Abstract
Background
Studies show that exposure to air pollution damages human health, but the mechanisms are not fully understood. One suggested pathway is via oxidative stress.
Objectives
This study is to examine associations between exposure to air pollution and oxidative DNA damage, as indicated by urinary 8-hydroxy-2’-deoxyguanosine (8-OHdG) concentrations in aging participants during 2006-2008.
Methods
We fit linear regression models to examine associations between air pollutants and 8-OHdG adjusting for potential confounders.
Results
8-OHdG was significantly associated with ambient particulate matter ≤ 2.5 μm in aerodynamic diameter (PM2.5), the number of particles (PN), nitrogen dioxide (NO2), maximal 1-hour ozone (O3), sulfate (SO42-) and organic carbon (OC), but not with black carbon (BC), carbon monoxide (CO) or elemental carbon (EC). Effects were more apparent with multi-week averages of exposures. Per IQR increases of 21-day averages of PM2.5, PN, BC, EC, OC, CO, SO42-, NO2 and maximal 1-hour O3 were associated with 30.8% (95% confidence interval (CI): 9.3%, 52.2%), -13.1% (95%CI: -41.7%, 15.5%), 3.0% (95% CI: -19.8%, 25.8%), 5.3% (95% CI: -23.6%, 34.2%), 24.4% (95% CI: 1.8%, 47.1%), -2.0% (95% CI: -12.4%, 8.3%), 29.8% (95% CI: 6.3%, 53.3%), 32.2% (95% CI: 7.4%, 56.9%) and 47.7% (95% CI: 3.6%, 91.7%) changes in 8-OHdG, respectively.
Conclusions
This study suggests that aging participants experienced an increased risk of developing oxidative DNA injury after exposure to the secondary, but not primary ambient pollutants.
Keywords: 8-Hydroxy-2′-Deoxyguanosine, air pollution, DNA damage, oxidative stress, biomarker
Introduction
Growing evidence suggests that reactive oxygen species (ROS) play a vital role in human disease development. [1-5] ROS refer to oxidation via multiple chemicals that are oxidizing agents and/or are easily converted into radicals such as ozone, peroxynitrite, HOCl, hydrogen peroxide and singlet oxygen. [5] ROS have been shown to possess many characteristics of carcinogens and can result in endothelial dysfunction as well as DNA damage.[2] ROS can cause DNA structural alteration, including base pair rearrangements, mutations, insertions, deletions and sequence amplification.[6] They can also influence cytoplasmic and nuclear signal transduction pathways,[7] modulate the activities of genes and proteins responding to oxidative stress and regulate the genes related to cell proliferation, differentiation and apoptosis.[5]
8-Hydroxy-2’-deoxyguanosine (8-OHdG) is a common biomarker of DNA lesion that is induced by the reaction of hydroxyl radicals with 2’-deoxyguanosine at the C-8 position in DNA.[8] 8-OHdG is released into the circulation system after the DNA repair by the DNA base excision repair pathway and then is excreted into urine.[8, 9] Studies show that the urinary concentration of 8-OHdG is not influenced directly by either diet or cell turnover. Therefore, it is a good biomarker for ROS or oxidative stress.[9]
A large literature has shown that exposures to air pollutants such as PM2.5 and ozone are related to human diseases.[10-15] Many studies have shown that oxidative stress is one important pathway through which particulate matter and ozone execute their effects on cardiovascular and other diseases.[16-19] Exposure to concentrated air particles can result in oxidative stress in both the lung and the heart.[18, 20] A limited number of studies have examined associations of 8-OHdG with exposures to indoor and ambient pollution or smoking, but they were usually conducted among a small number of children or occupationally exposed employees.[1, 21, 22] The present study was designed to investigate whether exposure to a rich set of ambient pollutants was associated with urinary 8-OHdG among a larger cohort of elderly individuals from the Normative Aging Study (NAS), and whether the pattern of the associations was informative about which types of pollutants produced these effects. The pollutants involved in this study included ambient particulate matter ≤ 2.5 μm in aerodynamic diameter (PM2.5), nitrogen dioxide (NO2), ozone (O3), carbon monoxide (CO), sulfate (SO42-), black carbon (BC), organic carbon (OC), number of particles (PN) and elemental carbon (EC).
Methods
Study population
The NAS, a longitudinal study of aging, was established by the Veterans Administration (VA) in 1961 when 2,280 men from the greater Boston area who were free of known chronic medical conditions were enrolled.[23] Participants underwent detailed examinations every 3 to 5 years, including routine physical examination, laboratory tests, collection of medical history, social status information, and administration of questionnaires on smoking history, food intake and other factors that may influence health. Between January 2006 and December 2008, all 320 participants who appeared for examination were evaluated for urinary 8-OHdG and other covariates. The present investigation was approved by the Institutional Review Boards of all participating institutions.
Plasma analysis of B vitamins, creatinine and 8-hydroxy-2’-deoxyguanosine
Fasting plasma samples were drawn at the VA field site and stored at -80 °C. Urinary samples were collected on the same morning of the visit and stored at -80 °C until analysis. Folate, vitamin B6 and B12 in fasting plasma were measured at the USDA Human Nutrition Research Center on Aging at Tufts University. Folate and vitamin B12 were assessed by radioassay using a commercially available kit from Bio-Rad (Hercules, CA); vitamin B6 (as pyridoxal-5-phosphate) by an enzymatic method using tyrosine decarboxylase. Further details are described elsewhere.[24, 25] Blood and urinary creatinine was measured with urine 8-OHdG using spectrophotmetric assay. The method has been described elsewhere in details.[26]
A competitive enzyme-linked immunosorbent assay was used to determine urinary 8-OHdG using ELISA (Genox Corp, Baltimore, MD).[27, 28] In brief, 50 μl of urine samples, quality control (QC) and standards were added micro-titer plates pre-coated with 8-OHdG protein conjugate. After washing out thrice with 250 μl wash solution, an enzyme-labeled secondary antibody (100 μl) was added to plates for incubation of 1 hour at 37°C. After washing as the above, 100 μl of the chromatic substrate, (3,3’,5,5’)-tetramethylbenzidene, was added to the plates and then allowed to react for 15 min at room temperature. The intensity of color produced for each sample was measured at an optical density of 490 nm in standards, QC and urine samples. The concentration of 8-OHdG was calculated based on the color intensity. A pooled urine sample from several healthy adults was used as the quality control samples. For each standard 96 well microplate, 6-9 QC samples were randomly placed along with the unknown samples. The measured QC values were averaged and compared with the established QC value. For each unknown sample, either a duplicate or triplicate measurement was performed. The average, Std. Deviation, and CV (%) were calculated and any sample with CV (%) equal or greater than 20% was re-tested.
Air pollution and Weather Data
Continuous hourly PM2.5, PN, NO2, O3, CO, SO42-, BC, OC and EC were measured at a stationary monitoring site 1 mile from the examination. They were divided into two groups, the primary and secondary pollutants. The primary pollutants refer to those that were directly emitted into the atmosphere. The secondary pollutants refer to those produced after the primary pollutants react or interact with other components in the atmosphere. Some pollutants may be both primary and secondary: that is, they are both emitted directly and formed from other primary pollutants. In this study, CO, EC and BC are the primary pollutants. NO2, O3 and SO42- were categorized into the secondary pollutants. PM2.5 and OC are the mixtures of the primary and secondary pollutants, which we categorized into the secondary. PN is the number of particles, highly related to the secondary pollutant PM2.5. BC was measured using an aethalometer (Magee Scientific, Berkeley, CA), and PM2.5 was measured using a Tapered Element Oscillating Microbalance (model 1400A; Rupprecht & Pataschnick Co., East Greenbush, NY), operated at 50 degrees with two 4 liter per minute PM2.5 impactors before the inlet. CO was measured using a gas filter correlation carbon monoxide analyzer by comparing infrared energy absorption (Model 300E; Teledyne Advanced Pollution Instrumentation Inc. San Diego, CA). O3 was measured using a UV absorption ozone analyzer (Model 400E; Teledyne Advanced Pollution Instrumentation Inc. San Diego, CA). NO2 was measured using the proven chemiluminescence detection principle, coupled with state-of-the-art microprocessor technology to provide sensitivity and stability (Model 200E; Teledyne Advanced Pollution Instrumentation Inc. San Diego, CA). EC and OC were measured with a Rupprecht & Patashnick Ambient Carbon Particulate Monitor (Model 5400).[29] Sulfate was measured based on XRF determination of the Sulfur on the particle filters. Daily averages of their concentrations were used for this study during 2006-2008. Data for OC and EC were only available between January 2007 and December 2008. Gaseous air pollutant data were provided by the Massachusetts Department of Environmental Protection. The moving averages of daily PM2.5, NO2, SO42-, CO, BC, EC, OC and maximal 1-hour O3 up to 4 weeks before the visit were used as the exposure indices. To adjust for outdoor weather, we used apparent temperature as an index, defined as a person’s perceived air temperature, given the humidity.[30]
Statistical analyses
Statistical analyses were performed with R V2.7.2. We fitted linear regression models to separately examine the association of a single pollutant with urinary 8-OHdG using different moving averages of the exposure. We calculated the Pearson correlations between pollutants. Because we felt that longer averaging times were likely more appropriate for a marker of oxidative damage, we considered moving averages of exposure up to 28 days. Because primary analyses showed a skewed distribution of 8-OHdG, we used the log transformation of 8-OHdG concentrations to improve the normality of residuals and to stabilize the variance. We identified a priori the following variables as important determinants of 8-OHdG, based on our previous NAS studies and other studies because they might confound the associations between air pollution and 8-OHdG: age, body mass index (BMI), smoking status (never, former, current), pack-years of cigarettes smoked, alcohol consumption (≥ 2 drinks/day; yes/no), use of statin medication (yes/no), season, plasma folate, vitamin B6 and B12.[19, 31] We adjusted for age, BMI, pack-years of cigarettes smoked, plasma folate, vitamin B6 and B12 as continuous variables. We adjusted for smoking status, alcohol consumption, use of statin medication and season as categorical variables. Because of the potential nonlinear relationship between temperature and 8-OHdG, we also adjusted for 3-day moving average of apparent temperature using both linear and quadratic terms. In addition, because the concentration of 8-OHdG was related to kidney function, we adjusted for creatinine clearance rate using the Cockcroft-Gault formula ([140 - age(year)]* weight(kg)]/[72*serum creatinine(mg/dL)]).[32] We also adjusted for chronic disease status (cardiovascular disease or chronic respiratory diseases) as a dummy variable. To compare with day-moving average effects of pollution, we also examined the accumulative lag effects of each pollutant up to 4 weeks using unconstrained distributed lag methods.
Results
Table 1 and table 2 presents the study population characteristics and average concentrations of pollutants. The study population consisted of 320 men and 309 (97.5%) of them were non-Hispanic white. Their age ranged from 63 to 96 years old, with mean ± standard deviation (SD) of 76.7 ± 6.1 when they visited. On average, the 8-OHdG concentration was 20.8 ± 12.3 ng/ml, with the log-transformation 2.81 ± 0.78 log ng/ml. 68.8% of the participants ever smoked, 29.1% never smoked and only 2.2% still smoked when they visited. The means of daily concentrations of pollutants were shown in Table 2.
Table 1.
Descriptive statistics of the demographic, health variables of participants at visit (n = 320)
| Variable | Values * |
|---|---|
| Average 8-hydroxy-2’-Deoxyguanosine, ng/ml (log) | 2.81 (0.78) |
| Age, years | 76.7 (6.1) |
| Body mass index, kg/m2 | 28.0 (4.5) |
| Systolic blood pressure, mmHg | 124 (18) |
| Plasma folate, ng/mL | 21.6 (12.7) |
| Plasma pyridoxal-5-phosphate, nmol/L | 101 (105.) |
| Plasma vitamin B12, pg/mL | 590 (273) |
| Plasma creatinine, mg/dL | 1.19 (0.33) |
| Cumulative cigarette package years | 19.8 (23.4) |
| Alcohol intake (≥ 2/day), n (%) | 61 (19.4) |
| Use of statin, n (%) | 180 (56.6) |
| Smoking status, n (%) | |
| Never smoker | 93 (29.1) |
| Current smoker | 7 (2.2) |
| Former smoker | 220 (68.8) |
Values are mean ± SD when appropriate.
Table 2.
Daily averages of air pollution and temperature at visits (n = 320)
| Pollutants | Values (SD) |
|---|---|
| Carbon monoxide, ppm | 0.30 (0.13) |
| Black carbon, μg/m3 | 0.88 (0.50) |
| Elemental carbon, μg/m3 | 0.44 (0.24) |
| Maximal 1-hour ozone, ppb | 39.2 (15.9) |
| Sulfate, μg/m3 | 2.68 (2.14) |
| Nitrogen dioxide, ppb | 17.8 (5.0) |
| PM2.5, μg/m3 | 13.0 (8.5) |
| Organic carbon, μg/m3 | 3.43 (1.31) |
| Particle number (×1000) | 14.8 (6.4) |
| Apparent temperature, °C | 13.2 (9.8) |
The Pearson correlation coefficients between all pollutants on the day of the participants’ visit show that PM2.5 was highly correlated with SO42-, BC and OC, and also moderately correlated with O3 and EC (Table 3). O3 was only moderately or poorly correlated with most of other pollutants. EC and OC also tended to be highly correlated with the other pollutants.
Table 3.
Pearson Correlation Coefficients of air pollutants on the current days of participant visits
| OC | CO | BC | EC | O3-1 hr | SO42- | NO2 | PM2.5 | |
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| CO | 0.47 | |||||||
| <.001 | ||||||||
|
| ||||||||
| BC | 0.54 | 0.56 | ||||||
| <.001 | <.001 | |||||||
|
| ||||||||
| EC | 0.60 | 0.60 | 0.72 | |||||
| <.001 | <.001 | <.001 | ||||||
|
| ||||||||
| O3-1 hr | 0.35 | 0.07 | 0.07 | -0.13 | ||||
| <.001 | 0.190 | 0.197 | 0.054 | |||||
|
| ||||||||
| SO42- | 0.51 | 0.35 | 0.45 | 0.31 | 0.48 | |||
| <.001 | <.001 | <.001 | <.001 | <.001 | ||||
|
| ||||||||
| NO2 | 0.53 | 0.44 | 0.66 | 0.64 | -0.09 | 0.33 | ||
| <.001 | <.001 | <.001 | <.001 | 0.109 | <.001 | |||
|
| ||||||||
| PM2.5 | 0.67 | 0.22 | 0.58 | 0.48 | 0.49 | 0.75 | 0.31 | |
| <.001 | <.001 | <.001 | <.001 | <.001 | <.001 | <.001 | ||
|
| ||||||||
| PN | -0.12 | 0.12 | 0.10 | 0.19 | -0.44 | -0.18 | 0.41 | -0.2 |
| 0.083 | 0.032 | 0.071 | 0.006 | <0.001 | 0.002 | <0.001 | <0.001 | |
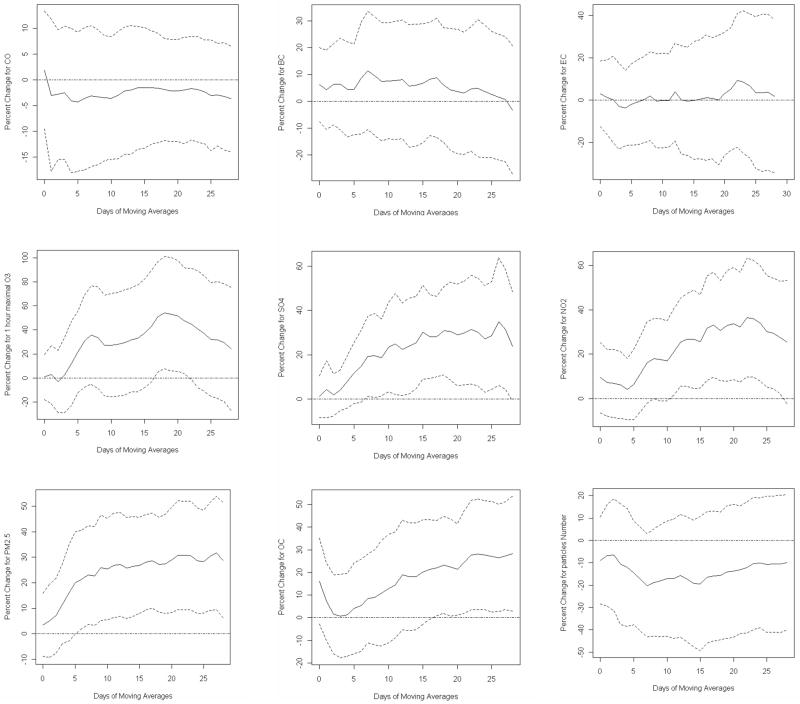
We fit models to separately estimate associations between a single pollutant and 8-OHdG using different day moving averages up to 4 weeks. Table 4 shows the percent changes in 8-OHdG per interquatile range (IQR, calculated separately for each averaging period) increase in each pollutant for the current day, 7-, 14- and 21-day moving averages. Results for 28-day averages were similar to, but usually less significant than the 21-day results, and are omitted to keep the table size manageable. They are shown in Figure 1. PM2.5, maximal 1-hour O3, SO42-, NO2 and OC were significantly associated with 8-OHdG at various moving averages, but there were no significant associations between 8-OHdG and CO, EC or BC. Across the IQR in 3-week moving averages of daily concentrations of PM2.5, PN, BC, EC, OC, CO, SO42-, NO2 and maximal 1-hour O3, urinary 8-OHdG increased by 30.8% (95% CI: 9.3%, 52.2%), -13.1% (95% CI: -41.7%. 15.5%), 3.0% (95% CI: -19.8%, 25.8%), 5.3% (95% CI: -23.6%, 34.2%), 24.4% (95% CI: 1.8%, 47.1%), -2.0% (95% CI: -12.4%, 8.3%), 29.8% (95% CI: 6.3%, 53.3%), 32.2% (95% CI: 7.4%, 56.9%) and 47.7% (95% CI: 3.6%, 91.7%), respectively. Overall, significant associations were found for more than 10-days moving averages of the secondary pollutants except for OC where significant effect only appeared after 16-day moving averages. There were no significant effects for the primary pollutants. 3-week moving averages showed stronger associations for the secondary pollutants in spite of the variation across pollutants (Figure 1).
Table 4.
Adjusted percent change (95% confidence interval) in 8-OHdG associated with per IQR increases of the moving averages of each pollutants on different period (days)
| Pollutant | Period (days) | IQR | P value | Percent changes per IQR |
|---|---|---|---|---|
| CO (ppm) | Current day | 0.145 | 0.746 | 1.9 (-9.6, 13.4) |
| 7 | 0.119 | 0.653 | -3.1 (-16.9, 10.6) | |
| 14 | 0.095 | 0.802 | -1.5 (-13.5, 10.4) | |
| 21 | 0.078 | 0.698 | -2.0 (-12.4, 8.3) | |
| BC (μg/m3) | Current day | 0.608 | 0.387 | 6.1 (-7.7, 20.0) |
| 7 | 0.355 | 0.310 | 11.4 (-10.6, 33.5) | |
| 14 | 0.292 | 0.605 | 6.0 (-16.7, 28.7) | |
| 21 | 0.271 | 0.795 | 3.0 (-19.8, 25.8) | |
| EC (μg/m3) | Current day | 0.284 | 0.707 | 3.0 (-12.5, 18.4) |
| 7 | 0.135 | 0.985 | 0.2 (-20.2, 20.6) | |
| 14 | 0.122 | 0.970 | -0.5 (-25.9, 24.9) | |
| 21 | 0.123 | 0.720 | 5.3 (-23.6, 34.2) | |
| Max 1-h O3 (ppb) | Current day | 19.563 | 0.927 | 0.9 (-17.7, 19.4) |
| 7 | 18.657 | 0.089 | 35.7 (-5.3, 76.7) | |
| 14 | 17.563 | 0.145 | 33.1 (-11.3, 77.4) | |
| 21 | 15.287 | 0.035 | 47.7 (3.6, 91.7) | |
| SO42- (μg/m3) | Current day | 1.682 | 0.826 | 1.1 (-8.4, 10.5) |
| 7 | 1.561 | 0.039 | 19.2 (1.1, 37.4) | |
| 14 | 1.351 | 0.017 | 25.5 (4.7, 46.2) | |
| 21 | 1.281 | 0.014 | 29.8 (6.3, 53.3) | |
| NO2 (ppb) | Current day | 6.702 | 0.249 | 9.4 (-6.5, 25.2) |
| 7 | 3.631 | 0.086 | 16.2 (-2.2, 34.5) | |
| 14 | 3.244 | 0.019 | 26.7 (4.6, 48.9) | |
| 21 | 3.268 | 0.012 | 32.2 (7.4, 56.9) | |
| PM2.5 (μg/m3) | Current day | 9.270 | 0.577 | 3.5 (-8.8, 15.8) |
| 7 | 7.521 | 0.020 | 23.0 (3.7, 42.3) | |
| 14 | 6.803 | 0.008 | 26.4 (6.9, 45.8) | |
| 21 | 6.885 | 0.005 | 30.8 (9.3, 52.2) | |
| OC (μg/m3) | Current day | 1.889 | 0.097 | 16.2 (-2.8, 35.2) |
| 7 | 1.036 | 0.400 | 8.4 (-11.1, 28) | |
| 14 | 1.029 | 0.134 | 18.2 (-5.5, 41.9) | |
| 21 | 0.884 | 0.037 | 24.4 (1.8, 47.1) | |
| Number of Particle (×1000) | Current day | 8.345 | 0.367 | -9.0 (-28.4, 10.5) |
| 7 | 6.402 | 0.088 | -20.2 (-43.2, 2.9) | |
| 14 | 6.840 | 0.186 | -19.3 (-47.8, 9.2) | |
| 21 | 6.653 | 0.370 | -13.1 (-41.7, 15.5) |
Fig 1.
Adjusted percent changes of urinary 8-hydroxy-2’-deoxyguanosine for ambient particulate matter ≤ 2.5 μm in aerodynamic diameter (PM2.5), black carbon (BC), elemental carbon (EC), organic carbon (OC), carbon monoxide (CO), nitrogen dioxide (NO2), sulfate (SO42-), maximal 1-hour ozone (O3) and particle number (PN) at various moving averages. Solid lines refer to estimates and dashed lines represents 95% confidence intervals
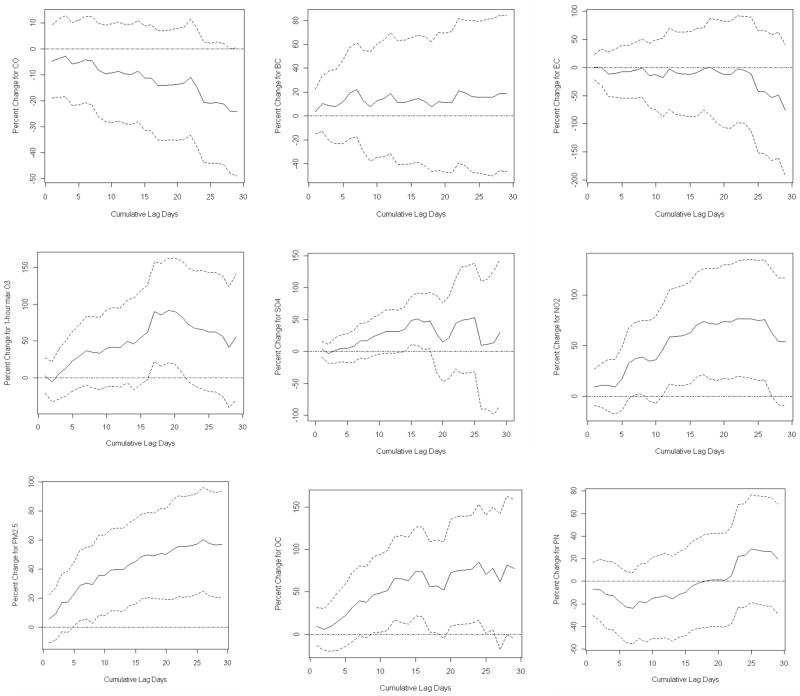
The trends of accumulative lag effects of PM2.5, OC, SO42- and maximal 1-hour O3 showed significant associations at different days (Figure 2). Accumulative lag effects of PM2.5 and OC slowly escalated with fluctuation within the exploration period. The accumulative effect of SO42- increased and fluctuated. The trends of NO2 and 1-hour maximal O3 escalated and then declined. There was a declining trend for CO, but there was no significant accumulative effect. There were no accumulative effects for BC or EC. These findings are consistent with effects of moving averages of pollutants.
Fig 2.
Adjusted percent changes of urinary 8-hyroxy-2’-deoxyguanosine for ambient particulate matter ≤ 2.5 μm in aerodynamic diameter (PM2.5), Black carbon (BC), sulfate (SO42-), nitrogen dioxide (NO2), element carbon (EC), organic carbon (OC), maximal 1-hour ozone (O3), and at cumulative lag effects on different days. Solid lines refer to estimates and dashed lines represents 95% confidence intervals.
As a sensitivity analysis, we also adjusted for different day moving averages of apparent temperature up to 3 weeks including its linear and quadratic terms as above. Overall, the estimates were slightly attenuated when using only the current day of apparent temperature, but were basically unchanged using the two or more-day moving averages. In addition, after adjusting for urinary creatinine, results were a little varied but the trend was the same.
Discussion
Results show that exposure to the secondary pollutants or mixtures of the primary and secondary pollutants (PM2.5, NO2, OC, SO42- and maximal 1-hr O3) was significantly associated with oxidative stress biomarker urinary 8-OHdG for more than 10 day moving averages, but no significant associations were observed for the primary pollutants (CO, BC and EC) in the Normative Aging Study population (Table 4 and Figure 1, 2). Significant associations between the secondary pollutants and 8-OHdG were found for both moving-day averages and accumulative lag effects of these pollutants. Overall, the 21-day moving averages of the secondary pollutants showed the strongest effects.
The use of moving averages of air pollution provides statistical stability in estimating longer term effects, but by constraining the estimates to effectively be the same for each lag in the average, may miss part of the pattern. Unconstrained distributed lag models, in contrast, allow the pattern of association to vary by lag, but because of the multicollinearity of the different lags, are quite unstable. The sum of the effects over all lags is more stable, and provides a reasonable check on the moving average estimates to make sure they are not biased by the use of simple moving averages.[33] This study used both approach to estimate associations between an array of air pollutants and 8-OHdG and we found consistent estimates for both methods (Figure 1 and 2). As expected, there were more fluctuations for accumulative distributed lag effects, as compared to separately modeled lagged daily averages. We estimated the effect of each pollutant up to 4 weeks (daily-moving average or accumulative lag effect) because we think this period would be long enough for acute oxidative or inflammatory responses of human beings to exposures to pollutants, and we stopped at that point because the effect sizes were falling, and longer averages begin to conflict with control for season.
ROS in living cells are continuously generated as the consequence of metabolic reactions. For example, mitochondria (oxidative phosphorylation), leukocytes (oxidative burst), peroxisomes (degradation of fatty acids) and the cytochrome p450 system (mixed function oxidation system) all release ROS. Under normal physiological status, generation and deletion of ROS is in a balance status, known as homeostasis. If the generation of endogenous or exogenous oxidants increase or antioxidants decreases, the global oxidative stress occurs.[34] Inhaled pollutants containing oxidants or oxidant-related components can cause physiological oxidative stress. Oxidative stress can cause impaired physiological functions, resulting in DNA damage, aging, diseases.[35, 36] Oxidative stress can cause the oxidization of 2-deoxyguanosine in DNA into 8-OHdG. Damaged DNA is repaired by DNA excision repair system and 8-OHdG is released into the circulation system.[1, 31] Urinary 8-OHdG can be used as a biomarker of oxidative stress because it is not influenced directly by diet or cell turnover. Its measure is noninvasive.[5, 37]
In urban areas, primary pollutants such as NO, CO, BC or EC are mainly produced by gasoline or diesel fuel combustion. NO and CO are not oxidants. The ability of BC or EC to produce reactive oxygen species is not well known. In contrast, secondary pollutants are formed mostly by atmospheric photochemical processes. Photochemical reactions could result in the formation of strong oxidative molecular, such as ozone and hydrogen peroxide as well as organic radicals and bi-radicals. Therefore, it is anticipated that when concentrations of important secondary pollutants such ozone, sulfate and nitrate, among others are high concentrations of many gaseous and particulate oxidant species are elevated.[38, 39] For example, NO2 is mainly produced from NO via oxidative atmospheric chemical reactions. Ozone is produced by complicated photochemical procedures via chemical reactions of oxygen with nitrogen oxide, organic peroxy radicals, hydrocarbon, etc under sunlight. It is a strong ROS.[38, 39] PM2.5 is a mixture of fine aerosol particles, of which sulfate and organic carbon account for the largest fraction in Eastern Massachusetts. Heavy metals, especially Fe and Cd, are also important components of PM2.5, and they are strong ROS.[38-40]
A limited number of studies have reported that exposures to ambient and indoor pollution or smoking are associated with 8-OHdG.[1, 20, 41] Chuang et al. investigated associations of pollutants with oxidative stress among 76 students, aged 18-25 years, and found that the increases in blood 8-OHdG were significantly associated with the averages of SO42-, O3 and NOx on various days (1-3 day).[21] Kim et al. reported that urinary 8-OHdG was significantly associated with fine particle exposure among 20 boilermakers working at a power plant during an overhaul of oil-fired boilers.[31] Calderón-Garcidueñas et al. found that primary school students exposed to higher air pollution had 2-3 fold of 8-OHdG in the nasal respiratory epithelium compared to those who exposed to lower air pollution.[1] Similar associations have also been found in other populations who were exposed to other pollution sources, such as cigarette use, office employees and restaurant workers.[22, 41, 42] Our study found evidence that 8-OHdG was significantly associated with exposures over 10-day averages of secondary pollutants, but not primary pollutants among 320 aging participants.
Some studies from other fields also support for these results. For example, using a florescent marker of reactive oxygen species, Gurgueira and coworkers reported that exposure of animals to concentrated air particles induced oxidative stress, and conversely moving them from room air to filtered air reduced reactive oxygen species in multiple organs, in vivo.[43] Air pollution exposure has also been associated with thiobarbituric acid reactive substances, a ROS.[44] Sørensen et al. reported that personal exposure to PM2.5 was associated with 7-hydro-8oxy-2′-deoxyguanosine, a DNA damage biomarker.[45] On the other hand, unlike other studies, e.g. Delfino et al.,[46] we did not find associations with the primary pollutants, but rather with the secondary pollutants. Whether this is due to population differences, pollution differences between southern California and Boston, averaging time, the more precise exposure measures used in Delfino et al.[46] or chance remains to be determined.
One limitation is that we used air pollution concentrations from a single monitoring site as surrogates for recent personal pollution exposure, which may lead to exposure misclassification. Although we were not able to quantify the impact of exposure error, on our results, previous studies suggest that exposure error will be greater for primary pollutants such as BC, as compared to secondary pollutants such as PM2.5 and ozone.[47] This differential exposure error likely results from spatial variability in outdoor concentrations, which have been shown to be greater for primary pollutants. For example, outdoor spatial variability in concentrations of the secondary pollutants ozone, sulfate, and PM2.5 is limited within urban areas.[48, 49] In contrast, outdoor CO and BC concentrations have been shown to vary by as much as a factor of three.[50, 51] This may overstate the relative differences in the impacts of measurement error on the effect estimates, however. To see this, consider the following. If Xijt is the exposure of the ith subject, in the jth neighborhood, at time t, then we have the following identity:[52]
where Ut is the measurement at the central monitor, and X̄jt is the average exposure in neighborhood j, on day t.
While traffic pollutants have much more spatial variation, that occurs on a very fine scale, with noticeable changes between an address on a busy street and one around the corner on a side street. That is, much of the spatial variation in traffic pollution will be seen within neighborhoods, between subjects. This is the third term of the equation above. And it is Berkson error, which does not bias downward the regression coefficient. Secondary pollutants vary much more slowly spatially. Therefore a larger fraction of their spatial measurement error is captured in the second term. Hence, while spatial variation overall is larger for traffic pollutants, much of that is on a fine enough scale to be Berkson, and not downwardly bias effect estimates. The second term, which includes classical error, does produce bias, but the relative difference in spatial measurement error on the neighborhood scale between traffic and non-traffic pollutants is much lower than the overall difference, and hence we believe focusing on the overall spatial variability overstates the potential for greater bias in the coefficients from traffic pollution. Nevertheless, greater downward bias is still likely for traffic pollutants. Despite this greater measurement error, most of the previous reports from this cohort have found a stronger association with primary pollutants. For example in Mordukhovitch et al.,[53] we reported an association between BC and blood pressure, and did not observe an association with the secondary pollutants. In Madrigano et al.,[54] we reported BC was associated with increases in levels of vascular cellular adhesion molecules. While there are substantial spatial gradients in primary pollutants, the analyses in this study was based on temporary variation or day-to-day fluctuations in pollution concentrations in Boston, which were primarily driven by meteorology. Hence, while our analysis has missed the additional gradient concentrations of primary particles that occurs over space, it captures the temporal gradient.
Conclusion
This study found that exposure to secondary pollutants was significantly associated with 8-OHdG (PM2.5, NO2, OC, SO42- and maximal 1-hr O3), but not for primary pollutants (CO, EC and BC) in the Normative Aging Study population. These effects were more apparent with multi-week averages of exposures.
What this paper adds?
Particulate air pollution is associated with health outcomes but the mechanism remains to be clarified.
One of suggested mechanistic pathways is via oxidative stress. However, a limited number of studies have directly examined associations between air pollution and oxidative DNA damage.
Results show that 8-OhdG, a biomarker of DNA damage, was significantly associated with the secondary pollutants, but not with the primary pollutants among a aging population.
This delivers an important message that exposure to secondary pollution may further increase risk to chronic human diseases such as ageing, cancer and other degenerative diseases.
Acknowledgments
Grant support: This work was supported by the National Institute of Environmental Health Sciences grants ES014663, ES 15172, and ES-00002, by U.S. Environmental Protection Agency grant R832416 and USDA Contract 58-1950-7-707. The Normative Aging Study is supported by the Cooperative Studies Program/Epidemiology Research and Information Center of the U.S. Department of Veterans Affairs, and is a component of the Massachusetts Veterans Epidemiology Research and Information Center. It is partially supported by Harvard-NIOSH ERC Pilot (T42 OH008416).
List of Abbreviation
- NAS
Normative Aging Study
- 8-OHdG
8-hydroxy-2’-deoxyguanosine
- PM2.5
particulate matter ≤ 2.5 μm in aerodynamic diameter
- NO2
nitrogen dioxide
- O3
ozone
- SO42-
sulfate
- OC
organic carbon
- BC
black carbon
- CO
carbon monoxide
- EC
elemental carbon
- PN
the number of particles
Footnotes
Competing Interest: None declared.
References
- 1.Calderón-Garcidueñas L, Wen-Wang L, Zhang Y, Rodriguez-Alcaraz A, Osnaya N, Villarreal-Calderón, Santilla R. 8-hydroxy-2’-deoxyguanosine, a major mutagenic oxidative DNA lesion, and DNA strand breaks in nasal respiratory epithelium of children exposed to urban pollution. Envion Health Perspect. 1999;107:469–74. doi: 10.1289/ehp.107-1566580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Higashi Y, Noma K, Yoshizumi M, et al. Endothelial function and oxidative stress in cardiovascular diseases. Circ J. 2009;73:411–8. doi: 10.1253/circj.cj-08-1102. [DOI] [PubMed] [Google Scholar]
- 3.Koh KK, OH PC, Quon MJ. Does reversal of oxidative stress and inflammation provide vascular protection? Cardiovasc Res. 2009;81:649–59. doi: 10.1093/cvr/cvn354. [DOI] [PubMed] [Google Scholar]
- 4.Roberts CK, Sindhu KK. Oxidative stress and metabolic syndrome. Life Sci. 2009;84:705–12. doi: 10.1016/j.lfs.2009.02.026. [DOI] [PubMed] [Google Scholar]
- 5.Wiseman H, Halliwell B. Damage to DNA by reactive oxygen and nitrogen species: role in inflammatory disease and progression to cancer. Biochem J. 1996;313:17–29. doi: 10.1042/bj3130017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cerutti PA. Oxy-radicals and cancer. Lancet. 1994;344:862–3. doi: 10.1016/s0140-6736(94)92832-0. [DOI] [PubMed] [Google Scholar]
- 7.Burdon RH. Superoxide and hydrogen peroxide in relation to mammalian cell proliferation. Free Radic Biol Med. 1995;18:775–94. doi: 10.1016/0891-5849(94)00198-s. [DOI] [PubMed] [Google Scholar]
- 8.Kasai H, Crain PF, Kuchino Y, et al. Formation of 8-hydroxygunine moiety in cellular DNA by agents producing oxygen radicals and evidence for its repair. Carcinogenesis. 1986;7:1849–51. doi: 10.1093/carcin/7.11.1849. [DOI] [PubMed] [Google Scholar]
- 9.Cooke MS, Evans MD, Dove R, et al. DNA repair is responsible for the presence of oxidative damaged DNA lesions in urine. Mutat Res. 2005;574:58–66. doi: 10.1016/j.mrfmmm.2005.01.022. [DOI] [PubMed] [Google Scholar]
- 10.Ren C, Williams GM, Mengersen K, et al. Does temperature modify short-term effects of ozone on total mortality in 60 large eastern US Communities? – an assessment using NMMAPS data. Environ Int. 2008;34:451–8. doi: 10.1016/j.envint.2007.10.001. [DOI] [PubMed] [Google Scholar]
- 11.Ren C, Williams GM, Morawska L, et al. Ozone modifies associations between temperature and cardiovascular mortality: analysis the NMMAPS data. Occup Environ Med. 2008;65:255–60. doi: 10.1136/oem.2007.033878. [DOI] [PubMed] [Google Scholar]
- 12.Schwartz J. The effects of particulate air pollution on daily deaths: a multi-city case-crossover analysis. Occup Environ Med. 2004;61:956–61. doi: 10.1136/oem.2003.008250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zanobetti A, Schwartz J. Particulate air pollution, progression, survival after myocardial infarction. Environ Health Perspect. 2007;115:769–75. doi: 10.1289/ehp.9201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dominici F, Peng RD, Bell ML, et al. Fine particulate air pollution and hospital admission for cardiovascular and respiratory diseases. JAMA. 2006;295:1127–34. doi: 10.1001/jama.295.10.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bell ML, McDermott A, Zeger SL, et al. Ozone and short-term mortality in 95 US urban communities, 1987-2000. JAMA. 2004;292:2372–8. doi: 10.1001/jama.292.19.2372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brook RD. Cardiovascular effects of air pollution. Clin Sci. 2008;115:175–87. doi: 10.1042/CS20070444. [DOI] [PubMed] [Google Scholar]
- 17.Park SK, O’Neill MS, Wright RO, et al. HFE genotype, particulate air pollution, and heart rate variability – a gene-environment interaction. Circulation. 2006;114:2798–805. doi: 10.1161/CIRCULATIONAHA.106.643197. [DOI] [PubMed] [Google Scholar]
- 18.González-Flecha B. Oxidative mechanisms in response to ambient air particles. Mol Aspects Med. 2004;25:169–82. doi: 10.1016/j.mam.2004.02.017. [DOI] [PubMed] [Google Scholar]
- 19.Schwartz J, Park SK, O’Neill MS, et al. Glutathione-S-Transferase M1, obesity, statins and automonic effects of particles. Am J Respir Crit Care Med. 2005;172:1529–33. doi: 10.1164/rccm.200412-1698OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gurgueira SA, Lawrence J, Coull B, et al. Rapid increases in the steady-state concentration of reactive oxygen species in the lungs and heart after particulate air pollution inhalation. Environ Health Perspect. 2002;110:749–55. doi: 10.1289/ehp.02110749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chuang KJ, Chang CC, Su TC, et al. The effect of urban air pollution on inflammation, oxidative stress, coagulation, and autonomic dysfunction in young adults. Am J Respir Crit Care Med. 2007;176:370–6. doi: 10.1164/rccm.200611-1627OC. [DOI] [PubMed] [Google Scholar]
- 22.Lu CY, Ma YC, Lin JM, et al. Oxidative DNA damage estimated by urinary 8-hydroxydeoxyguanosine and indoor air pollution among non-smoking office employees. Environ Res. 2007;103:331–7. doi: 10.1016/j.envres.2006.08.009. [DOI] [PubMed] [Google Scholar]
- 23.Bell B, Rose C, Damon A. The veterans Administration longitudinal study of healthly aging. Gerontologist. 1996;6:179–84. doi: 10.1093/geront/6.4.179. [DOI] [PubMed] [Google Scholar]
- 24.Park SK, O’Neill MS, Vokonas PS, et al. Traffic-related particles are associated with elevated homocysteine – the VA Normative Aging Study. Am J Respir Crit Care Med. 2008;178:283–9. doi: 10.1164/rccm.200708-1286OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tucker KL, Qiao N, Scott T, et al. High homocysteine and low B vitamins predict cognitive decline in aging men: the Veterans Affairs Normative Aging Study. Am J Clin Nutr. 2005;82:627–35. doi: 10.1093/ajcn.82.3.627. [DOI] [PubMed] [Google Scholar]
- 26.Bowers L, Wong E. Kinetic serum creatinine assays. II. A critcal evaluation and review. Clin Chem. 1980;26:555. [PubMed] [Google Scholar]
- 27.Erhola M, Toyokuni S, Okada K, et al. Biomarker evidence of DNA oxidation in lung cancer patients: association of urinary 8-hydroxy-2’-deoxyguanosine excretion with radiotherapy, chemotherapy, and response to treatment. FEBS Lett. 1997;409:287–91. doi: 10.1016/s0014-5793(97)00523-1. [DOI] [PubMed] [Google Scholar]
- 28.Leinonen J, Lehtimaki T, Toyokuni S, et al. New biomarker evidence of oxidative DNA damage in patients with non-insulin-dependent diabetes mellitus. FEBS Lett. 1997;417:150–2. doi: 10.1016/s0014-5793(97)01273-8. [DOI] [PubMed] [Google Scholar]
- 29.Watson JG, Chow JC. Atmospheric Scinces Data Center at NASA Langley Research Center; Hampton, Virginia, U.S.A: 2003. NARSTO EPA_SS_FRESNO PM2.5 Organic and Elemental Carbon Data. Available: http://eosweb.larc.nasa.gov/PRODOCS/narsto/table_narsto.html at the. [Google Scholar]
- 30.Kalkstein L, Valamont K. An evaluation of summer discomfort in the United States using a relative climatologic index. Bull Am Meteorol Soc. 1986;67:842–8. [Google Scholar]
- 31.Kim JY, Mukherjee S, Ngo L, et al. Urinary 8-hydroxy-2’-deoxyguanosine as a biomarker of oxidative DNA damage in workers exposure to fine particles. Environ Health Perspect. 2004;112:666–71. doi: 10.1289/ehp.6827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cockcroft DW, Gault MH. Prediction of creatinine clearance from serum creatinine. Nephron. 1976;16:31–41. doi: 10.1159/000180580. [DOI] [PubMed] [Google Scholar]
- 33.Schwartz J. The distributed lag between air pollution and daily deaths. Epidemiology. 2000;11:320–26. doi: 10.1097/00001648-200005000-00016. [DOI] [PubMed] [Google Scholar]
- 34.Halliwell B, Gutteridge JMC. Free radicals in biology and medicine. 3. Oxford: Oxford Univ. Press; 1999. [Google Scholar]
- 35.Finkel T, Holbrook NJ. Oxidants, oxidative stress and biological ageing. Nature. 2000;408:239–47. doi: 10.1038/35041687. [DOI] [PubMed] [Google Scholar]
- 36.Loft S, Vistisen K, Ewertz M, et al. Oxidative DNA damage estimated by 8-hyroxydeoxyguanosine excretion in humans: influence of smoking, gender and body mass index. Carcinogenesis. 1992;13:2241–7. doi: 10.1093/carcin/13.12.2241. [DOI] [PubMed] [Google Scholar]
- 37.Wu LL, Chiou CC, Chang PY, et al. Urinary 8-OHdG: a mark of oxidative stress to DNA and a risk factor for cancer, atherosclerosis and diabetics. Clin Chim Acta. 2004;339:1–9. doi: 10.1016/j.cccn.2003.09.010. [DOI] [PubMed] [Google Scholar]
- 38.Derwent RG. Atmospheric chemistry. In: Holgate ST, Samet JM, Koren HS, Maynard RL, editors. In air pollution and health. Academic Press; Boston: 1999. pp. 51–62. [Google Scholar]
- 39.Seinfeld HJ, Pandis SN. Atmospheric chemistry and physics: from air pollution to climate change. Wiley; New York: 1998. [Google Scholar]
- 40.Johri N, Jacquillet G, Unwin R. Heavy metal poisoning: the effects of cadmium on the kidney. Biometals. 2010 doi: 10.1007/s10534-010-9328-y. Online: March 31, 2010. [DOI] [PubMed] [Google Scholar]
- 41.Pan CH, Chan CC, Wu KY. Effects on Chinese restaurant workers of exposure to cooking oil fumes: a cautionary note on urinary 8-hydroxy-2’-deoxyguanosine. Cancer Epidemiol Biomarkers Prev. 2008;17:3351–7. doi: 10.1158/1055-9965.EPI-08-0075. [DOI] [PubMed] [Google Scholar]
- 42.Xi ZG, Chao FH, Yang DF, et al. 8-hydroxydeoxyguanosine as a biomarker of oxidative DNA damage induced by environmental tobacco side-stream smoke and its mechanism. Biomed Environ Sci. 2005;18:43–7. [PubMed] [Google Scholar]
- 43.Evelson P, González-Flecha B. Time course and quantitative analysis of the adaptive response to 85% oxygen in the rat lung and heart. Bichim Biophys Acta. 2000;1532:209–16. doi: 10.1016/s0304-4165(00)00124-0. [DOI] [PubMed] [Google Scholar]
- 44.Liu L, Poon R, Chen L, et al. Acute effects of air pollution on pulmonary function, airway inflammation, and oxidative stress in asthmatic children. Environ Health Perspect. 2009;117:668–74. doi: 10.1289/ehp11813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sørensen M, Autrup H, Hertel O, et al. Personal exposure to PM2.5 and Biomarkers of DNA damage. Cancer Epidemiol Biomarkers Prev. 2003;12:191–96. [PubMed] [Google Scholar]
- 46.Delfino RJ, Staimer N, Tjoa T, et al. Circulating biomarkers of inflammation, antioxidant activity, and platelet activation are associated with primary combustion aerosols in subjects with coronary artery disease. Environ Health Perspect. 2008;116:898–906. doi: 10.1289/ehp.11189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Suh HH, Zanobetti A. Exposure error masks the relationship between traffic-related air pollution and heart rate variability. J Occup Environ Med. 2010;52:685–92. doi: 10.1097/JOM.0b013e3181e8071f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Suh HH, Koutrakis P, Allen GA, et al. Spatial variation in outdoor acidic sulfate and ammonia concentrations within metropolitan Philadelphia. J Air Waste Manage Assoc. 1995;45:442–52. [Google Scholar]
- 49.Suh HH, Nishioka Y, Koutrakis P, et al. The metropolitan acid aerosol characterization study: results from Washington, DC. Environ Health Perspect. 1997;105:826–34. doi: 10.1289/ehp.97105826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gryparis A, Coull BA, Schwartz J, et al. Semiparametric regression models for spatio-temporal modeling of mobile source particles in the greater Boston area. Appl Stat. 2007;56(Part 2):183–209. [Google Scholar]
- 51.Brown KW, Sarnat JA, Suh HH, et al. Ambient site, home outdoor and home indoor particulate concentrations as proxies of personal exposures. J Environ Monit. 2008;10:1041–51. doi: 10.1039/b805991h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zeger SL, Thomas D, Dominici F, et al. Exposure measurement error in time-series studies of air pollution: Concepts and Consequences. Environ Health Perspect. 2000;108:419–26. doi: 10.1289/ehp.00108419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mordukhovich I, Wilker E, Suh H, et al. Black carbon exposure, oxidative stress genes, and blood pressure in a repeated-measures study. Environ Health Perspect. 2009;117:1767–72. doi: 10.1289/ehp.0900591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Madrigano J, Baccarelli A, Wright RO, et al. Air pollution, obesity, genes and cellular adhesion molecules. Occup Environ Med. 2010;67:312–7. doi: 10.1136/oem.2009.046193. [DOI] [PMC free article] [PubMed] [Google Scholar]