Abstract
Recent data in humans and animals suggest that assisted reproductive technology (ART) might affect the epigenetics of early embryogenesis and might cause birth defects. We report the first evidence, to our knowledge, that ART is associated with a human overgrowth syndrome—namely, Beckwith-Wiedemann syndrome (BWS). In a prospective study, the prevalence of ART was 4.6% (3 of 65), versus the background rate of 0.8% in the United States. A total of seven children with BWS were born after ART—five of whom were conceived after intracytoplasmic sperm injection. Molecular studies of six of the children indicate that five of the six have specific epigenetic alterations associated with BWS—four at LIT1 and one at both LIT1 and H19. We discuss the implications of our finding that ART is associated with human overgrowth, similar to the large offspring syndrome reported in ruminants.
Introduction
Beckwith-Wiedemann syndrome (BWS [MIM 130650]) is a congenital disorder that involves overgrowth and neoplasia. The primary features are macrosomia, macroglossia, midline abdominal wall defects, and predisposition to embryonal cancer (for review, see DeBaun et al. 2002). BWS has garnered considerable recent interest, because the molecular cause in most cases is epigenetic, rather than genetic (i.e., it involves stable alterations in DNA other than the sequence itself). In particular, BWS has been shown to involve loss of imprinting (LOI) of a group of imprinted genes on 11p15 (i.e., genes that show preferential expression from a specific parental allele). Approximately 15% of patients with BWS have aberrant methylation and imprinting of H19 (MIM 103280), an untranslated RNA, and IGF2 (MIM 147470), an autocrine growth factor (Weksberg et al. 1993; Reik et al. 1995); almost half of patients with BWS have aberrant methylation and imprinting of LIT1 (MIM 604115), an untranslated RNA within the KVLQT1 gene (MIM 192500) (Lee et al. 1999; Smilinich et al. 1999).
LOI can involve hypomethylation or hypermethylation, depending on the gene. In the case of H19, patients with BWS who have LOI show hypermethylation, which likely leads to aberrant activation of IGF2 (Moulton et al. 1994; Steenman et al. 1994). In the case of LIT1, patients with BWS show hypomethylation, which likely leads to aberrant silencing of p57KIP2 (MIM 600856) (Horike et al. 2000; Cleary et al. 2001), a cyclin-dependent kinase inhibitor. LIT1 is normally methylated on the maternal allele and unmethylated on the paternal allele, and, in patients with BWS who have an imprinting defect, the maternal allele is aberrantly hypomethylated. Recently, we performed an epigenotype-phenotype study of BWS and found that aberrant methylation of LIT1 is specifically associated with overgrowth and birth defects, and aberrant methylation of H19 is specifically associated with cancer risk (DeBaun et al. 2002).
Recent data suggest that assisted reproductive technology (ART) might affect the epigenetics of early embryogenesis and might cause birth defects—specifically, Angelman syndrome (MIM 105830) (Cox et al. 2002). After nuclear transfer or even exposure to in vitro environments by tissue culture, unusually large offspring have been born, and this has been referred to as “large offspring syndrome” (LOS) (Young et al. 1998). Direct evidence that overgrowth in ART offspring of animals may be linked to imprinting abnormalities came initially from studies showing that sheep affected with LOS that are derived from cultured embryos undergo LOI of the IGF2 receptor gene (Young et al. 2001), although this gene is not imprinted in humans. Also, preimplantation mouse embryos cultured in a chemically defined medium (M16) undergo epigenetic alterations in the H19, IGF2, Grb10, and Grb7 genes (Khosla et al. 2001); mouse embryos cultured in Whitten’s medium can undergo LOI of H19 (Doherty et al. 2000); and cloned mice show variations in several imprinted genes in the resulting embryos (Humpherys et al. 2001). Taken together, these results suggest that imprinting mutations might occur more frequently in infants that are born after ART. Some data from human ART also support the idea that birth defects may be increased. Specifically, low birth weight has been described after ART (Schieve et al. 2002), and intracytoplasmic sperm injection (ICSI) may be associated with an increased risk of major birth defects (Hansen et al. 2002). A small case-control series of ART/cryopreserved offspring reported a single case of BWS among 91 cases (Sutcliffe et al. 1995).
In 1994, we initiated the BWS Registry, to better define the phenotype, the incidence of cancer, and the genotype-phenotype correlations, if any. A detailed questionnaire was used, and clinical features were verified with medical records and pathology reports. After the start of the Registry, we noted several patients with BWS who were born after ART. However, we were unable to determine if the association was a chance occurrence, since the original BWS questionnaire did not ask specific questions regarding ART and since we did not know how many children with BWS were born after ART. We subsequently designed a new questionnaire, beginning in 2001, to systematically assess the method of conception, including questions about ART in children with BWS. Furthermore, to support our hypothesis, we assessed each patient born after ART for the presence of imprinting alterations associated with BWS.
Subjects and Methods
We established the BWS Registry in 1994 at the Genetic Epidemiology Branch of the National Cancer Institute (NCI), to understand the natural history of this syndrome and to determine if genotype-phenotype relationships exist. For those families who agreed to participate, a comprehensive questionnaire was completed; informed consent and medical-information–release documents were obtained. For the purposes of the present study, we defined a patient as having BWS if a clinical diagnosis of BWS had been made by a physician and the patient had at least two of the five most common features associated with BWS (Pettenati et al. 1986): (1) macroglossia, (2) birth weight and length >90th percentile, (3) hypoglycemia in the 1st mo of life, (4) ear creases or ear pits, and (5) midline abdominal wall defects (e.g., omphalocele, diastasis recti, and umbilical hernia). All positive phenotypic features were diagnosed by a physician and were documented in the questionnaire. The local physician defined “hemihypertrophy.” If a cancer was reported, the diagnosis was confirmed by review of the final pathology report and medical records.
In June 2001, the Registry was moved to the Washington University School of Medicine, where the Human Studies Committee approved the new questionnaire. We used the same research definition for both the Washington University BWS Registry and the NCI BWS Registry. The only difference between the two questionnaires is that new questions were added that pertained to the method of conception (natural vs. ART) and the types of ART—specifically, artificial insemination using biological father's sperm, artificial insemination using donor sperm, in vitro fertilization (IVF) using biological mother's egg and biological father's sperm, IVF using biological mother's egg and donor sperm, IVF using donor egg and biological father's sperm, and IVF using donor egg and donor sperm. If IVF was used, we asked what type of ICSI was used—ICSI using ejaculated sperm, ICSI using testicular sperm extraction (TESE), or ICSI using microsurgical epididymal sperm aspiration. For the reasons described above, we did not include BWS Registry patients from before 2001 in the prevalence estimate; however, we did include biological samples from these patients in our analysis of imprinting alterations.
Genomic DNA was prepared from peripheral-blood lymphocytes or biopsy samples, by standard proteinase K digestion and phenol extraction (Cui et al. 1998). This material is suitable for methylation assays involving DNA. A 1.8-kb PstI fragment of the H19 gene was analyzed for DNA methylation, by digestion with PstI and SmaI. For H19 (GenBank accession number M32053), 5 μg of DNA was digested at 25°C overnight with 40 U of SmaI, followed by an additional incubation at 37°C for 4 h with 40 U of PstI. The digested DNA was electrophoresed on a 1% agarose gel, was transferred to Zeta-Probe GT blotting membranes (Biorad), and was hybridized with the 1-kb PstI+SmaI fragment isolated from an H19 genomic clone and oligolabeled with [α-32P]dATP (DeBaun et al. 2002). For LIT1 (GenBank accession number AA155639), 5 μg of DNA was digested with BamHI and NotI at 37°C overnight. The digested DNA was electrophoresed on a 1% agarose gel, was transferred to Zeta-Probe GT blotting membranes (Biorad), and was hybridized with an EST 592241 probe oligolabeled with [α-32P]dATP (DeBaun et al. 2002). Uniparental disomy was excluded, as described elsewhere (DeBaun et al. 2002).
Results
Increased Frequency of IVF among Children with BWS
A total of seven children with BWS were identified as having been born after ART. All represented sporadic cases, with no family history. Six of the seven children were born after IVF using biological mother's egg and biological father's sperm. One child was born after IVF using donor egg and biological father’s sperm. Four of the seven children were born after ICSI using ejaculated sperm, one was born after ICSI using TESE, and two did not involve ICSI.
Three patients were identified from the more recently established Washington University BWS Registry in 2001, and the remaining four children were identified from the NCI BWS Registry. In the Washington University BWS Registry, the prevalence of ART was 4.6% (3 of 65). In the United States in 1999, for which the most recent data are available, there were 0.76% (30,285 of 3,959,417) live births resulting from ART (see the Centers for Disease Control [CDC] Web site [CDC's Reproductive Health Information Source, 1999 Assisted Reproductive Technology Success Rates]) (Ventura et al. 2001, p. 25). On the basis of this background rate, we estimate at least a sixfold increase in BWS in children born after ART, compared with the general population. Five of the patients with BWS who were born after IVF were conceived by ICSI, and 42% of IVF cycles in the general population were ICSI related (see the CDC Web site [CDC's Reproductive Health Information Source, 1999 Assisted Reproductive Technology Success Rates]). Despite identifying four patients born after ART in the NCI BWS Registry, we were unable to ascertain the prevalence of ART in the NCI group because of the nonsystematic assessment of ART in probands (n=279).
Clinical Features of Children with BWS Who Were Born after ART
No clinical feature occurred at a different proportion than expected when comparing between the children with BWS who were born after ART and the children in the BWS Registry as a whole. The frequencies of macrosomia (71% [5 of 7]; P=.587), hypoglycemia (29% [2 of 7]; P=.39), abdominal wall defects (86% [6 of 7]; P=.77), macroglossia (86% [6 of 7]; P=.957), ear creases or ear pits (43% [3 of 7]; P=.306), and hemihypertrophy (43% [3 of 7]; P=.90) were no different than previously reported from the BWS Registry (DeBaun et al. 2002).
Imprinting Alterations in Patients with BWS Who Were Born after ART
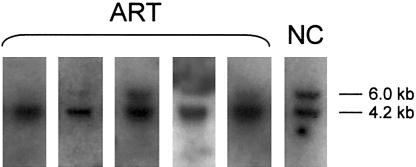
Samples were obtained from six of seven children with BWS who were born after ART. Five of the six children showed abnormal imprinting of LIT1, as indicated by hypomethylation of the LIT1 differentially methylated region (DMR) (table 1 and fig. 1). One child of six tested also showed abnormal imprinting of H19, as indicated by hypermethylation of the H19 DMR (data not shown). Only one of the six children with BWS who were born after ART had a normal methylation pattern at both LIT1 and H19.
Table 1.
Clinical and Molecular Characteristics of Patients with BWS Who Were Conceived by IVF[Note]
|
AberrantMethylation |
||||||||
| Patient | Macrosomia | Hypoglycemia | Macroglossia | Ear Anomalies | Abdominal Wall Defect | Hemihypertrophy | LIT1 | H19 |
| 1 | Yes | No | Yes | Yes | Yes | No | Yes | No |
| 2 | No | No | Yes | No | Yes | No | Yes | No |
| 3 | Yes | No | Yes | No | No | Yes | Yes | Yes |
| 4 | Yes | Yes | Yes | No | Yes | No | Yes | No |
| 5 | Yes | Yes | No | Yes | Yes | Yes | No | No |
| 6 | No | No | Yes | Yes | Yes | Yes | ND | ND |
| 7 | Yes | Yes | Yes | Yes | Yes | No | Yes | No |
Note.— ND = not done (no sample available).
Figure 1.
Analysis of methylation at the Lit1 CpG island. Methylation-sensitive Southern blots of LIT1 on DNA from five children with BWS who were conceived using ART and from a normal control (NC). The upper band (6.0 kb) is methylated, and the lower band (4.2 kb) is unmethylated.
Discussion
We have provided the first evidence that ART is associated with a human overgrowth syndrome, BWS. In the Washington University BWS Registry, ∼5% (3 of 65) of the children with BWS were born after ART, whereas, if no association existed, we would have expected no children with BWS to have been born after ART, suggesting at least a sixfold increase in the rate of ART in children with BWS. Further support for the association between BWS and ART is based on the observation that four additional patients with BWS in the NCI Registry were conceived after ART—an observation made despite the fact that this Registry was not designed to determine if there was an association between BWS and ART. We believe that a 5% prevalence of children conceived after ART is most likely an underestimate, in that the BWS questionnaire has asked specific questions regarding ART in only 2 of the past 8 years of the BWS Registry’s existence. In addition to the epidemiologic evidence that ART is associated with BWS, we have shown biological data strengthening this association. Five of the six children with BWS who were born after ART had imprinting mutations of the LIT1 and/or H19 genes, two distinct methylation alterations specific to BWS. These results confirm the clinical diagnosis of BWS and demonstrate a specific imprinting defect in most patients with BWS who were born after ART.
On the basis of our findings, as well as the observation that Angelman syndrome occurred after ICSI (Cox et al. 2002), sufficient evidence exists to suggest that some aspect of the ART procedure increases the frequency of epigenetic abnormalities leading to congenital malformation syndromes. The incidence of cancer in children with BWS who are <4 years of age is 0.02 per 100 patient years (DeBaun and Tucker 1998). Thus, the small number of patients with BWS who were born after ART did not allow assessment of an association between ART and cancer. However, given the known association between BWS, cancer, and an increased frequency of H19 methylation abnormalities in children with BWS, we postulate that ART is associated with embryonal cancers of childhood in which methylation abnormalities of H19 have been implicated (Wilms tumor, hepatoblastoma, and rhabdomyosarcoma).
In both Angelman syndrome (Cox et al. 2002) and BWS after ICSI, it is the maternal allele that is affected (unmethylated). This makes it unlikely that the problem is related to sperm differentiation. It is more likely that ICSI or some other aspect of the ART used disturbs methylation of the maternal genome in the oocyte or early embryo. Given that 42% of ART procedures in the general population are ICSI related, we cannot discriminate between ICSI and other aspects of the procedure in the association with BWS; this must come from a large-scale study.
As expected when a rare congenital malformation syndrome is studied with a registry, certain limitations are inherent in our analysis. We could not exclude the possibility that parents of children with BWS who were born after ART were more likely to join the Registry than parents born after natural birth were. However, we made no effort to recruit parents of children born after ART for the Washington University BWS Registry. Another limitation is the small number of patients in the Washington University BWS Registry (n=65), raising the possibility of a chance association between ART and BWS. Although the Washington University BWS Registry has a small number of patients, we created the new questionnaire specifically to test the hypothesis that BWS was associated with ART. We believe strongly that our data reflect a real association and warrant further investigation into the biological basis, since, at this point, we do not know what specific step (or steps) of ART is responsible for the association with BWS. Potential factors could include culture conditions for the ovum, length of exposure to specific media or growth factors therein, the stage of differentiation of sperm at the time of ICSI, or ICSI itself, since some of the patients had undergone ICSI as part of the ART procedure.
The clinical implications of our finding that ART is associated with a congenital malformation syndrome, in addition to recent data from other studies, are unknown at present. Further biological studies will be required in order to understand the pathogenesis of this association and what precautions can be taken to prevent the occurrence of these syndromes.
Acknowledgments
This work was supported by grant CA54358 from the National Institutes of Health and grant FY00-668 from the Birth Defects March of Dimes Foundation (both to A.P.F.), by the Robert Wood Johnson Minority Faculty Development Program and the Doris Duke Clinical Scientific Foundation (support to M.R.D.), and by support of the NCI BWS Registry from the Genetic Epidemiology Branch (NCI). We thank C. Terrill and M. Graber for assistance.
Electronic-Database Information
Accession numbers and URLs for data presented herein is as follows:
- CDC's Reproductive Health Information Source, 1999 Assisted Reproductive Technology Success Rates, http://www.cdc.gov/nccdphp/drh/ART99/99nation.htm
- GenBank, http://www.ncbi.nlm.nih.gov/Genbank/ (for human H19 probe [accession number M32053] and human LIT1 probe [accession number AA155639])
- Online Mendelian Inheritance in Man (OMIM), http://www.ncbi.nlm.nih.gov/Omim/ (for BWS [MIM 130650], IGF2 [MIM 147470], H19 [MIM 103280], p57KIP2 [MIM 600856], KVLQT1 [MIM 192500], LIT1 [MIM 604115], and Angelman syndrome [MIM 105830])
References
- Cleary MA, van Raamsdonk CD, Levorse J, Zheng BH, Bradley A, Tilghman SM (2001) Disruption of an imprinted gene cluster by a targeted chromosomal translocation in mice. Nat Genet 29:78–82 [DOI] [PubMed] [Google Scholar]
- Cox GF, Burger J, Lip V, Mau UA, Sperling K, Wu BL, Horsthemke B (2002) Intracytoplasmic sperm injection may increase the risk of imprinting defects. Am J Hum Genet 71:162–164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui H, Horon IL, Ohlsson R, Hamilton SR, Feinberg AP (1998) Loss of Imprinting in normal tissue of colorectal cancer patients with microsatellite instability. Nat Med 4:1276–1280 [DOI] [PubMed] [Google Scholar]
- DeBaun MR, Niemitz EL, McNeil DE, Brandenburg SA, Lee MP, Feinberg AP (2002) Epigenetic alterations of H19 and LIT1 distinguish patients with Beckwith-Wiedemann syndrome with cancer and birth defects. Am J Hum Genet 70:604–611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeBaun MR, Tucker MA (1998) Risk of cancer during the first four years of life in children from the Beckwith-Wiedemann Syndrome Registry. J Pediatr 132:398–400 [DOI] [PubMed] [Google Scholar]
- Doherty AS, Mann MR, Tremblay KD, Bartolomei MS, Schultz RM (2000) Differential effects of culture on imprinted H19 expression in the preimplantation mouse embryo. Biol Reprod 62:1526–1535 [DOI] [PubMed] [Google Scholar]
- Hansen M, Kurinczuk JJ, Bower C, Webb S (2002) The risk of major birth defects after intracytoplasmic sperm injection and in vitro fertilization. N Engl J Med 346:725–730 [DOI] [PubMed] [Google Scholar]
- Horike S, Mitsuya K, Meguro M, Kotobuki N, Kashiwagi A, Notsu T, Schulz TC, Shirayoshi Y, Oshimura M (2000) Targeted disruption of the human LIT1 locus defines a putative imprinting control element playing an essential role in Beckwith-Wiedemann syndrome. Hum Mol Genet 9:2075–2083 [DOI] [PubMed] [Google Scholar]
- Humpherys D, Eggan K, Akutsu H, Hochedlinger K, Rideout WM III, Biniszkiewicz D, Yanagimachi R, Jaenisch R (2001) Epigenetic instability in ES cells and cloned mice. Science 293:95–97 [DOI] [PubMed] [Google Scholar]
- Khosla S, Dean W, Brown D, Reik W, Feil R (2001) Culture of preimplantation mouse embryos affects fetal development and the expression of imprinted genes. Biol Reprod 64:918–926 [DOI] [PubMed] [Google Scholar]
- Lee MP, DeBaun MR, Mitsuya K, Galonek HL, Brandenburg S, Oshimura M, Feinberg AP (1999) Loss of imprinting of a paternally expressed transcript, with antisense orientation to KVLQT1, occurs frequently in Beckwith-Wiedemann syndrome and is independent of insulin-like growth factor II imprinting. Proc Natl Acad Sci USA 96:5203–5208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moulton T, Crenshaw T, Hao Y, Moosikasuwan J, Lin N, Dembitzer F, Hensle T, Weiss L, McMorrow L, Loew T, Kraus W, Gerald W, Tycko B (1994) Epigenetic lesions at the H19 locus in Wilms' tumour patients. Nat Genet 7:440–447 [DOI] [PubMed] [Google Scholar]
- Pettenati MJ, Haines JL, Higgins RR, Wappner RS, Palmer CG, Weaver DD (1986) Wiedemann-Beckwith syndrome: presentation of clinical and cytogenetic data on 22 new cases and review of the literature. Hum Genet 74:143–154 [DOI] [PubMed] [Google Scholar]
- Reik W, Brown KW, Schneid H, Le Bouc Y, Bickmore W, Maher ER (1995) Imprinting mutations in the Beckwith-Wiedemann syndrome suggested by an altered imprinting pattern in the IGF2-H19 domain. Hum Mol Genet 4:2379–2385 [DOI] [PubMed] [Google Scholar]
- Schieve LA, Meikle SF, Ferre C, Peterson HB, Jeng G, Wilcox LS (2002) Low and very low birth weight in infants conceived with use of assisted reproductive technology. N Engl J Med 346:731–737 [DOI] [PubMed] [Google Scholar]
- Smilinich NJ, Day CD, Fitzpatrick GV, Caldwell GM, Lossie AC, Cooper PR, Smallwood AC, Joyce JA, Schofield PN, Reik W, Nicholls RD, Weksberg R, Driscoll DJ, Maher ER, Shows TB, Higgins MJ (1999) A maternally methylated CpG island in KvLQT1 is associated with an antisense paternal transcript and loss of imprinting in Beckwith-Wiedemann syndrome. Proc Natl Acad Sci USA 96:8064–8069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steenman MJ, Rainier S, Dobry CJ, Grundy P, Horon IL, Feinberg AP (1994) Loss of imprinting of IGF2 is linked to reduced expression and abnormal methylation of H19 in Wilms' tumour. Nat Genet 7:433–439 [DOI] [PubMed] [Google Scholar]
- Sutcliffe AG, D'Souza SW, Cadman J, Richards B, McKinlay IA, Lieberman B (1995) Minor congenital anomalies, major congenital malformations and development in children conceived from cryopreserved embryos. Hum Reprod 10:3332–3337 [DOI] [PubMed] [Google Scholar]
- Ventura SJ, Martin JA, Curtin SC, Menacker F, Hamilton BE (2001) Births: final data for 1999. Natl Vital Stat Rep 49:1–100 [PubMed] [Google Scholar]
- Weksberg R, Shen DR, Fei YL, Song QL, Squire J (1993) Disruption of insulin-like growth factor 2 imprinting in Beckwith-Wiedemann syndrome. Nat Genet 5:143–150 [DOI] [PubMed] [Google Scholar]
- Young LE, Fernandes K, McEvoy TG, Butterwith SC, Gutierrez CG, Carolan C, Broadbent PJ, Robinson JJ, Wilmut I, Sinclair KD (2001) Epigenetic change in IGF2R is associated with fetal overgrowth after sheep embryo culture. Nat Genet 27:153–154 [DOI] [PubMed] [Google Scholar]
- Young LE, Sinclair KD, Wilmut I (1998) Large offspring syndrome in cattle and sheep. Rev Reprod 3:155–163 [DOI] [PubMed] [Google Scholar]