SUMMARY
Postnatal and adult human and monkey fibroblasts were infected with Sendai virus containing the Yamanaka factors for 24 hr, then they were cultured in a chemically defined medium containing leukemia inhibitory factor (LIF), transforming growth factor (TGF)-β inhibitor SB431542, and glycogen synthase kinase (GSK)-3β inhibitor CHIR99021 at 39°C for inactivation of the virus. Induced neural progenitor (iNP) colonies appeared as early as day 13 and can be expanded for >20 passages. Under the same defined condition, no induced pluripotent stem cell (iPSC) colonies formed at either 37°Cor 39°C. The iNPs predominantly express hindbrain genes and differentiate into hindbrain neurons, and when caudalized, they produced an enriched population of spinal motor neurons. Following transplantation into the forebrain, the iNP-derived cells retained the hindbrain identity. The ability to generate defined, integration-free iNPs from adult primate fibroblasts under a defined condition with predictable fate choices will facilitate disease modeling and therapeutic development.
INTRODUCTION
Induction of pluripotent stem cells (iPSCs) from differentiated somatic cells by defined transcription factors represents a major breakthrough in cellular reprogramming (Takahashi and Yamanaka, 2006; Takahashi et al., 2007; Yu et al., 2007). Extension of this concept has led to transdifferentiation from one differentiated cell type into another through overexpression of lineagerelated transcription factors (Caiazzo et al., 2011; Efe et al., 2011; Huang et al., 2011; Ieda et al., 2010; Pang et al., 2011; Pfisterer et al., 2011; Vierbuchen et al., 2010; Yoo et al., 2011). Although those reprogrammed somatic cell populations offer useful tools for understanding disease processes and discovering therapeutics, they usually have little or no proliferation potential, limiting their utilities. Direct conversion of somatic cells to lineage-committed stem/progenitor cells, such as neural stem/progenitor cells, would allow production of sufficient cells for downstream research or application and overcome the potential risk for tumor formation by iPSCs.
Recently, neural stem/progenitor cells have been generated from mouse fibroblasts by overexpressing Yamanaka factors or neural transcription factors (Kim et al., 2011; Han et al., 2012; Lujan et al., 2012; Thier et al., 2012). Similar neural stem cells were also generated from human fibroblasts by retroviralmediated gene transduction (Kumar et al., 2012; Ring et al., 2012). All these induced neural stem/progenitor (iNP) cells are generated by the use of integrating lentiviruses or retroviruses, which could disrupt endogenous gene expression and are associated with the risk for tumor formation due to potential spontaneous reactivation of the viral transgenes (Okita et al., 2007). Furthermore, the regional and functional identity of the iNPs has not been carefully examined, limiting their utility. For application in humans, it will be ideal and more practical to induce patients’ somatic cells instead of fetal tissue into iNPs in an integration-free manner.
In the present study, we generated iNPs from young and old human and monkey fibroblasts using nonintegrating Sendai (RNA) virus containing OCT4, SOX2, KLF4, and MYC (refer to as OSKM). Infection with the Sendai virus (SeV) for 24 hr followed by culturing in a chemically defined medium (CDM) containing leukemia inhibitory factor (LIF), transforming growth factor (TGF)-β inhibitor SB431542, and glycogen synthase kinase (GSK)-3β inhibitor CHIR99021, and inactivation of the virus at 39°C resulted in generation of neuroepithelial colonies as early as day 13, but not iPSCs up to day 30. These integration-free iNPs exhibit characteristic morphology, gene expression patterns, growth rate, as well as predictable in vitro and in vivo differentiation potentials. Importantly, the stable expandable iNP lines carry a hindbrain identity and can differentiate into hindbrain neurons and, when caudalized, an enriched population of spinal cord motor neurons.
RESULTS
Human iNPs Are Generated from Postnatal and Adult Fibroblasts by a Nonintegration Method
To generate human iNPs from nonfetal tissues with nonintegrating methods, we infected fibroblasts derived from three individuals at different ages (cell line GM02504 from a 1-month-old infant, cell line GM03815 from an adult, and cell line ND32947 from a 64-year-old adult; Table S1) with SeV containing four reprogramming factors (OSKM) or GFP (as a control). Twenty-four hours later, the cultures were incubated at 39°C, a condition that facilitates the removal of SeV (TS15) (Ban et al., 2011). At the same time, the medium was changed to a CDM supplemented with recombinant human LIF, SB431542 (TGF-β inhibitor), and CHIR99021 (GSK3β inhibitor) (refer to as LSC), a condition that promotes long-term self-renewal of neural precursors (Li et al., 2011) (Figure 1A). From day 3, the fibroblasts retracted their processes and became square or round in morphology with a large clear nucleus and two to three nucleoli in the OSKM group, but not in the GFP group (Figure 1B, Day 3). On day 7, cell death became obvious, and the surviving cells were reseeded as single cells or small clusters on laminin-coated plates to form colonies. On day 12, GFP disappeared, indicating loss of viral genes (Figure S1). We therefore switched the culture temperature back to 37°C on day 13. At this stage, colonies with columnar epithelial morphology were observed in the OSKM group treated with LSC (Figure 1B, Day 13 OSKM+LSC), but not in either the OSKM group not treated with LSC (Figure 1B, Day 13 OSKM) or the GFP group treated with LSC (Figure 1B, Day 13 GFP+LSC). These colonies grew rapidly in the following week, and by day 20, they were large enough to be selected manually (Figure 1C). The selected neuroepithelial colonies, confirmed by expression of neural stem cell marker SOX1 (Figure 1D), were mechanically triturated into small cell clusters and plated onto poly-L-ornithine (PO) and laminin-coated coverslips for immunostaining or onto laminin-coated 96-well-plates for cell expansion. We obtained 25 and 20 SOX1-expressing colonies from the fibroblast line GM02504 (two independent experiments), 22 and 21 SOX1-expressing colonies from the fibroblast line GM03815 (two independent experiments), and 10 SOX1-expressing colonies from fibroblast line ND32947, respectively (Table S1). Hence, the efficiency of human iNP generation is around 0.03%–0.08% as determined from the initial number of fibroblasts used in the reprogramming experiments. Q-PCR analysis indicated that SeV genomes were absent in the human iNP lines (Figure 1E). Dropout experiments for the four transgenes OSKM showed that no iNP colony could be observed if any one of the four transgenes was removed from the system (data not shown), indicating that none of them is negligible in this system. Dropout experiments for the three small molecules LSC showed that omission of LIF did not affect the efficiency of iNP generation but decreased the long-term expansion capacity (Table S2). Thus, nonintegrating iNPs can be readily generated from fibroblasts of human individuals with different ages under the chemically defined conditions.
Figure 1. Generation of iNPs from Human Fibroblasts.
(A) Schematic representation of iNP generation process.
(B) Morphological changes during human iNP generation are shown. Fibroblasts were infected with SeV containing OSKM or GFP in the absence (upper row) or presence (middle and lower rows) of LSC.
(C) A neural epithelium-like colony was observed in the OSKM+LSC group.
(D) Cells from selected colonies were SOX1+ (green), but NANOG− (red). Hoechst staining is blue.
(E) Q-PCR of existing SeV genomes in noninfected parent fibroblasts (SeV−, negative control), nine human iNP cell lines, and SeV-infected parent fibroblasts (SeV+, positive control). Data are represented as mean ± SEM.
(F) Q-PCR of human ESC markers (OCT4, NANOG, and REX1) during the re-programming process (from the reprogramming experiments of fibroblast cell line GM03815; data from other fibroblast cell lines follow the similar trends, not shown). H9, human ESC line; Days 0–13, days after SeV infection. Data are represented as mean ± SEM.
(G) Q-PCR of neural markers (SOX1 and N-Cadherin) during the reprogramming process (from the reprogramming experiments of fibroblast cell line GM03815; data from other fibroblast cell lines follow the similar trends, not shown). Data are represented as mean ± SEM.
Scale bars,50 µm (B and D). See also Figure S1 and Tables S1, S2, S5, and S6.
Given that OSKM have been used to generate iPSCs, we asked if iPSCs may be generated under this condition. No iPSC colonies were observed at day 20 when the infected cells were cultured in CDM (with LSC) on laminin-coated plates either at 37°C or 39°C. When the cultures were replaced with the conditioned medium (CM) from γ-radiated mouse embryonic fibroblasts (MEFs) cultured in human embryonic stem cell (ESC) culture medium (ESCM) and with 4 ng/ml FGF2, a condition for generating iPSCs (Takahashi et al., 2007), we observed two to four iPSC colonies when the cultures were maintained at 37°C throughout, but not at 39°C from days 1 to 12 (Table 1). Q-PCR analysis indicated that neural markers N-Cadherin and SOX1 were upregulated around the time of the first appearance of iNP colonies, whereas the expression of pluripotent genes NANOG and REX1 was not altered throughout the process with a brief increased expression of OCT4 (Figures 1F and 1G). These results indicate that inactivation of SeV from day 1 to 12 and lack of MEF CM and FGF prevent the formation of iPSC colonies.
Table 1.
Generation of iPSC and iNP Colonies under Different Culture Conditions
| Culture Conditions |
Number of iPSC Colonies (Day 20/30) |
Number of iNP Colonies (Day 20) |
||||||
|---|---|---|---|---|---|---|---|---|
| Temperature |
Medium |
|||||||
| (Days 1–12) | (Days 1–7) | (Day 8) | GM02504 | GM03815 | R47 | GM02504 | GM03815 | R47 |
| 37°C | CM | CM | 0/2 | 0/4 | 0/0 | 0 | 0 | 0 |
| CDM | 0/0 | 0/0 | 0/0 | 0 | 0 | 0 | ||
| CDM | CM | 0/0 | 0/0 | 0/0 | 0 | 0 | 0 | |
| CDM | 0/0 | 0/0 | 0/0 | 30 | 19 | 78 | ||
| 39° C | CM | CM | 0/0 | 0/0 | 0/0 | 0 | 0 | 0 |
| CDM | 0/0 | 0/0 | 0/0 | 0 | 0 | 0 | ||
| CDM | CM | 0/0 | 0/0 | 0/0 | 0 | 0 | 0 | |
| CDM | 0/0 | 0/0 | 0/0 | 25 | 22 | 57 | ||
| 37°C | FM | CM+MEF | 89/NA | 157/NA | 0/0 | 0 | 0 | 0 |
A total of 30,000 fibroblast cells were infected with SeV (OSKM) and cultured with different media (CM, CDM, or FM) for 1 week, then cells were reseeded onto laminin-coated or MEF-coated plates and cultured with different media (CM, CM, or CDM). CM, CM from γ-radiated MEFs cultured in human ESCM and with the treatment of 4 ng/ml FGF2; CM+MEF, cells reseeded onto MEF and cultured in CM; CDM, CDM with LSC; FM, fibroblast growth medium (DMEM with 10% FBS); NA, not analyzed.
Human iNPs Exhibit Characteristics of Neural Progenitors
The iNPs displayed a columnar epithelial morphology and organized into a rosette form. However, the rosette did not have a clear lumen (Figures 2A, 2B, S2A, and S2H), resembling the primitive neuroepithelia generated from ESCs and iPSCs (Pankratz et al., 2007). They expressed neural progenitor markers, including NESTIN, SOX1, SOX2, FABP7, NOTCH1, PAX6, and HES5, as shown by immunocytochemistry and RT-PCR (Figures 2C–2G, 2I, S2B–S2D, S2F, S2I–S2K, and S2M). The iNPs were highly proliferative, as evidenced by expression of Ki67 in a large proportion of the cells (65.3% ± 8.1%) (Figures 2H, S2E, and S2L). Quantitative measurement of the population expansion showed exponential growth of the iNPs over several passages (Figures 2J, S2G, and S2N). These cells have now been expanded for more than 20 passages, thus generating a large amount (trillions) of cells. At passage 20, the iNP cell lines retained a normal karyotype (Figure 2K). Thus, human iNPs exhibit characteristics of neural progenitors.
Figure 2. Characterization of Human iNPs.
(A) Phase-contrast image for established human iNP cell line 15-LA-24 (passage 5).
(B) High-magnification image of cells in the inset in (A).
(C–G) Human iNPs were stained positively for NESTIN (C, green), SOX1 (D, red), SOX2 (E, red), FABP7 (F, green), and NOTCH1 (G, green).Hoechst staining is blue.
(H) Immunostaining for Ki67 (red). Hoechst staining is blue.
(I) RT-PCR shows human iNPs expressed neural progenitor markers: SOX1, SOX2, FABP7, PAX6, and HES5, GAPDH was used as an internal control. RNA was extracted from noninfected parent fibroblasts (Fibroblasts, negative control), three human iNP cell lines (GM15-LA-24, GM15-LA-25, and GM15-LA-01), early passage (passage 5), and late passage (passage 20) of human iNP cell line GM15-LA-24.
(J) Growth curve of human iNP cell lines GM15-LA-24, GM15-LA-25, and GM15-LA-01. Cells at passage 5 were used as initial cells.
(K) Representative karyotype of human iNP cell line 15-LA-24 at passage 20.
Scale bars, 50 mm (A–H). See also Figure S2 and Tables S5 and S6.
Human iNPs Differentiate to Functional Neurons and Glia In Vitro
To assess the differentiation potential, the iNPs were dissociated into single cells and cultured on laminin substrate in a neural differentiation medium. After 60 days of differentiation, process-bearing cells were obvious, and most of the cells were Tuj1+ and MAP2+ neurons, some cells were GFAP+ astrocytes (Figures 3A and 3B), and less than 1% of cells were Peripherin+ or Brn-3a+ neural crest cells (Figure S3). In addition, iNP-derived neurons expressed synapsin along neurites in a punctate manner (Figure 3A, arrowheads). The majority of neurons were γ-aminobutyric acid positive (GABA+) (75.0% ± 2.5% of Tuj1+ cells), whereas few (1.1% ± 0.4% of Tuj1+ cells) were TH+ (Figure 3C). However, O4+ oligodendrocytes were not observed in the culture (data not shown). Because the majority of oligodendrocytes are derived from ventral neural progenitors (Hu et al., 2009), we treated the human iNPs with SHH (500 ng/ml) for 1 week. A small population (8.3% ± 3.1% of total cells) of NKX2.2 and OLIG2 double-positive preoligodendrocyte precursor cells (pre-OPC) was observed in the culture (Figure 3D). When further culturing in the glial differentiation medium for 10 weeks, O4+ oligodendrocytes were detected (Figure 3E).
Figure 3. In Vitro Differentiation Potential of Human iNPs.
(A) Human iNP-derived neural cells showed typical neuronal morphology and expressed markers both for neurons (Tuj1, MAP2, and synapsin) and for astrocytes (GFAP) after 60 days of differentiation.
(B) Quantification of MAP2+ neurons and GFAP+ astrocytes among total cells. Data are represented as mean ± SEM.
(C) The major population of human iNP-derived neurons was GABA+, and only a small population of neurons was TH+.
(D) NKX2.2 (green) and OLIG2 (red) double-positive pre-OPCs were observed in the culture of human iNPs treated with SHH (500 ng/ml) for 1 week.
(E) After 10 weeks of differentiation in the glial differentiation medium, O4+ oligodendrocytes were observed in cultures that were treated with SHH (500 ng/ml).
(F) Physiological properties of human iNP-derived neurons assessed by whole-cell patch-clamp recordings. (a) Whole-cell recording on a neuron cultured for 10 weeks in vitro. (b) Electrophysiological characteristics of human iNP-derived neurons. (c) Inward Na+ and outward K+ currents were triggered upon −50 to +50 mV voltage steps. The initial currents were enlarged in the inset panel. (d) Action potentials were induced from −60 to +60-pA-injected current steps. (e) Neurons displayed a low rate of spontaneous action potential spiking with a subthreshold oscillatory potential (−30 mV). (f) Both excitatory (inward current) and inhibitory (outward current) spontaneous postsynaptic currents were recorded when neurons were held at 0 mV. The excitatory responses were eliminated after treatment with CNQX, and the inhibitory responses were further eliminated upon the presence of additive bicuculline in the extracellular solution. The asterisks indicate individual excitatory (sEPSCs) and inhibitory (sIPSCs) events.
Scale bars, 50 mm (A, C, D, and E). See also Figure S3 and Table S6.
The functional properties of iNP-derived neurons were analyzed by whole-cell patch-clamp recordings on cultures after 10 weeks of differentiation (Figure 3F). In the 12 neurons tested, the mean cell capacitance (Cap) was 35.2 ± 4.5 pF, resting membrane potential (RMP) was −47 ± 6 mV, and the spontaneous action potential frequency (APs Freq.) was 2.6 ± 0.2 Hz (Figure 3Fb). Both inward Na+ and outward K+ currents were observed in these cells by voltage clamp (Figure 3Fc). Action potentials can be triggered by injection of current steps from −60 to +60 pA (Figure 3Fd). Neurons displayed a low rate of spontaneous action potential spikes with a slow, subthreshold oscillatory potential (−30 mV, Figure 3Fe). The spontaneous postsynaptic responses were observed, indicating formation of functional synaptic network with surrounding neurons (Figure 3Ff). The synaptic activity was partially blocked by CNQX and further eliminated by combination of CNQX and bicuculline (Figure 3Ff) unveiling the excitatory (glutamatergic) and inhibitory (GABAergic) neurotransmission inputs, respectively. Thus, the iNPs possess the potential to differentiate into astrocytes, oligodendrocytes, and functional neurons in vitro.
iNPs Can Be Generated from Monkey Fibroblasts in a Similar Manner
To assess whether other primate fibroblasts could also be reprogrammed into iNPs in a similar manner and to build a primate model for assessing disease process and cell therapy through autologous transplantation, we obtained fibroblasts from an 8-year-old rhesus monkey (Xi et al., 2012) and repeated the experiments described above. As shown in Figure 4, monkey iNPs can be similarly generated from fibroblasts, and the iNPs can differentiate into functional neurons and glial cells in vitro. It is interesting to note that whereas iPSCs were generated from the monkey fibroblasts with retroviral methods (Xi et al., 2012), they were not produced with the SeV under the conventional culture condition (Table 1).
Figure 4. Generation and Characterization of iNPs from Monkey Fibroblasts.
(A) A SOX1+ (green) and NANOG− (red) neuroepithelial colony was observed in the reprogramming culture from monkey fibroblasts.
(B) Phase-contrast image of monkey iNPs. Inset is the high-magnification image.
(C–F) Monkey iNPs were NESTIN+ (C, green), SOX1+ (D, red), SOX2+ (E, red), and FABP7+ (F, green).
(G) Immunostaining for Ki67 (red).
(H) Growth curve from monkey iNP cell lines (47-LA-01, 47-LA-05, 47-LA-11)and from human iNP cell lines (GM15-LA-24, GM15-LA-24, GM15-LA-24). Cells at passage 5 were used as initial cells.
(I) Q-PCR of existing SeV genomes in noninfected parent fibroblasts (Fibroblast, negative control), three monkey iNP cell lines (47-LA-01, 47-LA-05, 47-LA-11), and SeV-infected human fibroblasts (SeV, positive control). Data are represented as mean ± SEM.
(J) Monkey iNP-derived neural cells showed typical neuronal morphology and expressed markers both for neurons (Tuj1, MAP2, and synapsin) and for astrocytes (GFAP) after 8 weeks of differentiation. O4+ oligodendrocytes were observed in differentiation cultures from ventralized monkey iNPs.
(K) Physiological properties of monkey iNP-derived neurons assessed by whole-cell patch-clamp recordings. (a) Whole-cell recording on a neuron at 10 weeks in vitro. (b) Electrophysiological characteristics of monkey iNP-derived neurons. (c) Inward Na+ and outward K+ currents were triggered upon −50 to +50 mV voltage steps. The initial currents were enlarged in the inset panel. (d) Action potentials were induced from −60 to +60-pA-injected current steps. (e) Neurons displayed a low rate of spontaneous action potential spiking with a subthreshold oscillatory potential (−30 mV). (f) Both excitatory (inward current) and inhibitory (outward current) spontaneous postsynaptic currents were recorded when neurons were held at 0 mV. The excitatory responses were eliminated after treatment with CNQX, and the inhibitory responses were further eliminated upon the presence of additive bicuculline in extracellular solution. The asterisks indicate individual excitatory (sEPSCs) and inhibitory (sIPSCs) events.
Scale bars, 50 µm (A–G and J).
Human iNPs Can Be Patterned to Region-Specific Neural Subtypes
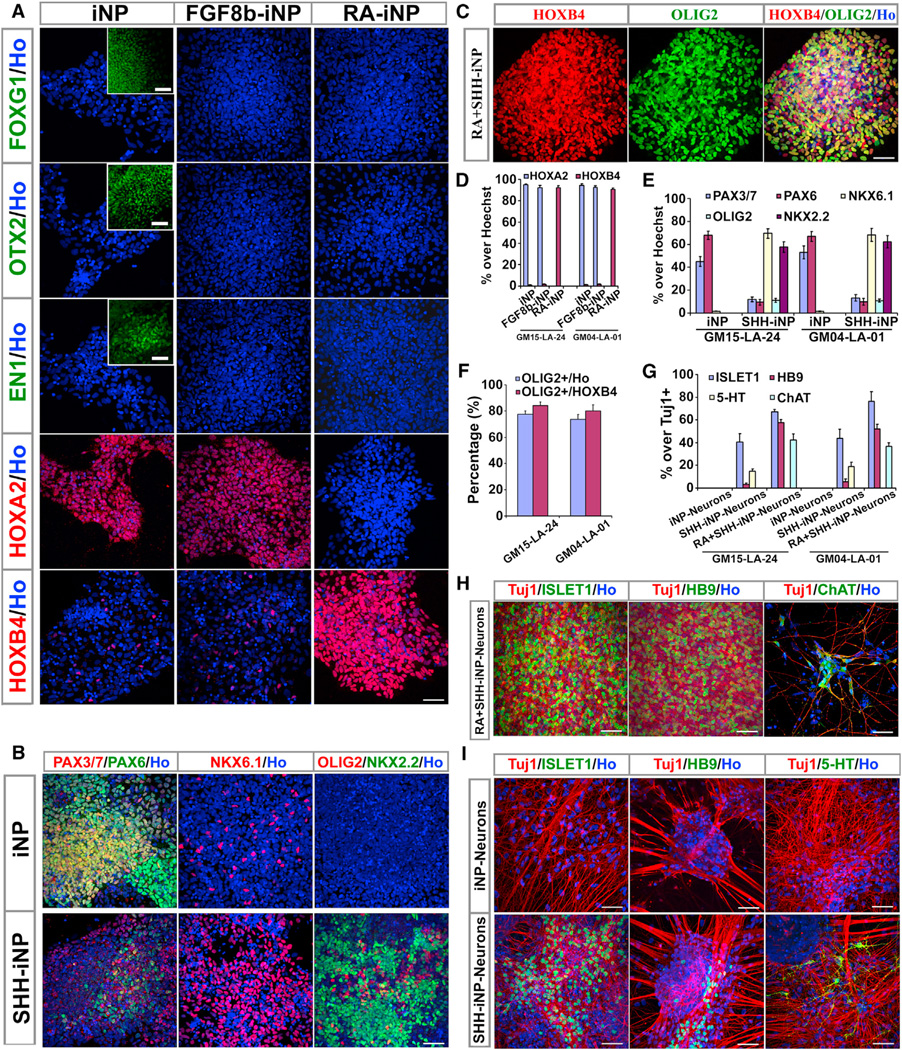
Neural progenitors differentiated from PSCs often carry a specific regional identity depending on the presence of morphogens. We asked if the iNPs carry a particular regional identity under the culture condition. The isolated iNP colonies were expanded to multiple new colonies for 1 week (refer to as passage 1) and stained for homeodomain markers. We found that the new colonies were either stained positive for forebrain markers FOXG1 and/or OTX2 or hindbrain markers HOXA2 and/or HOXB4, but not for midbrain marker EN1 (Figures S4A–S4H; Table S3; data not shown). After around 1 month expansion (passage 5), all iNP colonies lost their anterior identity and uniformly showed posterior identity. RT-PCR and immunostaining showed that the human iNP cell lines at passage 5 and passage 20 predominantly expressed hindbrain markers, including GBX2, KROX20, HOXA2, HOXA3, and HOXB2, but not forebrain (such as FOXG1, EMX1, and OTX2), midbrain (such as PAX2 and EN1), or spinal cord (such as HOXB6 and HOXB8) markers (Figures 5A, 5D, and S4I). The stable human iNP lines also predominantly expressed markers in the dorsal and middle parts of the hindbrain, including PAX7, IRX3, PAX6, and NKX6.1, but not markers of the ventral hindbrain, including OLIG2 and NKX2.2 (Figures 5B, 5E, and S4I). Therefore, the stable iNP lines are predominantly dorsal hindbrain progenitors. This pattern of homeodomain gene expression was retained when comparing cells from late stage (passage 20) to those from early stage (passage 5) (Figure S4I), indicating the maintenance of the iNP regional identity over cellular expansion.
Figure 5. Identity and Generation of Region-Specific Progenitors and Neurons from iNPs.
(A) Human iNPs predominantly expressed hindbrain marker HOXA2, and only a small portion of cells expressed marker for posterior hindbrain or spinal cord (HOXB4), but not anterior markers FOXG1, OTX2, and EN1. When treated with FGF8b (100 ng/ml, FGF8b-iNP), the regional identity was not altered. When treated with RA (0.1 µM, RA-iNP), human iNPs were posteriorly patterned and predominantly expressed marker for posterior hindbrain or cervical spinal cord (HOXB4). Insets show positive controls for FOXG1, OTX2, and EN1, respectively.
(B) Human iNPs predominantly expressed marker for dorsal (PAX3/PAX7) and marker for middle part of hindbrain (PAX6), and only a small portion of cells expressed marker for ventral hindbrain (NKX6.1). When treated with SHH (1,000 ng/ml, SHH-iNP), human iNPs were ventrally patterned and predominantly expressed markers for ventral hindbrain (NKX6.1 and NKX2.2).
(C) When treated with RA (0.1 µM) and SHH (500 ng/ml) (RA+SHH-iNP), most human iNP cells became OLIG2+ and HOXB4+ spinal cord motor neuron precursors.
(D) Quantification of cells expressing HOXA2 and HOXB4 in (A). Data are represented as mean ± SEM.
(E) Quantification of cells in (B). Data are represented as mean ± SEM.
(F) Quantification of cells in (C). Data are represented as mean ± SEM.
(G) Quantification of cells in (H) and (I). Data are represented as mean ± SEM.
(H) The major neuronal population in the RA and SHH-treated iNP cultures (RA+SHH-iNP-Neurons) was HB9+, ISLET1+, and ChAT+ spinal cord motor neurons.
(I) The ventralized neural progenitors differentiated to ISLET1+, HB9+, and 5-HT+ ventral neurons (SHH-iNP-Neurons).
Cell nuclei were stained with Hoechst (blue).
Scale bars, 50 µm (A–C, H, and I). See also Figure S4 and Tables S3 and S6.
We then asked if such a regional fate may be altered by treating the stable iNP lines with morphogens. Although treatment of early-stage iNPs (at passage 1) with FGF8b (100 ng/ml) plus SHH (200 ng/ml) drove some of the clones to express midbrain marker EN1, treatment of iNPs (at passage 5) with FGF8b or FGF8b plus SHH did not drive the human iNPs to express forebrain or midbrain markers (Table S4; data not shown). However, addition of posterior patterning reagent retinoic acid (RA; 0.1 µM) turned off the expression of HOXA2 but turned on the expression of HOXB4 (Figures 5A and 5D), suggesting the acquisition of the upper spinal cord fate. When treated with a high dosage of SHH (1,000 ng/ml), the human iNPs were ventralized by expressing the ventral hindbrain marker NKX2.2 (Figures 5B, 5E, S4J, and S4K). Treatment with both RA (0.1 µM) and SHH (500 ng/ml) resulted in more than 80% human iNP cells to express OLIG2 and HOXB4, indicative of spinal cord motor neuron precursors (Figures 5C and 5F). Indeed, with further differentiation of RA and SHH-treated iNPs, a large proportion of the Tuj1+ neurons expressed ISLET1, HB9, and ChAT (Figures 5G and 5H), indicative of motor neurons; with further differentiation of SHH (1,000 ng/ml)-treated iNPs, some neurons expressed a serotonin neuronal marker 5-HT (Figures 5G and 5I). It is noted that during long-term passaging, all human iNP cell lines gradually lost the capability of being respecified, and fewer and fewer cells were patterned to NKX2.2+ ventral cells or OLIG2+ spinal cord cells (Figures S4L–S4O; data not shown). Together, these results indicate that the iNPs are of dorsal hindbrain identity but can be respecified to ventral and more caudal neural types and further differentiate into hindbrain neurons and an enriched population of spinal cord motor neurons.
Human iNPs Generate Neurons and Glia and Maintain the Hindbrain Identity In Vivo
To assess the in vivo differentiation potential of human iNPs, the iNP cell line 15-LA-24 (at passage 5), which was derived from fibroblast cell line GM03815, was transplanted into the forebrain of newborn mice (Figure 6). One month after transplantation, most of the grafted cells, identified by human-specific antibody STEM121, were found in the ventricle (n = 6 mice) (Figure 6A). The majority of the grafted cells were Tuj1+ neurons (Figure 6C). Some cells expressed GFAP, as indicated by a human-specific GFAP antibody STEM123, indicating astrocytes (Figure 6E). However, few cells were NeuN+ mature neurons, and no MBP+ oligodendrocytes were observed (Figures 6G and 6I). Four months after transplantation, some grafted cells migrated into the parenchyma in the subventricular area (n = 8 mice) (Figure 6B, inset). In addition to Tuj1+ neurons (Figure 6D) and GFAP+ astrocytes (Figure 6F), some human cells became positive for NeuN (Figure 6H, arrowheads; Figure S5A), indicative of neuronal maturation. Furthermore, patches of MBP+ profiles were also detected in the grafts, suggestive of myelination (Figures 6J and S5B). Because human PSC-derived OPCs often become mature oligodendrocytes and produce myelin sheaths 3–4 months after transplantation (Hu et al., 2009), we observed MBP staining in some fibers as well as some cell bodies (Figure 6J). More importantly, even though cells were transplanted into the forebrain for 4 months, most of the cells still expressed hindbrain markers HOXA2 and HOXB4, but not forebrain markers FOXG1 or OTX2 (Figures 6K–6N). No Ki67+ human cells were observed in the graft, and no tumors were found in the mice 4 months posttransplantation. Thus, human iNPs possess the potential to differentiate into mature neurons, astrocytes, and oligodendrocytes and keep the hindbrain identity in vivo.
Figure 6. In Vivo Differentiation Potential of Human iNPs.
(A, C, E, G, and I) One month posttransplantation, human cells, stained for STEM121, were present in the lateral ventricle (A) and were positive for Tuj1 (C), GFAP (E) but rarely for NeuN (G), and not for MBP (I).
(B, D, F, H, and J) Four months posttransplantation, the human cells were also in parenchyma tissues near the ventricle (B) and were positive for Tuj1 (D), GFAP (F), NeuN (H, arrowheads), and MBP (J, arrows indicate MBP+ fibers).
(K–N) Four months posttransplantation, the human cells were detected as FOXG1- (K), OTX2-(L), HOXA2+ (M), and HOXB4+ (N).
Nuclei were stained with Hoechst (blue). Scale bars, 200 µm (A and B) and 50 µm (C–N). See also Figure S5 and Table S6.
DISCUSSION
In this study, we have developed a strategy for generation of iNPs from human and monkey individuals at different ages using the nonintegrating SeVs. Importantly, these iNPs can be expanded to a large amount in a CDM with a cocktail of growth factors and small molecules but without the presence of viral and reprogramming genes. The expanded iNPs stably carry the predictable dorsal hindbrain identity. Interestingly, this regional identity can be modified to produce more caudal cell types, including enriched populations of spinal motor neurons. Upon removal of the growth factor cocktail or transplantation into the mouse brain, the iNPs differentiate into astrocytes, oligodendrocytes, and functional neurons, and they retain the expected hindbrain identity. These results open the possibility of generating consistent and predictable iNPs from individuals for drug discovery and potential cell therapy.
The Yamanaka factors are generally used to induce pluripotency. Our study was hence based on the hypothesis that neural progenitors may be derived during the process of pluripotency induction, although mouse fibroblasts were reported to convert to neural stem cells by bypassing the iPSC stage using the same factors (Kim et al., 2011). To our surprise, the iNP colonies were observable as early as day 13, whereas no iPSC colonies form 3–4 weeks after viral infection under the chemically defined condition or even with the addition of MEF CM and FGF2, a culture condition for ESCs and iPSCs. It has been shown that the exogenous transgenes need to be expressed for at least 12 days in order to generate mouse iPSCs (Brambrink et al., 2008). In our culture system, we raised temperature to remove transgenes 1 day after infection for 12 days, which potentially disturbed the process of iPSC generation. Furthermore, the expression of pluripotent genes, Nanog and Rex1, was not significantly activated throughout the process. We reason that the lack of upregulation of pluripotent genes is likely due to our unique induction condition in which the viral genes are being inactivated by raised temperature, and the infected cells are grown in a chemically defined condition from day 1. This is supported by the fact that iPSC colonies indeed formed when the cultures were maintained at 37°C and supplemented with MEF CM and FGF2. Therefore, the formation of iNPs under our present culture condition is unlikely going through the pluripotent stage. This conclusion is further supported by the fact that when the infected cells were cultured in MEF CM and FGF2 for the first week and then reseeded in CDM (with LSC), no iNPs could be observed (Table 1). Taken together, these results suggest that generation of iPSCs under our chemically defined condition at 39°C within 2 weeks is very unlikely and that iNPs are most likely generated by direct conversion rather than through rapid differentiation of iPSCs.
Although iPSCs have been established using nonintegrating methods (Ban et al., 2011; Okita et al., 2008; Stadtfeld et al., 2008; Yusa et al., 2009; Zhou et al., 2009), direct conversion of fibroblasts to neurons or iNPs, especially the maintenance of iNPs, often depends on the presence of reprogramming genes. Hence, the methods employed are largely based on integrating viruses to date (Han et al., 2012; Kim et al., 2011; Lujan et al., 2012; Ring et al., 2012; Thier et al., 2012). One of the potential reasons is lack of a condition that would support the survival and/or maintenance of dedifferentiated or reprogrammed iNPs. Indeed, in the presence of the LSC cocktail, which has been shown by Ding’s group to sustain the long-term self-renewal of human ESC-derived neural stem cells (Li et al., 2011), iNPs have been consistently generated with the nonintegrating SeV from fibroblasts of both human and monkey at various ages (human) in this study. The importance of the LSC cocktail is also evident by the lack of iNP colonies in the cultures without the LSC cocktail (Figure 1B). The presence of LSC cocktail, however, has driven the iNP cells to acquire a hindbrain fate, which is quite different from the human ESC-derived neural stem cells reported by Ding’s group (Li et al., 2011). In Ding’s report, the neural stem/progenitor cells differentiated from ESCs under the same LSC cocktail carry a forebrain/midbrain fate by expressing Forse1 and OTX2 (Li et al., 2011). It is not clear why there is such a discrepancy. We have also differentiated human ESCs/iPSCs under the LSC cocktail, and the resultant neural progenitors almost always carry a hindbrain character. We reason that besides its proliferative effect, CHIR also exerts its dose-dependent caudalizing (patterning) effect as indicated by us and others (Kirkeby et al., 2012; Xi et al., 2012). That explains why at the formation of iNP colonies some carry forebrain/midbrain markers, but they quickly become stable hindbrain progenitors.
Neural stem/progenitor cells often change their identities over the course of in vitro expansion. One important feature of the human and monkey iNPs generated in the present study is their stable phenotypes after long-term expansion. In particular, the dorsal hindbrain identity is not altered even after 20 passages. This allows generation of large quantities of uniform cell populations with predictable phenotypes. The conversion and maintenance of the iNP phenotype are likely due to the LSC cocktail, especially CHIR99021, the GSK3β inhibitor. It has been shown that inhibition of GSK3β renders neural progenitors in the entire neuraxis in a state of self-renewal (Kim et al., 2009). The dorsal hindbrain identity of the iNPs is likely due to the effect of CHIR99021 on WNT. By inhibiting GSK3β, CHIR99021 activates the WNT pathway (Frame and Cohen, 2001), thus caudalizing and dorsalizing the iNPs. Indeed, we and others have shown that CHIR99021 induces the mid-hindbrain phenotypes of human ESC- and iPSC-derived neuroepithelia in a dose-dependent manner (Kirkeby et al., 2012; Kriks et al., 2011; Xi et al., 2012). In that regard, it will be interesting to identify a condition that would induce/maintain iNPs without altering the regional identity.
The dorsal hindbrain phenotype acquired during the iNP generation and expansion appears permanent because treatment with FGF8b does not push the cells back to the telencephalic or mesencephalic fate. Nevertheless, our iNPs retain the ability to be respecified to more caudal fates. In particular, with the combined treatment of RA and SHH, the iNPs are specified to OLIG2+ and HOXB4+ spinal motor neuron progenitors, which subsequently become an enriched population of spinal cord motor neurons. This phenomenon suggests that if a specific cocktail is applied during reprogramming, iNPs with a particular regional identity and predictable differentiation potential may be generated. This will allow generation of large quantities of custom-designed iNPs, facilitating drug testing and potential application in cell therapy. It should be noted that the iNPs gradually lose their ability to be respecified to other fates during long-term passaging (>20). This indicates that a simple culture cocktail without persistent transgene expression may not be sufficient to maintain the “plasticity” of the iNPs even though the iNPs can still be expanded. This phenomenon also suggests that perhaps we could “fix” the identity of the iNPs early on, thus increasing the predictability of the fate of iNPs.
iNPs have been generated from mouse fibroblasts. In humans, some samples were used to generate iNPs by the retroviral approach (Kumar et al., 2012; Ring et al., 2012). Although single transgene (SOX2) seems sufficient to generate iNPs from fetal foreskin tissue samples via retroviral infection (Ring et al., 2012), there still exist the risks for disruption of endogenous gene expression and tumor formation due to potential spontaneous reactivation of the viral transgenes (Okita et al., 2007). Most recently, Pei and colleagues reported generation of iNPs from urine cells of humans (10, 25, and 37 years) using a similar nonintegrating method (oriP/EBNA episomal vectors) for a similar period (12 days) (Wang et al., 2013). The finding coincides with our report. In that report, they used a set of six factors that have been used for iPSC generation (Li et al., 2009), and the iNPs were generated on Matrigel without raising temperature to inactivate the transgenes. Interestingly, it is stated in the report that that protocol does not apply to human dermal fibroblasts. It is not clear why they could not generate iNPs from dermal fibroblasts because our dropout analysis indicated that only three factors (LSC) are sufficient. More importantly, that report does not address if the iNPs are generated through direct conversion or via iPSCs, nor does it analyze the regional identity and the plasticity of the iNPs, key issues addressed in our present report.
The strategy described in the present study allows efficient generation of iNPs (for human cells, 0.03%–0.08%; for monkey cells, around 0.19%) from primate species by a nonintegrating method. This will make it possible to generate iNPs from patients’ skin tissues directly. We chose SeV because to date, there is no report of pathogenicity associated with SeV in primates, and its safety could be further enhanced by the F-deficiency (Nagai et al., 2011). SeV-based vector has been used in clinical gene therapy for cystic fibrosis (Ferrari et al., 2007; Yonemitsu et al., 2000) and vaccine delivery (Takeda et al., 2008). Additionally, the temperature-sensitive nature of the RNA virus (Ban et al., 2011) offers another safeguard step to ensure removal of viral genomes, as shown in the present study. Therefore, our transgene integration-free and expandable primate iNPs provide a safe and robust cellular platform for the generation of patient-specific neural cells for biomedical applications.
EXPERIMENTAL PROCEDURES
Generation of iNPs from Human and Monkey Fibroblasts
Information for human and monkey fibroblasts is summarized in Table S1, and the process for iNP generation is shown in Figure 1A. NIH guidelines for laboratory animal care and safety were strictly followed. On day −1, around 30,000 fibroblasts were seeded in one well of a 24-well-plate (Nunc) and cultured in the fibroblast medium (FM; Dulbecco’s modified Eagle’s medium [DMEM] supplemented with 10% FBS). On day 0, the fibroblasts were infected with SeVs (TS15) containing OSKM, or GFP(kindly provided by DNAVEC, Japan) according to the manufacturer’s instruction. On day 1, the medium was changed to a CDM consisting of DMEM/F12: Neurobasal (1:1), 1 3 N2,1 3 B27,1%GlutaMAX (all from Life Technologies). In some cultures, 10 ng/ml recombinant human LIF, 3 µM CHIR99021, and 2 µM SB431542 (all from Stemgent) were added. All the cultures were incubated at 39°C. On day 7, the cells were dissociated with EDTA in the presence of ROCK inhibitor (Y-27632, 5 µM; Tocris Bioscience) and then reseeded onto one well of a 6-well-plate (Nunc), which was precoated with laminin (Life Technologies).On day 13, GFP, an indicator of viral presence, disappeared, and neuroepithelium-like colonies began to appear in the culture. The cultures were then transferred back to 37°C. On day 20, cell colonies were selected, mechanically triturated into small clusters, and reseeded onto PO (Sigma-Aldrich) and laminin-precoated 12mmglass coverslip for immunostaining or laminin-coated 96-well-plates for cell expansion. iNP cells were passed on laminin-coated plates and cultured in the same medium used for the generation of iNPs. Human ESC medium used in the study was composed of DMEM/F12 containing 20% knockout serum replacement (KSR; Life Technologies), 1% GlutaMAX, 1% Nonessential Amino Acids (NEAA; Life Technologies), 0.1 mM 2-ME (Life Technologies), and 4 ng/ml bFGF (PeproTech).
Regional Patterning and Neural Differentiation of iNPs
To pattern the iNPs, LSC were removed from CDM, and morphogens were added to the culture for 1 week, including FGF8b (PeproTech; 100 ng/ml), RA (Sigma-Aldrich; 0.1 µM), and/or SHH (C24II, R&D Systems; 200–1,000 ng/ml). For terminal differentiation, iNPs were seeded at a density of 10,000–15,000 cells per 12 mm glass coverslip that was coated with PO and laminin in a neural differentiation medium (Neurobasal with 1 × N2, 1 × B27, 1 × NEAA supplemented with 1 µg/ml laminin, 0.2 mM vitamin C [Tocris Bioscience], 300 ng/ml cAMP [Sigma-Aldrich], 10 ng/ml GDNF, 10 ng/ml BDNF, and 10 ng/ml IGF-I [all from PeproTech]). For oligodendrocyte differentiation, glia differentiation medium was applied as described before by Hu et al. (2009). Briefly, SHH-treated iNPs were seeded at a density of 10,000–15,000 cells per 12 mm glass coverslip that was coated with PO and laminin in a glial differentiation medium (DMEM:F12, N1 supplement, T3 [60 ng/ml], biotin [100 ng/ml], and cAMP [1 µM] [all from Sigma-Aldrich] supplemented with a cocktail of cytokines consisting of the PDGF-AA isoform, insulin-like growth factor 1 [IGF1], and neurotrophin 3 [NT3] [all at 10 ng/ml from R&D Systems]).
RNA Extraction and RT-PCR
RNA extraction and RT-PCR analysis were described before (Zhang et al., 2010). To compare the expression level of different genes, probes were normalized to glyceraldehyde 3-phosphate dehydrogenase (GAPDH) by performing 15, 20, and 25 cycles. PCR conditions and cycle numbers were established by using commercially available human fetal brain (single donor, male, 19 weeks of gestation) (Agilent Technologies). Q-PCR was performed by using the Power SYBR Green kit (Applied Biosystems, UK). Primers are listed in Table S5.
Immunocytochemistry
Immunostaining was performed as described previously (Zhang et al., 2010). In brief, cells were fixed in 4% neutral-buffered paraformaldehyde (PFA) for 20 min at room temperature. For detection of GABA, 0.05% glutaraldehyde (Electron Microscopy Sciences) was included in the fixative. All antibodies, sources, and dilutions are listed in Table S6. Cell populations were counted among total cells (Hoechst labeled) or neurons (Tuj1+) using the ImageJ software. At least five fields of each coverslip were chosen randomly, and three coverslips in each group were counted. Data were expressed as mean ± SEM.
Electrophysiology
Whole-cell patch-clamp recordings were performed on human or monkey iNP-derived neurons after 10 weeks of differentiation, as previously described by Ma et al. (2012).
Cell Transplantation and Histology
Transplantation studies were conducted following protocols approved by the Animal Care and Use Committees at the University of Wisconsin-Madison. iNPs (105 cells in 2 ml artificial cerebral spinal fluid, Harvard Apparatus) were injected 1 mm from the midline between the bregma and lambda and 1 mm deep into the lateral ventricles of both hemispheres of newborn severe combined immunodeficiency (SCID)-beige (Taconic) mice. One and 4 months after transplantation, animals were processed for histological analysis as described before by Ma et al. (2012). All antibodies, sources, and dilutions are listed in Table S6.
Statistical Analysis
Values were expressed as mean ± SEM. Differences between means were assessed by t test. A p value <0.05 was considered statistically significant.
Supplementary Material
ACKNOWLEDGMENTS
This study was supported by the NIH-NINDS (NS04596, NS064578, and NS074189), the Bleser Family Foundation, the Busta Foundation, and in part by NICHD (P30 HD03352). We thank DNAVEC Corporation for providing us with Sendai virus kits.
Footnotes
SUPPLEMENTAL INFORMATION
Supplemental Information includes five figures and six tables and can be found with this article online at http://dx.doi.org/10.1016/j.celrep.2013.04.004.
LICENSING INFORMATION
This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
REFERENCES
- Ban H, Nishishita N, Fusaki N, Tabata T, Saeki K, Shikamura M, Takada N, Inoue M, Hasegawa M, Kawamata S, Nishikawa S. Efficient generation of transgene-free human induced pluripotent stem cells (iPSCs) by temperature-sensitive Sendai virus vectors. Proc. Natl. Acad. Sci. USA. 2011;108:14234–14239. doi: 10.1073/pnas.1103509108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brambrink T, Foreman R, Welstead GG, Lengner CJ, Wernig M, Suh H, Jaenisch R. Sequential expression of pluripotency markers during direct reprogramming of mouse somatic cells. Cell Stem Cell. 2008;2:151–159. doi: 10.1016/j.stem.2008.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caiazzo M, Dell’Anno MT, Dvoretskova E, Lazarevic D, Taverna S, Leo D, Sotnikova TD, Menegon A, Roncaglia P, Colciago G, et al. Direct generation of functional dopaminergic neurons from mouse and human fibroblasts. Nature. 2011;476:224–227. doi: 10.1038/nature10284. [DOI] [PubMed] [Google Scholar]
- Efe JA, Hilcove S, Kim J, Zhou H, Ouyang K, Wang G, Chen J, Ding S. Conversion of mouse fibroblasts into cardiomyocytes using a direct reprogramming strategy. Nat. Cell Biol. 2011;13:215–222. doi: 10.1038/ncb2164. [DOI] [PubMed] [Google Scholar]
- Ferrari S, Griesenbach U, Iida A, Farley R, Wright AM, Zhu J, Munkonge FM, Smith SN, You J, Ban H, et al. Sendai virus-mediated CFTR gene transfer to the airway epithelium. Gene Ther. 2007;14:1371–1379. doi: 10.1038/sj.gt.3302991. [DOI] [PubMed] [Google Scholar]
- Frame S, Cohen P. GSK3 takes centre stage more than 20 years after its discovery. Biochem. J. 2001;359:1–16. doi: 10.1042/0264-6021:3590001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han DW, Tapia N, Hermann A, Hemmer K, Höing S, Araúzo-Bravo MJ, Zaehres H, Wu G, Frank S, Moritz S, et al. Direct reprogramming of fibroblasts into neural stem cells by defined factors. Cell Stem Cell. 2012;10:465–472. doi: 10.1016/j.stem.2012.02.021. [DOI] [PubMed] [Google Scholar]
- Hu BY, Du ZW, Li XJ, Ayala M, Zhang SC. Human oligodendrocytes from embryonic stem cells: conserved SHH signaling networks and divergent FGF effects. Development. 2009;136:1443–1452. doi: 10.1242/dev.029447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang P, He Z, Ji S, Sun H, Xiang D, Liu C, Hu Y, Wang X, Hui L. Induction of functional hepatocyte-like cells from mouse fibroblasts by defined factors. Nature. 2011;475:386–389. doi: 10.1038/nature10116. [DOI] [PubMed] [Google Scholar]
- Ieda M, Fu JD, Delgado-Olguin P, Vedantham V, Hayashi Y, Bruneau BG, Srivastava D. Direct reprogramming of fibroblasts into functional cardiomyocytes by defined factors. Cell. 2010;142:375–386. doi: 10.1016/j.cell.2010.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim WY, Wang X, Wu Y, Doble BW, Patel S, Woodgett JR, Snider WD. GSK-3 is a master regulator of neural progenitor homeostasis. Nat. Neurosci. 2009;12:1390–1397. doi: 10.1038/nn.2408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Efe JA, Zhu S, Talantova M, Yuan X, Wang S, Lipton SA, Zhang K, Ding S. Direct reprogramming of mouse fibroblasts to neural progenitors. Proc. Natl. Acad. Sci. USA. 2011;108:7838–7843. doi: 10.1073/pnas.1103113108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkeby A, Grealish S, Wolf DA, Nelander J, Wood J, Lundblad M, Lindvall O, Parmar M. Generation of regionally specified neural progenitors and functional neurons from human embryonic stem cells under defined conditions. Cell Rep. 2012;1:703–714. doi: 10.1016/j.celrep.2012.04.009. [DOI] [PubMed] [Google Scholar]
- Kriks S, Shim JW, Piao J, Ganat YM, Wakeman DR, Xie Z, Carrillo-Reid L, Auyeung G, Antonacci C, Buch A, et al. Dopamine neurons derived from human ES cells efficiently engraft in animal models of Parkinson’s disease. Nature. 2011;480:547–551. doi: 10.1038/nature10648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar A, Declercq J, Eggermont K, Agirre X, Prosper F, Verfaillie CM. Zic3 induces conversion of human fibroblasts to stable neural progenitor-like cells. J. Mol. Cell Biol. 2012;4:252–255. doi: 10.1093/jmcb/mjs015. [DOI] [PubMed] [Google Scholar]
- Li W, Wei W, Zhu S, Zhu J, Shi Y, Lin T, Hao E, Hayek A, Deng H, Ding S. Generation of rat and human induced pluripotent stem cells by combining genetic reprogramming and chemical inhibitors. Cell Stem Cell. 2009;4:16–19. doi: 10.1016/j.stem.2008.11.014. [DOI] [PubMed] [Google Scholar]
- Li W, Sun W, Zhang Y, Wei W, Ambasudhan R, Xia P, Talantova M, Lin T, Kim J, Wang X, et al. Rapid induction and long-term self-renewal of primitive neural precursors from human embryonic stem cells by small molecule inhibitors. Proc. Natl. Acad. Sci. USA. 2011;108:8299–8304. doi: 10.1073/pnas.1014041108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lujan E, Chanda S, Ahlenius H, Südhof TC, Wernig M. Direct conversion of mouse fibroblasts to self-renewing, tripotent neural precursor cells. Proc. Natl. Acad. Sci. USA. 2012;109:2527–2532. doi: 10.1073/pnas.1121003109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma L, Hu B, Liu Y, Vermilyea SC, Liu H, Gao L, Sun Y, Zhang X, Zhang SC. Human embryonic stem cell-derived GABA neurons correct locomotion deficits in quinolinic acid-lesioned mice. Cell Stem Cell. 2012;10:455–464. doi: 10.1016/j.stem.2012.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagai Y, Takakura A, Irie T, Yonemitsu M, Gotoh B. Evolution of Sendai virus: the journey from mouse pathogen to a state-of the-art tool in virus research and biotechnology. In: Samal SK, editor. The Biology of Paramyxoviruses. Norwich, UK: Horizon Scientific Press; 2011. pp. 115–173. [Google Scholar]
- Okita K, Ichisaka T, Yamanaka S. Generation of germline-competent induced pluripotent stem cells. Nature. 2007;448:313–317. doi: 10.1038/nature05934. [DOI] [PubMed] [Google Scholar]
- Okita K, Nakagawa M, Hyenjong H, Ichisaka T, Yamanaka S. Generation of mouse induced pluripotent stem cells without viral vectors. Science. 2008;322:949–953. doi: 10.1126/science.1164270. [DOI] [PubMed] [Google Scholar]
- Pang ZP, Yang N, Vierbuchen T, Ostermeier A, Fuentes DR, Yang TQ, Citri A, Sebastiano V, Marro S, Südhof TC, Wernig M. Induction of human neuronal cells by defined transcription factors. Nature. 2011;476:220–223. doi: 10.1038/nature10202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pankratz MT, Li XJ, Lavaute TM, Lyons EA, Chen X, Zhang SC. Directed neural differentiation of human embryonic stem cells via an obligated primitive anterior stage. Stem Cells. 2007;25:1511–1520. doi: 10.1634/stemcells.2006-0707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfisterer U, Kirkeby A, Torper O, Wood J, Nelander J, Dufour A, Björklund A, Lindvall O, Jakobsson J, Parmar M. Direct conversion of human fibroblasts to dopaminergic neurons. Proc. Natl. Acad. Sci. USA. 2011;108:10343–10348. doi: 10.1073/pnas.1105135108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ring KL, Tong LM, Balestra ME, Javier R, Andrews-Zwilling Y, Li G, Walker D, Zhang WR, Kreitzer AC, Huang Y. Direct reprogramming of mouse and human fibroblasts into multipotent neural stem cells with a single factor. Cell Stem Cell. 2012;11:100–109. doi: 10.1016/j.stem.2012.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stadtfeld M, Nagaya M, Utikal J, Weir G, Hochedlinger K. Induced pluripotent stem cells generated without viral integration. Science. 2008;322:945–949. doi: 10.1126/science.1162494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, Yamanaka S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861–872. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- Takeda A, Igarashi H, Kawada M, Tsukamoto T, Yamamoto H, Inoue M, Iida A, Shu T, Hasegawa M, Matano T. Evaluation of the immunogenicity of replication-competent V-knocked-out and replication-defective F-deleted Sendai virus vector-based vaccines in macaques. Vaccine. 2008;26:6839–6843. doi: 10.1016/j.vaccine.2008.09.074. [DOI] [PubMed] [Google Scholar]
- Thier M, Wörsdörfer P, Lakes YB, Gorris R, Herms S, Opitz T, Seiferling D, Quandel T, Hoffmann P, Nöthen MM, et al. Direct conversion of fibroblasts into stably expandable neural stem cells. Cell Stem Cell. 2012;10:473–479. doi: 10.1016/j.stem.2012.03.003. [DOI] [PubMed] [Google Scholar]
- Vierbuchen T, Ostermeier A, Pang ZP, Kokubu Y, Südhof TC, Wernig M. Direct conversion of fibroblasts to functional neurons by defined factors. Nature. 2010;463:1035–1041. doi: 10.1038/nature08797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Wang L, Huang W, Su H, Xue Y, Su Z, Liao B, Wang H, Bao X, Qin D, et al. Generation of integration-free neural progenitor cells from cells in human urine. Nat. Methods. 2013;10:84–89. doi: 10.1038/nmeth.2283. [DOI] [PubMed] [Google Scholar]
- Xi J, Liu Y, Liu H, Chen H, Emborg ME, Zhang SC. Specification of midbrain dopamine neurons from primate pluripotent stem cells. Stem Cells. 2012;30:1655–1663. doi: 10.1002/stem.1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yonemitsu Y, Kitson C, Ferrari S, Farley R, Griesenbach U, Judd D, Steel R, Scheid P, Zhu J, Jeffery PK, et al. Efficient gene transfer to airway epithelium using recombinant Sendai virus. Nat. Biotechnol. 2000;18:970–973. doi: 10.1038/79463. [DOI] [PubMed] [Google Scholar]
- Yoo AS, Sun AX, Li L, Shcheglovitov A, Portmann T, Li Y, Lee-Messer C, Dolmetsch RE, Tsien RW, Crabtree GR. MicroRNA-mediated conversion of human fibroblasts to neurons. Nature. 2011;476:228–231. doi: 10.1038/nature10323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu J, Vodyanik MA, Smuga-Otto K, Antosiewicz-Bourget J, Frane JL, Tian S, Nie J, Jonsdottir GA, Ruotti V, Stewart R, et al. Induced pluripotent stem cell lines derived from human somatic cells. Science. 2007;318:1917–1920. doi: 10.1126/science.1151526. [DOI] [PubMed] [Google Scholar]
- Yusa K, Rad R, Takeda J, Bradley A. Generation of transgene-free induced pluripotent mouse stem cells by the piggyBac transposon. Nat. Methods. 2009;6:363–369. doi: 10.1038/nmeth.1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Huang CT, Chen J, Pankratz MT, Xi J, Li J, Yang Y, Lavaute TM, Li XJ, Ayala M, et al. Pax6 is a human neuroectoderm cell fate determinant. Cell Stem Cell. 2010;7:90–100. doi: 10.1016/j.stem.2010.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou H, Wu S, Joo JY, Zhu S, Han DW, Lin T, Trauger S, Bien G, Yao S, Zhu Y, et al. Generation of induced pluripotent stem cells using recombinant proteins. Cell Stem Cell. 2009;4:381–384. doi: 10.1016/j.stem.2009.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.