Abstract
Background
Acute encephalitis syndrome (AES) is a constellation of symptoms that includes fever and altered mental status. Most cases are attributed to viral encephalitis (VE), occurring either in outbreaks or sporadically. We conducted hospital-based surveillance for sporadic adult-AES in rural Central India in order to describe its incidence, spatial and temporal distribution, clinical profile, etiology and predictors of mortality.
Methods
All consecutive hospital admissions during the study period were screened to identify adult-AES cases and were followed until 30-days of hospitalization. We estimated incidence by administrative sub-division of residence and described the temporal distribution of cases. We performed viral diagnostic studies on cerebrospinal fluid (CSF) samples to determine the etiology of AES. The diagnostic tests included RT-PCR (for enteroviruses, HSV 1 and 2), conventional PCR (for flaviviruses), CSF IgM capture ELISA (for Japanese encephalitis virus, dengue, West Nile virus, Varicella zoster virus, measles, and mumps). We compared demographic and clinical variables across etiologic subtypes and estimated predictors of 30-day mortality.
Results
A total of 183 AES cases were identified between January and October 2007, representing 2.38% of all admissions. The incidence of adult AES in the administrative subdivisions closest to the hospital was 16 per 100,000. Of the 183 cases, a non-viral etiology was confirmed in 31 (16.9%) and the remaining 152 were considered as VE suspects. Of the VE suspects, we could confirm a viral etiology in 31 cases: 17 (11.2%) enterovirus; 8 (5.2%) flavivirus; 3 (1.9%) Varicella zoster; 1 (0.6%) herpesvirus; and 2 (1.3%) mixed etiology); the etiology remained unknown in remaining 121 (79.6%) cases. 53 (36%) of the AES patients died; the case fatality proportion was similar in patients with a confirmed and unknown viral etiology (45.1 and 33.6% respectively). A requirement for assisted ventilation significantly increased mortality (HR 2.14 (95% CI 1.0–4.77)), while a high Glasgow coma score (HR 0.76 (95% CI 0.69–0.83)), and longer duration of hospitalization (HR 0.88 (95% CI 0.83–0.94)) were protective.
Conclusion
This study is the first description of the etiology of adult-AES in India, and provides a framework for future surveillance programs in India.
Keywords: Acute encephalitis syndrome, Viral encephalitis, Adults, Rural, Central India
1. Introduction
Acute encephalitis syndrome (AES) is defined as the acute onset of fever and a change in mental status (including symptoms such as confusion, disorientation, coma, or inability to talk) and/or new onset of seizures (excluding simple febrile seizures) in a person of any age at any time of year [1]. This broad syndromic definition is mainly used for disease surveillance because the clinical presentation of AES caused by different etiologic agents is similar. AES occurs in explosive epidemics or in a non-epidemic (sporadic) form. A systematic review of non-epidemic studies in children, all-age groups, and adults reported incidences of at least 10, 6, and 2 per 100,000 in children, all-age groups and adults respectively; these results have been suggested as levels for effective AES surveillance [2].
A large number of neurotropic viruses and non-viral etiologies can produce AES, making specific diagnosis difficult. Epidemics in India have been attributed to Japanese encephalitis virus (JEV) infection and have predominantly affected children [3–6]. Various novel agents have been reported more recently as causes of AES including enteroviruses [7], Chandipura virus [8,9] and Nipah virus [10]. Only a few surveillance studies have been performed on sporadic AES, all of them in children [11–13]. JEV, enteroviruses, and Chandipura virus were the primary etiologic agents in these studies. There are no previous surveillance studies on adult AES from India. In a previous study by our group, 16% of adults hospitalized with acute fever had AES, and 21% of them died during hospitalization [14].
While epidemics have a singular etiology, sporadic cases are more likely to be due to multiple etiologies, which require testing for multiple pathogens for effective surveillance. Despite advances made in virology in recent decades, the technology to detect these agents is expensive and often not available outside a select group of reference laboratories. This makes periodic hospital-based epidemiological investigations essential to determine the spectrum of agents that cause AES. This information can be used to develop preventive measures against specific etiologic agents. We designed this prospective study to answer three specific research questions (a) what is the incidence and distribution (spatial and temporal) of AES cases in Central India?; (b) what is the spectrum of etiological agents causing viral encephalitis in Central India?; and (c) what are the predictors of mortality in patients with AES?
2. Methods
2.1. Design
We performed a prospective hospital based surveillance of adult-AES patients with a 30-day follow up from date of hospital admission.
2.2. Setting
Mahatma Gandhi Institute of Medical Sciences (MGIMS) is a 760 bed teaching hospital located at Sevagram, in Wardha district of Maharashtra state in India. About 40,000 patients are admitted to the in-patient services of the hospital annually and 10,000 of these are cared for in the medicine wards. Most adult patients with AES are referred to the hospital from primary and secondary care facilities in and around the district, and are admitted in the medicine wards. As part of the standard treatment protocol of AES in the hospital, treating physicians perform a lumbar puncture and send cerebrospinal fluid (CSF) sample for microscopy, biochemistry (CSF sugar and proteins), and bacterial cultures. The hospital has an electronic patient medical record system, where clinical summary, investigation results, and diagnoses on discharge or death were available to study investigators for purpose of surveillance.
The hospital has a catchment area of about 72,000 square kilometers (between latitudes19 and 22 N, and longitudes 77 and 80 E), with an adult population of about 14 million (2001 census). This catchment area consists of about 70 administrative subdivisions in districts of Wardha, Yeotmal, Chandrapur, Amravati, Nagpur, Gadchiroli, Nanded, Washim, and Adilabad. MGIMS hospital is one of two teaching hospitals in the Wardha district, the only locations where mechanical ventilation is available. MGIMS hospital is a state-funded hospital, in contrast to the other teaching hospital, which is privately funded.
2.3. Patients: initial screening
At the onset of the study, emergency medical officers and house-residents were sensitized about the study, to ensure that all adults with AES are admitted to the hospital, standard management protocols are followed, and a member of the study team is contacted when any patient with AES is admitted, and lumbar puncture is planned. A member of the surveillance team (usually a trained laboratory technician or a social worker) attended to all lumbar puncture procedures, to confirm eligibility of participants, obtain consent, and to ensure proper collection of additional CSF sample required for the study. Treating physicians and house-residents screened all consecutive hospital admissions to identify adult patients with (a) fever of 14 or fewer days and (b) a change in mental status (including symptoms such as irritability, somnolence or abnormal behavior, confusion, disorientation, coma, or inability to talk), with fever having preceded the onset of the change in mental status. Patients were excluded if (a) a peripheral blood-smear and/or serology for malaria was positive, (b) an alternate explanation for fever (such as a definite localized infection such as abnormal chest X-ray suggestive of pneumonia or tuberculosis, positive acid fast bacilli in respiratory secretions, urinary tract infection, soft-tissue infection with sepsis etc.) was plausible (c) there was biochemical or clinical evidence of a metabolic encephalopathy (including but not limited to hyponatremia, hepatic dysfunction, hypoglycemia, or alcohol intoxication). All patients who satisfied the above eligibility criteria were classified as having AES and were approached for participation in the study. Similar case definitions have been used in previous studies [9,13]. In addition a study investigator (RJ) with access to electronic hospital information system maintained a log of all consecutive admissions and their discharge diagnosis from the medical wards.
2.4. Informed consent process
Because patients with AES are cognitively compromised, surrogate written informed-consent was sought from an available closest caregiver of the patient (the order of closeness being spouse, parent, offspring, sibling, friend, and other relation or friend) for administration of the questionnaire, to obtain additional serum and CSF samples, and follow up. We sought a second consent from patients, as and if they became cognitively competent. All informed consent materials were available in the local language Marathi. The study design was approved by the institutional review boards of participating institutes (MGIMS Sevagram, Bhopal Memorial hospital and research center, Bhopal and University of California, Berkeley).
2.5. Additional exclusion criteria
Patients were excluded from the study, after obtaining the informed consent, if there was definite evidence of a non-viral etiology for encephalitis. The additional exclusion criteria were: (a) CSF suggestive of bacterial meningitis, based on either a positive culture for pathogenic bacteria, five or more polymorphonuclear cells in CSF, CSF glucose <40 mg/dL, or a CSF/blood glucose ratio <0.25; (b) positive mycobacterial cultures for tubercular meningitis (mycobacterial cultures were done with 1 mL of the freshly collected CSF, which was inoculated in BACTEC), (c) positive cryptococcal antigen test in CSF sample suggestive of cryptococcal meningitis. This test was done in patients who were HIV positive, (d) presence of a; (c) cryptococcal meningitis (i.e. presence of cryptococcal antigen in CSF, a test performed in HIV-positive individuals only); (d) brain imaging, if performed suggested an intracranial lesion compatible with a non-AES etiology; or (e) a definite metabolic or known infectious etiology for the illness detected in course of hospital stay. After these additional exclusions for non-viral etiologies, patients with AES were classified as viral encephalitis (VE) suspects.
2.6. Study procedures
2.6.1. Questionnaire
We administered a questionnaire to all included patients to record residential address, the time of onset of AES, demographic (age, gender) and on-admission characteristics (symptom duration, presence of seizures, Glasgow coma score (GCS), clinical meningitis). Daily chart review of all cases was performed to record in-hospital course e.g. requirement for assisted ventilation, gastro-intestinal bleed, hypotension, days of hospital stay, and further in-hospital investigations. Gender, presence of seizures, clinical meningitis, requirement for assisted ventilation, gastro-intestinal bleed, and hypotension were collected as binary variables (coded 0,1), while the remaining variables were continuous measures. We followed all patients included in the study every day until discharge from the hospital, and at 30 days from the date of symptom onset. Mortality was defined as death after in-hospital admission; the time between the symptom onset and the date of death was considered as the survival time. Home-visits were performed for all patients at day-30, to verify location of their residence, estimate their socio-economic score [15], collect a follow-up convalescent serum sample, and to assess cognitive disability by mini-mental status examination (MMSE), with score less than 25 considered as indicative of cognitive impairment.
2.6.2. Laboratory investigations
At the time of the initial lumbar puncture (after obtaining informed consent, but before applying all exclusions), we obtained 3 mL of additional CSF sample. This sample was collected in three 1.0 mL aliquots, and stored at −70 °C untill further testing. In addition a serum sample was obtained at the same time as the initial CSF fluid collection.
A battery of investigations to determine the etiology of AES were performed and interpreted in a priori defined order: CSF RT-PCR, followed by CSF-PCR, CSF IgM ELISA and serum IgM ELISA. We used one CSF aliquot (1.0 mL) to perform nucleic acid extraction with Qiagen DNA and RNA extraction kits, using standard techniques. We performed RT-PCR on extracted RNA for enteroviruses (artus enterovirus LC RT PCR kit, which amplifies the 114 bp region, analytic sensitivity 3.2 copies per microliter) and on extracted DNA for herpesviruses (Artus HSV 1/2 LC PCR kit, which amplifies the 148 bp region, analytic sensitivity 1 copy per microliter). We performed conventional PCR for flaviviruses (consensus primers YF1, YF3 expected product size 390 bp). Extracted nucleic acids from a subset of all samples (in patients who had died) were also tested for Chandipura virus (CHPG F2, and CHPG R2, expected product size 200 bp), and Nipah viruses (NF1 and NF22 primers, expected product size 1596 bp) by conventional PCR. We used a second aliquot of the CSF sample (volume 1.0 mL) to test for IgM antibodies against Japanese encephalitis virus, dengue virus, West Nile virus, and Varicella zoster virus using commercial IgM capture ELISA kits manufactured by PanBio, Brisbane, Australia. The second line tests for measles and mumps were performed with an IgM ELISA on CSF samples using commercial kits (Serion, Germany). All IgM capture ELISA tests were done in 1:10 CSF dilution, with remaining steps as per the manufacturer’s guidelines. If the CSF RT-PCR was positive for an etiologic agent, it was considered as diagnostic. CSF IgM ELISA results were interpreted for PCR negative cases, and serum IgM ELISA tests were considered as diagnostic only if all CSF-based test results were negative. Patients with AES who had two or more positive tests using the same testing technique and sample were classified as having a mixed infection. Patients in whom all test results were negative were classified as having AES of unknown etiology.
We screened all participants for antibodies against HIV 1 & 2 in serum, using a rapid immuno-chromatographic test (Retrochek HIV, Tulip laboratories, Goa, India). We confirmed all positive tests by ELISA. A separate consent was obtained and pre and post-test counseling was provided for HIV testing.
2.7. Statistical analysis
We described the frequency distribution of all cases per week, aligned with mean weekly temperature and rainfall data. We mapped AES cases based on point-location of their villages and estimated cumulative yearly incidence per 100,000 adult population for each administrative subdivision. We performed a simple linear regression of distance of subdivision headquarter and AES incidence in subdivision, to understand if incidence was different in geographically closer or farther subdivisions.
We compared the demographic, admission, and in-hospital characteristics of survivors and non-survivors using the t-test for continuous and chi-square test for binary variables. We considered the time to event for each individual in the study, using ‘survival analysis’ methods. The Kaplan–Meier product limit estimator was used to estimate survival and for the time-to-event plot. Event-free individuals were right-censored on day 30 after symptom onset. We used the log rank test to identify those predictors with the most significant independent influence on prognosis. Crude hazard ratios were computed to assess the strength of association between potential prognostic factors and outcomes (mortality, and mortality or disability on day 30). We used Cox proportional-hazards regression models for analyses of multiple predictor variables for the study outcomes. These models measured the hazard ratio – the relative effect of a predictive factor on an outcome – by assuming that this relation is constant over time. Because many of the risk factors were correlated, co-linearity was evaluated by generating correlation matrices and handled by eliminating one of the two collinear variables. A backward stepwise technique was used for model selection. For a variable to be removed from the model, the p value had to be >0.1. Both the crude and the adjusted hazard ratio estimates were computed along with 95% confidence intervals (CI). While mortality events were recorded on the day of their occurrence, cognitive disability was recorded using mini-mental status examination on day 30. Thus occurrence of this event is skewed, and assumption of constant occurrence over time is violated. Hence, for composite outcome of mortality and disability on day 30 we also performed logistic regression to understand variables contributing to magnitude of risk, without being contingent on time to event.
After virologic testing, we divided all cases into three etiologic subtypes: confirmed non-viral etiology, confirmed viral etiology, and AES of unknown etiology. We used the CDC criteria [16] to classify a confirmed VE case, with either of the following features: (a) demonstration of specific viral antigen or genomic sequences in CSF; (b) virus-specific immunoglobulin M (IgM) antibodies demonstrated in CSF by antibody-capture enzyme immunoassay; or (c) fourfold or greater change in virus-specific serum antibody titer. We determined the proportion of cases in each of these three etiologic subtypes, and compared demographic, clinical, and survival characteristics across them. All statistical analysis were performed using STATA (version 12, Stata corp. Lakeway drive TX).
3. Results
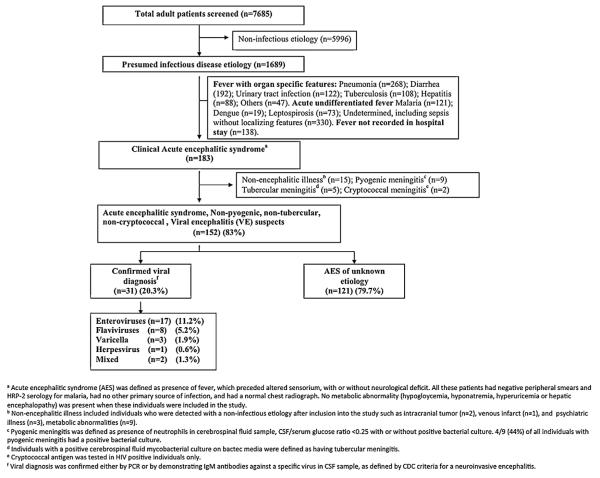
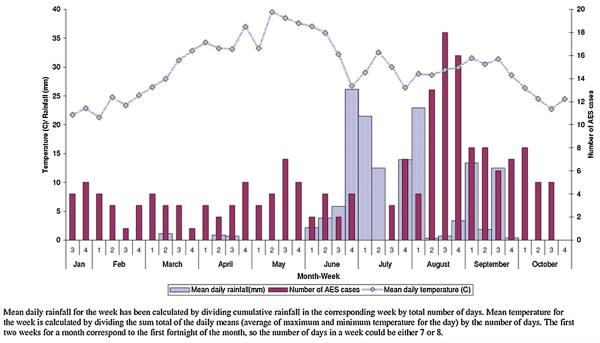
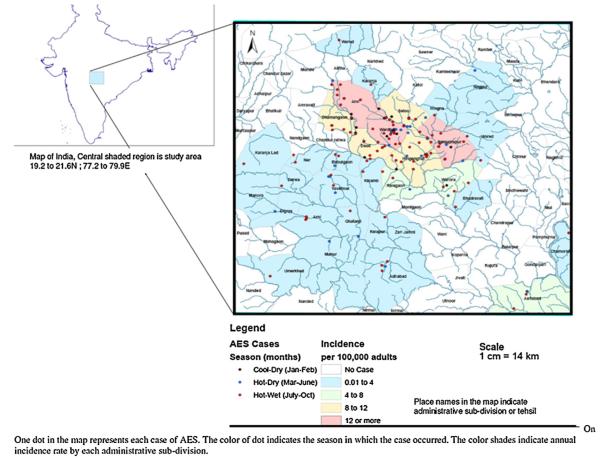
Altogether 7685 patients were admitted to the medicine wards between January and October 2007; 1689 (21.9%) of these had an infectious disease diagnosis. Of these 1689 patients 183 (10.8%) had symptoms suggestive of AES and were included in the study (Fig. 1). Most AES cases were seen in the hot and wet months between July and October (Table S1, and Fig. 2), and were from Wardha district (97/183; 53%) (Fig. 3). The incidence of AES was between 10 and 16 per 100,000 adults in sub-divisions within Wardha district, and averaged 4 per 100,000 adults in sub-divisions of neighboring districts. This difference in incidence is likely to be due to referral bias. Of 183 AES cases, 31 (16.9%) were confirmed to be due to non-viral etiologies, and the remaining 152 (83%) were viral encephalitis (VE) suspects (Fig. 1). Cases with confirmed non-viral AES had a longer duration of fever and headache; higher proportion of individuals with neck stiffness; lower CSF glucose levels and higher CSF protein concentration, and were more likely to be HIV positive as compared to those who were classified as viral encephalitis suspects (Table 1).
Fig. 1.
Study flow chart.
Fig. 2.
Temporal profile of all acute encephalitis syndrome cases (n = 183).
Fig. 3.
Spatial distribution of acute encephalitis syndrome cases and mapping by administrative sub-divisions (n = 183).
Table 1.
Characteristics of patients defined as viral encephalitis suspects and those with conformed non-viral acute encephalitis syndrome (n = 183).
| Variable | AES viral encephalitis suspect, N = 152 | AES with confirmed non-viral etiology, N = 31 | P value |
|---|---|---|---|
| Age (years) | 40.2 (18.3) | 37.8 (18.3) | 0.49 |
| Male gender, n (%) | 90 (59%)a | 17 (54%)a | 0.65 |
| Socioeconomic score | 19.38 (7.02) | 18.70 (7.28) | 0.62 |
| Fever duration (days) | 8.9 (7.2) | 18.9 (29.4) | <0.01 |
| Headache duration (days) | 4.8 (5.2) | 11.6 (25.5) | <0.01 |
| Altered behavior duration (days) | 1.4 (2.2) | 0.8 (1.6) | 0.26 |
| Seizures, n (%) | 34 (22.4)a | 5 (19.1)a | 0.72 |
| Glasgow coma scale (on admission) | 9.4 (3.8) | 10.2 (3.2) | 0.38 |
| Neck stiffness, n (%) | 47 (30.9)a | 15 (60)a | <0.01 |
| Hypotension, n (%) | 11(7.2)a | 2 (8)a | 0.89 |
| Need for assisted ventilation, n (%) | 33 (21.7)a | 4(16.0)a | 0.51 |
| Hospital stay (days) | 10.0 (7.5) | 9.2 (8.0) | 0.61 |
| Hemoglobin, g/dL | 10.7 (2.4) | 11.0 (2.8) | 0.52 |
| Total Leukocyte count (× 103/mm3) | 8.35 (3.5) | 4.78 (2.5) | 0.61 |
| Platelet count (× 106/mm3) | 2.29 (1.26) | 2.27 (1.32) | 0.94 |
| CSF cell count (per mm3) | 432 (1519) | 921 (1935) | 0.14 |
| CSF sugar (mg/dl) | 63.7 (23.6) | 52.5 (38.1) | 0.03 |
| CSF proteins (mg/dL) | 137.3 (166.8) | 246.9 (297.4) | <0.01 |
| HIV positivity n (%) | 6 (3.9)a | 4 (12.9)a | 0.04 |
| Mortality n (%) | 53 (36.0)a | 10 (58.8)a | 0.06 |
AES = acute encephalitis syndrome, CSF = cerebrospinal fluid, HIV = human immunodeficiency virus.
All values indicate mean (SD), and difference in their distribution is tested using Student’s t-test, unless indicated.
These values are n (%), and difference in their distribution is tested using chi-square test.
The patients who were suspected of having VE were young (mean age 40.2, SD (18.3) years), of a low socio-economic class (mean SES score 19.4, SD (7.0)), and presented to the hospital after a mean of 5.9 days of symptoms. These patients stayed in hospital for an average 10 days (SD 7.5). We could confirm a viral etiology in 31 (20.3%) of the 152 viral encephalitis suspects (17 (11.2%) enterovirus; 8 (5.2%) flavivirus; 3 (1.9%) Varicella zoster; 1 (0.6%) herpesvirus; and 2 (1.3%) mixed etiology) and all of the viral diagnostic tests were negative in the remaining 121 (79.7%). The basis of diagnosis of etiology in patients with a confirmed viral etiology was RT-PCR in 17 patients with enteroviral one with herpesvirus encephalitis. The remaining 13 patients had anti-viral IgM antibodies in their CSF samples of whom eight had flaviviral encephalitis (four JEV, three dengue, and one with both), three had Varicella-zoster encephalitis, and another two had a mixed CSF serology (positive for both varicella and either Japanese encephalitis and/or dengue). None of the patients had a positive CSF serology for West-Nile virus, measles, and mumps. None of the CSF samples tested for Chandipura virus and Nipah virus were positive (Fig. 1). The demographic and clinical characteristics of those with a confirmed viral etiology were similar to those in whom a viral etiology was not identified, except for the presence of neck-stiffness, which was more frequent in those with a confirmed viral etiology. There were no significant differences in the clinical and demographic characteristics between those with enteroviral or flaviviral AES and those in whom etiology remained unknown (Table 2).
Table 2.
Subgroup analysis of patients who are viral encephalitis suspects (n = 152) of known viral etiology (n = 31) or of unknown etiology (n = 121).
| Variable | Etiologic subtypes of acute encephalitis syndrome (AES) |
P value |
|||||
|---|---|---|---|---|---|---|---|
| Unknown | Confirmed viral etiology |
Enteroviral confirmed |
Flaviviral confirmed |
Confirmed vs. unknown |
Enteroviral vs. unknown |
Flaviviral vs. unknown |
|
| Number | 121 | 31 | 16 | 8 | |||
| Age (years) | 39.9 (17.9) | 41.2 (19.5) | 45.2 (20.7) | 44.2 (18.4) | 0.72 | 0.33 | 0.55 |
| Male gender, n (%) | 76 (62.3)a | 14 (46.6)a | 9 (52.9)a | 5 (62.5)a | 0.11 | 0.18 | 0.66 |
| Socioeconomic score | 19.4 (7.0) | 18.8 (7.3) | 18.8 (7.6) | 21.0 (7.5) | 0.65 | 0.58 | 0.69 |
| Fever duration (days) | 9.0 (7.8) | 8.4 (3.7) | 7.4 (3.0) | 8.8 (3.7) | 0.67 | 0.40 | 0.85 |
| Headache duration (days) | 4.7 (5.5) | 5.3 (4.1) | 4.2 (3.3) | 6.5 (6.1) | 0.59 | 0.82 | 0.26 |
| Altered behavior duration (days) | 1.3 (2.2) | 1.9 (2.3) | 1.7 (2.0) | 2.8(3.2) | 0.15 | 0.44 | 0.07 |
| Seizures, n (%) | 29 (23.6)a | 5 (16.6)a | 4 (23.6)a | 0a | 0.35 | 0.70 | 0.17 |
| Glasgow coma scale (on admission) | 9.5 (3.6) | 9.0 (4.3) | 9.0 (4.7) | 8.3 (3.6) | 0.52 | 0.94 | 0.69 |
| Neck stiffness, n (%) | 31 (25.6)a | 16 (51.1)a | 9 (52.9)a | 3 (37.5)a | 0.01 | 0.10 | 0.73 |
| Hypotension, n (%) | 8 (6.6)a | 3 (9.6)a | 2 (11.7)a | 1 (12.5)a | 0.55 | 0.88 | 0.86 |
| Need for assisted ventilation, n (%) | 27 (22.3)a | 6 (19.3)a | 3 (17.6)a | 2 (25)a | 0.72 | 0.32 | 0.77 |
| Hospital stay (days) | 9.7 (7.1) | 11 (8.4) | 12.7 (10.7) | 8.1 (4.2) | 0.41 | 0.23 | 0.54 |
| Hemoglobin, g/dL | 10.8 (2.3) | 10.2 (2.7) | 11 (2.5) | 10.3 (2.7) | 0.21 | 0.95 | 0.48 |
| Total Leukocyte count (×103/mm3) | 8.1 (3.9) | 9.3 (4.4) | 14.1 (8.2) | 3.2 (8.1) | 0.87 | 0.37 | 0.21 |
| Platelet count (×106/mm3) | 2.2 (1.1) | 2.5 (1.8) | 2.5 (1.2) | 2.8 (2.7) | 0.24 | 0.31 | 0.23 |
| CSF cell count (per mm3) | 317 (770) | 904 (360) | 389 (689) | 2226 (5570) | 0.07 | 0.85 | 0.03 |
| CSF sugar (mg/dl) | 64.8 (23.7) | 59.2 (22.9) | 57.82 (25.5) | 53.6 (13.8) | 0.24 | 0.37 | 0.25 |
| CSF proteins (mg/dL) | 135.5 (175.6) | 144.7 (126.9) | 86.5 (56.5) | 266.8 (226.7) | 0.78 | 0.12 | 0.15 |
| HIV positivity, n (%) | 5 (4.1)a | 1 (3.2)a | 1 (5.8)a | 0a | 0.81 | 0.87 | 0.43 |
| Mortality, n (%) | 39 (33.6)a | 14 (45.1)a | 8 (47)a | 4 (50)a | 0.23 | 0.88 | 0.96 |
AES = acute encephalitis syndrome, CSF = cerebrospinal fluid, HIV = human immunodeficiency virus.
All values indicate mean (SD), and difference in their distribution is tested using Student’s t-test, unless indicated.
These values are n (%), and difference in their distribution is tested using chi-square test.
On 30-day follow up, 53 patients (34.8%) had died, and 99 (65.1%) survived. Of those who survived, 34 (34%) had significant cognitive disability (MMSE less than 25). Thus, of all VE suspects, only 65 (42.7%) patients were free of death or disability at one month. All deaths occurred in the first 30 days after symptom onset (Fig. 4). In Cox proportional hazards multivariable regression models, four variables significantly increased the hazard for the outcome of 30-day mortality namely age, Glasgow coma score (GCS) on admission, duration of hospital stay, and requirement for assisted ventilation (Table 3). Same variables were associated with composite outcome of 30-day mortality or disability by both Cox proportional hazard and logistic regression models (Table S2). Higher GCS on admission, and longer duration of hospital stay were associated with a lower risk of mortality, with hazard ratios of 0.76 (95% CI 0.69–0.83), and 0.88 (95% CI 0.83–0.94) respectively. The need for assisted ventilation was significantly associated with mortality (hazard ratios 2.14 (95% CI 1.0–4.77)). These hazard ratios imply that the risk of death is reduced by 24% and 12% for every one point elevation in GCS, and for every additional day spent in the hospital respectively. The risk of death was increased by more than twofold if the patient required assisted ventilation.
Fig. 4.
Kaplan Meier survival curve for the cohort of AES cases, who were VE suspects (n = 152).
Table 3.
Unadjusted and adjusted hazard ratios for 30-day mortality among patients with AES, who are viral encephalitis suspects (n = 152).
| Variable | Survived till day 30 N = 99 |
Died before day 30 N = 53 |
Unadjusted Hazard ratio (95% CI) |
Adjustedb Hazard ratio (95% CI) |
|---|---|---|---|---|
| Demographic variables | ||||
| Age (y) | 37.5 (17.1) | 45.3 (19.5) | 1.01 (1.01–1.03) | 1.02 (1.00–1.03) |
| Male gendera | 50 (50.5)a | 40 (75.4)a | 2.57 (1.37–4.82) | |
| Socioeconomic score | 19.6 (7.1) | 18.8 (6.9) | 0.98 (0.94–1.02) | |
| On-admission variable | ||||
| Duration of symptoms (days) | 6.4 (5.0) | 5.2 (3.4) | 0.94 (0.88–1.01) | |
| Presence of seizuresa | 23 (23.2)a | 11 (20.7)a | 0.81 (0.41–1.57) | |
| GCS on admission | 11.2 (2.5) | 6.2 (3.7) | 0.73 (0.68–0.79) | 0.76 (0.69–0.83) |
| Clinical signs of meningitisa | 30 (30.3)a | 17 (32.0)a | 1.10 (0.62–1.95) | |
| In-hospital stay and complications | ||||
| Hospital stay | 11.5 (8.0) | 7.1 (5.3) | 0.86 (0.80–0.93) | 0.88 (0.83–0.94) |
| Gastro-intestinal bleeda | 1 (1.8)a | 1 (1.0)a | 1.41 (0.19–10.2) | |
| Hypotensiona | 0 (0)a | 11 (20.7)a | 5.90 (2.96–11.76) | |
| Requirement for assisted ventilationa | 5 (5.0)a | 28 (52.8)a | 7.51 (4.30–13.10) | 2.14 (1.0–4.77) |
| Investigations | ||||
| Hemoglobin (g/dL) | 10.6 (2.3) | 10.8 (2.7) | 1.04 (0.93–1.18) | |
| Total leukocyte count (×103 mm3) | 7.0 (30.9) | 10.9 (4.4) | 1.00 (0.98–1.00) | |
| Platelet count (×106/mm3) | 2.4 (1.3) | 2.1 (1.2) | 0.99 (0.99–1.00) | |
| Positive HIV test | 2 (2.0) | 4 (7.5) | 1.99 (0.72–5.55) | |
| CSF cell count (per mm3) | 303 (742) | 716 (2485) | 1.00 (1.00–1.00) | |
| CSF sugar (mg/dL) | 61.1 (20.7) | 68.5 (27.8) | 1.01(1.00–1.02) | |
| CSF proteins (g/dL) | 114.8 (140.8) | 179.5 (201.7) | 1.00 (1.00–1.00) | |
| Obtaining brain imaginga | 38 (38.3)a | 21 (39.6)a | 1.04 (0.60–1.81) |
AES = acute encephalitis syndrome, CSF = cerebrospinal fluid, GCS = glasgow coma scale, HIV = human immunodeficiency virus.
These variables are dichotomous, and these values represent number (percent); remaining variables are continuous and these values represent mean (SD).
These are adjusted hazard ratios obtained after a multivariable regression using Cox proportional hazards model.
4. Discussion
This study of hospitalized adults with AES from rural Central India, satisfied the minimum incidence criterion for effective surveillance (our incidence 16/100,000 adult-population in subdivisions closer to the hospital is well above the suggested minimum surveillance definition of 2 per 100,000 for adults [2]). Despite extensive testing for multiple etiologic agents, in four-fifth of the VE suspects the etiology remained unknown. Most of the confirmed viral encephalitis cases were due to an enterovirus. Mortality was similar in confirmed viral and unknown etiology groups. A requirement for assisted ventilation was associated with a significantly increased the hazard of mortality; high admission Glasgow coma score and longer duration of hospitalization were associated with a lower risk of mortality.
The AES spectrum consists of patients with confirmed non-viral, viral, and unknown etiologies. We found some clinical differences across these subtypes. Cases with confirmed non-viral etiologies had a longer duration of fever before they developed altered sensorium, were more likely to have a stiff-neck, and had higher CSF protein levels. Typically in tubercular and cryptococcal meningitis, the clinical course is long and indolent before neurological deterioration. In-contrast, as compared to patients in with an unknown etiology, patients with a confirmed viral etiology were more likely to have a stiff-neck, and CSF pleocytosis suggestive of meningeal inflammation. Clinical and survival characteristics of patients with known viral and unknown etiologies were similar. It is plausible that patients with known viral encephalitis, had higher viral loads, and consequently more meningeal inflammation, and those classified as unknown etiology were due to an unidentified viral agent with lower viral-loads.
The diagnostic yield in testing for AES cases is typically low. In previous studies, despite the use of a wide array of diagnostic tests, at least a third of all cases remained undiagnosed. For example, in the California Encephalitis Project, a total of 1571 patients with encephalitis were evaluated over a seven year period, and an infectious etiology could be identified in only 15% of them; 73 cases (4.6%) had enteroviral encephalitis [17]. In another study from Finland, [18] which included 144 consecutive patients over a four year period, about 34% of the cases remained undiagnosed, despite extensive use of PCR based diagnostic tests. In our study 26% of all patients had enteroviral disease and 17% had herpesvirus as an etiology. Recently two other studies in the pediatric age-range have also reported enteroviruses as a predominant causative agent. In an outbreak investigation of 306 patients from northern India, evidence of enteroviral infection was seen in 66 (21%) of all patients [7]. In a hospital-based surveillance study from north India, 20 (13%) of all 151 AES cases were reported to have an evidence of enteroviral infection [11]. Enteroviruses, a diverse group of about 70 viruses, are usually present in the CSF only briefly, and in later stages, translocate to brain parenchyma [17]. Thus, it is likely that some patients with encephalitis of unknown etiology may have had enteroviral encephalitis, which could have been detected only by tissue-PCR techniques.
Most AES in India is attributed by clinicians and public-health professionals, to vector-borne flaviviruses, particularly JEV. In JEV endemic districts of India, JEV positivity has been reported to be high even in inter-epidemic periods. However, more recently this pattern has been changing. In JEV hyper-endemic districts of India, where annual epidemics occur, and a recent outbreak investigation failed to show JEV positivity [7]. A common argument for this epidemiologic shift is the widespread use of JE vaccine, which may have introduced herd immunity against JEV. In our study area, JE-vaccination campaigns have never been launched. Low yield of JEV in our study is likely because it excluded children.
There are important global variations in case-fatality proportion due to AES. In South and South-east Asia, various studies have reported a high mortality, ranging from 17 to 50% [19–21]. In-contrast, AES related mortality is low in developed countries (less than 5%), but overall disability is as much as 40% [22–24]. Part of this difference in outcomes may be due to variations in the quality of available neuro-intensive care facilities. Low Glasgow coma score (GCS), and need for mechanical ventilation have been associated with a poor prognosis in VE in many studies [24–31], as well in tubercular meningitis [32], and pneumococcal meningitis [33]. Patients with AES, who either have a brain-stem encephalitis, or cerebral herniation leading to brain-stem compression have compromised respiratory drive, and need assisted ventilation. Herniation and brain-stem involvement in AES is known to have a poor prognosis [34]. High admission Glasgow coma score suggests less severe neurologic involvement and is protective. Other predictors of mortality described in literature include a short prodromal phase [31], the presence of seizures [25], the presence of oculocephalic responses [24], decerebrate posturing [31], CSF pleocytosis [27], elevated CSF protein levels [35], and hyponatremia [36]. Longer hospital stay in our study was associated with a lower risk of mortality, largely because death occurs early in course of disease, and survivors with disability have a longer in-hospital stay.
The high incidence rate of AES among adults in our study could suggest persistent circulation of the etiologic agents in the community. Most AES cases occurred in the hot and humid months. This finding, consistent with previous reports from India, suggests a climate dependant transmission cycle. This season is characterized by water-logging, filling up of the perennial rivers and streams, and increased agricultural activity in the rural areas. These conditions have the potential to enhance vector densities (due to increased mosquito breeding in hot and humid climate), contamination of drinking water supplies (due to groundwater contaminating wells or perennial rivers washing pathogens downstream), and increase in outdoor exposures (which further increases cutaneous contact with water, soil and water). Transmission of both water-borne enteroviruses and vector-borne flaviviruses is likely to increase during this time of the year.
Our study is a comprehensive clinical and virologic descripton of adult-AES, in a setting with high quality hospital-based surveillance. Our study also has certain limitations, first, the definition of AES is clinical and subject to clinical interpretation. This is likely to result in over-inclusion of non-encephalitic cases as AES. However, this is an operational definition, and it is difficult to have a high degree of accuracy to define true encephalitis. Second, the risk of disease and hazard of mortality or disability is likely to be etiology-specific, and most of the AES cases in our study did not have a specific etiology identified. However this limitation is true of many similar studies. Third, all AES cases included in this study were sampled from a single hospital, and it is likely that we missed those individuals who never sought medical care for their symptoms or sought care at another facility. Although patient load in both hospitals in the district, which have mechanical ventilation is comparable, individuals with a lower socio-economic status are likely to prefer state-funded facility where the study was conducted. Since AES is more likely to affect lower socio-economic population, we expect sampling bias to only moderately underestimate the overall incidence. Overall hospital-based nature of the study can lead to underestimation of the true incidence of AES since milder, non-hospitalized cases are not captured. However our incidence rate of 16/100,000 (in the sub-divisions of the same district as the hospital) is well above the suggested minimum surveillance definition of 2 per 100,000 for adults [2]. Future efforts to estimate the community burden of AES are needed. Fourth, we used only commercially available diagnostics to look for common organisms which cause AES. We did not test for Rabies, Simian retroviruses, and other fungal or parasitic organisms. Also, we did not perform necropsy or post-mortem examination in cases with mortality. It is likely that many AES cases of unknown etiology are due to less common or as yet unknown agents. Future studies on stored CSF and serum samples may help in understanding etiology of some of these cases.
We believe that hospital based surveillance systems can help track changes in encephalitis patterns over the long term [37,38]. While viral diagnostics are resource intensive, we can incorporate standard protocols for identification of AES and basic CSF based diagnostics into routine clinical care, as has happened after completion of study at this hospital. Periodic diagnostic testing can provide valuable etiologic and epidemiologic information. In India, an Integrated Disease Surveillence Program has been developed and implemented by the government, but needs to be strengthened [39,40]. This system is designed to collect and electronically integrate information from front-line workers, primary health centers, and diagnostic laboratories across the country. Our results demonstrate, however, that for this highly fatal condition a hospital-based monitoring system with periodic diagnostic testing is feasible and can provide information useful for clinicians as well as public health planners.
Supplementary Material
Acknowledgements
We thank Santosh Chavhan, BSW, Prashant Raut, BA, Rekha Zade MSc, DMLT and Vinod Kulkarni MSW for helping with data collection and entry.
Financial support RJ was a recipient of a training fellowship from the Fogarty AIDS International Training Program [AITRP] (grant 1-D43-TW00003-17), USA. This study was part of his Ph.D. dissertation. Fogarty AIRTP had no role in the design, conduct or review of this manuscript.
Footnotes
Appendix A. Supplementary data Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.clineuro.2013.04.008.
References
- [1].Solomon T, Thao TT, Lewthwaite P, Ooi MH, Kneen R, Dung NM, et al. A cohort study to assess the new WHO Japanese encephalitis surveillance standards. Bulletin of the World Health Organization. 2008;86:178–86. doi: 10.2471/BLT.07.043307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Jmor F, Emsley HC, Fischer M, Solomon T, Lewthwaite P. The incidence of acute encephalitis syndrome in Western industrialised and tropical countries. Virology Journal. 2008;5:134. doi: 10.1186/1743-422X-5-134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Mathur A, Chaturvedi UC, Tandon HO, Agarwal AK, Mathur GP, Nag D, et al. Japanese encephalitis epidemic in Uttar Pradesh, India during 1978. Indian Journal of Medical Research. 1982;75:161–9. [PubMed] [Google Scholar]
- [4].Vajpayee A, Dey PN, Chakraborty AK, Chakraborty MS. Study of the outbreak of Japanese encephalitis in Lakhimpur district of Assam in 1989. Journal of the Indian Medical Association. 1992;90:114–5. [PubMed] [Google Scholar]
- [5].Kar NJ, Saxena VK. Some epidemiological characteristics of Japanese encephalitis in Haryana state of northern India. Journal of Communicable Diseases. 1998;30:129–31. [PubMed] [Google Scholar]
- [6].Parida M, Dash PK, Tripathi NK, Ambuj, Sannarangaiah S, Saxena P, et al. Japanese encephalitis outbreak, India, 2005. Emerging Infectious Diseases. 2006;12:1427–30. doi: 10.3201/eid1209.060200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Sapkal GN, Bondre VP, Fulmali PV, Patil P, Gopalkrishna V, Dadhania V, et al. Enteroviruses in patients with acute encephalitis, Uttar Pradesh, India. Emerging Infectious Diseases. 2009;15:295–8. doi: 10.3201/eid1502.080865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Chadha MS, Arankalle VA, Jadi RS, Joshi MV, Thakare JP, Mahadev PV, et al. An outbreak of Chandipura virus encephalitis in the eastern districts of Gujarat state, India. American Journal of Tropical Medicine and Hygiene. 2005;73:566–70. [PubMed] [Google Scholar]
- [9].Rao BL, Basu A, Wairagkar NS, Gore MM, Arankalle VA, Thakare JP, et al. A large outbreak of acute encephalitis with high fatality rate in children in Andhra Pradesh, India, in 2003, associated with Chandipura virus. Lancet. 2004;364:869–74. doi: 10.1016/S0140-6736(04)16982-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Chadha MS, Comer JA, Lowe L, Rota PA, Rollin PE, Bellini WJ, et al. Nipah virus-associated encephalitis outbreak, Siliguri, India. Emerging Infectious Diseases. 2006;12:235–40. doi: 10.3201/eid1202.051247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Karmarkar SA, Aneja S, Khare S, Saini A, Seth A, Chauhan BK. A study of acute febrile encephalopathy with special reference to viral etiology. Indian Journal of Pediatrics. 2008;75:801–5. doi: 10.1007/s12098-008-0150-2. [DOI] [PubMed] [Google Scholar]
- [12].Saxena V, Dhole TN. Preventive strategies for frequent outbreaks of Japanese encephalitis in Northern India. Journal of Biosciences. 2008;33:505–14. doi: 10.1007/s12038-008-0069-9. [DOI] [PubMed] [Google Scholar]
- [13].Tandale BV, Tikute SS, Arankalle VA, Sathe PS, Joshi MV, Ranadive SN, et al. Chandipura virus: a major cause of acute encephalitis in children in North Telangana, Andhra Pradesh. Indian Journal of Medical Virology. 2008;80:118–24. doi: 10.1002/jmv.21041. [DOI] [PubMed] [Google Scholar]
- [14].Joshi R, Colford JM, Reingold AL, Kalantri S. Nonmalarial acute undifferentiated fever in a rural hospital in Central India: diagnostic uncertainty and overtreatment with antimalarial agents. American Journal of Tropical Medicine and Hygiene. 2008;78:393–9. [PubMed] [Google Scholar]
- [15].Tiwari SC, Kumar A, Kumar A. Development and standardization of a scale to measure socio-economic status in urban and rural communities in India. Indian Journal of Medical Research. 2005;122:300–14. [PubMed] [Google Scholar]
- [16].Center for Disease Control . Neuroinvasive and non-neuroinvasive domestic arboviral diseases. vol. 2009. CDC; Atlanta: 2004. Case definitions for infectious conditions under public health surveillance. [Google Scholar]
- [17].Fowlkes AL, Honarmand S, Glaser C, Yagi S, Schnurr D, Oberste MS, et al. Enterovirus-associated encephalitis in the California encephalitis project, 1998–2005. Journal of Infectious Diseases. 2008;198:1685–91. doi: 10.1086/592988. [DOI] [PubMed] [Google Scholar]
- [18].Kupila L, Vuorinen T, Vainionpaa R, Hukkanen V, Marttila RJ, Kotilainen P. Etiology of aseptic meningitis and encephalitis in an adult population. Neurology. 2006;66:75–80. doi: 10.1212/01.wnl.0000191407.81333.00. [DOI] [PubMed] [Google Scholar]
- [19].Burke DS, Lorsomrudee W, Leake CJ, Hoke CH, Nisalak A, Chongswasdi V, et al. Fatal outcome in Japanese encephalitis. American Journal of Tropical Medicine and Hygiene. 1985;34:1203–10. doi: 10.4269/ajtmh.1985.34.1203. [DOI] [PubMed] [Google Scholar]
- [20].Poneprasert B. Japanese encephalitis in children in northern Thailand. Southeast Asian Journal of Tropical Medicine and Public Health. 1989;20:599–603. [PubMed] [Google Scholar]
- [21].Maha MS, Moniaga VA, Hills SL, Widjaya A, Sasmito A, Hariati R, et al. Outcome and extent of disability following Japanese encephalitis in Indonesian children. International Journal of Infectious Diseases. 2009 doi: 10.1016/j.ijid.2009.01.009. [DOI] [PubMed] [Google Scholar]
- [22].Iff T, Donati F, Vassella F, Schaad UB, Bianchetti MG. Acute encephalitis in Swiss children: aetiology and outcome. European Journal of Paediatric Neurology. 1998;2:233–7. doi: 10.1016/s1090-3798(98)80036-6. [DOI] [PubMed] [Google Scholar]
- [23].Fowler A, Stodberg T, Eriksson M, Wickstrom R. Childhood encephalitis in Sweden: etiology, clinical presentation and outcome. European Journal of Paediatric Neurology. 2008;12:484–90. doi: 10.1016/j.ejpn.2007.12.009. [DOI] [PubMed] [Google Scholar]
- [24].Kennedy CR, Duffy SW, Smith R, Robinson RO. Clinical predictors of outcome in encephalitis. Archives of Disease in Childhood. 1987;62:1156–62. doi: 10.1136/adc.62.11.1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Bhutto E, Naim M, Ehtesham M, Rehman M, Siddique MA, Jehan I. Prognostic indicators of childhood acute viral encephalitis. Journal of Pakistan Medical Association. 1999;49:311–6. [PubMed] [Google Scholar]
- [26].Misra UK, Kalita J, Srivastava M. Prognosis of Japanese encephalitis: a multivariate analysis. Journal of the Neurological Sciences. 1998;161:143–7. doi: 10.1016/s0022-510x(98)00265-2. [DOI] [PubMed] [Google Scholar]
- [27].Luo D, Song J, Ying H, Yao R, Wang Z. Prognostic factors of early sequelae and fatal outcome of Japanese encephalitis. Southeast Asian Journal of Tropical Medicine and Public Health. 1995;26:694–8. [PubMed] [Google Scholar]
- [28].Klein SK, Hom DL, Anderson MR, Latrizza AT, Toltzis P. Predictive factors of short-term neurologic outcome in children with encephalitis. Pediatric Neurology. 1994;11:308–12. doi: 10.1016/0887-8994(94)90007-8. [DOI] [PubMed] [Google Scholar]
- [29].Huy BV, Tu HC, Luan TV, Lindqvist R. Early mental and neurological sequelae after Japanese B encephalitis. Southeast Asian Journal of Tropical Medicine and Public Health. 1994;25:549–53. [PubMed] [Google Scholar]
- [30].McCallum JD. Japanese encephalitis in southeastern Nepal: clinical aspects in the 1986 epidemic. Journal of the Royal Army Medical Corps. 1991;137:8–13. doi: 10.1136/jramc-137-01-03. [DOI] [PubMed] [Google Scholar]
- [31].Kumar R, Mathur A, Kumar A, Sharma S, Chakraborty S, Chaturvedi UC. Clinical features and prognostic indicators of Japanese encephalitis in children in Lucknow (India) Indian Journal of Medical Research. 1990;91:321–7. [PubMed] [Google Scholar]
- [32].Kalita J, Misra UK. Outcome of tuberculous meningitis at 6 and 12 months: a multiple regression analysis. International Journal of Tuberculosis and Lung Disease. 1999;3:261–5. [PubMed] [Google Scholar]
- [33].Singhi P, Bansal A, Geeta P, Singhi S. Predictors of long term neurological outcome in bacterial meningitis. Indian Journal of Pediatrics. 2007;74:369–74. doi: 10.1007/s12098-007-0062-6. [DOI] [PubMed] [Google Scholar]
- [34].Solomon T, Dung NM, Kneen R, Thao le TT, Gainsborough M, Nisalak A, et al. Seizures and raised intracranial pressure in Vietnamese patients with Japanese encephalitis. Brain. 2002;125:1084–93. doi: 10.1093/brain/awf116. [DOI] [PubMed] [Google Scholar]
- [35].Libraty DH, Nisalak A, Endy TP, Suntayakorn S, Vaughn DW, Innis BL. Clinical and immunological risk factors for severe disease in Japanese encephalitis. Transactions of the Royal Society of Tropical Medicine and Hygiene. 2002;96:173–8. doi: 10.1016/s0035-9203(02)90294-4. [DOI] [PubMed] [Google Scholar]
- [36].Tiroumourougane SV, Raghava P, Srinivasana Badrinath S. Management parameters affecting the outcome of Japanese encephalitis. Journal of Tropical Pediatrics. 2003;49:153–6. doi: 10.1093/tropej/49.3.153. [DOI] [PubMed] [Google Scholar]
- [37].Wong SC, Ooi MH, Abdullah AR, Wong SY, Krishnan S, Tio PH, et al. A decade of Japanese encephalitis surveillance in Sarawak, Malaysia: 1997–2006. Tropical Medicine and International Health. 2008;13:52–5. doi: 10.1111/j.1365-3156.2007.01967.x. [DOI] [PubMed] [Google Scholar]
- [38].Kari K, Liu W, Gautama K, Mammen MP, Jr, Clemens JD, Nisalak A, et al. A hospital-based surveillance for Japanese encephalitis in Bali, Indonesia. BMC Medicine. 2006;4:8. doi: 10.1186/1741-7015-4-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Prasad MN, Mithilesh M, Ramanarao GV. Public health surveillence in India – challenges and opportunities. Indian Emergency Journal. 2010;5:21–6. [Google Scholar]
- [40].Gupta SK, Gupta P, Sharma P, Srivastava A, Soni SK. Emerging and re-emerging infectious diseases, future challenges and strategy. Journal of Clinical and Diagnostic Research. 2012;6:1095–100. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.