Abstract
The ability of HIV-1 to rapidly accumulate mutations provides the virus with an effective means of escaping CD8+ cytotoxic T lymphocyte (CTL) responses. Here we describe how subtle alterations in CTL epitopes expressed by naturally occurring HIV-1 variants can result in an incomplete escape from CTL recognition, providing the virus with a selective advantage. Rather than paralyzing the CTL response, these epitope modifications selectively induce the CTL to produce pro-inflammatory cytokines in the absence of target killing. Importantly, instead of dampening the immune response through CTL elimination of variant antigen-expressing immature dendritic cells (iDC), a positive CTL-to-DC immune feedback loop dominates whereby the iDC differentiate into mature pro-inflammatory DC. Moreover, these CTL-programmed DC exhibit a superior capacity to mediate HIV-1 trans-infection of T cells. This discordant induction of CTL helper activity in the absence of killing likely contributes to the chronic immune activation associated with HIV-1 infection, and can be utilized by HIV-1 to promote viral dissemination and persistence. Our findings highlight the need to address the detrimental potential of eliciting dysfunctional cross-reactive memory CTL responses when designing and implementing anti-HIV-1 immunotherapies.
Introduction
Effective CD8+ cytotoxic T lymphocyte (CTL) responses are critical for the control of HIV-1 infection (1). Early in HIV-1 infection, the immune system responds vigorously and seemingly appropriately with the induction of strong CTL responses that coincide with the resolution of acute viremia (2). In primate models of SIV as well as HIV-1 in humans, those who limit progression and maintain relatively low virus loads mount and maintain potent and long-term anti-viral CTL activity. However, in most cases of HIV-1 infection, the virus escapes from immune control, causing a variety of complications that directly and indirectly negatively impact the cellular immune response, creating a state of persistent immune activation, eventually leading to T cell senescence and disease progression (3).
One way HIV-1 evades immune elimination is through its ability to rapidly mutate. CTL epitopes resulting from divergence of the infecting viral strains during viral replication have been shown to occur during acute and chronic stages of infection, emerging due to immune selective pressure provided by antigen specific CTL responses (4). While these genetic changes pose an inherent risk of adversely impacting viral fitness (5), their establishment likely provides the pathogen with a selective advantage. Minor viral protein modifications within CTL epitopes have been shown to contribute to immune escape by causing changes in antigen processing, reduced capacity to bind to the MHC class-I molecule, and alterations in the ability of T cell receptors to interact with the presented peptides, all of which can reduce the effectiveness of memory CTL responses (6).
There is, however, evidence that contrasts with the concept that viral mutations driven by CTL immune pressure lead to the establishment of viral escape variants that paralyze antigen-specific CTL responders. Cale et al. (7) found CTL epitope variants of SIV arise during the acute stages of infection while in the presence of pre-existing variant reactive CTL. While these CTL efficiently recognize newly acquired variants, they fail to control the evolving and eventual fixation of mutant escape epitopes. Similarly, during chronic stages of HIV-1 infection, highly avid antigen specific CTL responses against autologous virus can be maintained without impact on viral evolution (8, 9). These CTL responses diminish as the subjects received antiviral therapy, suggesting that CTL were indeed actively responding to virus (8). Moreover, the presence of active antigen-cognizant CTL that fail to impact viral evolution or epitope divergence can be found in high frequency along with high viral load during progression to AIDS (8, 10).
It remains unclear why the pre-existing antigen-reactive CTL described in these studies lack the ability to provide sufficient immune pressure to either influence further viral evolution or impede the establishment of the recognized variants. It is conceivable that the CTL detected ex-vivo are dysfunctional or suppressed in vivo as a result of the harsh environmental conditions associated with chronic viral assault. Furthermore, it is possible that any continued change to these epitopes might be more detrimental to the overall fitness and survival of the virus than the CTL response itself. However, data from previous reports suggest that HIV-1 can evolve directly into the path of pre-existing antigen responsive CTL rather than evolve away from CTL pressure, even when viral fitness would permit (7, 11). Another plausible explanation is that the presentation of some altered peptide variants can simply trigger detectable yet ineffective responses from previously established cross-reactive CTL. Nevertheless, the potential benefit that may exist for the virus to evade such ineffective CTL activity is apparently outweighed by the advantage maintaining it.
In the present study, we explore the notion that incomplete immune escape from sub-optimal CTL responses could provide an advantage for the pathogen. Using an in vitro DC-based CTL priming system, we show that minor viral changes in CTL epitopes can selectively induce the helper rather than killer function of cross-reactive CTL to promote their dysfunctional dialogue with HIV-1 antigen-expressing DC. As a result, an inflammatory state continues, promoting DC survival and acquisition of characteristics ideal for mediating HIV-1 dissemination through trans infection of CD4+ T cells.
Materials and Methods
Media, reagents and cell lines
Cell cultures and lines were maintained in Iscove’s Modified Dulbecco’s Medium (IMDM; Invitrogen) containing 10% heat inactivated FBS (Gemini Bio Products) and 1% penicillin/streptomycin (Invitrogen). The following factors were used: rhGM-CSF (Leukine®, Bayer), rhIL-2 (Proleukin® Chiron), IFN-α (Intron® A, Schering-Plough), rhIL-4, rhIL-6, rhIL-7, rhIL-15, rhTNF-α, rhIL-1β and rhIFN-γ (R&D Systems). The CD40L-transfected J558 cell line (J558-CD40L) was a gift from Dr. P. Lane, University of Birmingham, UK. The HLA A2 expressing T2 cell line was provided by Dr. Walter Storkus, University of Pittsburgh.
Human subjects
This research was part of the Pittsburgh portion of the Multicenter Aids Cohort Study (MACS) (12), and was approved by the University of Pittsburgh Institutional Review Board. Plasma and PBMC were collected and cryopreserved at biannual MACS visits beginning at the time of enrollment, and plasma viral RNA copies/ml and T cell counts were determined. Whole blood products (buffy coats) from healthy, anonymous, HIV-1 negative donors were purchased from the Central Blood Bank of Pittsburgh. HIV-1 screening was performed as part of the product release criteria.
Selection of HIV-1 epitopes
Families of CTL epitope peptides chosen for this study were identified through extensive sequence analysis of plasma derived viral RNA samples collected throughout the course of infection from 3 HIV-1 infected MACS subjects. These sequence analyses allowed for the identification of autologous viral epitopes and determination of the appearance and establishment of epitope variants. Synthetic MHC class 1-restricted epitope and variant peptides were then generated. PBMC from each collection time point were screened by routine IFN-γ ELISPOT assays for CTL reactivity against each peptide target. Reactivity of the pre-existing CTL responders against later established variants within certain epitope families was noted in each of the 3 subjects tested (data from one representative donor shown in Fig. S1, Table S1). Founder virus sequences from 3 epitope families, i.e., Gag77–85 (SLFNTVATL), Gag151–159 (TLNAWVKVV) and Nef72–80 (FPVRPQVPL), identified from one subject of a common HLA type (A*0201 / B*0702 positive) were selected to initiate in vitro CTL priming in MHC class I single allele- matched HIV-1 naïve donors.
HIV-1 genetic sequencing
Plasma HIV-1 RNA was isolated and amplified as previously described (13). Viral sequences were derived from 5′ and 3′ half genomes from 12, 16, and 9 time points for gag-p17, –p24, env-gp160, and nef respectively. An average of 12 clonal sequences was obtained per time point. Sequences bearing open reading frames were aligned with the Pileup program in the GCG suite (Genetics Computer Group, Madison, WI) (5).
Dengue virus epitope sequences
The dengue virus serotype 3 (DENV3) epitope NS3399–407 (KLNDWDFVV) was identified through use of the human HLA A*0201 transgenic mouse model previously described (14). The DENV2 (RTNDWDFVV) and DENV4 (KLTDWDFVV) serotype variants were identified from the NCBI Entrez Protein Database.
Synthetic peptides
The Los Alamos HIV Molecular Immunology Database was used to identify optimal MHC class I epitopes, whereas predicted MHC class I epitopes were defined based on the presence of known HLA specific anchor residues. HIV-1 associated peptide sequences were synthesized by Sigma-Aldrich and SynBioSci, whereas the DENV associated peptides were synthesized by GenScript (Piscataway, NJ).
Analysis of peptide: MHC class 1 affinity
A previously described (15) fluorescence polarization competitive binding assay was used to experimentally determine MHC class I peptide affinity (Pure Protein LLC, Oklahoma City, OK). Epitope variant peptides were classified as previously reported (15) based on the log IC50 as being binders with high affinity <3.7, medium affinity 4.7> and low/no affinity > 5.5.
HLA typing
High-resolution HLA molecular typing was performed by the University of Pittsburgh Medical Center Tissue Typing Laboratory. The in vitro priming studies were limited to HIV-1 seronegative donors confirmed to be HLA-A*0201 and/or HLA-B*0702 positive.
Generation of DC
Monocytes were isolated and cultured for 5–7 days in IMDM containing 10% FBS in the presence of GM-CSF and IL-4 (both 1000 IU/ml) (R&D systems) to generate immature DC (iDC) as previously described (16). To induce CD83+ mature DC, immature DC were differentially exposed, on day 5, to activation factors for 48h. Mature type-1 polarized DC used for CTL priming experiments were generated using the αDC1 maturation cocktail previously described (16); TNF-α (50 ng/ml) was used as a single maturation factor for ‘TNF-α-matured’ DC.
Induction and expansion of primary CTL
Induction and expansion of primary CTL from HIV-1 naïve donors was done using a protocol similar to that previously described (16). Briefly, CD8+ T cells were isolated from HIV-1 seronegative donors using the EasySep (StemCell Technologies) negative selection isolation system. These T cells were plated at 7.5×105 cells/well in 48 well plates and sensitized with 9mer peptide-pulsed αDC1 (7.5×104 cells/well). γ-irradiated (3000 Rad) J558-CD40L cells were added to the cultures (5×104 cells/well). At day 4, rhIL-2 (50 IU/ml), rhIL-7 (10 ng/ml), and rhIL15 (1 ng/ml) were added to the cultures. Long term CTL lines were maintained with increased concentration of rIL-2 (200 IU/ml) and rhIL-7 (10 ng/ml) and re-sensitized with the priming relevant peptide-pulsed γirradiated (3000 Rad) HLA-A2+ T2 cells at a stimulator to T cell responder ratio of 1:5 every 14 days.
Short term expansion of memory CTL
Memory CTL from HIV-1 positive subjects were generated using a short term expansion method previously described method with slight modifications (17). Briefly, PBMC (1×105 /well) were cultured in the presence of 9mer peptide antigens (1 μg/ml) for 3 days and then supplemented with rIL-2 (200 IU/ml) and rhIL-7 (10 ng/ml) for an additional 7 days, at which time they were analyzed or maintained.
CTL and DC co-cultures
CTL (2×105 cells/well) were added directly to day 5 autologous iDC cultures in the absence or presence of antigenic 9mer peptides (1μg/ml). An irrelevant DENV associated HLA-A*0201 restricted peptide (KLNDWDFVV) was also included as control where stated. Exposure time depended on the individual experiment. Human soluble TNF receptor I (sTNF-RI; 0.1 μg/ml; R&D Systems) or IFN-γ receptor 1 (IFNγ-R1; 1 μg/ml; R&D Systems) were added for blocking studies.
HIV-1 infection and transmission assay
HIV-Ba-L (R5 tropic virus) was propagated in PHA and IL-2 activated, normal donor PBMC and purified as described (18). Virus titers were determined by p24 ELISA (SAIC-Frederick). DC were differentially activated by addition of autologous CTL in the presence of an irrelevant epitope peptide (KLNDWDFVV- iDCCTL) or the relevant epitope specific variant (PLN9- mDCCTL), or TNF-α (mDCTNFα). T cells were removed from the co-cultures prior to use of DC in HIV-1 transmission assay using the positive isolation system EasySep (StemCell Technologies). The DC were incubated with virus at 37°C for 2hr at an MOI of 10−4, an MOI not sufficient to directly infect activated CD4+ T cells, as previously shown (19). HIV-1 loaded DC were then washed extensively and incubated with autologous, activated CD4+ T cells at a 1:10 ratio respectively. After 4 days, cell free supernatants were collected and measured for viral p24 by ELISA.
Flow cytometry
Anti-human CD86 (FITC), CD83 (PE) (Immunotech), anti-CD3 mAb (APC-Cy7, BD Bioscience), anti-CD8 mAb (PerCP-Cy5.5, BD Biosciences), anti-CD107a (PC5, Pharmingen), anti-TNF-α (eFluor 450, eBiosciences), anti-IFN-γ (FITC, eBioscience), anti-IL-2 (APC Pharmingen) mAb reagents were used. Anti-CD28/CD49d (FastImmune) (BD Biosciences), Golgistop™ (BD Biosciences), Golgiplug™ (BD Biosciences) reagents were used for intracellular staining. Viability was determined using the LIVE/DEAD® Aqua Kit (Invitrogen) per manufacturer’s instructions. Data was acquired using a LSR-II 12-color flow cytometer (BD Biosciences) and analyzed using FlowJo 7.6 (TreeStar Inc.). Polyfunctional responses were determined using a previously described multi-parameter gating strategy (20) and displayed using the SPICE (5.2) program (Mario Roederer, NIAID, NIH).
ELISPOT assays
PBMC (1×105/well) and cultured CTL (3×104/well) were tested for reactivity to 9mer peptide antigens by ELISPOT assay as previously described with minor modifications (17). HLA-A2+ T2 target cells (1×104 /well) were added to the assay as antigen presenting cells when testing HLA-A2+ restricted CTL. Spots were counted with an automated ELISPOT reader (AID GmbH). All data presented as spot forming units (SFUs) per 105 cells.
Cytotoxicity assays
CD4+ T cell targets: PBMC were activated and cultured for 5 days in the presence of PHA (1μg/ml) and rIL-2 (100 IU/ml). The pre-activated CD4+ T-cell targets were purified by negative selection (EasySep, StemCell Technologies) and pulsed with 1μg/mL target peptide for 1h and then washed. Peptide-pulsed targets were cultured at 37°C with CTL for 18h at ratios (CTL: target) of 3:1, 1:1, 0.3:1, and 0:1. Following incubation, cells were washed and stained with anti-CD4-V450 (BD Biosciences) and anti-CD8-APC-Cy7 (BD Biosciences). Cells were fixed and permeabilized with BD Cytofix/Cytoperm™ Fixation/Permeabilization Solution Kit (BD Bioscience). DNA of permeabilized cells was stained with 7-amino-actinomycin D (7-AAD) (BD Pharmingen) for 20 minutes at 37°C. To assess target cell death, DNA content was measured by gating on the singlet, CD4+ target cells and analyzing 7-AAD intensity on a linear scale. Immature DC targets: Peptide-pulsed autologous immature DC were used as targets in standard 4 h 51Cr-release assays as previously described (16). Flow cytometry was used to measure the percent cell loss in CTL: DC co-cultures by determining the ratio of cell types recovered as determined by gating based on light scatter properties and expression of CD3 and CD86 on T cells and DC, respectively. Further analysis of cell viability on the DC gated events was achieved using LIVE/DEAD™ Aqua amine-binding dye. CTL:DC exposure times varied.
DC production of immune mediators
Cytokine and chemokine production of DC was induced as previously described (16). DC were plated (2×104 cells/well) in a 96-well flat bottom plate and stimulated with J558-CD40L cells (5×104 /well). Supernatants were collected at 24 h and tested by specific ELISA (Thermo Fisher) for the presence of IL-12p70, IL-6, CCL5, and CXCL10.
Scanning electron microscopy (SEM)
Sterile 12 mm round glass coverslips were placed in the bottom of the well of day 5 iDC culture suspensions. CTL (2×105/well) and specified peptides (1 μg/ml) were added to the cultures. The glass coverslips were removed at 24h, fixed in 2.5% glutaraldehyde in PBS and post-fixed in aqueous 1% osmium tetroxide and washed with PBS. Samples were dehydrated through a graded ethanol series, critical point dried, and coated with 3.5 nm gold palladium. A JEOL JSM-6330F SEM was used at 3 kV for imaging.
Live cell imaging
Imaging was performed using a Perkin Elmer Ultraview spinning disk confocal microscope equipped with a Nikon TE 2000E camera. A Metamorph (Molecular Devices) was used to collect all data and to drive the microscope. All images were collected using a 1.3 NA oil immersion 40× objective with a 1× coupler between the microscope and either the confocal or wide-field cameras. Cells were maintained at 37°C in the microscope using a Harvard Apparatus heated stage insert. The XYZ stage used was made by ASI.
Statistical analysis
Data was analyzed using either paired Student’s t-test (two-tailed) or one way ANOVA followed by a Tukey post hoc test. Significance was determined at an α of 0.05.
Results
Use of DC to induce broadly cross-reactive HIV-1 specific CTL from HIV-1 naïve donors
Previous studies have shown that viral antigen specific CTL can actively persist and respond to certain target epitopes throughout the course of both SIV and HIV infection without providing sufficient immune pressure to induce selective change in their establishment (7–11). We hypothesize that such ineffective immune responses could represent the activity of previously established memory CTL sub-optimally cross-reacting to epitope variants. To ascertain whether early CTL responders generated against founder HIV-1 epitopes could indeed differentially cross-react to viral epitope variants arising at later time points of infection, we developed an in vitro strategy to study and characterize cross-reactive CTL responses to natural HIV-1 epitope variants. Our approach was to recapitulate the in vivo anti-HIV-1 CTL response using a previously described autologous dendritic cell-based in vitro model (15) to induce primary CTL responses in HIV-1 naïve donors using founder epitopes identified through the analysis of virus collected from MHC-class 1 matched HIV-1 positive donors. These CTL could be expanded and subsequently characterized for reactivity to naturally occurring epitope variants.
Founder virus sequences from 3 epitope families identified from one particular HIV-1 positive subject having a common HLA type (A*0201 / B*0702 positive) were selected to initiate in vitro CTL priming in HIV-1 naïve donors (see Materials and Methods, Table S1, and Fig. S1). These were Gag77–85 (SLFNTVATL), Gag151–159 (TLNAWVKVV) and Nef72–80 (FPVRPQVPL). The SLFNTVATL and TLNAWVKVV peptides are HLA-A*0201 restricted, and the FPVRPQVPL peptide is HLA-B*0702 restricted. Using this strategy, HIV-1 antigen-specific CTL responses against all 3 epitopes were successfully generated as determined by IFN-γ ELISPOT assay (Fig. 1 and Fig. S2).
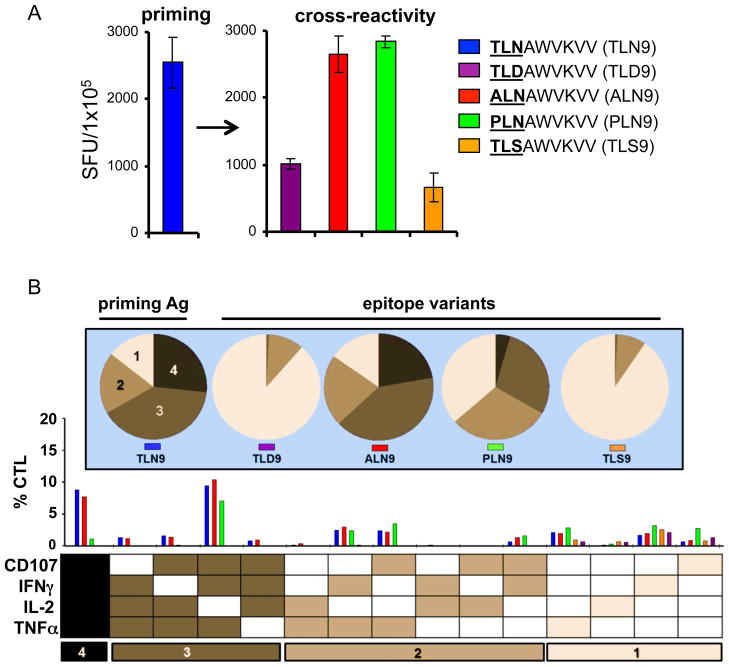
Fig. 1. In vitro induction of broadly reactive primary HIV-1 specific CTL.
Primary CTL from an HIV naïve donor were generated against the HLA-A2 restricted, HIV-1 associated epitope p24 Gag151–159 TLNAWVKVV (TLN9), maintained in culture, and tested for compared responsiveness to natural epitope variants. (A) IFNγ ELISPOT results recorded as net spot forming units (SFU) /105 cells (non-specific background subtracted). Error bars represent ± standard deviation of assay replicates. (B) Polyfunctional analysis of CTL cross-reactivity using intracellular cytokine staining and multi-parameter flow cytometry. The bar graph represents the percentage of CD3+CD8+ T cells induced to express any combination of the 4 immune factors, IFN-γ, TNF-α, IL-2 and CD107 (below), and were color coded based on the peptide used for stimulation. Pie charts (above) indicate the relative amount of polyfunctional responses to the individual peptide, with darker shades having higher degrees of polyfunctionality. The numbers at the bottom delineate groups with the indicated numbers of responses. Responses within each of these groups are summed to obtain the fractions shown in the pie charts. The data sets shown were generated using one representative CTL culture of 6 independently established each generated from different donors.
We next tested the ability of these CTL to respond to the respective natural epitope variants. In general, we found that all the CTL lines generated showed some degree of cross-reactivity to epitope variants, with some responses being comparable to that of the priming peptide (Fig. 1A and Fig. S2). For example, TLNAWVKVV (TLN9) primed CTL induced responses of similar magnitude when re-challenged with either the cognate peptide or variant peptides ALNAWVKVV (ALN9) and PLNAWVKVV (PLN9), with more apparent differences found only with decreasing antigen concentration (Fig S2 C). Similar cross-reactivity was found with CTL lines generated against Gag77–85 (SLFNTVATL) and Nef72–80 (FPVRPQVPL) (Fig. S2A and B). For sake of transparency, we chose to focus the rest of this report on CTL generated against the TLN9 epitope. However it is important to note that the findings we report translate across the peptide families tested.
Phenotypic characterization of cross-reactive CTL lines
We used intracellular cytokine staining (ICS) and flow cytometry analysis to compare the cytokine producing capacity and polyfunctional profiles of the CTL stimulated with either cognate or variant peptides. In doing so, we found that the peptides that induced the highest magnitude of responses also induced the most polyfunctional responses in the CTL (Fig. 1A and B). Specifically, the TLN9-induced CTL reacted comparably in magnitude and polyfunctionality when exposed to the cross-reactive variants ALN9 and PLN9 as they did to the cognate TLN9 peptide, but reacted to the TLD9 and TLS9 epitope variants with less magnitude and polyfunctionality.
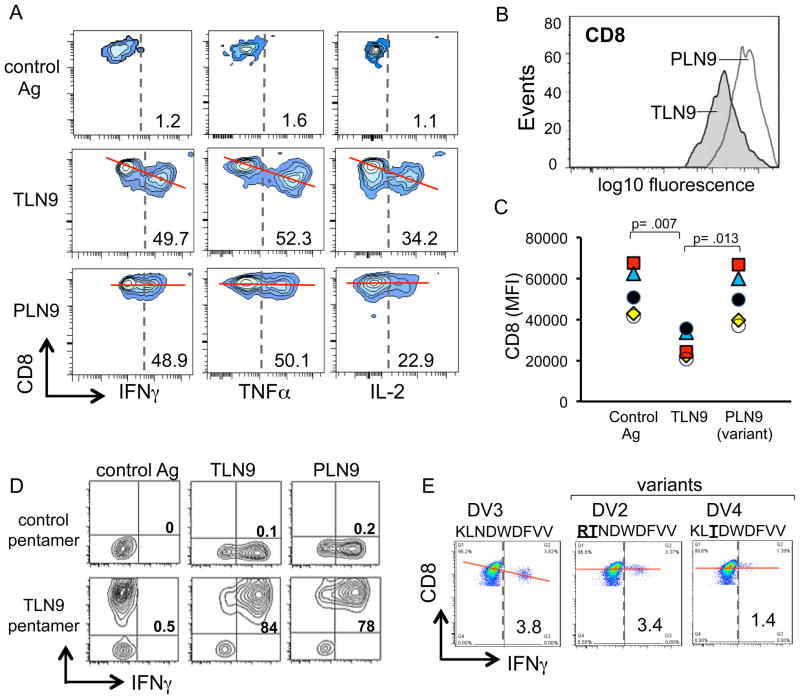
Even though the magnitude and quality of the CTL responses to some of the epitope variants seemed comparable to that of the cognate priming peptide, we noted subtle phenotypic differences. For example, when the priming peptide TLN9 or late viral variant PLN9 was used as the peptide stimulator, the cytokine profile and overall percentage of the responding CTL were similar (Fig. 1 and Fig. 2A). However, when stimulated with the priming peptide TLN9, the surface expression of CD8 on the peptide reactive CTL decreased, while its expression was maintained on the CTL exposed to the cross-reactive variant peptide PLN9 (Fig. 2A and B). The differences observed in CD8 expression of CTL stimulated by the cognate TLN9 peptide compared to the variant PLN9 peptide was found to be consistent and statistically significant among CTL lines generated from 5 different donors (Fig. 2C). Although not as striking, the expression level of CD3 followed the same pattern, with the variant peptide failing to down-regulate the expression of this surface marker (data not shown).
Fig. 2. Differential expression of the T cell co-receptor CD8 on cross-reactive CTL.
(A) HIV-1 p24 Gag151–159 specific CTL were stimulated with either the relevant priming peptide TLNAWVKVV (TLN9), the epitope variant peptide PLNAWVKVV (PLN9), or an irrelevant DENV3 associated HLA-A*0201 restricted control peptide (KLNDWDFVV) and assessed for dual expression of CD8 and each of the cytokines TNF-α, IFN-γ, and IL-2. (B) Single parameter histogram display comparing CD8 expression of differential peptide-induced IFN-γ producing CTL. (C) Comparison of CD8 expression on TLN9 specific CTL following their differential stimulation with either their cognate priming peptide TLN9, the epitope variant PLN9, and an irrelevant peptide control. Data plotted as mean fluorescence intensity (MFI) using CTL generated from 5 different HIV-1 naïve donors (each represented by a unique symbol). Peptide responsive cells were analyzed by gating on the IFN-γ producing T cells. Statistical significance was determined by one-way ANOVA followed by a Tukey post hoc test. (D) TLN9-specific CTL assessed for dual expression of the TCR specific MHC class 1 pentamer (A*0201, TLNAWVKVV) stain and IFN-γ following differential peptide stimulation. (E) HLA-A*0201 restricted DENV3 NS3339–407 epitope specific CTL were stimulated with either the priming peptide (KLNDWDFVV) or inter-serotype (DENV2 and DENV4) associated variant peptides and assessed for dual expression levels of CD8 and IFN-γ. Red lines included a visual reference to compare relative differences in CD8 expression of antigen responsive CTL.
To verify that these differential responses were in fact occurring in cross-reactive CTL, and not the result of a simultaneous outgrowth of distinct T cell clones having different specificities, we further analyzed the CTL by flow cytometry using a fluorochrome labeled T cell receptor (TCR)-specific pentamer designed to recognize a TCR having specificity for the peptide TLN9 in the context of the MHC molecule HLA-A*2010. The specific binding of this pentamer to those cells producing cytokines in response to either the TLN9 or the PLN9 peptide, and not the irrelevant peptide, clearly demonstrated that the same cell population was in fact cross-reactive to both of the related HIV-1 peptides (Fig. 2D).
These findings compelled us to revisit the HIV-1 positive donor samples we originally used for virus sequencing and epitope selection to see if a similar pattern in CD8 expression could be found in CTL obtained from natural infection (Table. S1, Fig. S1). We focused attention on the Gag77–85 (SLFNTVATL) epitope family which demonstrated the most dominant and potentially cross-reactive early responses to the late variant SLYNTVATL. Indeed, CTL expanded from early PBMC samples obtained from this donor 6 months post seroconversion which reacted to the late dominant variant SLYNTVATL failed to down-regulation CD8 expression, while down-regulation occurred with exposure to the founder epitope peptide SLFNTVATL (Fig. S3).
To determine if the observed relationship between CTL cross-reactivity and surface expression of CD8 was unique to HIV-1, we repeated CTL priming experiments using a dengue virus serotype 3 (DENV3)-associated HLA-A*0201 restricted CTL epitope peptide (NS3399–407, KLNDWDFVV). The CTL generated against the DENV3 epitope were then tested for reactivity to the altered peptide sequences naturally associated with different DENV serotypes. Similar to the findings with the HIV-1 specific CTL, the DENV3 specific CTL displayed intense cytokine expression and down-regulation of CD8 in response to the relevant priming peptide KLNDWDFVV, while the CD8 expression was maintained on the cells cross-reacting to the respective DENV2 and DENV4 serotype peptide variants RTNDWDFVV and KLTDWDFVV (Fig. 2E). Therefore, the association between CTL expression of CD8 and cross-reactivity to natural viral variants appears to represent a basic immunological phenomenon.
Cytolytic function of cross-reactive HIV-1 specific CTL
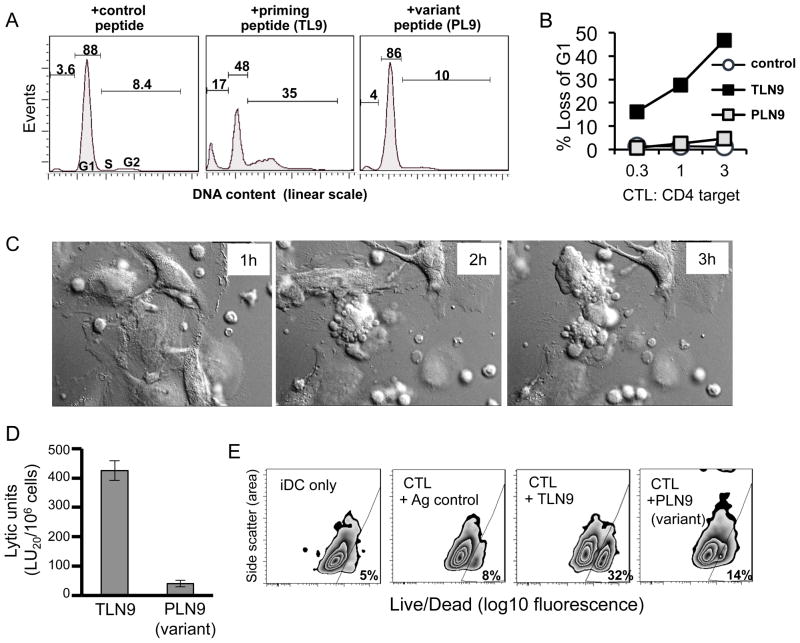
A critical function of CTL is their ability to eliminate antigen expressing targets. Therefore, we studied the cytotoxic effector responses of the CTL against antigen expressing autologous CD4+ T cells as well as immature DC (iDC), both of which are known to be naturally targeted by HIV-1 (21, 22). To examine CTL-induced cytotoxicity of pre-activated antigen expressing, CD4+ T cells, we used DNA intercalating 7-aminoactinomycin D (7-AAD) to measure by flow cytometry the changes in DNA content associated with cell death. We detected a dramatic antigen specific decrease in the DNA histogram peak representing target cells in the diploid G1 phase of the cell cycle (Fig. 3A and B). This was accompanied by an early increase in the S/G2 region (within 5 hours, data not shown), and followed by an increase in the sub-diploid DNA apoptotic region after 16 h co-culture (Fig. 3A, middle panel). These results were indicative of a CTL-induced toxic arrest of the targets at the S/G2 phase of the target cell cycle. This cytotoxic activity and loss of DNA in the G1 peak increased in a CTL dose dependent manner (Fig. 3B). Interestingly, CD4+ T cell targets labeled with an optimal concentration of the variant peptide PLN9 were not effectively killed (Fig. 3A, right panel, and 3B).
Fig. 3. Dysfunctional cytolytic capacity of cross-reactive HIV-1 specific CTL.
Purified autologous pre-activated CD4+ T cells as well as immature DC that differentially expressed the cognate TLNAWVKVV (TLN9), variant PLNAWVKVV (PLN9), or irrelevant KLNDWDFVV peptide antigen were used as HIV-1 specific CTL targets in cytotoxicity assays. (A) Flow cytometry cell cycle analysis using 7-AAD staining to measure cellular DNA content of antigen expressing CD4+ T cell targets following an 18h co-culture with TLN9-specific CTL. Percent of target events within the G1 and S/G2 phase of the cell cycle and sub-G1 (apoptotic) regions are shown. Data shown from a representative experiment of 4 performed yielding similar results (B) Dose titration of CTL-induced cytotoxicity of antigen expressing CD4+ T cell following an 18h co-culture determined by flow cytometry analysis of DNA content. Results plotted as a percent loss of G1 compared to baseline target values in the absence of CTL. (C) Still frame pictures from a live cell time lapse (4h) imaging video showing HIV p24 Gag151–159 specific CTL lysis of autologous iDC target cells expressing the priming relevant peptide TLN9 (CTL:DC = 1:1). See also the respective Video 1. (D) A 4h 51Cr-release assay showing differential ability of the CTL to effectively kill autologous iDC expressing the priming relevant peptide TLN9 but not the cross-reactive variant peptide PLN9. (E) Flow cytometry viability assessment using LIVE/DEAD® Aqua Kit discrimination staining of autologous iDC co-cultured for 6h with the CTL at a 3:1 (CLT:DC) ratio in the absence or presence of either the irrelevant control peptide KLNDWDFVV, the priming relevant TLN9 peptide, or the epitope variant peptide PLN9.
A similar pattern of cytotoxicity was found when analyzing iDC targets. In addition to representing a normal CTL function of eliminating infected target cells, lysis of antigen-expressing iDC represents a negative feedback mechanism used by CTL to dampen successful type-1 immune responses (23). Using live cell microscopy, we were able to visualize the capacity of in vitro primed CTL to kill TLN-9 antigen expressing autologous iDC targets (video1. mov and Fig. 3C). Within 4 hours, at a 1:1 effector to target ratio, a substantial number of iDC targets were lysed. The ability of the CTL to kill antigen expressing iDC was tested by standard 4 h 51Cr-release cytotoxicity assays. As expected and in accord with previous reports (23, 24), the CTL showed lytic activity against iDC exposed to 1μg/ml of the priming cognate antigen. However, this effector function was dramatically reduced when iDC were labeled with the same concentration of the variant peptide PLN9 (Fig. 3D). This difference in killing activity was also apparent when analyzing antigen expressing iDC cultures by flow cytometry following their 6h exposure to CTL at a 3:1 (CTL:DC) ratio. In the representative experiment described, of the remaining iDC that could be recovered from priming peptide co-cultures, 32% were determined to be dead compared to only 14% and 8.3% recovered from the co-cultures containing the variant peptide and control peptide respectively (Fig. 3E). This was in addition to an initial 49% reduction in the total number of iDC recovered from the cultures that contained the priming peptide, compared to a reduction of only 8.5% from the cultures having the variant peptide as compared to control cultures (data not shown).
Activation and programming of variant antigen expressing DC by cross-reactive CTL
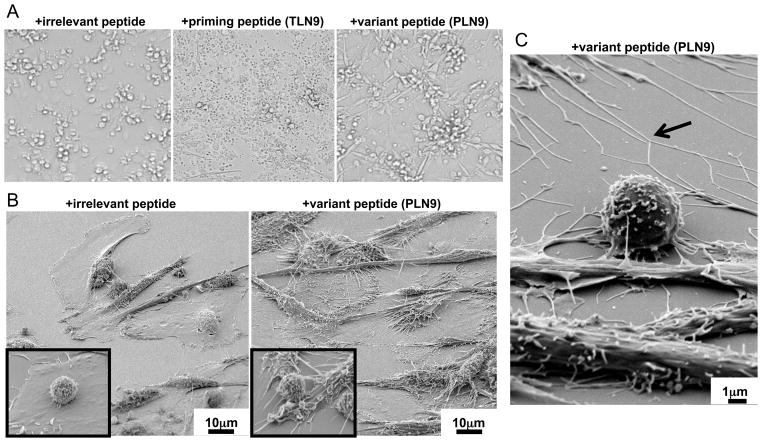
Although the CTL we evaluated showed a substantial reduction in killing capacity towards the iDC expressing PLN9 variant epitope, striking changes in DC morphology were seen after 24 h through light microscopic evaluation of the co-cultures, suggesting the occurrence of some level of CTL-induced DC activation. These DC partially adhere and developed a pronounced webbed network of long cellular extensions between neighboring cells (Fig. 4A, right panel). This was in sharp contrast to the iDC expressing the control irrelevant peptide (Fig. 4A, left panel) which went relatively unchanged, while the iDC expressing the TLN9 peptide failed to survive the extended exposure with the CTL (Fig. 4A, middle panel). Scanning electron microscopy (SEM) used to examine the changes in DC morphology in detail provided evidence of what appeared to be the induction of interconnected micro/nanotube formations exclusively in the DC:CTL co-cultures containing the variant peptide (Fig. 4B and C). These cellular formations appeared to be similar to the previously described tunneling membrane connections reported to facilitate intercellular communication and transfer of small molecules (25) as well as pathogens, including HIV-1 (26, 27).
Fig. 4. Cross-reactive CTL induce micro/nanotube extensions on DC expressing variant peptide.
(A) Light microscopy of iDC:CTL co-cultures following a 24h incubation in the presence of the irrelevant DENV3 associated peptide KLNDWDFVV (left panel), the relevant HIV-1 associated priming peptide TLNAWVKVV (TLN9) (middle panel), and the epitope variant peptide PLNAWVKVV (PLN9) (right panel). Cellular debris in the priming peptide culture indicates a massive degree CTL-induced DC death while the elongated adherent cells in the peptide variant culture suggest cross-reactive CTL dependent DC activation. (B) Scanning electron microscopy (SEM) images of HIV-1 peptide TLN9 specific CTL and autologous iDC co-cultures after 24h in the presence of an irrelevant DENV3 NS3339–407 (left) or the of epitope variant peptide PLN9 (600X) (right). Insets images are from the respective cultures showing CTL (smaller round cell) and DC in contact at an enhanced magnification (6000X). (C) SEM image of the iDC: CTL co-cultures after 24 h incubation in the presence of the HIV-1 epitope variant peptide PLN9 highlighting what appears to be intercellular-connecting micro/nanotube formations (arrow) induced by the cross-reactive CTL (6000X).
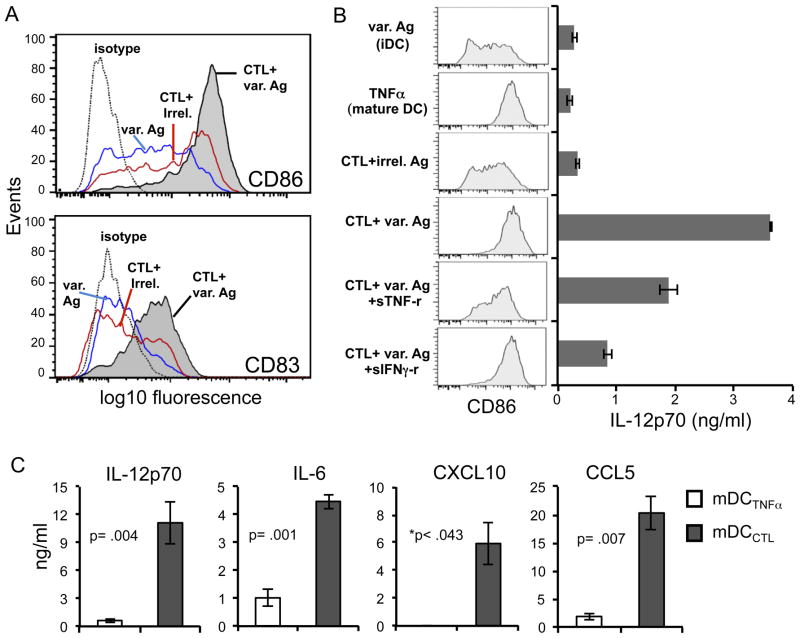
Flow cytometric analysis of the surface expression levels of the DC maturation markers CD86 and CD83 revealed that the cross-reactive CTL were in fact inducing maturation of those DC expressing the variant antigen compared to iDC exposed to peptide alone, or to CTL in the presence of an irrelevant peptide (Fig. 5A and B). While there were signs of low level non-specific CTL induced activation of DC, this was much less pronounced compared to the high degree of maturation that was dramatically induced by variant peptide (Fig. 5A and B). Of the factors produced by these CTL as measured by ICS flow cytometry (Fig. 1B and 2A), TNF-α and IFN-γ were the most likely candidates to contribute to this DC activation. Therefore we used the TNF-α and IFN-γ inhibitors, sTNF-rec1 and sIFNγ-rec1, respectively, to assess whether either factor was involved. While sIFNγ-rec1 did not inhibit the CTL-induced DC maturation, CD86 enhancement was partially reduced with the inclusion of sTNF-rec1 (Fig. 5B), suggesting that CTL-derived TNF-α played a role in the CTL-induced DC maturation. The fact that the CTL-induced DC enhancement of CD86 expression was not entirely blocked by the addition of sTNF-rec1 suggested that other factors along with TNF-α likely contributed to this DC maturation.
Fig. 5. Cross-reactive HIV-1 specific CTL induce mature pro-inflammatory ‘programmed’ DC.
DC were characterized following their 24h co-culture with HIV-1 antigen TLNAWVKVV (TLN9)-specific CTL in the presence of cognate or variant peptide. (A) Impact of cross-reactive CTL on the maturation status of variant peptide PLNAWVKVV (PLN9)-expressing iDC as determined by surface expression of CD86 and CD83. The blue line represents iDC exposed to PL9 peptide only (var. Ag). The red line denotes DC exposed to CTL and irrelevant control peptide KLNDWDFVV (CTL+irrel.) The shaded histogram represents DC exposed to both CTL and PLN9 peptide. The dashed line denotes the antibody isotype control stain of the CTL: PLN9 condition. For transparency, isotype control stains for the other conditions were recorded but not shown. These controls were set at the same MFI for all conditions. The conditions of iDC without peptide, and iDC+CTL in the absence of peptide are not shown, but values were overlapping with the blue and red lines respectively. (B) Impact of the presence of TNF-α blocker (sTNF-R1; 0.1μg/ml) or an IFN-γ blocker (sIFNγ-R1; 1μg/ml) on cross-reactive CTL-induced maturation of PLN9 (var. Ag) expressing DC. iDC and CTL were co-cultured for 48h, followed by assessment of DC surface expression of CD86 (column of shaded histograms on left) and capacity to produce IL-12p70 (bar graph on the right). The TNF-α (50ng/ml) stimulated DC served as a positive control for DC maturation and iDC exposed to PLN9 (var. Ag) peptide in the absence of CTL served as the iDC control. Error bars represent the standard deviation of the values obtained from assay triplicates. Data is from one experiment representative of 3 performed. (C) Comparison of cytokine and chemokine producing capacity of mature DC following a 48h exposure of iDC to either TNF-α (50ng/ml; mDCTNFα) to or variant peptide stimulated cross-reactive CTL (mDCCTL) in response to CD40L stimulation as determined by ELISA. Data presented as mean ± standard error of 4 independent experiments using 4 different DC and CTL donors. *Actual values for mDCTNFα were consistently below detection limits of the assay for CXCL10. Therefore, p values were recorded as < the calculated value substituting the assay detection limit as the DCTNFα value for CXCL10. Significance was determined using two-tailed paired t tests.
In addition to inducing DC maturation, the cross-reactive CTL triggered the type-1 polarization of the maturing DC, characterized by their enhanced IL-12p70 producing capacity upon subsequent stimulation with CD40 ligand (Fig. 5B). This was in stark contrast to DC matured with exposure to exogenous TNF-α (50 ng/ml) which similarly expressed high levels of CD86, but instead had a more IL-12p70 “exhausted” phenotype (28), in which the ability to produce this cytokine is curtailed (Fig. 5B and C). As was found with maturation, addition of sTNF-rec1 partially inhibited the CTL-dependent enhancement of DC IL-12p70 expression (Fig. 5B). Moreover, while IFN-γ did not appear to play a role in DC maturation with regard to surface expression of CD86 (Fig. 5B) and CD83 (not shown), the addition of sIFNγ-rec1 dramatically reduced their IL-12p70 producing capacity (Fig. 5B). When both blocking reagents were used simultaneously, additive effects were not observed (data not shown). The finding that either of these inhibitors could interfere with DC polarization is in line with previous studies showing that the combined exposure of iDC to both a maturation inducing stimulus and IFN-γ, but neither alone, can promote this high IL-12 producing mature DC phenotype (29). In addition to IL-12p70, when compared to the TNF-α-matured non-polarized DC, these CTL-programmed mature DC consistently produced higher levels of IL-6 as well as the pro-inflammatory chemokines CXCL10 and CCL5 (Fig. 5C), factors that can attract and promote their interaction with activated effector T cells, including CCR5 expressing CD4+ T cells typically targeted by HIV-1 (30).
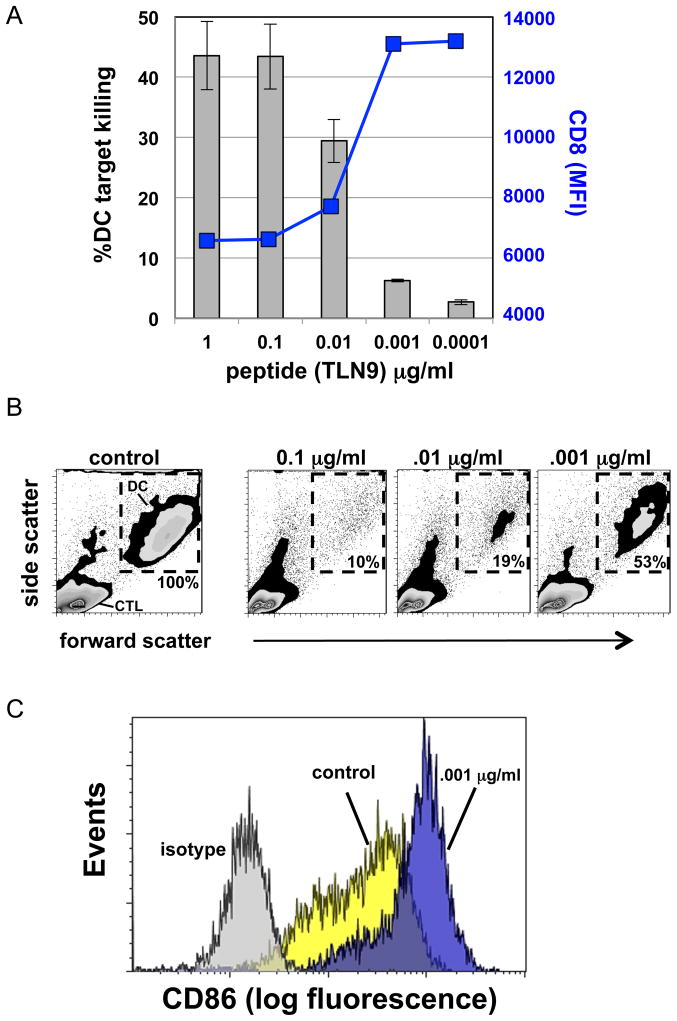
Expression level of cognate antigen can determine killer versus helper role of CTL
Low concentrations of viral antigenic peptides have been previously shown to activate CTL without causing down-regulation of TCR (31). Therefore, we questioned if the cognate peptide expression level could reach a threshold that would allow for the selective induction of CTL ‘help’ in the absence of iDC killing as observed with optimal expression of altered viral peptide variants. When CTL and iDC were co-cultured in the presence of high concentration (1.0 to .01 μg/ml) of the TLN9 peptide, iDC were effectively recognized as cytolytic targets as mentioned previously (Fig 6A and B). However, killing capacity of the CTL was reduced with decreasing concentration of cognate peptide, with a substantial drop in killing occurring at .001 μg/ml (Fig 6A and B). This decrease in killing capacity was inversely associated with an increase in CD8 expression (Fig 6A). When left in culture for 24 h, those DC that survived the exposure to CTL in the presence of .001 μg/ml of TLN9 peptide differentiated into high CD86 expressing mature DC (Fig. 6C), similar to when a high concentration of the PLN9 variant peptide was used (Fig 5A and B).
Fig. 6. Concentration of cognate antigen determines differential shift in CTL killer versus helper activity.
(A) Gray bars indicate percentage of iDC killed in a 6h co-culture with CTL at a 3:1 (CLT:DC) ratio by flow cytometry viability assessment using LIVE/DEAD® Aqua Kit discrimination. The blue line represents the relative CD8 expression (MFI) of TLN9 reactive CTL. Error bars represent ± standard error of three experiments. (B) Flow cytometry data showing percent DC recovery after overnight co-culture with CTL in relation to antigen concentration as determined by light scatter gating of DC region (dashed gates) compared to CTL in the presence of antigen control (0.1 μg/ml shown). (C) Relative expression of CD86 on surviving DC following 24h exposure to CTL and low concentration of either cognate (blue) or control (yellow) antigen.
CTL-programmed DC show enhanced ability to mediate HIV-1 transmission to T cells
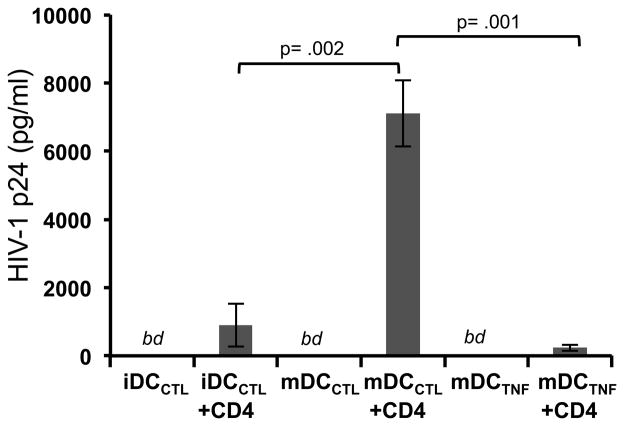
DC are known to play a critical role in HIV dissemination through trans infection of CD4+ T cells, with the efficiency of this trans-infection being greatly influenced by the status and mode of DC activation (32). Because the CTL programmed DC expressed the phenotypic, morphologic, and functional qualities ideal for activating and attracting activated CD4+ T cells, we speculated that they may be superior in mediating transmission of HIV-1 infection to CD4+ T cell. To test this, HIV-1 trans-infection assays were performed comparing the CTL-matured DC to both immature (CTL exposed) and TNF-α-matured DC. The differentially activated DC were purified and pulsed for a short time with a concentration of R5-tropic HIV-1 previously determined to be suboptimal for direct infection of activated CD4+ T cells (19), and subsequently co-incubated with autologous activated CD4+ T cells for 4 days. We found that the efficiency of HIV-1 trans infection was consistently greatest when using the CTL-matured DC (Fig. 7). Importantly, viral p24 concentrations remained below detectable levels in conditions where DC or CD4+ T cells alone (data not shown) were exposed to the suboptimal concentration of virus.
Fig. 7. DC matured by cross-reactive CTL are superior mediators of HIV-1 transmission to CD4+ T cells.
Immature DC were differentially stimulated for 48h with either CTL in the presence of the non CTL activating irrelevant peptide KLNDWDFVV (iDCCTL), CTL in the presence of the of the cognate priming peptide TLNAWVKVV (TLN9) (not shown), activating PLNAWVKVV (PLN9) epitope variant peptide (mDCCTL), or 50ng/ml of TNF-α (mDCTNFα). CTL were removed from the co-cultures and the differentially activated DC were incubated with HIV-1 R5 tropic virus at an MOI of 10−4 at 37°C for 2h. Virus loaded DC were then co-incubated with autologous pre-activated CD4+ T cells for 4 days at a DC:T cell ratio of 1:10. Cell free supernatants were tested by for levels of HIV-1 p24 by ELISA. DC initially present in co-cultures containing the CTL/TL9 peptide combination did not survive the 48h incubation (see Fig. 6A, middle panel) and therefore were not used in the trans-infection assay. Data presented as the combined mean concentration of p24 measured from supernatants collected from three separate trans-infection co-culture experiments ± standard error using autologous cells generated from one representative donor. Data was analyzed using a one-way ANOVA followed by a Tukey post hoc test. bd = below detection limits of the assay.
Discussion
CTL selective pressure is usually considered in the context of the antigen reactive CTL having the ability to kill infected target cells and inhibit viral production. CTL escape mutations that would allow infected cells to survive a cellular attack would thus provide an obvious selective advantage to the virus. However, the functions of antigen specific CTL are not limited to their killer effector role. They also play important immunoregulatory helper functions by producing cytokines and chemokines that influence the quality and character of the immune response (33). In addition, they can regulate the extent of the immune response by limiting the survival of antigen presenting cells (23). Therefore, it is likely that these functions are targeted by viruses and contribute to shaping the viral variant selection process.
We propose that the incomplete immune escape from CTL recognition through the establishment of partially activating epitope variants provides a selective advantage for the virus. Instead of totally bypassing the CTL response, these modifications can selectively promote the helper activity of the CTL while inhibiting their capacity to kill antigen expressing targets. Importantly, when the CTL effectors encounter such variant antigen presenting iDC, instead of dampening the immune response as a result of recognition of the iDC as cytolytic targets (23), a positive CTL to DC immune feedback loop dominates whereby the CTL provides helper signals to activate the HIV-1 antigen expressing DC, programming them to differentiate into a highly stimulatory, pro-inflammatory type of mature DC.
It is this viral ‘baiting’ of pre-existing CTL that allows the virus to utilize the CTL’s ability to program the phenotypic, morphologic, and functional character of the DC, the cell type that has been shown to be exploited by HIV-1 for dissemination and immune escape (21, 22). As shown in this study, iDC that survive the antigen-specific interaction with the cross-reactive effector CTL undergo a dramatic physical transformation in vitro. These DC rapidly sprout widespread micro- and nanotube-like extensions, allowing them to develop extensive interconnected cellular networks. Pathogens such as HIV-1 have been known utilize such cellular connections for cell-to-cell spread (26, 27). Therefore, altered CTL responses against epitope variants in vivo may actually promote the development of these complex cellular networks that can be exploited as an escape route by HIV-1, thereby allowing for intercellular spread without exposure to neutralizing antibodies, and establishment of a potentially latent reservoir.
Instead of having a diminished capacity to produce inflammatory factors upon maturation, DC that are programmed to mature by variant cross-reactive CTL have qualities consistent with type-1 polarized DC (DC1) (16), producing enhanced levels of cytokines, such as IL12p70 and IL-6, as well as chemokines such as CXCL10 and CCL5. Such characteristics provide the mature CTL-induced DC1 with the ability to efficiently interact with antigen specific naïve CD4+ T cells as well as attract activated CCR5+CD4+ T cells, the cells preferentially targeted by HIV-1 (34–36). Together, these characteristics likely contribute to the superior ability of CTL-induced DC1 to facilitate trans infection of CD4+ T cells as we report in this study. This notion supports a previous study that indicates DC1 to be effective mediators of HIV-1 trans infection (37). These results suggest that HIV-1 may utilize and benefit from the activity of cross-reactive CTL in vivo by promoting DC mediated viral spread.
The immunoregulatory helper roles of CD8+ T cells and their ability to modulate DC function have been previously described (33, 38–40). It is known that they can participate in heterologous immune responses to promote antiviral and anticancer immunity (41, 42), and contribute to a DC-mediated positive immunoregulatory feedback mechanism supporting success driven, type-1 immune responses (43). In our experience, however, the effectiveness of such direct DC-mediated CD8+ T cell ‘help’ greatly depends on the activation status of the T cells, and is limited to the activity of either naïve or resting memory CD8+ T cells (33, 42). The direct, antigen-specific interaction of fully activated CTL with DC typically results in a negative outcome with significant DC lysis (23, 24, 42), as we also have shown in this report. One novel finding from our study is that the presentation of altered peptide antigen can selectively promote the helper rather than killer function of the fully activated and otherwise potent effector CTL. The fact that these two CTL functions can occur either separately or simultaneously reinforces previous reports suggesting that data generated using common assays to measure and assess CTL function, such as ELISPOT and ICS flow cytometry, should be interpreted with caution (44, 45).
In this study, CTL killing activity was associated with the selective down-regulation and possible internalization of CD8 following antigenic stimulation as previously described (46). It has been suggested that the down-regulation of CD8 and CD3 serves to focus the CTL response on targets expressing high levels of antigen, limiting the response to protect against ‘self’ damage (46, 47). While exposure to low concentrations of viral antigenic peptides can activate CTL without causing down-regulation of TCR (31), we show that an altered viral peptide presented at high concentrations can likewise activate CTL without impacting TCR and CD8 expression. Importantly, we found the expression of the degranulation marker CD107a on the variant peptide activated CTL was not directly indicative of killing activity, a finding in line with that reported by others (48). Our data suggest that a decrease in CD8 expression is a reliable surrogate marker for measuring cytolytic function. Regardless of their lack of impact on CD8 expression, the variant peptides induced CTL production of cytokines at levels that had significant biological impact on DC function. Consistent with previous reports (49–51), this involvement of the T cell co-receptor CD8 appears to be a critical factor in determining the quality of the cross-reactive CTL response. The possibility that a virus may target the role of CD8 engagement during antigen specific interaction to selectively drive the helper versus killer function is intriguing.
We propose that an escape strategy in which the virus only partially evades, or even evolves towards partial CTL recognition to allow an ineffective CTL response to persist, provides the virus with a novel mechanism for survival. In contrast to what might be expected, the successful establishment of a CTL escape variant does not necessarily ensure an outcome favorable to the virus. In fact, it has been shown that the efficient selective escape from successful CTL responses by Gag epitope variants correlates with a decrease, rather than an increase in viral load, which was suspected to be due to loss in viral fitness (9, 52). Alternatively, the selective induction of an inefficient or incomplete CTL response could provide the virus with an immune ‘smoke screen’ to impede a more direct and effective antiviral CTL attack while limiting the need for excessive epitope modifications that would otherwise pose a threat to viral fitness. The proposed benefit of HIV-1 to selectively induce such a host response would suggest the likelihood that there would be an enrichment, at a population level, of certain epitope variants that would characteristically promote CTL helper activity in the absence of killing rather than completely abrogate CTL recognition. The immunodominant HLA-A*2010 restricted SL9 (SLYNTVATL) epitope, may represent such an example. While SL9 is widely targeted during chronic rather than acute infection (53), with often robust and potentially cross-reactive CTL responses, these attacks characteristically lack strong selective pressure (9, 53–55). This is true even when viral fitness pressure does not appear to be a major counteractive force to resist this immune pressure and epitope diversification (11). This suggests that these CTL responses are sub-optimal for target elimination, and that the potential benefit of the virus to evade this immune activity is outweighed by the advantage of maintaining it.
Our results fit with the “original antigenic sin” (56) model, whereby ineffective cross-reactive memory responses (in this case CTL memory) induced from an earlier exposure to virus hinders effective priming and expansion of naïve T cell responders specific against new viral variants. Such cross-reactive memory responses can interfere with the effectiveness of vaccines designed to elicit antigen specific cellular immunity, and may have partially contributed to the peculiar results of the STEP HIV-1 vaccine trial which showed a trend of increased HIV-1 infections observed in those receiving the vaccine (57). Genetic analysis of breakthrough virus in recipients of the STEP vaccine uncovered evidence for vaccine-associated divergence of Gag specific CTL epitopes (58). It is conceivable that narrow CTL responses elicited by this vaccine and the establishment of a limited pool of effector memory T cells allowed the virus to create an inflammatory response favorable for productive infection.
Our study adds a novel dimension to the idea of “original antigenic sin” by suggesting that in the setting of HIV-1 infection, the promiscuous nature of the CTL response is specifically exploited by an evolving virus to modulate the function of the DC to create an environment suitable for its spread and persistence within the host. Our results also point to the notion that this selective induction of CTL helper versus killer function is a general phenomenon, and not restricted to a specific virus, viral epitope, or HLA type, and therefore may be implicated in a broad range of diseases. This is supported by the fact that similar results were obtained when examining CTL responses to epitopes variants derived from DENV, a virus that upon secondary infection with heterotypic serotypes can lead to cross-reactive immune memory responses associated with severe disease including dengue hemorrhagic fever (59). Therefore, developing novel strategies to specifically disrupt or avoid this positive immune feedback loop may prove critical for the design of more effective immunotherapeutic therapies for a wide range of diseases including HIV-1 infection.
Supplementary Material
Acknowledgments
This work was supported by the NIAID grants U01 AI-35041, R37 AI-41870, and T32 AI-065380.
We thank Weimin Jiang, Blair Gleeson, Donna Stolz, Louise Borowski, Ravikumar Muthuswamy, Nancy McCarthy, Bill Buchanan and the volunteers of the Pittsburgh site of the MACS for their contributions to this report.
Footnotes
The authors have no conflicting financial interests to disclose.
References
- 1.Musey L, Hughes J, Schacker T, Shea T, Corey L, McElrath MJ. Cytotoxic-T-cell responses, viral load, and disease progression in early human immunodeficiency virus type 1 infection. The New England journal of medicine. 1997;337:1267–1274. doi: 10.1056/NEJM199710303371803. [DOI] [PubMed] [Google Scholar]
- 2.Koup RA, Safrit JT, Cao Y, Andrews CA, McLeod G, Borkowsky W, Farthing C, Ho DD. Temporal association of cellular immune responses with the initial control of viremia in primary human immunodeficiency virus type 1 syndrome. J Virol. 1994;68:4650–4655. doi: 10.1128/jvi.68.7.4650-4655.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Deeks SG. HIV infection, inflammation, immunosenescence, and aging. Annual review of medicine. 2011;62:141–155. doi: 10.1146/annurev-med-042909-093756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Goulder PJ, Watkins DI. HIV and SIV CTL escape: implications for vaccine design. Nat Rev Immunol. 2004;4:630–640. doi: 10.1038/nri1417. [DOI] [PubMed] [Google Scholar]
- 5.Liu Y, McNevin J, Zhao H, Tebit DM, Troyer RM, McSweyn M, Ghosh AK, Shriner D, Arts EJ, McElrath MJ, Mullins JI. Evolution of human immunodeficiency virus type 1 cytotoxic T-lymphocyte epitopes: fitness-balanced escape. J Virol. 2007;81:12179–12188. doi: 10.1128/JVI.01277-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Klenerman P, Wu Y, Phillips R. HIV: current opinion in escapology. Current opinion in microbiology. 2002;5:408–413. doi: 10.1016/s1369-5274(02)00339-9. [DOI] [PubMed] [Google Scholar]
- 7.Cale EM, Hraber P, Giorgi EE, Fischer W, Bhattacharya T, Leitner T, Yeh WW, Gleasner C, Green LD, Han CS, Korber B, Letvin NL. Epitope-specific CD8+ T lymphocytes cross-recognize mutant simian immunodeficiency virus (SIV) sequences but fail to contain very early evolution and eventual fixation of epitope escape mutations during SIV infection. J Virol. 2011;85:3746–3757. doi: 10.1128/JVI.02420-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Draenert R, Verrill CL, Tang Y, Allen TM, Wurcel AG, Boczanowski M, Lechner A, Kim AY, Suscovich T, Brown NV, Addo MM, Walker BD. Persistent recognition of autologous virus by high-avidity CD8 T cells in chronic, progressive human immunodeficiency virus type 1 infection. J Virol. 2004;78:630–641. doi: 10.1128/JVI.78.2.630-641.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Iversen AK, Stewart-Jones G, Learn GH, Christie N, Sylvester-Hviid C, Armitage AE, Kaul R, Beattie T, Lee JK, Li Y, Chotiyarnwong P, Dong T, Xu X, Luscher MA, MacDonald K, Ullum H, Klarlund-Pedersen B, Skinhoj P, Fugger L, Buus S, Mullins JI, Jones EY, van der Merwe PA, McMichael AJ. Conflicting selective forces affect T cell receptor contacts in an immunodominant human immunodeficiency virus epitope. Nature immunology. 2006;7:179–189. doi: 10.1038/ni1298. [DOI] [PubMed] [Google Scholar]
- 10.Hay CM, Ruhl DJ, Basgoz NO, Wilson CC, Billingsley JM, DePasquale MP, D’Aquila RT, Wolinsky SM, Crawford JM, Montefiori DC, Walker BD. Lack of viral escape and defective in vivo activation of human immunodeficiency virus type 1-specific cytotoxic T lymphocytes in rapidly progressive infection. J Virol. 1999;73:5509–5519. doi: 10.1128/jvi.73.7.5509-5519.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Christie NM, Willer DO, Lobritz MA, Chan JK, Arts EJ, Ostrowski MA, Cochrane A, Luscher MA, MacDonald KS. Viral fitness implications of variation within an immunodominant CD8+ T-cell epitope of HIV-1. Virology. 2009;388:137–146. doi: 10.1016/j.virol.2009.03.003. [DOI] [PubMed] [Google Scholar]
- 12.Detels R, Jacobson L, Margolick J, Martinez-Maza O, Munoz A, Phair J, Rinaldo C, Wolinsky S. The multicenter AIDS Cohort Study, 1983 to. Public health. 2012;126:196–198. doi: 10.1016/j.puhe.2011.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shankarappa R, Margolick JB, Gange SJ, Rodrigo AG, Upchurch D, Farzadegan H, Gupta P, Rinaldo CR, Learn GH, He X, Huang XL, Mullins JI. Consistent viral evolutionary changes associated with the progression of human immunodeficiency virus type 1 infection. J Virol. 1999;73:10489–10502. doi: 10.1128/jvi.73.12.10489-10502.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pascolo S, Bervas N, Ure JM, Smith AG, Lemonnier FA, Perarnau B. HLA-A2.1-restricted education and cytolytic activity of CD8(+) T lymphocytes from beta2 microglobulin (beta2m) HLA-A2.1 monochain transgenic H-2Db beta2m double knockout mice. The Journal of experimental medicine. 1997;185:2043–2051. doi: 10.1084/jem.185.12.2043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Buchli R, VanGundy RS, Hickman-Miller HD, Giberson CF, Bardet W, Hildebrand WH. Development and validation of a fluorescence polarization-based competitive peptide-binding assay for HLA-A*0201--a new tool for epitope discovery. Biochemistry. 2005;44:12491–12507. doi: 10.1021/bi050255v. [DOI] [PubMed] [Google Scholar]
- 16.Mailliard RB, Wankowicz-Kalinska A, Cai Q, Wesa A, Hilkens CM, Kapsenberg ML, Kirkwood JM, Storkus WJ, Kalinski P. alpha-type-1 polarized dendritic cells: a novel immunization tool with optimized CTL-inducing activity. Cancer Res. 2004;64:5934–5937. doi: 10.1158/0008-5472.CAN-04-1261. [DOI] [PubMed] [Google Scholar]
- 17.Keating SM, Bejon P, Berthoud T, Vuola JM, Todryk S, Webster DP, Dunachie SJ, Moorthy VS, McConkey SJ, Gilbert SC, Hill AV. Durable human memory T cells quantifiable by cultured enzyme-linked immunospot assays are induced by heterologous prime boost immunization and correlate with protection against malaria. J Immunol. 2005;175:5675–5680. doi: 10.4049/jimmunol.175.9.5675. [DOI] [PubMed] [Google Scholar]
- 18.Balachandran R, Thampatty P, Enrico A, Rinaldo C, Gupta P. Human immunodeficiency virus isolates from asymptomatic homosexual men and from AIDS patients have distinct biologic and genetic properties. Virology. 1991;180:229–238. doi: 10.1016/0042-6822(91)90027-9. [DOI] [PubMed] [Google Scholar]
- 19.Rappocciolo G, Piazza P, Fuller CL, Reinhart TA, Watkins SC, Rowe DT, Jais M, Gupta P, Rinaldo CR. DC-SIGN on B lymphocytes is required for transmission of HIV-1 to T lymphocytes. PLoS pathogens. 2006;2:e70. doi: 10.1371/journal.ppat.0020070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Seder RA, Darrah PA, Roederer M. T-cell quality in memory and protection: implications for vaccine design. Nat Rev Immunol. 2008;8:247–258. doi: 10.1038/nri2274. [DOI] [PubMed] [Google Scholar]
- 21.Geijtenbeek TB, Kwon DS, Torensma R, van Vliet SJ, van Duijnhoven GC, Middel J, Cornelissen IL, Nottet HS, KewalRamani VN, Littman DR, Figdor CG, van Kooyk Y. DC-SIGN, a dendritic cell-specific HIV-1-binding protein that enhances trans-infection of T cells. Cell. 2000;100:587–597. doi: 10.1016/s0092-8674(00)80694-7. [DOI] [PubMed] [Google Scholar]
- 22.Lekkerkerker AN, van Kooyk Y, Geijtenbeek TB. Viral piracy: HIV-1 targets dendritic cells for transmission. Current HIV research. 2006;4:169–176. doi: 10.2174/157016206776055020. [DOI] [PubMed] [Google Scholar]
- 23.Hermans IF, Ritchie DS, Yang J, Roberts JM, Ronchese F. CD8+ T cell-dependent elimination of dendritic cells in vivo limits the induction of antitumor immunity. J Immunol. 2000;164:3095–3101. doi: 10.4049/jimmunol.164.6.3095. [DOI] [PubMed] [Google Scholar]
- 24.Watchmaker PB, Urban JA, Berk E, Nakamura Y, Mailliard RB, Watkins SC, van Ham SM, Kalinski P. Memory CD8+ T cells protect dendritic cells from CTL killing. J Immunol. 2008;180:3857–3865. doi: 10.4049/jimmunol.180.6.3857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Watkins SC, Salter RD. Functional connectivity between immune cells mediated by tunneling nanotubules. Immunity. 2005;23:309–318. doi: 10.1016/j.immuni.2005.08.009. [DOI] [PubMed] [Google Scholar]
- 26.Sowinski S, Jolly C, Berninghausen O, Purbhoo MA, Chauveau A, Kohler K, Oddos S, Eissmann P, Brodsky FM, Hopkins C, Onfelt B, Sattentau Q, Davis DM. Membrane nanotubes physically connect T cells over long distances presenting a novel route for HIV-1 transmission. Nature cell biology. 2008;10:211–219. doi: 10.1038/ncb1682. [DOI] [PubMed] [Google Scholar]
- 27.Eugenin EA, Gaskill PJ, Berman JW. Tunneling nanotubes (TNT) are induced by HIV-infection of macrophages: a potential mechanism for intercellular HIV trafficking. Cellular immunology. 2009;254:142–148. doi: 10.1016/j.cellimm.2008.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Langenkamp A, Messi M, Lanzavecchia A, Sallusto F. Kinetics of dendritic cell activation: impact on priming of TH1, TH2 and nonpolarized T cells. Nature immunology. 2000;1:311–316. doi: 10.1038/79758. [DOI] [PubMed] [Google Scholar]
- 29.Kalinski P, Hilkens CM, Wierenga EA, Kapsenberg ML. T-cell priming by type-1 and type-2 polarized dendritic cells: the concept of a third signal. Immunol Today. 1999;20:561–567. doi: 10.1016/s0167-5699(99)01547-9. [DOI] [PubMed] [Google Scholar]
- 30.McDonald D, Wu L, Bohks SM, KewalRamani VN, Unutmaz D, Hope TJ. Recruitment of HIV and its receptors to dendritic cell-T cell junctions. Science. 2003;300:1295–1297. doi: 10.1126/science.1084238. [DOI] [PubMed] [Google Scholar]
- 31.Betts MR, Price DA, Brenchley JM, Lore K, Guenaga FJ, Smed-Sorensen A, Ambrozak DR, Migueles SA, Connors M, Roederer M, Douek DC, Koup RA. The functional profile of primary human antiviral CD8+ T cell effector activity is dictated by cognate peptide concentration. J Immunol. 2004;172:6407–6417. doi: 10.4049/jimmunol.172.10.6407. [DOI] [PubMed] [Google Scholar]
- 32.Wu L, KewalRamani VN. Dendritic-cell interactions with HIV: infection and viral dissemination. Nat Rev Immunol. 2006;6:859–868. doi: 10.1038/nri1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mailliard RB, Egawa S, Cai Q, Kalinska A, Bykovskaya SN, Lotze MT, Kapsenberg ML, Storkus WJ, Kalinski P. Complementary dendritic cell-activating function of CD8+ and CD4+ T cells: helper role of CD8+ T cells in the development of T helper type 1 responses. The Journal of experimental medicine. 2002;195:473–483. doi: 10.1084/jem.20011662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dragic T, Litwin V, Allaway GP, Martin SR, Huang Y, Nagashima KA, Cayanan C, Maddon PJ, Koup RA, Moore JP, Paxton WA. HIV-1 entry into CD4+ cells is mediated by the chemokine receptor CC-CKR-5. Nature. 1996;381:667–673. doi: 10.1038/381667a0. [DOI] [PubMed] [Google Scholar]
- 35.Deng H, Liu R, Ellmeier W, Choe S, Unutmaz D, Burkhart M, Di Marzio P, Marmon S, Sutton RE, Hill CM, Davis CB, Peiper SC, Schall TJ, Littman DR, Landau NR. Identification of a major co-receptor for primary isolates of HIV-1. Nature. 1996;381:661–666. doi: 10.1038/381661a0. [DOI] [PubMed] [Google Scholar]
- 36.Lore K, Smed-Sorensen A, Vasudevan J, Mascola JR, Koup RA. Myeloid and plasmacytoid dendritic cells transfer HIV-1 preferentially to antigen-specific CD4+ T cells. The Journal of experimental medicine. 2005;201:2023–2033. doi: 10.1084/jem.20042413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sanders RW, de Jong EC, Baldwin CE, Schuitemaker JH, Kapsenberg ML, Berkhout B. Differential transmission of human immunodeficiency virus type 1 by distinct subsets of effector dendritic cells. J Virol. 2002;76:7812–7821. doi: 10.1128/JVI.76.15.7812-7821.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ruedl C, Kopf M, Bachmann MF. CD8(+) T cells mediate CD40-independent maturation of dendritic cells in vivo. The Journal of experimental medicine. 1999;189:1875–1884. doi: 10.1084/jem.189.12.1875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gurunathan S, Stobie L, Prussin C, Sacks DL, Glaichenhaus N, Iwasaki A, Fowell DJ, Locksley RM, Chang JT, Wu CY, Seder RA. Requirements for the maintenance of Th1 immunity in vivo following DNA vaccination: a potential immunoregulatory role for CD8+ T cells. J Immunol. 2000;165:915–924. doi: 10.4049/jimmunol.165.2.915. [DOI] [PubMed] [Google Scholar]
- 40.Thomas MJ, Noble A, Sawicka E, Askenase PW, Kemeny DM. CD8 T cells inhibit IgE via dendritic cell IL-12 induction that promotes Th1 T cell counter-regulation. J Immunol. 2002;168:216–223. doi: 10.4049/jimmunol.168.1.216. [DOI] [PubMed] [Google Scholar]
- 41.Welsh RM, Che JW, Brehm MA, Selin LK. Heterologous immunity between viruses. Immunological reviews. 2010;235:244–266. doi: 10.1111/j.0105-2896.2010.00897.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nakamura Y, Watchmaker P, Urban J, Sheridan B, Giermasz A, Nishimura F, Sasaki K, Cumberland R, Muthuswamy R, Mailliard RB, Larregina AT, Falo LD, Gooding W, Storkus WJ, Okada H, Hendricks RL, Kalinski P. Helper function of memory CD8+ T cells: heterologous CD8+ T cells support the induction of therapeutic cancer immunity. Cancer Res. 2007;67:10012–10018. doi: 10.1158/0008-5472.CAN-07-1735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kalinski P, Moser M. Consensual immunity: success-driven development of T-helper-1 and T-helper-2 responses. Nat Rev Immunol. 2005;5:251–260. doi: 10.1038/nri1569. [DOI] [PubMed] [Google Scholar]
- 44.Valentine LE, Piaskowski SM, Rakasz EG, Henry NL, Wilson NA, Watkins DI. Recognition of escape variants in ELISPOT does not always predict CD8+ T-cell recognition of simian immunodeficiency virus-infected cells expressing the same variant sequences. J Virol. 2008;82:575–581. doi: 10.1128/JVI.00275-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Loffredo JT, Burwitz BJ, Rakasz EG, Spencer SP, Stephany JJ, Vela JP, Martin SR, Reed J, Piaskowski SM, Furlott J, Weisgrau KL, Rodrigues DS, Soma T, Napoe G, Friedrich TC, Wilson NA, Kallas EG, Watkins DI. The antiviral efficacy of simian immunodeficiency virus-specific CD8+ T cells is unrelated to epitope specificity and is abrogated by viral escape. J Virol. 2007;81:2624–2634. doi: 10.1128/JVI.01912-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Maile R, Siler CA, Kerry SE, Midkiff KE, Collins EJ, Frelinger JA. Peripheral “CD8 tuning” dynamically modulates the size and responsiveness of an antigen-specific T cell pool in vivo. J Immunol. 2005;174:619–627. doi: 10.4049/jimmunol.174.2.619. [DOI] [PubMed] [Google Scholar]
- 47.Xiao Z, Mescher MF, Jameson SC. Detuning CD8 T cells: down-regulation of CD8 expression, tetramer binding, and response during CTL activation. The Journal of experimental medicine. 2007;204:2667–2677. doi: 10.1084/jem.20062376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wolint P, Betts MR, Koup RA, Oxenius A. Immediate cytotoxicity but not degranulation distinguishes effector and memory subsets of CD8+ T cells. The Journal of experimental medicine. 2004;199:925–936. doi: 10.1084/jem.20031799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Daniels MA, Jameson SC. Critical role for CD8 in T cell receptor binding and activation by peptide/major histocompatibility complex multimers. The Journal of experimental medicine. 2000;191:335–346. doi: 10.1084/jem.191.2.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yachi PP, Ampudia J, Zal T, Gascoigne NR. Altered peptide ligands induce delayed CD8-T cell receptor interaction--a role for CD8 in distinguishing antigen quality. Immunity. 2006;25:203–211. doi: 10.1016/j.immuni.2006.05.015. [DOI] [PubMed] [Google Scholar]
- 51.Wooldridge L, Laugel B, Ekeruche J, Clement M, van den Berg HA, Price DA, Sewell AK. CD8 controls T cell cross-reactivity. J Immunol. 2010;185:4625–4632. doi: 10.4049/jimmunol.1001480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Brockman MA, Brumme ZL, Brumme CJ, Miura T, Sela J, Rosato PC, Kadie CM, Carlson JM, Markle TJ, Streeck H, Kelleher AD, Markowitz M, Jessen H, Rosenberg E, Altfeld M, Harrigan PR, Heckerman D, Walker BD, Allen TM. Early selection in Gag by protective HLA alleles contributes to reduced HIV-1 replication capacity that may be largely compensated for in chronic infection. J Virol. 2010;84:11937–11949. doi: 10.1128/JVI.01086-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Goulder PJ, Altfeld MA, Rosenberg ES, Nguyen T, Tang Y, Eldridge RL, Addo MM, He S, Mukherjee JS, Phillips MN, Bunce M, Kalams SA, Sekaly RP, Walker BD, Brander C. Substantial differences in specificity of HIV-specific cytotoxic T cells in acute and chronic HIV infection. The Journal of experimental medicine. 2001;193:181–194. doi: 10.1084/jem.193.2.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Brander C, Hartman KE, Trocha AK, Jones NG, Johnson RP, Korber B, Wentworth P, Buchbinder SP, Wolinsky S, Walker BD, Kalams SA. Lack of strong immune selection pressure by the immunodominant, HLA-A*0201-restricted cytotoxic T lymphocyte response in chronic human immunodeficiency virus-1 infection. The Journal of clinical investigation. 1998;101:2559–2566. doi: 10.1172/JCI2405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Brander C, Goulder PJ, Luzuriaga K, Yang OO, Hartman KE, Jones NG, Walker BD, Kalams SA. Persistent HIV-1-specific CTL clonal expansion despite high viral burden post in utero HIV-1 infection. J Immunol. 1999;162:4796–4800. [PubMed] [Google Scholar]
- 56.Klenerman P, Zinkernagel RM. Original antigenic sin impairs cytotoxic T lymphocyte responses to viruses bearing variant epitopes. Nature. 1998;394:482–485. doi: 10.1038/28860. [DOI] [PubMed] [Google Scholar]
- 57.Duerr A, Huang Y, Buchbinder S, Coombs RW, Sanchez J, Del Rio C, Casapia M, Santiago S, Gilbert P, Corey L, Robertson MN. Extended Follow-up Confirms Early Vaccine-Enhanced Risk of HIV Acquisition and Demonstrates Waning Effect Over Time Among Participants in a Randomized Trial of Recombinant Adenovirus HIV Vaccine (Step Study) The Journal of infectious diseases. 2012 doi: 10.1093/infdis/jis342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rolland M, Tovanabutra S, deCamp AC, Frahm N, Gilbert PB, Sanders-Buell E, Heath L, Magaret CA, Bose M, Bradfield A, O’Sullivan A, Crossler J, Jones T, Nau M, Wong K, Zhao H, Raugi DN, Sorensen S, Stoddard JN, Maust BS, Deng W, Hural J, Dubey S, Michael NL, Shiver J, Corey L, Li F, Self SG, Kim J, Buchbinder S, Casimiro DR, Robertson MN, Duerr A, McElrath MJ, McCutchan FE, Mullins JI. Genetic impact of vaccination on breakthrough HIV-1 sequences from the STEP trial. Nature medicine. 2011;17:366–371. doi: 10.1038/nm.2316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rothman AL. Immunity to dengue virus: a tale of original antigenic sin and tropical cytokine storms. Nat Rev Immunol. 2011;11:532–543. doi: 10.1038/nri3014. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.