Abstract
Purpose
To describe brain CT findings in retinopathy-confirmed, paediatric cerebral malaria.
Materials & Methods
In this outcomes study of paediatric cerebral malaria, a subset of children with protracted coma during initial presentation was scanned acutely. Survivors experiencing adverse neurological outcomes also underwent a head CT. All children had ophthalmological examination to confirm the presence of the retinopathy specific for cerebral malaria. Independent interpretation of CT images was provided by two neuroradiologists.
Results
Acute brain CT findings in three children included diffuse oedema with obstructive hydrocephalus (2), acute cerebral infarctions in multiple large vessel distributions with secondary oedema and herniation (1), and oedema of thalamic grey matter (1). One child who was reportedly normal prior to admission had parenchymal atrophy suggestive of pre-existing CNS injury.
Among 56 survivors (9-84 months old), 15 had adverse neurologic outcomes--11/15 had a follow-up head CT, 3/15 died and 1/15 refused CT. Follow-up head CTs obtained 7-18 months after the acute infection revealed focal and multifocal lobar atrophy correlating to regions affected by focal seizures during the acute infection (5/11). Other findings were communicating hydrocephalus (2/11), vermian atrophy (1/11) and normal studies (3/11).
Conclusions
The identification of pre-existing imaging abnormalities in acute cerebral malaria suggests that population-based studies are required to establish the rate and nature of incidental imaging abnormalities in Malawi. Children with focal seizures during acute cerebral malaria developed focal cortical atrophy in these regions at follow-up. Longitudinal studies are needed to further elucidate mechanisms of CNS injury and death in this common fatal disease.
Keywords: head CT, neuroimaging, diagnostic certainty, cerebral malaria, outcomes
Introduction
Cerebral malaria has historically been defined clinically as P. falciparum parasitemia with coma in the absence of an identifiable alternative cause of altered consciousness (1, 2). In regions with high rates of asymptomatic parasitemia and limited facilities for elucidating other aetiologies for coma, the clinical diagnosis lacks specificity and may result in a >20% misclassification rate. (3). Recent research indicates that a unique retinopathy associated with severe malaria can be identified during the acute illness and can play a key role in confirming the clinical diagnoses of cerebral malaria (95% sensitivity and 100% specificity in fatal cases)(4-7). Cerebral malaria retinopathy includes macular whitening, vessel discoloration, and white-centered hemorrhages. These are illustrated in figures 1-3.
Figure 1.

Fundus of child with cerebral malaria. Note the macular whitening. Hemorrhages with whitening in the center are also evident.
Figure 3.
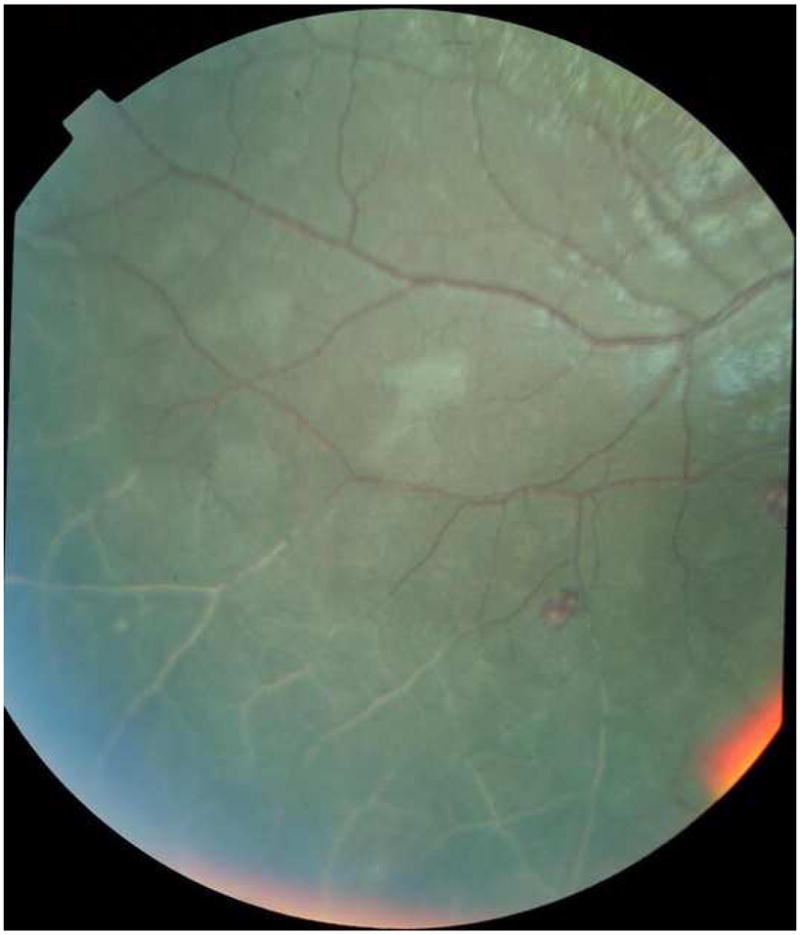
Fundus of a child with cerebral malaria. Peripheral retinal whitening and white retinal vessels evident. White vessel have sequestered, parasitized red blood cells. The hemoglobin has been consumed by the p. falciparum.
Nearly all episodes of fatal malaria are due to P. falciparum, the majority of victims in high-transmission areas being young children. Since approximately 90 percent of paediatric cerebral malaria cases occur in Africa where recourse to imaging is limited, there have been few studies of the neuroimaging features of paediatric malaria. Severe malaria in adults, a condition more common to regions of Southeast Asia, is characterized by multi-system organ failure in addition to cerebral disease. A comprehensive PubMed search revealed only five published studies describing neuroimaging findings associated with paediatric cerebral malaria in a cumulative total of 27 patients (Table 1). These studies were conducted before ophthalmological diagnostic criteria were known and are thus likely to include a substantial proportion of patients with CNS pathology other than cerebral malaria.
Table 1.
| Study | Modality | Sample size | Imaging Findings |
|---|---|---|---|
| (16) | CT | 14 |
|
| (17) | CT (noncontrast) | 1 (case report) |
|
| (18) | MRI | 3 |
|
| (19) | CT | 8 |
|
| (20) | MRI | 1 (case report) |
|
PubMed search using the terms malaria AND MRI OR CT OR imaging with a subsequent search through the reference list of each cited article
Cases were clinically identified without recourse to ophthalmological confirmation
The case fatality rate of paediatric cerebral malaria ranges from 15% to 25%(8). Retrospective cohort and case-control studies have shown that paediatric cerebral malaria survivors are at increased risk of epilepsy (9, 10), developmental delay (9, 11, 12), and behavioral problems (9), but the mechanism(s), pathogenesis and neuroanatomical correlates of adverse neurologic outcomes in paediatric cerebral malaria are poorly understood. In vivo assessments to determine the neuroanatomical correlates of malaria-related coma and subsequent neurologic sequelae have been limited to gross clinical assessments. Autopsy studies have provided insights into the clinicopathologic correlates of fatal cases and indicate a range of pathological findings (3, 13). These include sequestration of parasitized red blood cells in brain capillaries with diffuse oedema, petechial haemorrhages in the brain parenchyma surrounding ruptured vessels, ring haemorrhages; parenchymal necrosis with perivascular oedema and neuronal cell loss and microglial aggregates (Durck’s granulomas). The exact pathophysiologic pathways and the consequences that lead to these findings have yet to be fully elucidated.
The Blantyre Malaria Project Epilepsy Study (BMPES) is an ongoing, prospective exposure control study of paediatric cerebral malaria in patients with ophthalmological assessments to confirm the presence of the malaria-specific retinopathy. CT scans are available in the QEC Hospital, but for economic and technical reasons can only be conducted on patients with a strong clinical indication. During BMPES recruitment, head CTs were obtained on a few children with acute cerebral malaria who had prolonged coma. Head CTs were also obtained on study patients who were determined to have adverse neurologic outcomes during follow-up. CT findings in children with retinopathy-confirmed cerebral malaria are presented here.
Materials & Methods
Children admitted to the Paediatric Research Ward at Queen Elizabeth Central Hospital in Blantyre, Malawi with coma (defined as a Blantyre Coma Score of <3), P. falciparum parasitemia on peripheral blood smear, malaria retinopathy, and no other aetiology for coma are eligible for BMPES recruitment.
All children received routine clinical care including supportive therapy as required (fluids, glucose, oxygen, blood transfusion, anticonvulsant drugs, antipyretics) and specific antimalarial treatment according to national protocols. Lumbar punctures were routinely performed to rule-out other CNS infections.
Neurodevelopmental status prior to the malarial infection was assessed by a structured questionnaire about a child’s competences, discussed with the parent or guardian who had been living with and responsible for the child. This instrument, known as ‘The Ten Questions’. has been shown to be valid in children as young as two years (14) and has been evaluated in similar environments with good reliability (kappa=0.67) and 85% sensitivity for detecting moderate neurodevelopmental disabilities (14). Serial clinical and laboratory assessments were made throughout the admission including EEGs, and documentation of any focal neurologic deficits or seizures. Survivors were reviewed at 1-month post discharge and quarterly thereafter. At follow-up, neurodevelopmental status was assessed using the same Ten Questions Screen (14) and any children with new developmental or behavioural problems were assessed by a neurologist (GLB).
During recruitment, head CTs were obtained on children with acute cerebral malaria and protracted coma. At follow-up, children determined to have adverse neurologic outcomes evident also underwent a brain CT. CTs were completed on a single slice Phillips CT Scanner Tomoscan, EG with images obtained in axial plane with 120kVp, 30mA and 4 second rotation time per slice (standard brain algorithm used), 5mm slice thickness and 5mm skip. Intravenous contrast was not available. Two fellowship trained radiologists with certificates of added qualification in neuroradiology and 14 and 16 years of clinical experience provided independent blinded interpretations of the CT images (MJP and JKD). This included a standard clinical report as well as completion of a detailed check list of the presence or absence of key categorical findings, grading the severity of any abnormality and clear localization of the findings (See Appendix for instrument).
This work was approved by Michigan State University’s Biomedical Institutional Review Board and Malawi’s College of Medicine Research Ethics Committee
Results
Four head CTs were obtained on three children with retinopathy-confirmed acute cerebral malaria and protracted coma, all of whom died. Among 56 retinopathy-confirmed cerebral malaria survivors (9-84 months old), 15 had adverse neurologic outcomes evident during follow-up. Mean follow-up was 15 months (range 3-31). Eleven of these 15 children had a head CT during follow-up. Three died and one withdrew from the study before a CT could be obtained. Among survivors, adverse outcomes included focal motor deficits, language regression, epilepsy, and symptoms consistent with attention deficit hyperactive disorder (ADHD). CT findings, demographic details and outcomes in survivors are outlined in Table 2.
Table 2.
CT Findings among Malawian Children with Retinopathy-Confirmed Cerebral Malaria
| Age (months) | Gender | Presentation | CT findings | Outcome | |
|---|---|---|---|---|---|
| Patient 1 | 84 | Male | Coma |
Acute (3 days after coma onset) Hydrocephalus and diffuse cerebral edema including the brainstem with poor gray-white delineation. Multi- focal cortical infarcts plus additional infarcts in the brainstem and left thalamus. Punctate focus of parenchymal hemorrhage in left frontal lobe at gray-white junction. Mass effect with uncal herniation. |
Died |
|
Subacute (14 days after coma onset) Progressive hydrocephalus including sulcal prominence consistent with communicating form. Resultant increasing mass effect and downward herniation. Evolving strokes in the left frontal, left thalamus, and brainstem. New strokes evident in the right frontal and left occipital regions. | |||||
| Patient 2 | 48 | Female | Multifocal seizures and coma |
Acute (6 days after coma onset) Diffuse cerebral edema most notable in the right ACA and right MCA distribution consistent with acute ischemia. Some focal parenchymal atrophy evident in the right ACA and MCA distribution. 3mm midline shift and herniation. |
Died |
| Patient 3 | 30 | Female | Coma, generalized seizures |
Acute (day 4 after coma onset) Moderate diffuse parenchymal atrophy sparing the posterior fossa |
Died |
| Patient 4 | 60 | Female | Left-sided focal motor seizures and coma |
Follow-up at 11 months after acute cerebral malaria Diffuse, moderate-severe parenchymal atrophy with moderate hydrocephalus including the 4th ventricle |
Spastic quadriparesis, cortically blind, epilepsy |
| Patient 5 | 26 | Female | Multifocal seizures |
Follow-up (7 months after acute cerebral malaria Mild-moderate, diffuse parenchymal atrophy including the brainstem with moderate hydrocephalus ex vacuo |
Spastic quadriparesis and epilepsy |
| Patient 6 | 49 | Male | Coma |
Follow-up at 9 months post acute cerebral malaria Normal |
Developmental motor delays, attention deficit, hyperactive disorder |
| Patient 7 | 53 | Male | Coma |
Follow-up 7 months after acute cerebral malaria Normal |
Right lower extremity weakness in an upper motor neuron distribution. |
| Patient 8 | 36 | Female | Coma |
Follow-up at 18 months after acute cerebral malaria Normal |
Developmental motor delays with epilepsy |
| Patient 9 | 50 | Male | Right-sided focal motor seizures and coma |
Follow-up 6 months after acute cerebral malaria Low attenuation lesion in the left parietal region and a well-defined area of increased attenuation (similar to dural sinuses) without mass effect, suggestive of a vascular malformation |
Language regression and slight right hemiparesis. |
| Patient 10 | 26 | Male | Right-sided focal motor seizures and coma |
Follow-up completed 10 months after acute cerebral malaria Left parietal and right occipital focal atrophy. |
Developmental motor delay |
| Patient 11 | 21 | Male | Right-sided focal motor seizures and coma |
Follow-up completed 13 months after acute cerebral malaria Focal atrophy of left parietal region |
Language regression |
| Patient 12 | 9 | Female | Coma with posturing |
Follow-up 6 months after acute cerebral malaria Atrophy limited to the posterior fossa |
Developmental motor delay |
| Patient 13 | 17 | Female | Multifocal seizures and coma |
Follow-up at 13 months after cerebral malaria Bifrontal atrophy with dilation of the right trigone |
Left lower extremity weakness |
| Patient 14 | 32 | Male | Left-sided focal motor seizures and coma |
Follow-up 11 months after acute cerebral malaria Isolated vermian atrophy |
Attention deficit hyperactive disorder |
Children in protracted coma and with ultimately fatal cerebral malaria had CT findings of posterior fossa and brain stem region oedema, multiple large vessel infarcts, and basal ganglia oedema. One child’s head CT was dominated by diffuse atrophy in excess to volume loss which can be associated with treatment. This is suggestive of chronic changes despite reportedly normal neurologic development prior to the illness.
Among survivors with neurological sequelae, focal and multifocal atrophy was identified. These areas were congruent with anatomical regions affected by seizures as localized by EEGs at the time of the acute CM infection. Focal posterior fossa atrophy was found in 3 subjects, two with cerebellar hemispheric defects and isolated vermian atrophy in one. Despite clearly abnormal neurologic examinations, 3 subjects had normal head CTs. Communicating hydrocephalus was apparent in two cases. Examples of the most common findings are provided in Figures 4-7.
Figure 4. Case 3 - Five days status post coma onset in this patient with severe retinopathy confirmed cerebral malaria.


a and b: Diffuse prominence of the supratentorial subarachnoid spaces consistent with diffuse cerebral atrophy sparing the posterior fossa suggests this brain abnormality preceded the acute cerebral malaria infection. The presence of ventriculomegaly, including the 4th ventricle, out of proportion to sulcal prominence suggests hydrocephalus. The lack of mass effect or transependymal reabsorption of cerebral spinal fluid could be compatible with long standing hydrocephalus, although central atrophy greater then cortical atrophy could also be considered. Both would represent a preexisting condition. Note that this patient was reportedly developmentally normal prior to admission. This underscores the challenge presented by the unknown incidence of imaging abnormalities in this pediatric population potentially exposed to multiple infections and environmental factors prior to their acute episode of cerebral malaria.
Figure 7. Case 1 - Imaging at two separate time points was available in one pediatric patient with retinopathy confirmed cerebral malaria and prolonged coma.


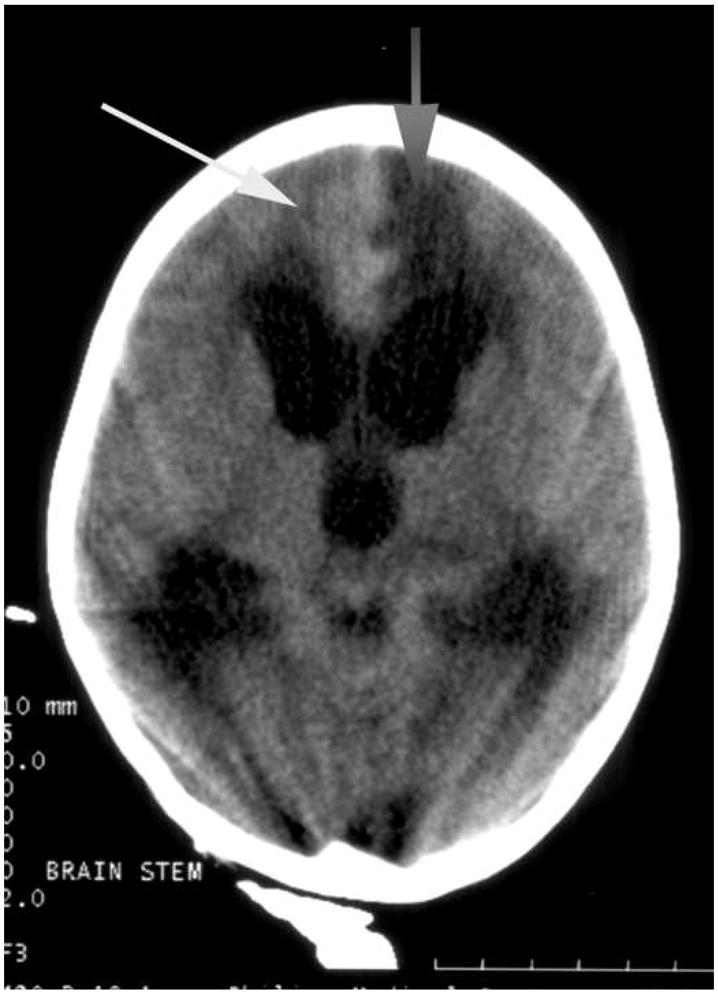
a. The initial scan three days after the onset of coma showed subacute cortical infarcts most notable in the left frontal region (gray arrow) with a small hemorrhagic component near the left frontal horn (white arrow).
b. The second scan eleven days later demonstrated worsening hydrocephalus. Note the prominent temporal tips and 4th ventricular enlargement consistent with a communicating form.
c. Also evident is evolution of the left frontal infarct (gray arrow) and interval development of additional infarcts including the right frontal lobe (white arrow). Evolution of the previously indentified acute hemorrhage in the left frontal lobe is also evident. No additional acute hemorrhages are noted.
Discussion
In this population of children with retinopathy-confirmed cerebral malaria, neuroimaging findings illustrated a range of pathologies consistent with the diversity described in autopsy studies (3). Among the three children imaged with protracted coma during their acute illness, large vessel infarcts, diffuse cerebral oedema involving the brainstem, and cerebral oedema sparing the brainstem were identified. The presence of atrophy on acute imaging in one child may represent incidental, pre-existing, and possibly unrelated CNS pathology. In the 11 children who survived cerebral malaria but had residual neurological sequelae, follow-up head CTs revealed structural lesions congruent with the initial presentation of focal and multifocal seizures.
Computerised tomography, although limited in what it can elucidate, offers some important insights in this group of paediatric patients. Animal models offer very limited insights into the pathogenesis of cerebral malaria, autopsy studies can only elucidate findings among non-survivors, and adult cerebral malaria, although more fully described in published imaging series, represents a different immunologic and pathologic process than paediatric cerebral malaria. Adverse neurologic outcomes in this population of paediatric CM patients occurred more commonly in those with acute seizures during their initial illness (15) and the CT findings indicate that both structural and functional deficits were associated with such seizures. More sophisticated techniques, including magnetic resonance imaging, with acute and follow-up studies are needed if we are to advance our understanding of the acute and chronic effects of severe malaria on the brain.
Conclusions
Fatal cerebral malaria is largely a paediatric condition characterized by CNS dysfunction and is unique from adult cerebral malaria which includes multisystem organ failure’s contribution to coma. This report offers unique insights into the imaging findings of paediatric cerebral malaria in a patient population in whom advanced diagnostic criteria were applied. It thus represents a more accurate perspective of cerebral malaria pathology than prior reports, which likely included a substantial proportion of other CNS disorders.
In patients with retinopathy-confirmed cerebral malaria, acute head CTs revealed findings consistent with autopsy studies. Follow-up images in survivors identified lesions that correlated with acute symptomatology and chronic deficits. More sophisticated imaging and longitudinal studies with serial images are needed to elucidate the pathogenic mechanisms of CNS injury in cerebral malaria.
Supplementary Material
Figure 2.

Fundus of a child with cerebral malaria. Papilledema with patches of peripheral retinal whitening.
Figure 5. Case 5 - Followup scan seven months after acute retinopathy confirmed cerebral malaria.
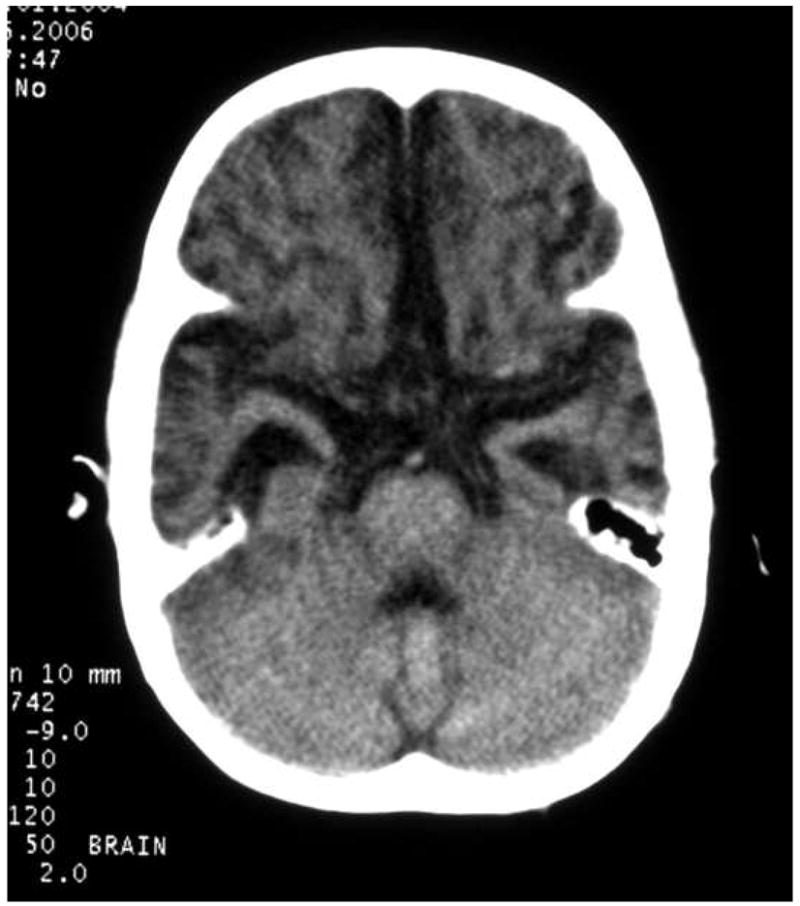

a. and b: Diffuse prominence of the supratentorial cisterns and sulci with ventriculomegaly. There were no clinical signs to suggest obstructive hydrocephalus, making supratentorial atrophy and hydrocephalus ex vacuo the favored interpretation. This is the most common imaging finding on follow-up imaging in pediatric patients who survived retinopathy confirmed cerebral malaria.
Figure 6. Case 2 - Acute imaging of retinopathy confirmed cerebral malaria.

Acute images were restricted to patients in prolonged coma and therefore likely reflect findings in the most severe cerebral malaria infections. Acute hydrocephalus with mass effect and cerebral edema were common findings in patients with prolonged coma from acute retinopathy confirmed cerebral malaria.
Acknowledgments
Funding Sources:
NIH/NINDS K23 NS046086-01
NIH/NIAID R01 AI034969-10AI
The Wellcome Trust (UK)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Warrell D, Gilles H. Essential Malariology. New York: Oxford University Press; 2002. [Google Scholar]
- 2.World Health Organization NP. Research Protocol for Measuring the Prevalence of Neurologic Disorders in Developing Countries. Geneva, Switzerland: World Health Organization; 1981. [Google Scholar]
- 3.Taylor TE, Fu WJ, Carr RA, et al. Differentiating the pathologies of cerebral malaria by postmortem parasite counts. Nat Med. 2004;10:143–145. doi: 10.1038/nm986. [DOI] [PubMed] [Google Scholar]
- 4.Lewallen S, Harding SP, Ajewole J, et al. A review of the spectrum of clinical ocular fundus findings in P. falciparum malaria in African children with a proposed classification and grading system. Trans R Soc Trop Med Hyg. 1999;93:619–622. doi: 10.1016/s0035-9203(99)90071-8. [DOI] [PubMed] [Google Scholar]
- 5.Lewallen S, White VA, Whitten RO, et al. Clinical-histopathological correlation of the abnormal retinal vessels in cerebral malaria. Arch Ophthalmol. 2000;118:924–928. [PubMed] [Google Scholar]
- 6.Beare NA, Taylor TE, Harding SP, Lewallen S, Molyneux ME. Malarial retinopathy: a newly established diagnostic sign in severe malaria. Am J Trop Med Hyg. 2006;75:790–797. [PMC free article] [PubMed] [Google Scholar]
- 7.Harding SP, Lewallen S, Beare NA, Smith A, Taylor TE, Molyneux ME. Classifying and grading retinal signs in severe malaria. Trop Doct. 2006;36(Suppl 1):1–13. doi: 10.1258/004947506776315781. [DOI] [PubMed] [Google Scholar]
- 8.CDC. Malaria. Atlanta. GA: 2006. [Google Scholar]
- 9.Birbeck G, Kaplan P, Molyneux M, et al. American Society of Tropical Medicine & Hygiene. Philadelphia, PA: Nov, 2007. The role of malaria retinopathy in cerebral malaria diagnosis and risk factors for adverse neuroligc outcomes. [Google Scholar]
- 10.Carter JA, Neville BG, White S, et al. Increased prevalence of epilepsy associated with severe falciparum malaria in children. Epilepsia. 2004;45:978–981. doi: 10.1111/j.0013-9580.2004.65103.x. [DOI] [PubMed] [Google Scholar]
- 11.Carter JA, Ross AJ, Neville BG, et al. Developmental impairments following severe falciparum malaria in children. Trop Med Int Health. 2005;10:3–10. doi: 10.1111/j.1365-3156.2004.01345.x. [DOI] [PubMed] [Google Scholar]
- 12.Idro R, Carter JA, Fegan G, Neville BG, Newton CR. Risk factors for persisting neurological and cognitive impairments following cerebral malaria. Arch Dis Child. 2006;91:142–148. doi: 10.1136/adc.2005.077784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Riganti M, Pongponratn E, Tegoshi T, Looareesuwan S, Punpoowong B, Aikawa M. Human cerebral malaria in Thailand: a clinico-pathological correlation. Immunol Lett. 1990;25:199–205. doi: 10.1016/0165-2478(90)90115-7. [DOI] [PubMed] [Google Scholar]
- 14.Durkin MS, Wang W, Shrout PE, et al. Evaluating a ten questions screen for childhood disability: reliability and internal structure in different cultures. J Clin Epidemiol. 1995;48:657–666. doi: 10.1016/0895-4356(94)00163-k. [DOI] [PubMed] [Google Scholar]
- 15.Birbeck G, Kaplan P, Molyneux M, et al. American Society of Tropical Medicine & Hygiene. Philadelphia, PA: 2007. The role of malaria retinopathy in cerebral malaria diagnosis and risk factors for adverse neuroligc outcomes. [Google Scholar]
- 16.Newton CR, Peshu N, Kendall B, et al. Brain swelling and ischaemia in Kenyans with cerebral malaria. Arch Dis Child. 1994;70:281–287. doi: 10.1136/adc.70.4.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Saavedra-Lozano J, Booth TN, Weprin BE, Ramilo O. Isolated cerebellar edema and obstructive hydrocephalus in a child with cerebral malaria. Pediatr Infect Dis J. 2001;20:908–911. doi: 10.1097/00006454-200109000-00017. [DOI] [PubMed] [Google Scholar]
- 18.Cordoliani YS, Sarrazin JL, Felten D, Caumes E, Leveque C, Fisch A. MR of cerebral malaria. AJNR Am J Neuroradiol. 1998;19:871–874. [PMC free article] [PubMed] [Google Scholar]
- 19.Ngoungou EB, Dulac O, Poudiougou B, et al. Epilepsy as a consequence of cerebral malaria in area in which malaria is endemic in Mali, West Africa. Epilepsia. 2006;47:873–879. doi: 10.1111/j.1528-1167.2006.00558.x. [DOI] [PubMed] [Google Scholar]
- 20.Gamanagatti S, Kandpal H. MR imaging of cerebral malaria in a child. Eur J Radiol. 2006;60:46–47. doi: 10.1016/j.ejrad.2006.05.014. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


