Abstract
Purpose
While trimodality therapy for esophageal cancer has improved patient outcomes, surgical complication rates remain high. The goal of this study was to identify modifiable factors associated with postoperative complications after neoadjuvant chemoradiation.
Methods and Materials
From 1998 to 2011, 444 patients were treated at our institution with surgical resection after chemoradiation. Postoperative (pulmonary, gastrointestinal [GI], cardiac, wound healing) complications were recorded up to 30 days postoperatively. Kruskal-Wallis tests and χ2 or Fisher exact tests were used to assess associations between continuous and categorical variables. Multivariate logistic regression tested the association between perioperative complications and patient or treatment factors that were significant on univariate analysis.
Results
The most frequent postoperative complications after trimodality therapy were pulmonary (25%) and GI (23%). Lung capacity and the type of radiation modality used were independent predictors of pulmonary and GI complications. After adjusting for confounding factors, pulmonary and GI complications were increased in patients treated with 3-dimensional conformal radiation therapy (3D-CRT) versus intensity modulated radiation therapy (IMRT; odds ratio [OR], 2.018; 95% confidence interval [CI], 1.104–3.688; OR, 1.704; 95% CI, 1.03–2.82, respectively) and for patients treated with 3D-CRT versus proton beam therapy (PBT; OR, 3.154; 95% CI, 1.365–7.289; OR, 1.55; 95% CI, 0.78–3.08, respectively). Mean lung radiation dose (MLD) was strongly associated with pulmonary complications, and the differences in toxicities seen for the radiation modalities could be fully accounted for by the MLD delivered by each of the modalities.
Conclusions
The radiation modality used can be a strong mitigating factor of postoperative complications after neoadjuvant chemoradiation.
Introduction
Esophageal carcinoma is relatively rare but deadly, causing 2% of all cancer-related deaths (1). Despite advances in diagnosis and treatment, the overall 5-year survival rate has improved from 3% to only 15% since the mid-1970s (1). Currently, radiation therapy, surgery, and chemotherapy are the main forms of treatment for esophageal cancer. While trimodality therapy for esophageal cancer has improved patient outcomes, surgical complication rates remain high. Up to 50% of all surgically treated esophageal cancer patients experience a severe postoperative complication within 30 days of surgery (2). Moreover, studies show that acute postoperative complications exert a long-lasting negative influence on quality of life (3), and also contribute to worse prognosis after surgical resection (4). These observations highlight the need to identify factors affecting not only survival, but also the risk of surgical complications.
In the era of neoadjuvant chemoradiation prior to surgical resection, little remains known regarding how chemoradiation (CRT) before surgery contributes to the risk of postoperative complications. One study suggested that patients with resectable esophageal cancer who undergo CRT before surgery are at substantial risk for postoperative pulmonary complications (5), which remain the most common serious morbidity and leading cause of postoperative mortality after esophagectomy (6). Given the concern with high morbidity of esophagectomy, especially in the era of trimodality therapy, many studies have attempted to determine risk factors for major postoperative morbidity (7, 8). Unfortunately, the predictors identified were largely unmodifiable patient characteristics such as functional capacity of the lung, age, and comorbidities.
The goal of this study was to identify modifiable clinical predictors of postoperative complications in esophageal cancer patients treated with trimodality therapy.
Methods and Materials
Study cohort
This was a retrospective analysis of 444 patients with esophageal cancer treated at our institution from 1998 to 2011 with surgical resection after CRT. Patients were included in the analyses if they had no distant metastases at presentation and were treated with preoperative concurrent CRT with or without induction chemotherapy followed by surgery. A total of 208, 164, and 72 patients received 3-dimensional conformal radiation therapy (3D-CRT; 1998–2008), intensity modulated RT (IMRT; 2004–2011), and proton beam therapy (PBT; 2006–2011), respectively. Staging was determined using the sixth edition (2002) of the American Joint Committee on Cancer TNM staging system.
Treatment and radiation planning
Patients were typically treated with neoadjuvant CRT with or without induction chemotherapy to a median dose of 50.4 Gy at 1.8 Gy/fraction. The 3D-CRT and IMRT plans were generated using the Pinnacle planning system (Phillips Medical Systems), with beam arrangements optimized for each patient. IMRT techniques have evolved over time at our institution, with the most recent approach using a modification of the “fire-fly technique” with posteriorly placed beam arrangements. For patients treated with PBT, treatment planning was performed with the Eclipse treatment planning system (Varian Medical Systems), based on passive scattering technique. The most common beam arrangement was a posterior-anterior and a left lateral beams. Chemotherapy was often administered in combinations of 5-flurouracil and taxane or with platinum-based compounds. At 5–6 weeks after neoadjuvant therapy completion, most patients underwent restaging and were evaluated for surgical management.
Outcome measures/perioperative complications
The primary end point for the present data analyses was the occurrence of perioperative complications. All perioperative complications, including pulmonary, gastrointestinal (GI), cardiac, and wound complications, were recorded up to 30 days postoperatively. Perioperative pulmonary complications were specifically noted to encompass pneumonia, Acute Respiratory Distress Syndrome (ARDS), pleural effusions, and/or respiratory insufficiency. Perioperative GI complications were defined as anastomotic leak, ileus, fistula, obstruction, or need for J-tube placement. Cardiac complications included atrial fibrillation, any non-specified arrhythmia, myocardial infarction, or development of congestive heart failure. Moreover, the median length of hospital stay, readmission to the hospital within 60 days, and death within 30 days of surgery were recorded.
Statistical analysis
Kruskal-Wallis, χ2, or Fisher exact test was used to assess associations between continuous and categorical variables and the radiation modalities, respectively. Multivariate logistic regression models were used to assess the association between perioperative complications and radiation modality, adjusting for other significant patient/disease characteristics. Only the patient characteristics that were found to have a P value of <.1 on univariate analyses were assessed. All pre- and postradiation forced expiratory volume in 1 second (FEV1) and pre- and postradiation diffusing capacity of the lung for carbon monoxide (DLCO) measurements were highly correlated. If more than one of these measurements were associated with any perioperative complication in bivariate analysis, then only the one variable with the most observations was used in the multivariate logistic regression models.
To identify the dosimetric predictors for the risk of perioperative pulmonary complications and to assess potential differences in risk based on radiation modality, lung and heart dose—volume histograms (DVH; n=392 patients) were generated using 1-Gydose bins. The mean lung dose (MLD) and mean heart dose (MHD) were computed for each patient using the DVH data provided. We used the mean dose in the final model because it optimally summarizes the entire DVH and is therefore a more accurate variable for analysis. The incidence of postoperative pulmonary toxicity was then plotted against the MLD for the entire cohort.
Results
Patient, tumor, and treatment characteristics
Table 1 summarizes patient, tumor, and treatment characteristics of this study cohort. Most patients received planned surgery with Ivor-Lewis esophagectomy, but other types of surgical procedures included 3-field, transhiatal, gastrectomy, transthoracic, and cervical surgery and minimally invasive esophagectomy (Table 1).
Table 1.
Patient, tumor, and treatment characteristics
| Characteristic | Overall cohort (n = 444) |
3D-CRT (n = 208) |
IMRT (n = 164) |
Protons (n = 72) |
P value |
|---|---|---|---|---|---|
| Median Age (range) | 61 (22–79) | 60 (22–79) | 60 (27–78) | 63 (29–76) | .13 |
| Sex | .93 | ||||
| Female | 44 (9.9%) | 22 (10.6%) | 17 (10.3%) | 5 (6.9%) | |
| Male | 400 (90.1%) | 186 (89.4%) | 148 (89.7%) | 67 (93.1%) | |
| Histology | .163 | ||||
| Adenocarcinoma | 395 (89.0%) | 179 (86.1%) | 149 (90.9%) | 67 (93.1%) | |
| Squamous cell carcinoma | 40 (9.0%) | 21 (10.1%) | 14 (8.5%) | 5 (6.9%) | |
| Other | 9 (2.0%) | 8 (3.8%) | 1 (0.6%) | 0 (0%) | |
| Lesion location | .314 | ||||
| Lower | 413 (93.0%) | 190 (91.3%) | 152 (92.7%) | 71 (98.6%) | |
| Middle | 27 (6.1%) | 16 (7.7%) | 10 (6.1%) | 1 (1.4%) | |
| Upper | 4 (0.9%) | 2 (1.0 %) | 2 (1.2%) | 0 (0%) | |
| KPS (%) | .085 | ||||
| 100 | 27 (6.1%) | 18 (8.7%) | 6 (3.7%) | 3 (4.2%) | |
| 90 | 219 (49.3%) | 104 (50%) | 75 (45.7%) | 40 (55.6%) | |
| 80 | 180 (40.5%) | 78 (37.5%) | 73 (44.5%) | 29 (40.3%) | |
| ≤70 | 18 (4.1%) | 8 (3.8%) | 10 (6.1%) | 0 (0%) | |
| No. of patients with stage shown (%) | |||||
| I | 7 (1.6%) | 1 (0.5%) | 3 (1.8%) | 3 (4.2%) | .319 |
| II | 164 (36.9%) | 83 (39.9%) | 56 (34.1%) | 25 (34.7%) | |
| III | 252 (56.8%) | 113 (54.3%) | 99 (60.4%) | 40 (55.6%) | |
| IVa | 21 (4.7%) | 11 (5.2%) | 6 (3.7%) | 4 (5.6%) | |
| No. of patients who received induction chemotherapy (%) | |||||
| Yes | 221 (49.8%) | 127 (61.1%) | 67 (40.9%) | 27 (37.5%) | <.001 |
| No | 223 (50.2%) | 81 (38.9%) | 97 (59.1%) | 45 (62.5%) | |
| Surgery intent | |||||
| Planned | 412 (92.8%) | 195 (93.8%) | 147 (89.1%) | 70 (97.2%) | >.06 |
| Salvage | 32 (7.2%) | 13 (6.3%) | 18 (10.9%) | 2 (2.8%) | |
| Median dose (range) | 50.4 Gy (41–59.4) | 50.4 Gy (41–59.4) | 50.4 Gy (45–50.4) | 50.4 CGE (45–50.4) | >.05 |
| Surgery type | <.001 | ||||
| Ivor-Lewis | 341 (76.8%) | 142 (70.2%) | 129 (78.7%) | 66 (91.7%) | |
| Other* | 103 (23.2%) | 62 (29.8%) | 35 (21.3%) | 6 (8.3%) |
Abbreviations: 3D-C\RT = 3-dimensional conformal radiation therapy; IMRT = intensity modulated radiation therapy; KPS = Karnofsky performance status.
Other surgery types include 3-field, transhiatal, gastrectomy, transthoracic, cervical, and minimally invasive esophagectomy.
Perioperative complications—overall outcomes
The 2 most common postoperative complications after esophagectomy in the entire cohort were: (1) pulmonary (25.2%); and (2) GI (23%). The overall incidence rates of cardiac and wound complications in the entire cohort were 15.3% and 9.9%, respectively. Approximately 13.5% of the patients in the study cohort required readmission to the hospital within 60 days after discharge. Eleven of 444 patients (2.5%) experienced postoperative death within 30 days (see supplementary Fig. E1).
Predictors of perioperative complications—univariate analysis
On univariate analysis, factors associated with pulmonary complications included preradiation DLCO (P=.0069), postradiation DLCO (P=.0105), postradiation FEV1 (P=.0266), and radiation modality (P=.019). Other factors, including age at diagnosis, comorbidities, smoking history, pretreatment body mass index, tumor stage, tumor location, and type of surgery and whether the patient received induction chemotherapy, were not significantly associated with the risk of pulmonary complications (supplementary Table E1).
In terms of GI complications, radiation modality (n=444) and preradiation FEV1 (n=299) were significantly associated with the incidence of GI complication (P=.04 and P=.0433, respectively). In addition, the location of the tumor (nondistal) and the type of surgery (not Ivor-Lewis) were statistically significantly associated with higher GI complication rate (P=.014 and P=.013, respectively) (supplementary Table E1).
Factors associated with cardiac complications included age at diagnosis (P<.0001), atrial fibrillation (P=.0004), and post-radiation FEV1 (P=.0194). On univariate analysis, no factors were found to be associated with wound complications.
We recognized that patients treated with various radiation modalities were treated in different time periods. To determine whether there were significant trends in the probability of complications over time, we examined the proportion of pulmonary or GI complications within 30 days of surgery over the time span of 1998–2011. There were no significant trends in complications over time (P>.05) (Data not shown).
Predictors of perioperative complications—multivariate analysis
Radiation modality (P=.0226) and preradiation DLCO (P=.0016) were found to be independent factors associated with the risk of postoperative pulmonary toxicity, after adjusting for radiation modality, preradiation DLCO, and year of surgery. When comparing among the 3 different types of radiation modalities, there was a significant increase in postoperative pulmonary complications for 3D-CRT compared to IMRT (odds ratio [OR], 4.097; 95% confidence interval [CI], 1.366–12.286) and for 3D-CRT versus PBT (OR, 9.127; 95% CI, 1.834–45.424) but not for IMRT compared to PBT (OR, 2.228; 95% CI, 0.863–5.755) after adjusting for pre-RT DLCO level (Table 2).
Table 2.
Comparison of risk of pulmonary and GI complications between radiation modalities
| Risk | Modality | OR, Estimate |
Lower 95% CL |
Upper 95% CL |
|---|---|---|---|---|
| Pulmonary complication* | 3DCRT vs IMRT | 4.097 | 1.366 | 12.286 |
| 3DCRT vs PBT | 9.127 | 1.834 | 45.424 | |
| IMRT vs PBT | 2.228 | 0.863 | 5.755 | |
| GI complication† | 3DCRT vs IMRT | 2.255 | 0.951 | 5.349 |
| 3DCRT vs PBT | 2.311 | 0.690 | 7.740 | |
| IMRT vs PBT | 1.025 | 0.467 | 2.249 |
Abbreviations: CL = confidence limit; GI = gastrointestinal; IMRT = intensity modulated radiation therapy; OR, = odds ratio; PBT = proton beam therapy.
Pulmonary multivariable model variables includes radiation modality, measurement of preradiation therapy diffusing capacity of the lung for carbon monoxide, and year of surgery.
GI multivariable model includes radiation modality, surgery type, tumor location, and year of surgery.
On multivariate analysis for GI complications, only radiation modality (P=.1818), type of surgery (P=.1033), and tumor locations (P=.1409) were found to be marginally associated with the rate of GI complications. After adjusting for tumor location, surgery type, radiation modality, and year of surgery, postoperative GI complications were marginally increased in patients treated with 3D-CRT versus those treated with IMRT (OR, 2.255; 95% CI, 0.951–5.349) and marginally increased in patients treated with 3D-CRT versus PBT (OR, 2.311; 95% CI, 0.690, 7.740), but not for IMRT versus PBT (OR, 1.025; 95% CI, 0.467–2.249) (Table 2). Cardiac and wound complications were not examined in multivariate analyse, because of the low rate of events.
Impact of radiation modality on perioperative complications
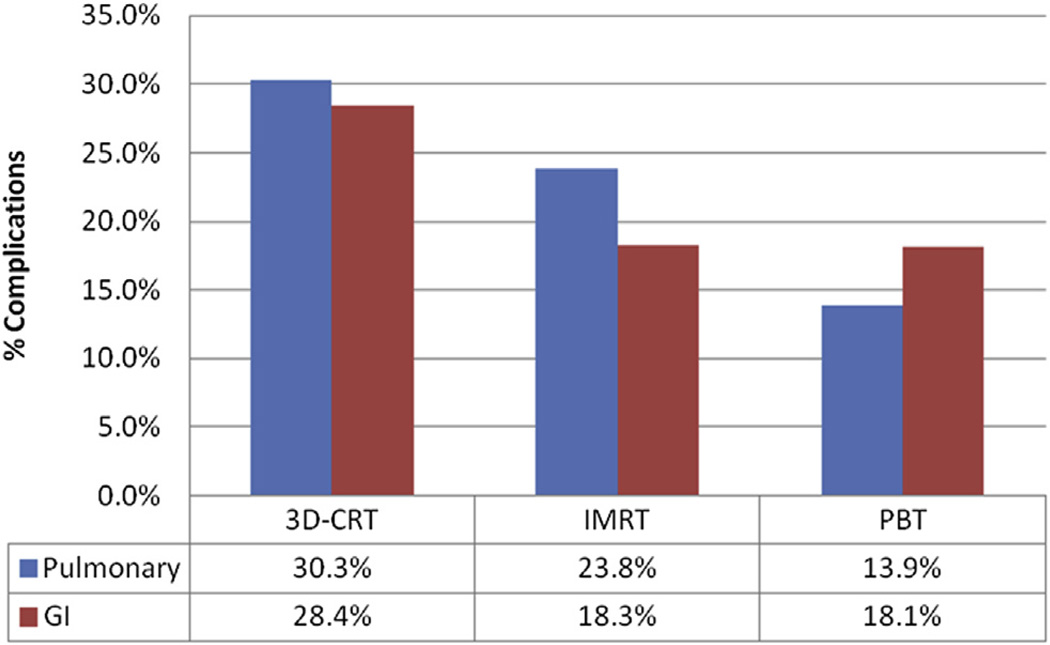
As shown in Figure 1, both GI and pulmonary complication rates were highest among the 3D-CRT group, second highest among the IMRT group, and lowest among those treated with PBT (P=.019 for pulmonary complications; P=.04 for GI complications on univariate analysis). Differences in specific pulmonary and GI complications rates stratified by radiation modality are shown in Table 3. Radiation modality was not associated with the risk of cardiac or wound complications within 30 days of surgery (P>.05). However, the median length of hospital stay was significantly different among radiation modalities (12, 10, and 8 days for 3D-CRT, IMRT, and PBT, respectively, P<.0001) (supplementary Table E2).
Fig. 1.
Percentage of incidence of pulmonary and GI complications.
Table 3.
Rates of specific pulmonary and GI complications by radiation modality
| Complication | Overall (n) %* |
3D-CRT (n) %† |
IMRT (n) %‡ |
PBT (n) %§ |
P Value |
|---|---|---|---|---|---|
| Pulmonary | |||||
| Pneumonia | (64) 14.4% | (30) 14.4% | (27) 16.5% | (7) 9.7% | .43 |
| ARDS | (9) 2.0% | (6) 2.9% | (2) 1.2% | (1) 1.4% | .604 |
| Pleural effusion | (40) 9.0% | (20) 9.6% | (16) 9.8% | (4) 5.6% | .598 |
| Respiratory insufficiency | (22) 4.9% | (18) 8.7% | (3) 1.8% | (1) 1.4% | .004 |
| GI | |||||
| Leakage | (49) 11.0% | (24) 11.5% | (16) 9.8% | (9) 12.5% | .004 |
| Ileus | (28) 6.3% | (20) 9.6% | (5) 3.0% | (3) 4.2% | .031 |
| Fistula | (5) 1.1% | (2) 1.0% | (3) 1.8% | (0) 0% | .588 |
| Obstruction | (5) 1.1% | (4) 1.9% | (1) 0.6% | (0) 0% | .477 |
| J-tube | (15) 3.4% | (9) 4.3% | (6) 3.7% | (0) 0% | .197 |
Abbreviation: ARDS = Acute Respiratory Distress Syndrome. All other abbreviations are shown in Table 2.
n=444.
n=208.
n=164.
n=72.
Impact of radiation modality on pulmonary complications
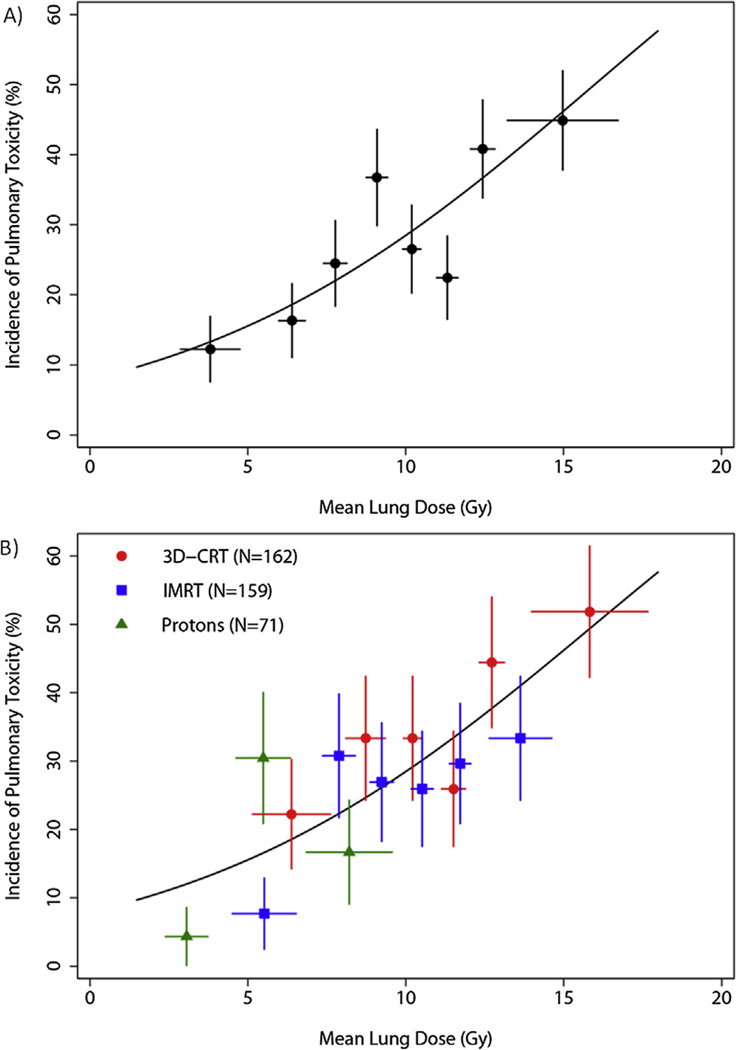
We hypothesized that the effect of radiation modality on the incidence of pulmonary complications may be mediated by MLD, rather than by the radiation modalities themselves. This is evident by the significant differences in the MLD between the different modalities (supplementary Fig. E2b). An even larger difference was seen in the heart dose, as shown by the differential MHDs, comparing the different modalities (supplementary Fig. E2c). We evaluated the relative contributions of the MHD and MLD to the risk of perioperative pulmonary complications. When MLD and MHD were added to the multivariate analysis after adjusting for DLCO and radiation modality, the effect of radiation modality was no longer significant (P=.189), whereas MLD was the only independent predictor of pulmonary complications (P=.044). We performed a logistic regression of the probability of pulmonary complications and the MLD, and determined that the overall probability of complications was fully related to the MLD contribution of each of the radiation modalities (Fig. 2).
Fig. 2.
Impact of mean lung dose and modality on perioperative pulmonary complications. (A) Incidence of pulmonary toxicity (PT) by MLD for the entire cohort with accessible DVH data (n=392). Data points show the observed incidence of PT in each group plotted at the mean value of MLD per group. Horizontal error bars show ±1 SD about the mean MLD per group. Vertical error bars show ±1 SEM of the incidence, computed using binomial statistics. (B) Incidence of PT by MLD, with data plotted separately for each treatment modality.
Discussion
Trimodality therapy is the current standard of care for the management of nonmetastatic esophageal cancer (9–11). However, the surgical procedure is extensive and entails a high risk of serious complications (3), which portends both poorer prognosis and worse quality of life (3, 4). In a number of large prospective trials examining neoadjuvant CRT followed by surgical resection (9–12), the postoperative pulmonary complication rate ranged as high as 33% to 46%, and the anastomotic leak rate ranged from 4% to 22% (Table 4). Large retrospective analyses (3, 13) similarly showed postoperative pulmonary complication rates as high as 45% and GI complication rates as high as 24% (Table 4).
Table 4.
Review of incidence of postoperative complications
| Study (reference) year | Rate of Pulmonary Complications |
Rate of GI Complications |
|---|---|---|
| Walsh et al (10) 1996 | 46.40% | 3.5%† |
| Daly et al (13) 2000 | 13.6%* | 24.40% |
| Urba et al (12) 2001 | Not reported | 14.9%† |
| Tepper et al (11) (CALGB trial) 2008 | 33.30% | 20.80% |
| Derogar et al (3) 2012 | 45.00% | 14.0%† |
| CROSS trial (9) 2012 | 46.00% | 22.3%† |
| Current Study | 25.2% (overall) | 23.0% (overall) |
| 30.3% (3D-CRT) | 28.4% (3D-CRT) | |
| 23.8% (IMRT) | 18.3% (IMRT) | |
| 13.9% (PBT) | 18.1% (PBT) |
Abbreviations are as shown in Table 2.
Did not include respiratory insufficiency.
Only GI complication was Anastomotic leak.
Our study thus validates previous studies in showing a high incidence of postoperative pulmonary (25.5%) and GI complications (23%) despite quality surgery at our high-volume center. While previously published studies have assessed risk factors for major postoperative morbidity (7, 8), the predictors identified were un-modifiable patient characteristics such as functional capacity of the lung, age, and underlying comorbidities. Our study similarly showed functional capacity of the lung (DLCO, pre-FEV1) and type of surgery as predictive of pulmonary and/or GI complications; however, we also discovered radiation modality as a major modifiable predictor of postoperative complications even after correcting for lung capacity, type of surgery, and tumor location. In fact, IMRT was associated with a pulmonary complication rate of 24% (compared to 33%–46% in previously published studies [Table 4]), and a GI complication rate of 18% (compared to rates of 22%–24% in other studies [9, 13]). PBT was associated with an even lower incidence of pulmonary complications of 14% and a GI complication rate of 18%. While a multitude of factors might contribute to the cumulative rate of postoperative complications, including various chemotherapy regimens, quality of surgery, and biases in patient selection factors, our results highlight advanced radiation technologies as one major modifiable factor that can mitigate postoperative pulmonary and GI complications in esophageal cancer patients undergoing trimodality therapy.
Because the impact of radiation modality appeared to be the greatest on postoperative pulmonary toxicity, we analyzed the dosimetric factors contributing to this effect. Previous studies of 3D-CRT in patients (14, 15) demonstrated that the risk of radiation pneumonitis was correlated with the MLD, and the percentage of lung volume receiving at least 20 Gy (V20), 13 Gy (V13), 10 Gy (V10), or 5 Gy (V5). Based on these studies, we hypothesized that MLD could explain the radiation modality effect. Thus, when MLD was added to the multivariate model for postoperative pulmonary complications, the effect of radiation modality was no longer significant. Since advanced radiation techniques are capable of delivering radiation doses more conformally to spare surrounding normal tissues from high radiation doses, the differences in the incidence of postoperative pulmonary complications were due largely to differences in MLD among the three radiation modalities.
While the median MLD between 3D-CRT and IMRT was significantly different (supplementary Fig. E2), these differences were somewhat disproportionate to the large clinical benefit seen. The average total lung DVH for 3D-CRT and IMRT were relatively similar compared to the much lower average total lung DVH curve for PBT (supplementary Fig. E2). One explanation could be a greater proportion of 3D-CRT patients having higher MLD, as can be seen from the distribution curve in Figure 2. Another contributing factor could be the effect of heart radiation dose on lung function contributing to pulmonary complications. Theoretically, damaged heart tissue could prevent adequate blood circulation and thus induce higher rates of pneumonia/pneumonitis, as shown in preclinical models (16). However, in our multivariate analysis, we did not find MHD to be a contributing factor to the incidence of postoperative pulmonary complications. While perioperative cardiac toxicities were not different among the 3 radiation modalities, perhaps late complications (such as myocardial infarction) could be affected by heart dose differences between 3D-CRT versus IMRT.
While the current standard of radiation treatment for esophageal cancer is still 3D-CRT, it is known to deliver substantial doses to normal organs at risk such as the lung and heart. IMRT and PBT are advanced forms of conformal radiation therapy, with the main dosimetric advantage of better sparing healthy organs at risk. Several studies have shown the dosimetric advantage of IMRT and PBT in reducing dose to healthy organs (17). The clinical utility of advanced radiation technologies has been shown in a few retrospective studies (18, 19). For example, Lin et al (18) compared IMRT versus 3D-CRT in 676 esophageal cancer patients treated with CRTand found that overall survival (OS), locoregional control, and noncancer-related mortality rates were significantly better in patients treated with IMRT than with 3D-CRT. The clinical experience with PBTis also limited. More recently, Lin et al (19) reported their clinical experience in 62 esophageal cancer patients treated with PBT and concurrent chemotherapy. That study demonstrated few severe toxicities and encouraging pathologic response and clinical outcomes. Our current study lends additional support to the clinical benefit of advanced radiation technologies, but also highlights for the first time radiation modality as a major modifiable predictor of postoperative complications.
Our study was limited by the retrospective nature of this analysis, which could contain inherent biases that we are not aware of despite our best efforts to control for potential confounders. The 3D-CRT patients were treated in an earlier time period (1998–2004), which may have accounted for increased toxicities with less modern surgical technique or postoperative care. However, this limitation may be somewhat mitigated by the fact that these patients all underwent surgery at a single institution, by many of the same surgeons, and our analysis also did not seem to indicate a difference in toxicity rates over time. Most patients in the modern era receive the Ivor-Lewis surgical procedure compared to those in earlier time periods when 3D-CRT was common. However, radiation modality was still an independent predictor for pulmonary and GI complications after correcting for these imbalances, including the year of surgery. We believe examining an early toxicity endpoint such as postoperative complications may be a more reliable measure of an effect of treatment rather than an endpoint such as survival, which can be more heavily influenced by lead-time bias and stage migration due to changes in disease staging over time.
Conclusions
Advanced radiation technologies using either IMRT or PBT significantly reduced postoperative pulmonary and GI complication rates compared to 3D-CRT in esophageal cancer patients. Although this result needs to be confirmed in prospective studies, we believe our results provide further evidence that the dosimetric advantages of IMRT and PBT in the radiation treatment of esophageal cancer can translate to improved clinical outcomes compared with traditional approaches.
Supplementary Material
Summary
While trimodality therapy for esophageal cancer has improved patient outcomes, surgical complication rates remain high. In our study, we identified radiation modality as a modifiable predictor of postoperative complications, with the use of advanced radiation technologies (intensity modulated radiation therapy and proton beam therapy) that significantly reduced pulmonary and gastrointestinal complication rates compared to those with 3-dimensional conformal radiation therapy. Our study highlights the importance of minimizing radiation dose to normal tissue via advanced radiation technologies as the major mechanism that mitigates postoperative complications.
Acknowledgments
This work was supported in part by The University of Texas MD Anderson Cancer Center and National Cancer Institute Cancer Center Support grant CA016672.
Footnotes
A portion of this work was presented in abstract form at the 54th Annual Meeting of the American Society for Radiation Oncology, Boston, MA, Oct 28–31, 2012.
Conflict of interest: none.
Supplementary material for this article can be found at www.redjournal.org.
References
- 1.Feig B, Berger BH, Fuhrman GM. The MD Anderson Surgical Oncology Handbook. Philadelphia, PA: Lippincott Williams & Wilkins; 2006. pp. 367–390. [Google Scholar]
- 2.Viklund P, Lindblad M, Lu M, et al. Risk factors for complications after esophageal cancer resection: a prospective population-based study in Sweden. Ann Surg. 2006;243:204–211. doi: 10.1097/01.sla.0000197698.17794.eb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Derogar M, Orsini N, Sadr-Azodi O, et al. Influence of major postoperative complications on health-related quality of life among long-term survivors of esophageal cancer surgery. J Clin Oncol. 2012;30:1615–1619. doi: 10.1200/JCO.2011.40.3568. [DOI] [PubMed] [Google Scholar]
- 4.Hirai T, Yamashita Y, Mukaida H, et al. Poor prognosis in esophageal cancer patients with postoperative complications. Surg Today. 1998;28:576–579. doi: 10.1007/s005950050187. [DOI] [PubMed] [Google Scholar]
- 5.Bosset JF, Gignoux M, Triboulet JP, et al. Chemoradiotherapy followed by surgery compared with surgery alone in squamous-cell cancer of the esophagus. N Engl J Med. 1997;337:161–167. doi: 10.1056/NEJM199707173370304. [DOI] [PubMed] [Google Scholar]
- 6.Law S, Wong KH, Kwok KF, et al. Predictive factors for postoperative pulmonary complications and mortality after esophagectomy for cancer. Ann Surg. 2004;240:791–800. doi: 10.1097/01.sla.0000143123.24556.1c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Aminian A, Panahi N, Mirsharifi R, et al. Predictors and outcome of cervical anastomotic leakage after esophageal cancer surgery. J Cancer Res Ther. 2011;7:448–453. doi: 10.4103/0973-1482.92016. [DOI] [PubMed] [Google Scholar]
- 8.Ferguson MK, Celauro AD, Prachand V. Prediction of major pulmonary complications after esophagectomy. Ann Thorac Surg. 2011;91:1494–1500. doi: 10.1016/j.athoracsur.2010.12.036. [DOI] [PubMed] [Google Scholar]
- 9.van Hagen P, Hulshof MC, van Lanschot JJ, et al. Preoperative chemoradiotherapy for esophageal or junctional cancer. N Engl J Med. 2012;366:2074–2084. doi: 10.1056/NEJMoa1112088. [DOI] [PubMed] [Google Scholar]
- 10.Walsh TN, Noonan N, Hollywood D, et al. A comparison of multimodal therapy and surgery for esophageal adenocarcinoma. N Engl J Med. 1996;335:462–467. doi: 10.1056/NEJM199608153350702. [DOI] [PubMed] [Google Scholar]
- 11.Tepper J, Krasna MJ, Niedzwiecki D, et al. Phase III trial of trimodality therapy with cisplatin, fluorouracil, radiotherapy, and surgery compared with surgery alone for esophageal cancer: CALGB 9781. J Clin Oncol. 2008;26:1086–1092. doi: 10.1200/JCO.2007.12.9593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Urba SG, Orringer MB, Turrisi A, et al. Randomized trial of preoperative chemoradiation versus surgery alone in patients with locoregional esophageal carcinoma. J Clin Oncol. 2001;19:305–313. doi: 10.1200/JCO.2001.19.2.305. [DOI] [PubMed] [Google Scholar]
- 13.Daly JM, Fry WA, Little AG, et al. Esophageal cancer: results of an American College of Surgeons patient care evaluation study. J Am Coll Surg. 2000;190:562–572. doi: 10.1016/s1072-7515(00)00238-6. [DOI] [PubMed] [Google Scholar]
- 14.Lee HK, Vaporciyan AA, Cox JD, et al. Postoperative pulmonary complications after preoperative chemoradiation for esophageal carcinoma: correlation with pulmonary dose-volume histogram parameters. Int J Radiat Oncol Biol Phys. 2003;57:1317–1322. doi: 10.1016/s0360-3016(03)01373-7. [DOI] [PubMed] [Google Scholar]
- 15.Tucker SL, Liu HH, Wang S, et al. Dose-volume modeling of the risk of postoperative pulmonary complications among esophageal cancer patients treated with concurrent chemoradiotherapy followed by surgery. Int J Radiat Oncol Biol Phys. 2006;66:754–761. doi: 10.1016/j.ijrobp.2006.06.002. [DOI] [PubMed] [Google Scholar]
- 16.Ghobadi G, van der Veen S, Bartelds B, et al. Physiological interaction of heart and lung in thoracic irradiation. Int J Radiat Oncol Biol Phys. 2012;84:e639–e646. doi: 10.1016/j.ijrobp.2012.07.2362. [DOI] [PubMed] [Google Scholar]
- 17.Welsh J, Gomez D, Palmer MB, et al. Intensity-modulated proton therapy further reduces normal tissue exposure during definitive therapy for locally advanced distal esophageal tumors: a dosimetric study. Int J Radiat Oncol Biol Phys. 2011;81:1336–1342. doi: 10.1016/j.ijrobp.2010.07.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lin SH, Wang L, Myles B, et al. Propensity score-based comparison of long-term outcomes with 3-dimensional conformal radiotherapy vs intensity-modulated radiotherapy for esophageal cancer. Int J Radiat Oncol Biol Phys. 2012;84:1078–1085. doi: 10.1016/j.ijrobp.2012.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lin SH, Komaki R, Liao Z, et al. Proton beam therapy and concurrent chemotherapy for esophageal cancer. Int J Radiat Oncol Biol Phys. 2012;83:e345–e351. doi: 10.1016/j.ijrobp.2012.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.