of maximum optical density was assigned the value 100%, and all others in that time course were expressed relative to that. Myf-5: myogenic factor-5; MYH-3: embryonic myosin heavy chain; CK: creatine kinase.
Quantitation of the immunoblot data (Fig 1B) confirmed that aldolase A is robustly expressed while CK is not in undifferentiated muscle cells, and early in differentiation. However, during the later stages of differentiation and thereafter, both aldolase A and CK are expressed at high levels.
Aldolase A mRNA is robustly expressed in undifferentiated myoblasts, whereas CKM mRNA levels are low
To define the kinetics of gene expression during muscle regeneration, we examined gene expression in cultured human myoblasts that were differentiated into myotubes. We examined the expression of well characterized markers of myoblast differentiation (MYF5 and MYH3, Fig 2A), as well as aldolase A (ALDOA) and CKM (Fig 2B). Myogenic factor 5 (MYF5) was expressed at highest levels in myoblasts (day 0), and was downregulated over time. In contrast, embryonic myosin heavy chain 3 (MYH3) displayed a reciprocal pattern, with low expression in myoblasts that was strikingly increased by day 2 and peaked by day 4. Interestingly, ALDOA was expressed at very high levels in myoblasts, and expression remained robust throughout differentiation. CKM was expressed at very low levels in myoblasts, but was significantly upregulated over the first 3 days and remained elevated thereafter. Thus the gene expression pattern of CKM was similar to MYH3, and is consistent with it being a marker of differentiating/mature muscle cells.
Figure 2. The expression of CKM is low in undifferntiated myoblasts but is highly induced during in vitro myoblast differentiation.

Gene expression in differentiating myoblasts was analyzed by qPCR. (A) Gene expressions of aldolase A (ALDOA) and creatine kinase M (CKM), and (B) myogenic factor-5 (MYF5) and embryonic myosin heavy chain (MYH3) are presented relative to the expression of a housekeeping gene (RPLP0) which is unchanged during differentiation.
Aldolase A protein expression is high in regenerating mouse muscle
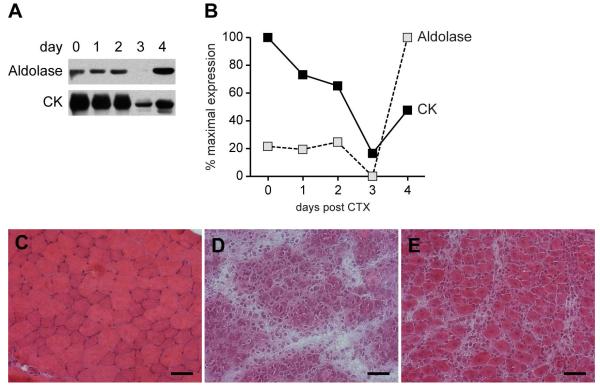
To evaluate whether our findings in the in vitro system are relevant in vivo, we used a mouse model of CTX-induced muscle damage and repair. In previous studies, we (11) and others have characterized and validated this mouse model of muscle injury and repair, in which there is widespread necrosis induced by CTX injection, followed by massive and synchronous muscle regeneration. Immunoblots were performed using equal protein amounts of lysates made from muscle harvested from uninjected mice, or from mice sacrificed at days 1 – 4 after injection. Aldolase levels remained constant until day 2 post-injection, were undetectable at day 3, and at day 4 were present at levels ~4-fold greater than those in the uninjected tissue (Fig 3 A & B). In contrast, CK levels were highest in uninjected muscle, decreased to their lowest level at day 3, with the level at day 4 being slightly higher than that at day 3, but still only ~50% of the level in uninjected muscle (Fig 3 A & B). H & E stains were performed on frozen sections cut from the same muscle pieces used to make the tissue lysates described in Fig 3A & B. These showed extensive degeneration/damage at day 3, characterized by myonecrosis and inflammatory infiltrates (Fig 3D). This was followed by a dramatic change at day 4, where exuberant regeneration was evidenced by large tracts of small fibers with centralized nuclei and the re-emergence of of normal fasicular architecture (Fig 3E).
Figure 3. Aldolase levels are high in early regenerating muscle cells in an in vivo mouse model of muscle damage and repair.
Uninjured (“day 0”) and CTX-injected anterior tibialis muscle samples were obtained by sacrificing the mice at days 1, 2, 3 and 4 post-injection/injury. (A) Lysates were made from the muscle biopsies, and equal protein amounts were immunoblotted with antibodies against aldolase A and CK. Similar data were obtained in 2 separate experiments. (B) The immunoblots were quantified by densitometry as described in Figure 1 legend. (C-E) Frozen sections of uninjured mouse muscle (panel C), or muscle harvested 3 (panel D) or 4 (panel E) days after CTX injection were stained with H & E and visualized with light microscopy. Scale bar: 50 microns. CK: creatine kinase; CTX: cardiotoxin.
In myositis biopsies, muscle cells with high aldolase A levels have features consistent with regeneration
The in vitro data shows that (i) undifferentiated myoblasts and muscle cells early in the differentiation process express high levels of aldolase A in the absence of detectable CK, and (ii) at subsequent stages of differentiation, aldolase A and CK are expressed together in the same cells. Of note, the time when cells express aldolase A in the absence of CK is a brief window during the differentiation process, making it likely that detection of this pattern in vivo would be less frequent than that of co-expression of these proteins. To address whether fibers preferentially expressing aldolase A are indeed found in vivo, we stained muscle paraffin sections from myositis patients with elevated aldolase A and normal CK levels. Sections were double-stained with a goat polyclonal antibody against aldolase A and a mouse monoclonal anti-CK antibody (Fig 4). Two distinct types of staining pattern were noted: (i) Muscle cells with strong aldolase A staining and no CK staining, and (ii) muscle cells expressing both aldolase A and CK, a pattern more common than the former. The aldolase A only staining was exclusively detected in muscle cells with large centralized nuclei, a feature typical of regenerating fibers. Fibers expressing aldolase A alone were not observed in normal muscle biopsies, which demonstrated overlapping patterns of aldolase A and CK (data not shown).
Figure 4. Muscle cells expressing robust amounts of aldolase A have features of regeneration in human myositis muscle biopsies.

Muscle paraffin sections from a DM patient with elevated aldolase A levels and normal CK were double-stained with antibodies against aldolase A (represented in green, top right panel) and CK (represented in red, lower right panel). The merged image is shown in the left panel. Nuclei were stained with DAPI (not shown as a single stain, but represented in blue in the merged image). In the merged image, examples of muscle cells expressing only aldolase A are annotated with asterisks, whereas those expressing both aldolase A and CK are marked with arrows. Scale bar: 50 microns. CK: creatine kinase.
Discussion
Detection of tissue-specific proteins in serum has been a classic method to identify and quantify tissue damage (examples include leakage of troponin I or CK-MB from myocardium, transaminases from the liver, and CK and aldolase A from skeletal muscle). Patterns of enzyme leakage are worthy of investigation because they can be associated with specific types of injury and may provide important insights into mechanisms and targets of damage. In this regard, although CK and aldolase A are frequently found together in the serum of patients with autoimmune myopathies as well as in juvenile DM (12), a substantial subpopulation of these patients have elevated serum levels of aldolase A in the absence of increases in CK. Since CK and aldolase A are both cytoplasmic and have similar molecular weights, the subpopulation of patients with isolated high levels of aldolase A only may provide important insights into the target of the immune attack in such patients – possibly, cells expressing aldolase A but not CK.
Recent findings that autoantigens targeted in myositis are present at elevated levels in muscle cells with features of regeneration (10, 11, 13) indicates that early regenerating muscle cells may be an important target of the ongoing immune response. Of note, muscle cell differentiation recapitulates embryonic development, with consecutive expression of gene cassettes guiding cells to the mature phenotype (14). We therefore sought to define whether expression levels of aldolase A and CK varied as a function of muscle cell differentiation.
Our in vitro data demonstrate clearly that aldolase A is expressed at highest levels in myoblasts and immature muscle cells, whereas CK is absent from the former, but becomes robustly expressed with progressive differentiation. We confirmed these findings in vivo using the CTX model of mouse muscle injury and repair (Fig 3). Our immunoblotting data showed that aldolase A is present in undamaged mouse muscle, but is expressed at levels ~4 –fold higher in muscle 4 days after injury, at a time when the repairing muscle consists almost exclusively of regenerating fibers. In contrast, highest CK levels are associated with undamaged muscle, consisting of mature, differentiated fibers. Additional in vivo validation of our findings was evidenced in the immunohistochemical staining performed on muscle biopsies from myositis patients. In some regenerating muscle cells with centralized nuclei, aldolase A was exclusively expressed without significant expression of CK in these fibers. Unlike the mouse model, these regenerating cells were infrequent, reflecting the asynchronous damage process evident in human biopsies.
Based on the in vitro immunoblotting data, aldolase expression is highest in early myoblasts, and remains robust throughout differentiation. Thus it is likely that detection of the skeletal muscle-specific aldolase isoenzyme in the serum of patients with myositis (in the absence of CK) most likely reflects damage restricted to that subset of cells which express aldolase A only and not yet CK. Importantly, it is not possible to ascertain whether differentiating muscle cells are targeted along with more mature muscle cells in patients with elevated serum levels of both aldolase A and CK. This is because the number of cells expressing aldolase A only as a proportion of cells in the myositis muscle appears to be low. Additionally, expression of aldolase A and CK occur together as differentiation proceeds, and damage to such cells would release both aldolase and CK, obscuring the aldolase-only population.
In conclusion, our findings indicate that isolated elevated levels of serum aldolase A in myositis patients reflects preferential damage of early regenerating muscle cells. In such patients, monitoring aldolase A levels may provide important information about the damage process. Additionally, aldolase A is potentially of use as a biomarker of damage targeting early muscle progenitors.
Acknowledgements and Funding
Dr Casciola-Rosen’s work was supported by the NIH (RO1-AR-044684). Dr Christopher-Stine’s work was supported by the NIH (K23-AR-053197). Dr Mammen’s work was supported by the NIH (grant K08-AR-054783) and a Passano Physician Scientist award. Dr Rosen’s work is supported by the NIH (grants R37-DE-12354 and P30-AR-053503). These studies were also supported by the Stabler Foundation (AR).
Abbreviations
- DM
dermatomyositis
- CK
creatine kinase
- CTX
cardiotoxin
- MYH-3
embryonic myosin heavy chain
- myf-5
myogenic factor-5
- PM
polymyositis
Footnotes
Conflict of Interest Statement
All of the authors declare no conflicts of interest.
References
- 1.GUSEINOVA D, CONSOLARO A, TRAIL L, et al. Comparison of clinical features and drug therapies among European and Latin American patients with juvenile dermatomyositis. Clin Exp Rheumatol. 2011;29:117–24. [PubMed] [Google Scholar]
- 2.BOHAN A, PETER JB. Polymyositis and dermatomyositis (second of two parts) N Engl J Med. 1975;292:403–7. doi: 10.1056/NEJM197502202920807. [DOI] [PubMed] [Google Scholar]
- 3.BOHAN A, PETER JB. Polymyositis and dermatomyositis (first of two parts) N Engl J Med. 1975;292:344–7. doi: 10.1056/NEJM197502132920706. [DOI] [PubMed] [Google Scholar]
- 4.LOTT JA, LANDESMAN PW. The enzymology of skeletal muscle disorders. Crit Rev Clin Lab Sci. 1984;20:153–90. doi: 10.3109/10408368409165773. [DOI] [PubMed] [Google Scholar]
- 5.FUJIMOTO M, SATO S, IHN H, KIKUCHI K, YAMADA N, TAKEHARA K. Serum aldolase level is a useful indicator of disease activity in eosinophilic fasciitis. J Rheumatol. 1995;22:563–5. [PubMed] [Google Scholar]
- 6.QUINTERO-DEL-RIO AI, PUNARO M, PASCUAL V. Faces of eosinophilic fasciitis in childhood. J Clin Rheumatol. 2002;8:99–103. doi: 10.1097/00124743-200204000-00007. [DOI] [PubMed] [Google Scholar]
- 7.CARTER JD, KANIK KS, VASEY FB, VALERIANO-MARCET J. Dermatomyositis with normal creatine kinase and elevated aldolase levels. J Rheumatol. 2001;28:2366–7. [PubMed] [Google Scholar]
- 8.SATO S, HIRAKATA M, KUWANA M, et al. Autoantibodies to a 140-kd polypeptide, CADM-140, in Japanese patients with clinically amyopathic dermatomyositis. Arthritis Rheum. 2005;52:1571–6. doi: 10.1002/art.21023. [DOI] [PubMed] [Google Scholar]
- 9.NOZAKI K, PESTRONK A. High aldolase with normal creatine kinase in serum predicts a myopathy with perimysial pathology. J Neurol Neurosurg Psychiatry. 2009;80:904–8. doi: 10.1136/jnnp.2008.161448. [DOI] [PubMed] [Google Scholar]
- 10.CASCIOLA-ROSEN L, NAGARAJU K, PLOTZ P, et al. Enhanced autoantigen expression in regenerating muscle cells in idiopathic inflammatory myopathy. J Exp Med. 2005;201:591–601. doi: 10.1084/jem.20041367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.MAMMEN AL, CASCIOLA-ROSEN LA, HALL JC, CHRISTOPHER-STINE L, CORSE AM, ROSEN A. Expression of the dermatomyositis autoantigen Mi-2 in regenerating muscle. Arthritis Rheum. 2009;60:3784–93. doi: 10.1002/art.24977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.MATHIESEN PR, ZAK M, HERLIN T, NIELSEN SM. Clinical features and outcome in a Danish cohort of juvenile dermatomyositis patients. Clin Exp Rheumatol. 2010;28:782–89. [PubMed] [Google Scholar]
- 13.MAMMEN AL, CHUNG T, CHRISTOPHER-STINE L, ROSEN P, ROSEN A, CASCIOLA-ROSEN LA. Autoantibodies against 3-hydroxy-3-methylglutaryl-coenzyme a reductase (HMGCR) in patients with statin-associated autoimmune myopathy. Arthritis Rheum. 2011;63:713–21. doi: 10.1002/art.30156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.ZHAO P, HOFFMAN EP. Embryonic myogenesis pathways in muscle regeneration. Dev Dyn. 2004;229:380–92. doi: 10.1002/dvdy.10457. [DOI] [PubMed] [Google Scholar]