Abstract
Background
The recent decline in US breast cancer incidence rates has been attributed to the marked reduction in use of postmenopausal hormone therapy (HT). An alternative explanation is that women who are not routinely seeking medical care to renew HT prescriptions may be less adherent to recommendations for mammographic screening. To investigate the latter possibility, we compared mammographic intervals according to HT use.
Methods
Using data (1995–2007) from the New Hampshire Mammography Network (NHMN), a state-based mammography registry, we assessed mammographic intervals corresponding to HT use or nonuse among postmenopausal women. We used linear mixed effects models to assess whether the length of screening mammogram intervals differed according to HT use.
Results
A total of 310,185 mammograms, representing 76,192 postmenopausal women and 319,353 person-years of screening, were included in the analysis. The median screening interval corresponding to HT use overall was 13.0 months (interquartile range: 12.4–15.1) and for nonuse was 13.1 (interquartile range: 12.4–15.8). Virtually, all screening mammograms occurred within a 2-year interval regardless of HT use or nonuse (98.5 and 98.1%, respectively).
Conclusions
Our findings indicate that screening mammography intervals are similar whether or not women are using HT. Thus, reduced utilization of screening mammography is unlikely to account for the decrease in breast cancer incidence seen soon after the WHI report.
Keywords: Mammography, Screening, Hormone therapy, Breast cancer
Introduction
The results of numerous observational studies have shown an association between postmenopausal hormone therapy (HT), particularly combined estrogen plus progesterone preparations, and increased breast cancer risk [1–5]. In 2002, the results of the Women’s health initiative (WHI) clinical trial confirmed that the use of combined regimens in postmenopausal women was associated with increased breast cancer risk as well as other adverse outcomes [6]. The highly publicized findings led to an immediate decrease in HT use in the United States, with estimates showing that up to 75% of women stopped using this medication [7–13].
In 2003, breast cancer incidence decreased by 6.7% in the United States, with rates leveling off since that time [14–16]. Studies in the United States [9, 12, 17, 18], Germany [19], and New Zealand [20] suggest a link between decreased HT use and declining breast cancer incidence, but lower incidence rates have not been seen in Sweden [21] or Norway [21], despite a sharp reduction of HT use. Data from Canada [22, 23] suggest that a decline in breast cancer incidence began prior to the drop in HT use and was significant only in women 75 years and older [24].
Of the few studies assessing the influence of HT use on mammography screening, one analysis based on screening data from four mammography registries found similar median screening intervals in women using or not using HT [18]. Also, an analysis of the Nurses’ Health Study restricted to women with prior screening showed only a small difference in the percent of HT users (93.6%) and nonusers (88.7%) who underwent subsequent screening [25]. No studies to our knowledge have explicitly sought to relate HT use with the length of screening mammography intervals, particularly in the same women, as they change their HT use patterns.
In 2008, an estimated 182,460 new cases of invasive and 67,770 new cases of noninvasive breast cancer will have been diagnosed with 40,480 deaths occurring from this disease in US women [15]. Mammography is the standard of care for breast cancer screening. At least six trials have found a 20–30% reduction in breast cancer mortality attributable to mammography screening in women [26–31]. Thus, it is important to know whether decreasing rates of breast cancer in the United States are real or an artifact of reduced screening and detection. To address this issue, we assessed screening intervals in a large state-based mammography registry. Our goal was to test the hypothesis that nonuse of HT is associated with longer intervals between screening mammograms.
Methods
Study population and data
We used data from the New Hampshire mammography network (NHMN) to assess screening intervals corresponding to use or nonuse of HT. Briefly, the NHMN is a state-based mammography registry consisting of more than 955,000 mammograms that occurred in New Hampshire since 1996 [32]. The NHMN records mammographic findings and provides linkage to pathology outcomes. Risk factor information, including HT use, is recorded at each visit through a self-administered questionnaire and a face-to-face interview conducted by the radiology technologist. The NHMN consistently has a consent rate exceeding 85%. Indication for exam is recorded by the radiologist for each mammogram, and was used to distinguish screening from diagnostic exams.
For the present report, the main analyses were based on consenting women with at least two postmenopausal screening mammograms from 1995 to 2007. Postmeno-pausal status was defined as natural menopause or bilateral oophorectomy as recorded on the radiology technologist form. Women with a mammogram at age less than 40 were excluded, as were women with a self-reported history of breast cancer, breast augmentation, or breast reduction prior to having two postmenopausal screening mammograms. Data for six women reporting ≥17 mammograms were removed. The majority of women (62%) in the study population had four or more mammograms.
Analysis variables
The unit of analysis was the interval between a pair of consecutive screening mammograms. In order to classify mammography intervals according to HT use or nonuse, postmenopausal women were required to have at least two consecutive screening mammograms for which HT use was recorded. A screening interval was classified as HT positive (HH) or HT negative (nn), when the two consecutive mammograms corresponded to use or nonuse of HT, respectively. Discordant pairs representing transitions in HT use were those in which the first mammogram was positive for HT, and the second was negative (Hn), or vice versa (nH). The outcome of interest was the median length of intervals between the pairs of consecutive screening mammograms as classified by HT use (HH, nn, Hn, nH). Each screening mammogram could define more than one consecutive pair. For example, in the setting of three consecutive screening mammograms, the middle mammogram would define the end of the first interval and the beginning of the second.
Woman-level characteristics considered as covariates and taken from the self-administered questionnaire included age in years, educational attainment (8th grade or less, some high school, associate degree or some college, college or postcollege graduate), marital status (single, separated, divorced, widowed), and family history of breast cancer (in first degree relative; yes or no). Covariates taken from the radiology technologist questionnaire included previous breast procedure (fine needle or cyst aspiration, needle biopsy, or surgical biopsy), and abnormal mammogram based on that exam (positive pathology, negative pathology, negative final assessment, unresolved). We categorized abnormal mammogram by Bi-RADS® score as follows: positive pathology, 4 or 5; negative pathology, 3 or 7; no pathology and negative final assessment, 0, 1, 2, or 3; unresolved, 0 or missing. Positive pathology was determined by the overall Bi-RADS category after follow-up. Woman-level characteristics were attributed to a mammogram pair based on information from the first mammogram in the pair, or (with the exception of HT use) forward filled as necessary. We searched forward 90 days from the date of the mammogram to capture pathology and final assessments. If a woman had an abnormal mammogram with positive pathology, we excluded from analysis all subsequent mammograms starting with the abnormal mammogram, due to the likelihood that a positive mammogram would influence women’s screening intervals.
Main analyses
The average screening interval was compared for the four types of intervals (HH, nn, Hn, nH) using a linear mixed effect model with random intercept for each woman to account for the multiplicity of intervals for some women, as well as an autocorrelation structure (AR1) to model the correlation of adjacent intervals (which was slightly negative, −0.07). The analyses were done with and without covariates: family history of breast cancer, educational level, marital status, age, self-reported breast abnormality, and abnormal mammogram at the start of the screening interval. Calendar time was additionally added as a covariate in a separate analysis. As a complement to these analysis that are based on HT status at the beginning and end of the interval, we used Kaplan–Meier analyses for recurrent events to examine time to the next screening based on HT status at the time of the beginning of the interval. This analysis used the right-censored information from women who were only screened once, as well as the interval of time from last screening to end of study for all other women.
Ancillary analyses
In addition to the analyses described earlier, we also conducted two subgroup analyses in which women served as their own controls, thus mimicking a crossover design. In the first subgroup analysis, we examined screening mammography intervals in postmenopausal women who had at least one pair of screening mammograms positive for HT use (HH) and one pair negative for HT use (nn). The second subgroup analysis was further restricted to women who had at least one HH interval and one nn interval after the dissemination of the WHI findings (i.e., after 31 July 2002).
Also, because women who had only one screening mammogram in the registry might differ from those included in our main analyses that required women to have at least two screening mammograms, we used methods for right-truncated data to study the association between HT status and time to next screening [33]. Specifically, we subtracted the interval from the time between the start of the interval and the end of study to create left-truncated data for which we used Cox’s model for left-truncated data with a random effect, i.e., gamma frailty, of women.
Results
The characteristics of 76,192 postmenopausal women who had at least two screening mammograms in the registry during the study period are shown in Table 1. On average (median), the women were 61.8 (63.5) years of age, the vast majority (91.8%) had completed high school, and 29.3% had completed college. The majority (69.5%) of women were married. About 30% of the women reported a family history of breast cancer. The minority of women (20.0%) had only two screening mammograms, 30.0% had 3–4, and 49.0% had at least 5. The women had a total of 310,185 screening mammograms in 319,353 person-years of screening. After excluding 2,866 mammograms (0.7%) due to positive pathology related to that exam, 310,185 mammograms (76,192 women) remained available for analysis.
Table 1.
Characteristics of postmenopausal women in the New Hampshire mammography network from 1995 to 2007 with at least two screening mammograms
| n (%) | |
|---|---|
| Total number of postmenopausal women | 76,192 (100) |
| Number of woman-years screened | 319,353 (100) |
| Number of screening mammogram intervals per woman |
|
| 1 | 4,408 (6) |
| 2 | 18,719 (25) |
| 3 | 13,667 (18) |
| 4 | 10,823 (14) |
| ≥5 | 28,575 (37) |
| Hormone therapy use statusa | |
| Never user | 42,252 (55) |
| Ever user | 33,940 (45) |
| Median (interquartile range) | |
| Age (years)b | 57 (52–66) |
Number of women with missing hormone therapy use
Age was recorded at the time of the first mammogram during the study period
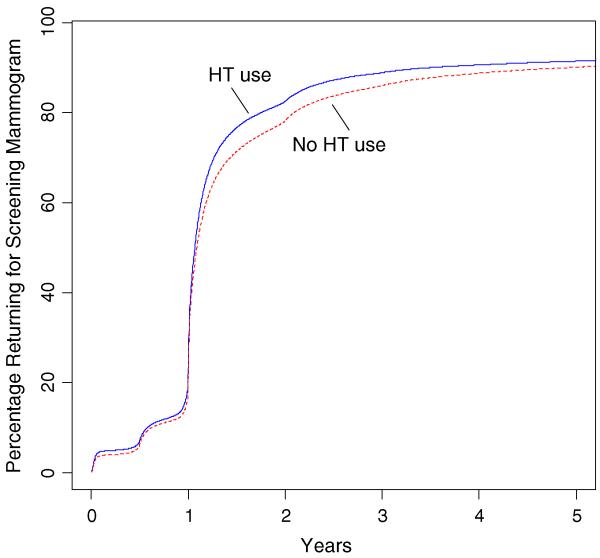
Overall, the mean and median lengths of screening mammographic intervals were similar regardless of HT use status (Table 2). The median (interquartile range; IQR) interval was 13.0 months (12.4–15.1) for pairs corresponding to HT use, and 13.1 months (12.4–15.8) for pairs corresponding to nonuse of HT. Adjusting for covariates did not result in notable differences from crude estimates (Fig. 1). Using Kaplan–Meier recurrent events analysis at the woman level, we found a similar proportion of women returning for screening mammography over time between HT users and nonusers (Fig. 2).
Table 2.
Median (interquartile range-IQR) and mean (standard deviation-SD) intervals (in months) between pairs of consecutive screening mammograms classified by hormone therapy use (HT); overall during the study period of 1995–2007, in the subgroup of women who had at least one pair corresponding to HT use and one pair corresponding to HT nonuse, and in the same subgroup further confined to mammograms occurring after publication of the 2002 WHI report
| HT usea for mammogram pair | Screening mammography interval length (months) |
|
|---|---|---|
| Median (IQR) | Mean (SD) | |
| Overall 1995–2007 (n = 218,930)b | ||
| HH | 13.0 (12.4–15.1) | 15.3 (6.4) |
| nn | 13.1 (12.4–15.8) | 16.2 (8.6) |
| Subgroup with at least one HH and one nn pair (n = 99,918)c | ||
| HH | 12.7 (12.3–14.3) | 13.6 (5.8) |
| nn | 12.5 (12.3–13.8) | 12.7 (5.6) |
| Subgroup with at least one HH and one nn pair after WHI report (n = 1,873)c | ||
| HH | 12.5 (12.3–13.7) | 13.2 (5.2) |
| nn | 12.5 (12.1–13.6) | 12.6 (5.9) |
HT use for consecutive mammogram pairs. HH: HT use in both mammograms; nn: nonuse in both mammograms
Analysis based on the mammographic interval using all pairs of consecutive screening mammograms
Analysis based on the mammographic interval among women who had at least one HH and one nn pair
Fig. 1.
Median mammographic screening intervals corresponding to HT status, adjusted for family history of breast cancer, education, marital status, age, self-reported prior breast abnormality, and abnormal mammogram in that interval; interquartile values (box) and 5th and 95th percentile values (vertical line)
Fig. 2.
Time to next screening by HT status estimated using Kaplan–Meier estimator for recurrent right-censored events
In subgroup analyses confined to mammograms of women who had at least one pair of consecutive mammograms corresponding to HT use and one pair corresponding to nonuse (n = 60,344) (Table 2), the median (IQR) interval was 12.7 (12.3–14.3) months with HT use and 12.5 (12.3–13.8) months with HT nonuse. Using the same approach, but limiting the analyses to mammograms occurring after July 2002 (n = 1,873), the median (IQR) interval was 12.5 (12.3–13.7) months with HT use and 12.5 (12.1–13.6) with nonuse (Table 2). We also examined transitions in HT use over the study period. The median Hn interval for screening mammograms among women who had at least one pair of HH and nn intervals (n = 99,918) was 12.7 months, with the 95th percentile at 25.1 months (Table 2).
We found no indication that the length of a screening interval was appreciably affected by an interim diagnostic mammogram regardless of whether a woman was using HT (median months: 13.8) or not (median months: 13.6). As expected, mammography intervals were generally shorter when one or both mammograms in a consecutive pair were diagnostic, but the interval length was not influenced by HT use. For example, the median number of months between two consecutive diagnostic mammograms ranged from 6.3 to 6.4 in all four scenarios (HH, Hn, nH, nn).
In the separate group of women who had only one screening mammogram in the registry, we compared the length of time elapsed between the single mammogram and the end of the study period. The median number of months elapsed for women using HT (26%) or not using HT (74%) was 98.5 and 91.2, respectively.
Discussion
In this population-based study, we found similar lengths of mammographic screening intervals corresponding to HT use and nonuse as reported at the time of the mammogram. The average difference in mammographic interval for the two groups was less than 1 month. Importantly, the vast majority of mammograms (>95%) occurred within the 2-year period recommended by US Preventive Services Task Force guidelines [34]. These findings strengthen the evidence that the relationship between declining HT use and decreased breast cancer incidence is not explained by reduced use of screening mammography.
Breast cancer incidence rates, and in particular rates of estrogen positive tumors decreased soon after the dramatic decline of HT use that directly followed the publication of the WHI clinical trial results [12]. Although a causal relationship was suspected, it was also recognized that discontinuing HT use might reduce adherence to screening mammography [12, 17, 18, 35]. To date, only a few studies have examined this possibility. One study based on a screened population represented in four mammography registries for the years 2000–2003 demonstrated concomitant declines in HT use and breast cancer rates that were not attributable to changes in mammography screening [18]. Data from Kaiser Permanente Northwest showed parallel trends for HT prescriptions and breast cancer incidence rates from 1980 to 2006, including an abrupt decline of both after the publication of the WHI findings, but mammography rates were comparable for the years surrounding the WHI report [17]. Similarly, a recent report based on the WHI clinical trial and observational study concluded that the decline in breast cancer incidence following HT cessation was not due to reductions in mammography screening [35]. Evidence from the WHI clinical trial, in which the frequency of mammographic screening was comparable per protocol in both arms, showed that breast cancer incidence increased with HT exposure and decreased in the postintervention period [35]. In the observational cohort study, the recommendation to discontinue HT use was followed by a 43% reduction in the annualized breast cancer incidence rate in the group of women with hormone use, whereas mammography use decreased by only 2%, a reduction insufficient to explain the decline in breast cancer rates [35]. Although these findings offer important evidence that reduced screening was not responsible for post-WHI declines in breast cancer rates, women participating in studies may be compliant with screening recommendations, a possibility that would limit the variability of screening intervals and the ability to assess factors influencing the intervals.
Our results, based on data recorded in a statewide mammography registry, should have direct relevance to the US screened population. Although our findings would not apply to unscreened women, we found similar intervals between a single mammogram and the end of the study period regardless of HT use, consistent with our main finding that HT use did not influence screening behavior. Moreover, chronically unscreened women cannot contribute to a decrease in breast cancer rates mediated by noncompliance with mammographic screening. Thus, a screened population provides the optimal setting for determining whether the decline in breast cancer rates seen after WHI was due to reduced use of mammography.
Further understanding of the tumor promoter effects of HT use on breast cancers, along with careful tracking of HT use and breast cancer incidence trends, should elucidate the large-scale trends to date and going forward. Given an estimated 52,401,000 postmenopausal women in the United States in 2010 [36], the public health implications for both HT use and breast cancer are enormous in terms of morbidity, mortality, and costs. For individual women and providers, weighing the risks and benefits of HT use can be complex and deserving of more evidence upon which to base clinical decisions. Health care systems and screening populations need rationale recommendations that account for competing risks in women’s health status.
Acknowledgments
This project was supported in part by the National Cancer Institute (U01CA86082).
Contributor Information
Tracy Onega, Department of Community and Family Medicine, Dartmouth Medical School, Hanover, NH, USA; Norris Cotton Cancer Center, Dartmouth Hitchcock Medical Center, Lebanon, NH, USA; Dartmouth Medical School, HB 7927, Rubin 8, One Medical Center Dr., Lebanon, NH 03755, USA.
Todd MacKenzie, Norris Cotton Cancer Center, Dartmouth Hitchcock Medical Center, Lebanon, NH, USA; Department of Medicine, Dartmouth Medical School, Hanover, NH, USA.
Julia Weiss, Department of Community and Family Medicine, Dartmouth Medical School, Hanover, NH, USA.
Martha Goodrich, Department of Community and Family Medicine, Dartmouth Medical School, Hanover, NH, USA; Norris Cotton Cancer Center, Dartmouth Hitchcock Medical Center, Lebanon, NH, USA.
Linda Titus-Ernstoff, Department of Community and Family Medicine, Dartmouth Medical School, Hanover, NH, USA; Norris Cotton Cancer Center, Dartmouth Hitchcock Medical Center, Lebanon, NH, USA; Department of Pediatrics, Dartmouth Medical School, Hanover, NH, USA.
References
- 1.Beral V. Breast cancer and hormone-replacement therapy in the million women study. Lancet. 2003;362(9382):419–427. doi: 10.1016/s0140-6736(03)14065-2. [DOI] [PubMed] [Google Scholar]
- 2.Chen WY, Manson JE, Hankinson SE, et al. Unopposed estrogen therapy and the risk of invasive breast cancer. Arch Intern Med. 2006;166(9):1027–1032. doi: 10.1001/archinte.166.9.1027. [DOI] [PubMed] [Google Scholar]
- 3.Colditz GA, Hankinson SE, Hunter DJ, et al. The use of estrogens and progestins and the risk of breast cancer in postmenopausal women. N Engl J Med. 1995;332(24):1589–1593. doi: 10.1056/NEJM199506153322401. [DOI] [PubMed] [Google Scholar]
- 4.Colditz GA, Stampfer MJ, Willett WC, et al. Type of postmenopausal hormone use and risk of breast cancer: 12-year follow-up from the nurses’ health study. Cancer Causes Control. 1992;3(5):433–439. doi: 10.1007/BF00051356. [DOI] [PubMed] [Google Scholar]
- 5.Grodstein F, Stampfer MJ, Colditz GA, et al. Postmeno-pausal hormone therapy and mortality. N Engl J Med. 1997;336(25):1769–1775. doi: 10.1056/NEJM199706193362501. [DOI] [PubMed] [Google Scholar]
- 6.Rossouw JE, Anderson GL, Prentice RL, et al. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results from the women’s health initiative randomized controlled trial. Jama. 2002;288(3):321–333. doi: 10.1001/jama.288.3.321. [DOI] [PubMed] [Google Scholar]
- 7.Breen N, AC K, Meissner HI, et al. Reported drop in mammography: is this cause for concern? Cancer. 2007;109(12):2405–2409. doi: 10.1002/cncr.22723. [DOI] [PubMed] [Google Scholar]
- 8.Buist DS, Newton KM, Miglioretti DL, et al. Hormone therapy prescribing patterns in the United States. Obstet Gynecol. 2004;104(5 Pt 1):1042–1050. doi: 10.1097/01.AOG.0000143826.38439.af. [DOI] [PubMed] [Google Scholar]
- 9.Clarke CA, Glaser SL, Uratsu CS, Selby JV, Kushi LH, Herrinton LJ. Recent declines in hormone therapy utilization and breast cancer incidence: clinical and population-based evidence. J Clin Oncol. 2006;24(33):e49–e50. doi: 10.1200/JCO.2006.08.6504. [DOI] [PubMed] [Google Scholar]
- 10.Hersh AL, Stefanick ML, Stafford RS. National use of postmenopausal hormone therapy: annual trends and response to recent evidence. Jama. 2004;291(1):47–53. doi: 10.1001/jama.291.1.47. [DOI] [PubMed] [Google Scholar]
- 11.Krieger N. Hormone therapy and the rise and perhaps fall of US breast cancer incidence rates: critical reflections. Int J Epidemiol. 2008;37(3):627–637. doi: 10.1093/ije/dyn055. [DOI] [PubMed] [Google Scholar]
- 12.Ravdin PM, Cronin KA, Howlader N, et al. The decrease in breast-cancer incidence in 2003 in the United States. N Engl J Med. 2007;356(16):1670–1674. doi: 10.1056/NEJMsr070105. [DOI] [PubMed] [Google Scholar]
- 13.Wei F, Miglioretti DL, Connelly MT, et al. Changes in women’s use of hormones after the women’s health initiative estrogen and progestin trial by race, education, and income. J Natl Cancer Inst Monogr. 2005;35:106–112. doi: 10.1093/jncimonographs/lgi047. [DOI] [PubMed] [Google Scholar]
- 14.Health, United States . With chartbook on trends in the health of Americans. National Center for Health Statistics; 2006. DHHS pub. no. 2006-1232. [PubMed] [Google Scholar]
- 15.Jemal A, Ward E, Thun MJ. Recent trends in breast cancer incidence rates by age and tumor characteristics among US women. Breast Cancer Res. 2007;9(3):1–6. doi: 10.1186/bcr1672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.US Cancer Statistics Working Group . United States Cancer Statistics (1999–2004) Incidence and mortality web-based report. US Department of Health and Human Services C; Atlanta: [Google Scholar]
- 17.Glass AG, Lacey JV, Jr, Carreon JD, Hoover RN. Breast cancer incidence, 1980–2006: combined roles of menopausal hormone therapy, screening mammography, and estrogen receptor status. J Natl Cancer Inst. 2007;99(15):1152–1161. doi: 10.1093/jnci/djm059. [DOI] [PubMed] [Google Scholar]
- 18.Kerlikowske K, Miglioretti DL, Buist DS, Walker R, Carney PA. Declines in invasive breast cancer and use of postmenopausal hormone therapy in a screening mammography population. J Natl Cancer Inst. 2007;99(17):1335–1339. doi: 10.1093/jnci/djm111. [DOI] [PubMed] [Google Scholar]
- 19.Katalinic A, Rawal R. Decline in breast cancer incidence after decrease in utilisation of hormone replacement therapy. Breast Cancer Res Treat. 2008;107(3):427–430. doi: 10.1007/s10549-007-9566-z. [DOI] [PubMed] [Google Scholar]
- 20. Accessed at http://www.smh.com.au/news/World/Breast-cancer-rates-drop-in-NZ/2006/12/20/1166290.
- 21.Zahl PH, Strand BH, Maehlen J. Incidence of breast cancer in Norway and Sweden during introduction of nationwide screening: prospective cohort study. BMJ. 2004;328(7445):921–924. doi: 10.1136/bmj.38044.666157.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. [Accessed 12 Sep 2007];Cancer Surveillance On-Line. 2007 http://dsol-smed.phac-aspc.gc.ca/dsol-smed/cancer/index_e.html.
- 23.The Ups and Downs of HRT 2006 Accessed at http://www.imshealth.com/vgn/images/portal/cit_40000873/6/1/79032750Insights01En061127.pdf.
- 24.Kliewer EV, Demers AA, Nugent ZJ. A decline in breast-cancer incidence. N Engl J Med. 2007;357(5):509–510. author reply 13. [PubMed] [Google Scholar]
- 25.Joffe MM, Byrne C, Colditz GA. Postmenopausal hormone use, screening, and breast cancer: characterization and control of a bias. Epidemiology. 2001;12(4):429–438. doi: 10.1097/00001648-200107000-00013. [DOI] [PubMed] [Google Scholar]
- 26.Andersson I, Aspegren K, Janzon L. Mammographic screening and mortality from breast cancer: the Malmo mammographic screening trial. BMJ. 1988;279:943–948. doi: 10.1136/bmj.297.6654.943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Frisell J, Eklund G, Hellstrom L, Lidbrink E, Rutqvist LE, Somell A. Randomized study of mammographic screening—preliminary report on mortality in the Stockholm trial. Breast Cancer Res Treat. 1991;18:49–56. doi: 10.1007/BF01975443. [DOI] [PubMed] [Google Scholar]
- 28.Nystrom L, Rutqvist LE, Wall S, et al. Breast cancer screening with mammography: overview of Swedish randomised trials. Lancet. 1993;341(8851):973–978. doi: 10.1016/0140-6736(93)91067-v. [DOI] [PubMed] [Google Scholar]
- 29.Roberts MM, Alexander F, Anderson TJ, et al. Edinburgh trial of screening for breast cancer: mortality at seven years. Lancet. 1990;335(8684):241–246. doi: 10.1016/0140-6736(90)90066-e. [DOI] [PubMed] [Google Scholar]
- 30.Shapiro S, Venet W, Strax P, Venet L. Periodic screening for breast cancer: the health insurance plan project and its sequelae, 1963-1986. The Johns Hopkins University Press; Baltimore: 1988. [Google Scholar]
- 31.Tabar L, Fagerberg G, Duffy SW, Day N, Gad A, Grontoft O. Update of the Swedish two-country program of mammographic screening for breast cancer. Radiol Clin North Am. 1992;30:187–210. [PubMed] [Google Scholar]
- 32.Carney PA, Poplack SP, Wells WA, Littenberg B. The New Hampshire mammography network: the development and design of a population-based registry. AJR. 1996;167(2):367–372. doi: 10.2214/ajr.167.2.8686606. [DOI] [PubMed] [Google Scholar]
- 33.Petersen L, TIA Sørensen, Nielsen GG, Andersen PK. Inference methods for correlated left truncated lifetimes: parent and offspring relations in an adoption study. Lifetime Data Anal. 2006;12(1):5–20. doi: 10.1007/s10985-005-7217-4. [DOI] [PubMed] [Google Scholar]
- 34.US Preventive Services Task Force . Screening for breast cancer. Agency for Healthcare Research and Quality; Rockville: [Accessed 08 Sep 2009]. 2002. http://www.ahrq.gov/clinic/3rduspstf/breastcancer/ [Google Scholar]
- 35.Chlebowski RT, Kuller LH, Prentice RL, Stefanick ML, Manson JE, Gass M, et al. Breast cancer after use of estrogen plus progestin in postmenopausal women. N Engl J Med. 2009;360(6):573–587. doi: 10.1056/NEJMoa0807684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.US Census [Accessed 08 Sep 2009];2009 http://www.allcountries.org/uscensus/14_resident_population_projections_by_sex_and.html.