Abstract
Migraine is a common and disabling neurological disease of unknown origin characterized by a remarkable clinical variability. It shows strong familial aggregation, suggesting that genetic factors are involved in its pathogenesis. Different approaches have been used to elucidate this hereditary component, but a unique transmission model and causative gene(s) have not yet been identified. We report clinical and molecular data from a large Italian pedigree in which migraine without aura (MO) segregates as an autosomal dominant trait. After exclusion of any association between MO and the known familial hemiplegic migraine and migraine with aura loci, we performed a genomewide linkage analysis using 482 polymorphic microsatellite markers. We obtained significant evidence of linkage between the MO phenotype and the marker D14S978 on 14q22.1 (maximum two-point LOD score of 3.70, at a recombination fraction of 0.01). Multipoint parametric analysis (maximum LOD score of 5.25 between markers D14S976 and D14S978) and haplotype construction showed strong evidence of linkage in a region of 10 cM flanked by markers D14S1027 and D14S980 on chromosome 14q21.2-q22.3. These results indicate the first evidence of a genetic locus associated with MO on chromosome 14.
Migraine (MIM 157300) is a complex and heterogeneous disease characterized by recurrent attacks of headache associated with autonomic and neurological symptoms, that affects ∼12% of the general population (Stewart et al. 1992). Diagnosis is based on clinical criteria, since biochemical or instrumental tests are not available (Headache Classification Committee of the International Headache Society [HCCIHS] 1988). Two primary types of migraine can be distinguished: migraine without aura (MO) and migraine with aura (MA). MO, the most common form of migraine, is characterized by unilateral pulsating headache of moderate to severe intensity, lasting 4–72 h. The attacks are associated with nausea, vomiting, and photo- and phonophobia and are aggravated by physical activity. In MA, headache attacks are preceded by transient focal neurological aura symptoms, which are usually visual. Frequency, duration, and severity of the attacks vary substantially among patients and also in the same patients. Moreover, these two forms of migraine can co-occur in up to 33% of migraineurs (Russell et al. 1995), but usually one type of attack prevails.
The etiopathogenesis of migraine remains largely unknown. Neurophysiological and neurofunctional studies support a primary neuronal theory, according to which a neuronal hyperexcitability is the biological basis for susceptibility to migraine (Hargreaves and Shepheard 1999). Neuronal instability should be responsible for neuroelectric (Goadsby and Edvinsson 1993; Lauritzen et al. 1994) and metabolic events (Goadsby et al. 1990) leading to migraine attack. Familial clustering suggests that migraine has a significant genetic component, but the mode of inheritance remains unclear (Mochi et al. 1993; Russell and Olesen 1993). Family-, twin-, and population-based studies strongly suggest that the two types of migraine are genetically determined, most likely with a multifactorial mode of inheritance (Honkasalo et al. 1995; Stewart et al. 1997; Gervil et al. 1999; Ulrich et al. 1999). However, in some families, a Mendelian pattern of inheritance cannot be excluded (Russell and Olesen 1995). At present, the only nosologic entity genetically characterized is familial hemiplegic migraine (FHM) (FHM1 [MIM 141500]), a rare autosomal dominantly inherited subtype of MA in which hemiplegia is the principal aura feature (HCCIHS 1988). It is considered a genetically heterogeneous disorder; half of the known families with FHM have been associated with mutations in the brain-specific P/Q type calcium channel alpha subunit (CACNA1A) located on 19p13 chromosome (MIM 601011) (Joutel et al. 1993; Ophoff et al. 1996; Ducros et al. 2001), and additional FHM linkage loci have been reported on chromosomes 1q21-q23 (FHM2 [MIM 602481]) (Ducros et al. 1997) and on 1q31 (Gardner et al. 1997). FHM can be considered part of the migraine spectrum and, thus, it has been used as a model to study the complex genetics of the common forms of migraine. Nevertheless, the role of 19p FHM locus in MO and MA is still debated (May et al. 1995; Hovatta et al. 1998; Nyholt et al. 1998). In familial typical migraine, linkage to 1q31 FHM locus (Lea et al. 2002) and to chromosome Xq24-q28 was recently established (MFTS [MIM 300125]) (Nyholt et al. 2000). Moreover, a new locus on chromosome 19p13, distinct from the CACNA1A gene, was found to be linked to MA (Jones et al. 2001), and a susceptibility locus for MA was identified on chromosome 4q24 (MGAU [MIM 300125]) by Wessman et al. (2002). Genetic association studies have suggested linkage between different genetic loci and either MO or MA (Pardo et al. 1995; Peroutka et al. 1997; Ogilvie et al. 1998; Lea et al. 2000; McCarthy et al. 2001, Tzourio et al. 2001), but the results of these studies often remain controversial and unconfirmed.
Identification of genes predisposing to migraine has been complicated by clinical and genetic heterogeneity of the disease. However, studies of large pedigrees with a clear pattern of inheritance might be extremely useful in identifying genes involved in migraine pathogenesis.
Here, we describe a large four-generation Italian family, originating from a restricted geographical area of northern Italy, in which MO appears to segregate as an autosomal dominant trait (fig. 1). After exclusion of any association between MO and the known FHM and MA loci, we performed a genomewide scan of the 22 autosomes to identify the putative genetic locus associated with MO.
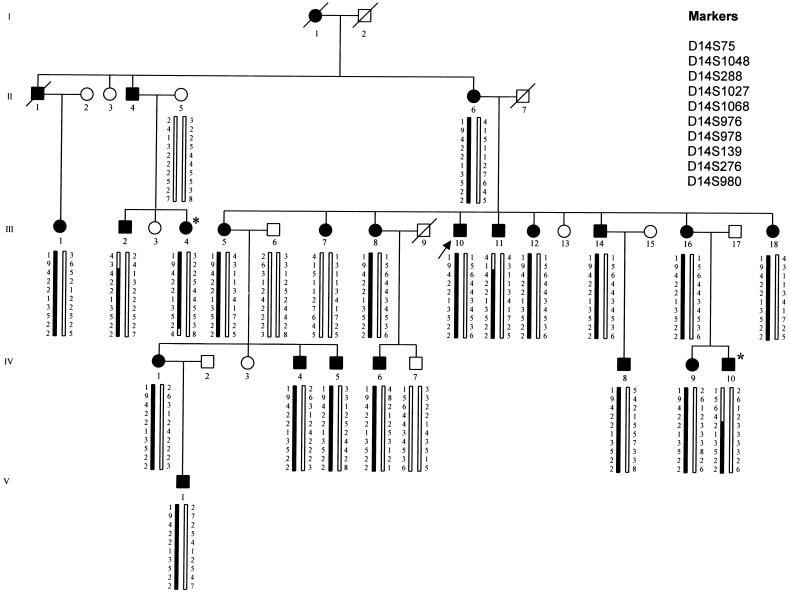
Figure 1.
MO family pedigree. Individuals available for typing are indicated by the generated genotype data. The disease haplotype for MO is indicated by the blackened bar. Blackened symbols denote affected individuals; unblackened symbols denote unaffected individuals. The arrow indicates the propositus; the asterisks indicate key recombinants (III:4 and IV:10), who determine the critical interval to be 10 cM, flanked by markers D14S1027 and D14S980.
The propositus (III:10), a 49-year-old man, was the fourth child of nonconsanguineous parents. Since his adolescence, he complained of migraine attacks occurring once per wk. The attacks were characterized by severe pulsating unilateral headache lasting ⩾12 h, accompanied by nausea and photo- and phonophobia. Neither premonitory symptoms nor triggering factors typical for migraine nor neurological aura was reported. The common antimigraine agents resulted only in a partial and brief relief of pain, and prophylactic headache medication (propanolol and amitriptyline) had low efficacy. General examination was normal; neurological examination disclosed the presence of diffuse brisk deep tendon reflexes without asymmetry. Electroencephalogram (EEG) and brain CT scan were within normal limits. Family history revealed that other family members suffered recurrent migraine attacks. A total of 33 subjects (27 family members and 6 spouses) were evaluated by a certified neurologist. Clinical evaluation included extensive history and physical and neurological examination. EEG and CT scan were performed in five individuals (II:6, III:5, III:7, III:12, and III:16). When HCCIHS (1988) criteria were used for diagnosing MO, 22 subjects were categorized as affected and 11 as unaffected (fig. 1). Among the 22 MO subjects, clinical features were almost homogeneous; attacks were characterized by moderate–severe pulsating unilateral headache lasting from a few hours to some days and accompanied by nausea and/or photo- and phonophobia. Migraine attack frequency was variable, from 1–2 attacks per mo to 2–3 attacks per wk. Two subjects (III:5 and III:16) occasionally experienced migraine attacks preceded by visual aura lasting a few minutes in addition to high frequency (>1 per wk) of MO attacks. In particular, subject III:5, who was affected with borderline hypertension, experienced two episodes of migraine with visual aura; individual III:16 presented only one attack of migraine with visual aura. We think that the isolated occurrence of MA attacks does not change the diagnosis of MO in these subjects. Disease onset was during childhood or adolescence in all individuals. All subjects were normal at the physical examination, although neurological examination disclosed the presence of a diffuse hyperactivity of the muscle stretch reflexes in eight affected individuals (II:6, III:5, III:7, III:8, III:10, III:12, IV:5, and IV:9). EEG and brain CT scan were within normal limits in the examined subjects.
We obtained informed consent from 21 subjects affected with MO and 3 unaffected subjects (1 family member, IV:7, and 2 spouses, II:5 and III:6), on whom we performed molecular analyses. Venous blood samples were used to extract genomic DNA and to establish permanent lymphoblastoid cell lines according to standard techniques.
First, we selected microsatellite markers from the putative candidate regions for FHM and MA on chromosomes 19p13, 1q21-23, 1q31, and 4q24. For these analyzed markers, we obtained two-point LOD score values <2 at a recombination fraction (θ) of 0.0 (data not shown).
To identify the genetic locus associated with MO in this large pedigree, we performed a genomewide search using 382 fluorescently labeled microsatellites from the ABI PRISM Linkage Mapping Set, version 2 (LMS2, Applied Biosystems). To improve the coverage of the whole genome, we used another 100 microsatellite markers chosen from the ABI PRISM Linkage Mapping set Version 5 (LMS5, Applied Biosystems). Using these 482 markers, we obtained a genome screening with a resolution of ∼5 cM. PCR reactions were performed according to the condition described in the LMS2 user’s manual. PCR products were run on a 7% denaturing polyacrylamide gel using an ABI373 automatic DNA sequencer, and the instrument’s output was analyzed using GENESCAN 672 1.2 and GENOTYPER 1.1 software applications (Applied Biosystems). Parametric LOD score for 482 markers used in the genomewide linkage analysis was calculated using the affected-only strategy, because it is the fittest method for complex diseases such as MO. Two-point LOD score values were calculated using the MLINK program of the LINKAGE package version 5.2 (Lathrop et al. 1984). Linkage analysis was performed assuming a dominant model with a complete penetrance, a phenocopy rate of 0.7% (Hovatta et al. 1994) and a disease allele frequency of 12% (Stewart et al. 1992). To assess the statistical significance of linkage, we considered a threshold value of 3.3, as suggested by Lander and Kruglyak (1995).
The highest two-point LOD score values were obtained for marker D14S288 (Z=2.76 at θ=0.10) and D14S276 (Z=2.44 at θ=0.05) on chromosome 14q21 (table 1). No other chromosomal regions presented statistical evidence of linkage. That region was further analyzed selecting eight additional unlabeled markers (D14S75, D14S1048, D14S1027, D14S1068, D14S976, D14S978, D14S139, and D14S980) according to the UCSC Human Genome Project Working Draft Database. Three markers gave a LOD score >3.3, with a maximum two-point LOD score value of 3.70 at θ=0.01 for marker D14S978 (table 1).
Table 1.
Two-Point LOD Scores for Migraine without Aura (MO) and Microsatellite Markers on Chromosome 14q
|
LOD at θ = |
|||||||
| Marker | .00 | .01 | .05 | .10 | .20 | .30 | .40 |
| D14S75 | −1.42 | −.70 | .17 | .47 | .54 | .35 | .11 |
| D14S1048 | −2.45 | −1.24 | .34 | 1.00 | 1.29 | 1.05 | .56 |
| D14S288 | 1.76 | 2.20 | 2.70 | 2.76 | 2.38 | 1.70 | .82 |
| D14S1027 | 2.47 | 2.54 | 2.61 | 2.51 | 2.04 | 1.39 | .63 |
| D14S1068 | 3.56 | 3.61 | 3.59 | 3.38 | 2.73 | 1.89 | .90 |
| D14S976 | 3.53 | 3.57 | 3.54 | 3.32 | 2.65 | 1.81 | .85 |
| D14S978 | 3.65 | 3.70 | 3.68 | 3.46 | 2.79 | 1.93 | .92 |
| D14S139 | 2.76 | 2.82 | 2.85 | 2.70 | 2.19 | 1.50 | .69 |
| D14S276 | 2.31 | 2.38 | 2.44 | 2.33 | 1.89 | 1.29 | .58 |
| D14S980 | 1.51 | 1.94 | 2.45 | 2.52 | 2.17 | 1.54 | .73 |
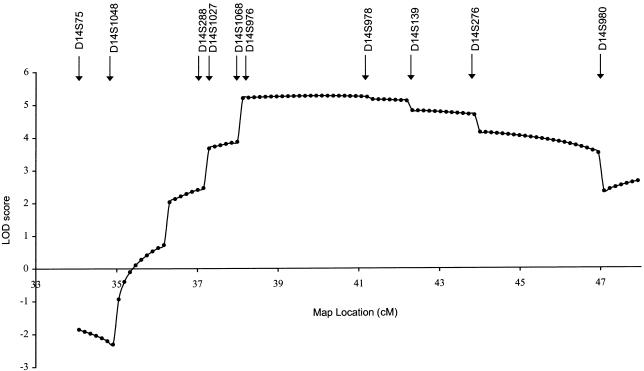
Multipoint analysis was performed by the FASTMAP program (Curtis et al. 1993), using intermarker genetic distances based on the sex-averaged distances reported by the Genetic Location Database. Multipoint linkage LOD score provided strong evidence of linkage between markers D14S1027 and D14S980, with a peak of 5.25 between D14S976 and D14S978 (fig. 2). The most likely haplotype cosegregating with the disease is displayed in figure 1 along with the pedigree. The upper and lower limits of the critical region were established by the recombination events occurring in individual IV:10 (between markers D14S1027 and D14S68) and individual III:4 (between markers D14S276 and D14S980) (fig. 1).
Figure 2.
Multipoint localization of MO. Genetic location (distance in cM) is plotted against the multipoint LOD score. The locations of markers used in the multipoint analysis are indicated. A maximum multipoint LOD score of 5.25 was obtained between markers D14S976 and D14S978.
Our results provide strong evidence of an MO susceptibility locus on chromosome 14q21.2-14q22.3 within a 10-cM interval flanked by markers D14S1027 and D14S980.
Statistically significant evidence of linkage was obtained with a parametric two-point linkage analysis and was supported by multipoint analyses. We chose to perform a parametric linkage analysis, since, in this family, MO segregates as an autosomal trait and presents with homogenous clinical features; therefore, it was plausible that a unique genetic defect contributes to risk of MO. Among the affected individuals, only III:7 does not share the affected haplotype and still presents with MO. Considering the high prevalence of migraine in the population and its complex etiology, subject III:7 may represent a phenocopy.
According to the UCSC Human Genome Project Working Draft database, the flanking markers D14S1027 and D14S980 span a 12-Mb candidate region, which contains 45 known genes and >100 predicted genes. To better refine the disease haplotype, SNPs in the known genes can be used. Moreover, the collection of other affected family members can be useful to identify more recombinations to further narrow the region. Meanwhile, interesting candidate genes will be screened according to their expression profiles, function, and possible involvement in MO pathogenesis.
Recently, several association genetic studies reported a possible association between different gene polymorphisms and either MA or MO. On the basis of these studies, neurotransmitter receptors (Peroutka et al. 1997; Del Zompo et al. 1998; Ogilvie et al. 1998; Lea et al. 2000), vasoconstrictor receptors, (Tzourio et al. 2001), ion channel genes (May et al. 1995; Terwindt et al. 2001), and the INSR gene (McCarthy et al. 2001) can be considered good candidates for migraine. Even if no genes with such features were present within the chromosomal region we have identified, there are some genes that can be related to migraine pathogenesis. At present, on the basis of their function and their expression profiles, probable candidate genes are the GTP cyclohydrolase 1 gene (GCH1 [MIM 600225]) and the atlastin gene (SPG3A [MIM 606439]). Mutations in these genes have been identified in Dopa-responsive dystonia (Ichinose et al. 1994) and spastic paraparesis (Zhao et al. 2001), respectively. Both of these genes are expressed in the CNS. SPG3A encodes a peptide showing homology with several GTPases that are involved in neurotransmission (Zhao et al. 2001), whereas GCH1 encodes GTP cyclohydrolase I, which catalyzes the rate-limiting step of tetrahydrobiopterin (Witter et al. 1996), cofactor of tyrosine and tryptophan hydroxylases, and nitric oxide synthase (Werner et al. 1996). The latter three are involved in the production of dopamine, serotonin, and nitric oxide, which are implicated in migraine pathogenesis (Hargreaves and Shepheard 1999). Moreover, prostaglandin D2 receptor (PTGDR [MIM 606687]) and prostaglandin E receptor 2 genes (PTGER2 [MIM 176804]), which are implicated in inflammatory processes and in pain responses (Funk et al. 2001), can be considered candidate genes. The bone morphogenetic protein 4 gene (BMP4 [MIM 112262]) encodes a cytokine belonging to the transforming growth factor-β superfamily and is implicated in the expression of the neuropeptide calcitonin gene-related protein (Ai et al. 1999), which mediates pain sensation and vasodilation (Goadsby et al. 1990).
In summary, we have identified an MO-susceptibility locus on chromosomes 14q21.2–14q22.3. Further studies are required to identify the causative MO gene in this family and to delineate the role of this locus in other families affected with MO.
The detection of genes involved in MO will allow the dissection of molecular mechanisms underlying migraine attacks to aid in diagnosis and to develop rational therapeutic tools.
Acknowledgments
This study was supported by Telethon Grant E.1252. We are grateful to the family who participated in this study. We thank Dr. F. Pampinella for her constructive criticism and helpful discussion during this study. We thank Dr. I. Ranzini for her secretarial support. A special thanks to the Riquier family, who supported D.S. in performing the research.
Electronic-Database Information
Accession numbers and URLs for data presented herein are as follows:
- Genetic Location Database, http://cedar.genetics.soton.ac.uk/public_html/ldb.html (for genetic-linkage distances)
- Online Mendelian Inheritance in Man (OMIM), http://www.ncbi.nlm.nih.gov/Omim/ (for migraine [MIM 157300], FHM1 [MIM 141500], CACNA1A [MIM 601011], FHM2 [MIM 602481], MFTS [MIM 300125], MGAU [MIM 300125], GCH1 [MIM 600225], SPG3A [MIM 606439], PTGDR [MIM 606687], PTGER2 [MIM 176804], and BMP4 [MIM 112262])
- UCSC Human Genome Project Working Draft, http://genome.ucsc.edu/ (for choosing the microsatellite markers on chromosome 14q)
References
- Ai X, Cappuzzello J, Hall AK (1999) Activin and bone morphogenetic proteins induce calcitonin gene-related peptide in embryonic sensory neurons in vitro. Mol Cell Neurosci 14:506–518 [DOI] [PubMed] [Google Scholar]
- Curtis D, Gurling H (1993) A procedure for combining two-point lod scores into a summary multipoint map. Hum Hered 43:173–185 [DOI] [PubMed] [Google Scholar]
- Del Zompo M, Cherchi A, Palmas MA, Ponti M, Bocchetta A, Gessa GL, Piccardi MP (1998) Association between dopamine receptor genes and migraine without aura in a Sardinian sample. Neurology 51:781–786 [DOI] [PubMed] [Google Scholar]
- Ducros A, Denier C, Joutel A, Cecillon M, Lescoat C, Vahedi K, Dracel F, Vicaut E, Bousser MG, Tournier-Lasserve E (2001) The clinical spectrum of familial hemiplegic migraine associated with mutations in a neuronal calcium channel. N Engl J Med 345:17–24 [DOI] [PubMed] [Google Scholar]
- Ducros A, Joutel A, Vahedi K, Cecillon M, Ferreira A, Bernard E, Verier A, Echenne B, Lopez de Manain A, Bousser MG, Tournier-Lasserve E (1997) Mapping of a second locus for familial hemiplegic migraine to 1q21–23 and evidence of further heterogeneity. Ann Neurol 42:885–890 [DOI] [PubMed] [Google Scholar]
- Funk CD (2001) Prostaglandins and leukotrienses: advances in ecosanoid biology. Science 294:1871–1875 [DOI] [PubMed] [Google Scholar]
- Gardner K, Barmada M, Ptacek LJ, Hoffman EP (1997) A new locus for hemiplegic migraine maps to chromosome 1q31. Neurology 49:1231–1238 [DOI] [PubMed] [Google Scholar]
- Gervil M, Ulrich V, Kyvik KO, Olesen J, Russell MB (1999) Migraine without aura: a population-based twin study. Ann Neurol 46:606–611 [DOI] [PubMed] [Google Scholar]
- Goadsby PJ, Edvinsson L (1993) The trigeminovascular system and migraine: studies characterizing cerebrovascular and neuropeptide changes seen in humans and cats. Ann Neurol 33:48–56 [DOI] [PubMed] [Google Scholar]
- Goadsby PJ, Edvinsson L, Ekman R (1990) Vasoactive neuropeptide release in the extracerebral circulation of humans during migraine headache. Ann Neurol 28:183–187 [DOI] [PubMed] [Google Scholar]
- Hargreaves RJ, Shepheard SL (1999) Pathophysiology of migraine—new insights. Can J Neurol Sci S12–S19 [DOI] [PubMed] [Google Scholar]
- Headache Classification Committee of the International Headache Society (1988) Classification and diagnostic criteria for headache disorders, cranial neuralgias and facial pain. Cephalalgia S1–S96 [PubMed] [Google Scholar]
- Honkasalo ML, Kaprio J, Winter T, Heikkila K, Sillanpaa M, Koskenvuo M (1995) Migraine and concomitant symptoms among 8167 adult twin pairs. Headache 35:70–78 [DOI] [PubMed] [Google Scholar]
- Hovatta I, Kallela M, Färkkilä M, Peltonen L (1994) Familial migraine: exclusion of the susceptibility gene from the reported locus of familial hemiplegic migraine on 19p. Genomics 23:707–709 [DOI] [PubMed] [Google Scholar]
- Ichinose H, Ohye T, Takahashi E, Seki N, Hori T, Segawa M, Nomura Y, Endo K, Tanaka H, Tsuji S, Fujita K, Nagatsu T (1994) Hereditary progressive dystonia with marked diurnal fluctuation caused by mutations in the GTP cyclohydrolase I gene. Nat Genet 8:236–242 [DOI] [PubMed] [Google Scholar]
- Jones KW, Ehm MG, Pericak-Vance MA, Haines JL, Boyd PR, Peroutka SJ (2001) Migraine with aura susceptibility locus on chromosome 19p13 is distinct from the familial hemiplegic migraine locus. Genomics 78:150–154 [DOI] [PubMed] [Google Scholar]
- Joutel A, Bousser MG, Biousse V, Labauge P, Chabriat H, Nibbio A, Maciazek J, Meyer B, Bach MA, Weissenbach J, Lathrop GM, Tournier-Lasserve E (1993) A gene for familial hemiplegic migraine maps to chromosome 19. Nat Genet 5:40–45 [DOI] [PubMed] [Google Scholar]
- Lander E, Kruglyak L (1995) Genetic dissection of complex traits: guidelines for interpreting and reporting linkage results. Nat Genet 11:241–247 [DOI] [PubMed] [Google Scholar]
- Lathrop G, Lalouel J (1984) Easy calculations of lod scores and genetic risks on small computers. Am J Hum Genet 36:460–465 [PMC free article] [PubMed] [Google Scholar]
- Lauritzen M (1994) Pathophysiology of migraine: the spreading depression theory. Brain 117:199–210 [DOI] [PubMed] [Google Scholar]
- Lea RA, Dohy A, Jordan K, Quinlan S, Brimage PJ, Griffiths LR (2000) Evidence for allelic association of the dopamine β-hydroxylase gene (DBH) with susceptibility to typical migraine. Neurogenetics 3:35–40 [DOI] [PubMed] [Google Scholar]
- Lea RA, Shepherd AG, Curtain RP, Nyholt DR, Quinlan S, Brimage PJ, Griffiths LR (2002) A typical migraine susceptibility region localizes to chromosome 1q31. Neurogenetics 4:17–22 [DOI] [PubMed] [Google Scholar]
- May A, Ophoff RA, Terwindt GM, Urban C, van Eijk R, Haan J, Diener HC, Lindhout D, Frants RR, Sandkuijl LA, Ferrari MD (1995) Familial hemiplegic migraine locus on 19p13 is involved in the common forms of migraine with and without aura. Hum Genet 96:604–608 [DOI] [PubMed] [Google Scholar]
- McCarthy LC, Hosford DA, Riley JH, Bird MI, White NJ, Hewett DR, Peroutka SJ, et al (2001) Single-nucleotide polymorphism alleles in the insulin receptor gene are associated with typical migraine. Genomics 78:135–149 [DOI] [PubMed] [Google Scholar]
- Mochi M, Sangiorgi S, Cortelli P, Carelli V, Scapoli C, Crisci M, Monari L, Pierangeli G, Montagna P (1993) Testing models for genetic determination of migraine. Cephalalgia 13:389–394 [DOI] [PubMed] [Google Scholar]
- Nyholt DR, Curtain RP, Griffiths LR (2000) Familial typical migraine: significant linkage and localization of a gene to Xq24-28. Hum Genet 107:18–23 [DOI] [PubMed] [Google Scholar]
- Nyholt DR, Lea R, Goadsby P, Brimage P, Griffiths L (1998) Familial typical migraine: linkage to chromosome 19p13 and evidence for genetic heterogeneity. Neurology 50: 1428–1432 [DOI] [PubMed] [Google Scholar]
- Ogilvie AD, Russell MB, Dhall P, Battersby S, Ulrich V, Smith CA, Goodwin GM, Harmar AJ, Olesen J (1998) Altered allelic distribution of the serotonin transporter gene in migraine without aura and migraine with aura. Cephalalgia 18:23–26 [DOI] [PubMed] [Google Scholar]
- Ophoff RA, Terwindt GM, Vergouwe MN, van Eijk R, Oefner PJ, Hoffman SM, Lamerdin JE, Mohrenweiser MW, Bulman DE, Ferrari M, Haan J, Linolhout D, van Ommen GJ, Hofker MH, Ferrari MD, Frants RR (1996) Familial hemiplegic migraine and episodic ataxia type-2 are caused by mutations in the Ca2+ channel gene CACNL1A4. Cell 87:543–552 [DOI] [PubMed] [Google Scholar]
- Pardo J, Carracedo A, Munoz I, Castillo J, Lema M, Noya M (1995) Genetic markers: association study in migraine. Cephalalgia 15:200–204 [DOI] [PubMed] [Google Scholar]
- Peroutka SJ, Wilhoit T, Jones K (1997) Clinical susceptibility to migraine with aura is modified by dopamine D2 receptor (DRD2) NcoI alleles. Neurology 49:201–206 [DOI] [PubMed] [Google Scholar]
- Russell MB, Olesen J (1993) The genetics of migraine without aura and migraine with aura. Cephalalgia 13:245–248 [DOI] [PubMed] [Google Scholar]
- ——— (1995) Inheritance of migraine investigated by complex segregation analysis. Hum Genet 96:726–730 [DOI] [PubMed] [Google Scholar]
- Russell MB, Rasmussen BK, Thornvaldsen P, Olesen J (1995) Prevalence and sex-ratio of the subtypes of migraine. Int J Epidemiol 24:612–618 [DOI] [PubMed] [Google Scholar]
- Stewart W, Lipton R, Celentano D, Reed M (1992) Prevalence of migraine headache in the United States. JAMA 267:64–69 [PubMed] [Google Scholar]
- Stewart WF, Staffa J, Lipton RB, Ottman R (1997) Familial risk of migraine: a population-based study. Ann Neurol 41:166–172 [DOI] [PubMed] [Google Scholar]
- Terwindt GM, Ophoff RA, van Eijk R, Vergouwe MN, Haan J, Frants RR, Sandkuijl LA, Ferrari MD (2001) Involvement of the CACNA1A gene containing region on 19p13 in migraine with and without aura. Neurology 56:1028–1032 [DOI] [PubMed] [Google Scholar]
- Tzourio C, El Amrani M, Poirier O, Nicaud V, Bousser MG, Alperovitch A (2001) Association between migraine and endothelin type A receptor (ETA −231 A/G) gene polymorphism. Neurology 56:1273–1277 [DOI] [PubMed] [Google Scholar]
- Ulrich V, Gervil M, Kyvik K, Olesen J, Russell MB (1999) Evidence of a genetic factor in migraine with aura: a population-based Danish twin study. Ann Neurol 45:242–246 [DOI] [PubMed] [Google Scholar]
- Werner ER, Werner-Felmayer G, Wachter H, Mayer B (1996) Biosynthesis of nitric oxide: dependence on pteridine metabolism. Rev Physiol Biochem Pharmacol 127:97–135 [DOI] [PubMed] [Google Scholar]
- Wessman M, Kallela M, Kaunisto MA, Marttila P, Sobel E, Hartiala J, Oswell G, Leal SM, Papp JC, Hämäläinen E, Broas P, Joslyn G, Hovatta I, Hiekkalinna T, Kaprio J, Ott J, Cantor RM, Zwart JA, Ilmavirta M, Havanka H, Färkkilä M, Peltonen L, Palotie A (2002) A susceptibility locus for migraine with aura, on chromosome 4q24. Am J Hum Genet 70:652–662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witter K, Werner T, Blusch JH, Schneider EM, Riess O, Ziegler I, Rödl W, Bacher A, Gütlich M (1996) Cloning, sequencing and functional studies of the gene encoding human GTP cyclohydrolase I. Gene 171:285–290 [DOI] [PubMed] [Google Scholar]
- Zhao X, Alvarado D, Rainier S, Lemons R, Hedera P, Weber CH, Tukel T, Apak M, Heiman-Patterson T, Ming L, Bui M, Fink JK (2001) Mutations in a newly identified GTPase gene cause autosomal dominant hereditary spastic paraplegia. Nat Genet 29:326–331 [DOI] [PubMed] [Google Scholar]