Abstract
Purpose
Many bioactive proteins including cytokines are reported to increase in dry eye disease although the specific profile and concentration of inflammatory mediators varies considerably from study to study. In part this variability results from inherent difficulties in quantifying low abundance proteins in a limited sample volume using relatively low sensitivity dot ELISA methods. Additional complexity comes with the use of pooled samples collected using a variety of techniques and intrinsic variation in the diurnal pattern of individual tear proteins. The current study describes a recent advance in the area of proteomics that has allowed the identification of dozens of low abundance proteins in human tear samples.
Methods
Commercially available stationary phase antibody protein arrays were adapted to improve suitability for use in small volume biological fluid analysis with particular emphasis on tear film proteomics. Arrays were adapted to allow simultaneous screening for a panel of inflammatory cytokines in low volume tear samples collected from individual eyes.
Results
A preliminary study comparing tear array results in a small population of Sjögren’s syndrome patients was conducted. The multiplex microplate array assays of cytokines in tear fluid present an unanticipated challenge due to the unique nature of tear fluid. The presence of factors that exhibit an affinity for plastic, capture antibodies and IgG and create a complex series of matrix effects profoundly impacting the reliability of dot ELISA, including with elevated levels of background reactivity and reduction in capacity to bind targeted protein.
Conclusions
Preliminary results using tears collected from patients with Sjögren’s syndrome reveal methodological advantages of protein array technology and support the concept that autoimmune-mediated dry eye disease has an inflammatory component. They also emphasize the inherent difficulties one can face when interpreting the results of micro-well arrays that result from blooming effects, matrix effects, image saturation and cross-talk between capture and probe antibodies that can greatly reduce signal-to-noise and limit the ability to obtain meaningful results.
Keywords: Sjögren’s Syndrome, tears, dry eye, protein array, cytokine
The ocular surface is bathed in a complex mixture of lipids, proteins and glycoproteins that serve to lubricate and protect the underlying mucosal epithelial surface and provide a smooth refractive surface. Characterization of pre-ocular tear film components has historically relied on classical ELISA to measure changes in the concentrations and distribution of specific proteins. This approach has restricted analysis to one or two specific proteins at a time with further limitations set forth by the availability of limited sample volumes and the low concentration of many tear film proteins.1,2
Recent advances in the area of proteomics have resulted in the identification of dozens of low abundance proteins in human tear samples that are bioactive even in trace quantities. An example of this approach is the use of stationary phase forms of antibody protein array technology to allow the simultaneous assay of multiple proteins by dot ELISA.3-5 Antibody array technology is a high throughput technique that allows one to screen biological fluids simultaneously for a wide range of possible biomarkers that can affect the innate and adaptive host defense systems, cell apoptosis, migration, wound healing and angiogenesis. While this approach is of considerable value in understanding the complex biological processes that accompany ocular surface biology and disease pathogenesis, the application of this technology to obtain quantitative data has proven difficult due to the presence in tear fluid of various factors that interfere with the binding and efficacy of both stationary arrays and the more popular form of moving bead array analysis.3,6,7
We have been working on techniques to circumvent many of the confounding problems with array technology and thereby enhance the signal-to-noise ratios of individual dot ELISA for use in the study of dry eye disease. With several modifications to standard protocols, we have been able to characterize changes in the distribution of many cytokines, chemokines, growth factors and angiogenic modulators in individual tear samples recovered during the normal diurnal cycle in normals and individuals with allergic ocular diseases.3, 4 In these earlier studies, tear fluid was collected by micro capillary tube and subjected to centrifugation to remove cells and debris. Of particular challenge has been the application of this technology to the study of dry eye syndromes including autoimmune-mediated dry eye diseases like Sjögren’s syndrome (SS). Pathological lymphocytic infiltration of the lacrimal gland in patients with SS greatly reduces the aqueous component of tears with significantly reduced wetting on non-anesthetized Schirmer tear testing.8, 9 The reduced pre-ocular tear volume and low fluid turnover in individuals with severe dry eye makes tear sampling by micro capillary tube especially difficult. Moreover, confounding tear specific matrix effects present in samples collected by micro capillary interfere with accurate assessment of tear proteins.
In this study we examined the use of Schirmer strips as a means of tear collection. The Schirmer test represents a well-known clinical procedure routinely used to test tear function in dry eye patients. While this method of tear collection is known to result in the inclusion of ocular surface tissue constituents, it is far more adaptable for use in dry eye patients. Moreover, the inclusion of proteins from the ocular surface could prove an advantage by providing an enriched sample for contrasting normal and pathological environments. An additional advantage of Schirmer strip-derived extracts is the disappearance of many (if not all) of the unknown factors responsible for tear specific matrix effects that appear to remain on the strip following extraction. This results in an extract with a greatly improved signal-to-noise ratio when subjected to micro well plate assays of a wide range of inflammatory cytokines.
We report preliminary studies of Schirmer strip-collected tears analyzed by micro well plate array. To illustrate both the inherent difficulties of this technology as well as the potential advantages, we show recent results from a small cohort of SS patients and normal controls. Our studies provide increasing evidence that dry eye syndrome is associated with ocular surface inflammation, with increased concentrations of several bioactive inflammatory proteins in the pre-ocular tear film that may augment the symptoms of dry eye and also elicit the disease process itself (reviewed in 10). The techniques developed and presented require further optimization in order to allow the screening of large numbers of trace proteins in a single tear sample and identification of several new biomarkers related to specific diseases of the ocular surface. With further work, this type of analysis will eventually prove to be useful for both diagnostic and therapeutic purposes. 3,4
MATERIALS AND METHODS
Human subject recruitment and tear collection
Patients with SS and normal control subjects were recruited from the University of California, San Francisco (UCSF) SS clinic with informed consent according to the guidelines established by the Association for Research in Vision and Ophthalmology. All procedures were approved by the UCSF Committee for Human Research and adhered to the tenets of the Declaration of Helsinki. Tear samples were collected by Schirmer strips from both eyes of each subject. Patients with SS currently using any topical ocular medications other than artificial tears were instructed not to instill any eye drops for three days prior to tear collection. All samples were collected in a standardized fashion (unanaesthetized eye with strip inserted in the inferior cul-de-sac for 5 minutes). Strips from both eyes were placed into a 1.5 ml eppendorf tube and snap frozen at −80°C until analysis for inflammatory mediators by antibody array. Two types of tear samples were collected, open eye tear fluid (OTF) and closed eye tear fluid (CTF). The latter was collected by the patient at home immediately upon awakening after overnight sleep. In addition OTF and CTF collected by glass micro capillary tube were used for comparison purposes. The micro capillary tube collection method has been described previously.11
Micro well plate array
Preliminary micro well plate studies were carried out using a laboratory-designed protocol (LDP) detailed earlier3 employing most of the components of two similar 9-plex arrays (SearchLight™ cytokine arrays, Pierce Rockford, IL) that varied in matrix design and the specific assays when purchased in kit form. The first was designed to simultaneously assay IL1α, IL1β, IL2, IL6, IL8, IL10, IL12, interferon gamma (IFNγ) and tumor necrosis factor alpha (TNFα) and the second was designed to assay simultaneously IL2, IL4, IL5, IL8, IL10, IL12, IL13, IFNγ and TNFα providing overlap for evaluative purposes. These assays have been validated for use in serum, urine, tissue culture media and other fluids. The sensitivities of each of the assays are detailed by the manufacturer based upon a classical dot sandwich ELISA protocol employing biotin-streptavidin-HRP amplification and chemiluminescence for detection. The details of this methodology are reported elsewhere.3
Further analysis was carried out on a larger 12-plex micro well plate array kit that was purchased from Quansys Biosciences (Logan, Utah). A total of 12 cytokines were analyzed in each well consisting of IL1α, IL1β, IL2, IL4, IL5, IL6, IL8, IL10, IL13, IFNγ, TNFα and TNFβ. The minimum sensitivity range for detection of each protein varied and is reported to range from 3.8 to 56 pg/ml. While this array is available as an “off–the-shelf” standard kit for this purpose, the kit was specifically modified at our request to contain a cocktail of all of the biotinylated secondary antibodies except IL8 and a second solution containing all the biotinylated secondary antibodies, including IL8. This allowed us to carry out a sequential set of assays described below to analyze all of the cytokines except for IL8 and image the plate long term to pick up low intensity signals and to then wash and re-assay the same plate with the addition of the biotinylated secondary antibody to IL8 and image for a short time frame.
Micro well plates were processed using a modification of a protocol detailed elsewhere. 3 Briefly the plates were pre-blocked using MEGA BLOCK 3™, a proprietary synthetic blocking agent (Cell Associates, Inc. Pearland, TX), for one hour at room temperature. Schirmer strips were used to collect tears rather than the micro capillary tube. The bottom 5 mm portion of each strip encompassing that which would have been wetted with approximately 5 μl of tear fluid was cut into fragments and placed in eppendorf tubes. These fragments were extracted with 150 μl of MEGA Block 3 with agitation at room temperature for one hour. The tubes were centrifuged at 11,000 rpm for 10 minutes and a 50 μl volume was collected and added to each well. To assess the blocking effects of tears, we tested the supplied recombinant protein standards spiked with eluted tears and all samples (i.e., tears alone, standards alone and tear-spiked standards) were added to individual wells (data not shown). In those samples in which the size of the collected tears was less than 5 mm from the bottom of the strip, a dilution factor was calculated. After incubation with agitation at room temperature for 60 min, the tear fluid and the standard protein fluids were decanted and reserved for future assays. The wells were washed six times as directed using the supplied wash buffer. A cocktail of diluted biotinylated secondary antibodies was added to each well and the plate was incubated for 30 min at room temperature with agitation. The residual solutions were discarded and the washing sequence was repeated. The wells were incubated for 30 min with 50 μl of the supplied streptavidin-peroxidase-linked reporter enzyme with agitation. The remaining fluid was discarded and the wells were washed as directed. To detect the bound complex, 50 μl of a freshly prepared solution of the long acting substrate ChemiGlow™ (Alpha Innotech, San Leandro, CA) was added to each well instead of the kit supplied substrate. After checking for the presence of interfering bubbles, the wells were visualized using a Chemdoc XRS image station.
Imaging was carried out without binning for periods ranging from 1 min to as long as 1 h. The images of each of the wells in the plate were assessed for background chemiluminescence and tear blocking effects. Densitometry was carried out using ImageMaster 2D Platinum. Images were uniformly adjusted for size, brightness and contrast and imported into the software. Using the SPOT tool, a user-defined area (corresponding to each cytokine on the array) was manually spotted. For each spot, the software generated a value for the volume of the spot representing the product of the area and the highest pixel value contained in that area. This value was representative of the total amount of signal generated for each cytokine. Spot volumes were similarly calculated for standards of known concentration and a standard graph was plotted. A baseline control array was generated with each assay to assess the background signal. This value was subtracted from the spot volume obtained for each sample and the standards. Using the standard graph, the concentration of each cytokine was calculated.
Initial analysis of individual tear samples revealed an intense signal for IL8 that at times obstructed the surrounding wells and made boundary determination for densitometric analysis difficult. To address this issue, a two-step protocol was adopted using micro well arrays, graciously designed and provided per our request by Quansys Biosciences. Extraction was carried out using a proprietary tear dilution buffer provided by the manufacturer. This buffer was developed to specifically block a wide range of tear matrix effects and was used as the blocking agent and dilution buffer. In “step one”, arrays were imaged using a secondary antibody cocktail that was devoid of IL8 antibody. This allowed detection and imaging of the 11 remaining cytokines. In “step two”, the same samples were re-assayed using a cocktail containing antibodies for all 12 cytokines, including IL8. Densitometric analysis was carried out using ImageMaster 2 D Platinum as described above.
Statistical Methods
Cytokine expression was normalized by subtracting background from each sample. Resulting values were divided by values from a standard concentration to allow for cross-array comparisons. Densitometry results for micro well arrays were analyzed using Principle Components Analysis (PCA) to quantify which proteins were most responsible for the variance in the samples. PCA represents the samples as a set of vectors with one dimension for each protein and uses linear algebra to find an axis of maximal variance. This new axis is not one of the original twelve dimensions; it is a new composite “eigenprotein” that indicates which proteins contribute the most variance to the experiment.
RESULTS
Matrix Effects Result in Array Variation
We have shown that multiplex microplate array assays of cytokines in tear fluid present an unanticipated challenge due to the unique nature of tear fluid. Specifically, the presence of many factors that exhibit an affinity for plastic, capture antibodies and IgG create a complex series of matrix effects that can profoundly impact the reliability of dot ELISA. Components with a high affinity for plastic cause an elevated level of background reactivity, while factors that bind to the capture antibodies reduce their capacity to bind the targeted protein. The tear-specific moieties responsible for these effects have not been identified and their deleterious effects are particularly evident if the assay protocol does not include an initial pre-blocking step (as in the case of Searchlight array assay protocols, validated for serum and culuture fluid). As shown in Figure 1A, tear specific matrix effects can preclude accurate cytokine assay of capillary tube collected tear samples and can vary with the nature (OTF vs. CTF) and volume of added tear fluid. Exclusion of these phenomenon is critical for development of a valid assay protocol.
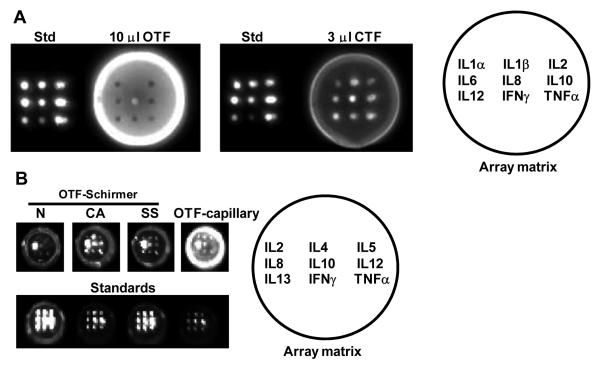
Figure 1.
(A) Matrix effects of human tear analyzed by micro well protein antibody array. Wells contain a cocktail of standard protein of known concentration (Std), 10μl OTF or 3μl CTF collected by micromicrocapillary tube. Figures demonstrate non specific binding and blocking activity which varies dramatically in the OTF and CTF samples from the same donor. More information on these phemonena are detailed elsewhere.3 (B) Background was reduced by addition of a pre-blocking step and modification of the collection protocol. Normal and pathological tears were collected by Schirmer strip and eluted in blocking buffer. Examples of cytokine expression are shown for tears collected from patients with chronic allergy (CA), Sjögren’s syndrome (SS) and controls (N, normal ocular surface) using the Schirmer strip. OTF-capillary sample demonstrates persistence of a residual level of matrix effect in tears collected by micro capillary tube despite the addition of a blocking step. The formats of the two arrays are depicted.
Reducing Background by Addition of Blocking Steps
Based upon extensive exploratory studies, a lab-designed protocol was developed 3 to include a blocking step that reduced but did not entirely eliminate the matrix effects observed in OTF collected by capillary tube. Quantification remained difficult because of the residual matrix effect and the very low levels of all probed cytokines except IL8. Switching to the Schirmer strip extracts for analysis revealed greatly improved array profile with non-specific background reactivity nearly totally eliminated and strong signals observed for many cytokines in the majority of the SS samples, even in extracts from strips containing as little as 2-3 mm of absorbed fluid (Figure 1B). An example of cytokine expression is shown for tears collected from a patient with chronic allergy (CA), SS and a control patient with a normal ocular surface (N) (Figure 1B, OTF-Schirmer). We speculate that the interfering factors remain bound to the strip resulting in an extract with a lower risk of background reactivity. The importance of the Schirmer strip in “absorbing” the protein moiety responsible for excessive background luminance can be demonstrated by the persistence of this matrix effect with micro capillary tube collected OTF (Figure 1B, OTF-capillary). Conversely, it is possible that some cytokines may selectively remain bound to the strip. The array format of the recombinant cytokine antibodies is depicted in the array matrix.
Analysis of IL8 Shielded Samples
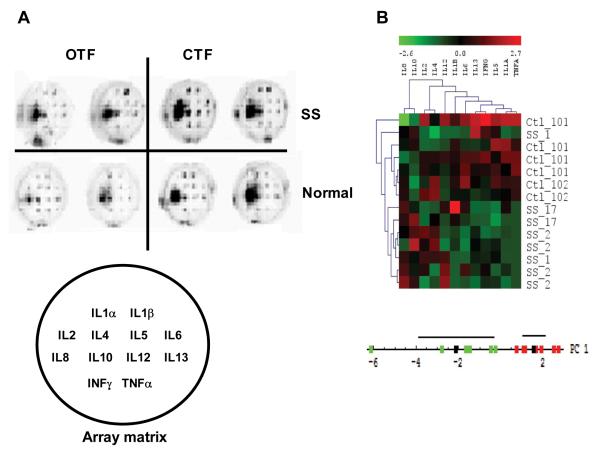
We used a 12-plex micro well plate array to study the cytokine profile in patients with SS dry eye. Samples were collected from patients with labial gland biopsies characteristic of SS and age-matched controls. Schirmer strips were used to collect tear samples in the office by the investigator (OTF) and at home by the patient immediately upon awakening from overnight sleep (CTF). These initial studies identified a shielding effect caused by high levels of luminescence in the IL8 wells in both normal and diseased tears. As shown in Figure 2A, this intense signal interfered with accurate readings of expression levels in adjacent wells. Automated spot boundary determination of these samples was difficult and manual localization and quantification of spots by a masked experienced observer was required. To analyze the data, we calculated the relative density of each spot as described in the methods. Using the volume calculations of density, we normalized the protein measurements by subtracting the mean values and dividing them by the standard deviation on a per sample basis. We performed unsupervised hierarchical clustering of the normalized results using the TMEV software package.12 This analysis revealed that with one exception, cases were most similar to other cases and controls were most similar to other controls (Figure 2B, upper panel). Same-patient samples from right and left eyes tended to be more similar to each other than to any other sample. The heat map graphic emphasized that TNFα, IL1α, and IL5 quantities were uniformly lower in SS than in controls while IL8, IL10 tended to be higher. We then used PCA to quantify which proteins are most responsible for the variance in the samples. The cases and controls were cleanly separated using the first principle component (Figure 2B, lower panel) with over ninety percent of the variance in the data set contributed by five proteins, ranked in descending order of impact: TNFα, IFNγ, IL1α, IL6, IL5. However, we were uncertain about the extent to which the IL8 shielding effect may have modified the assayed levels of cytokine expression in the array. This motivated the development of the two-step protocol.
Figure 2.
(A) Micro well plate arrays of SS and normal tear samples. Representative examples of open and closed eye tear samples from the same SS or normal patients are shown. Location of cytokine capture antibody spotted onto the array is indicated in the array matrix. Raw images of cytokine expression levels are shown. (B) Unsupervised hierarchical clustering of normalized protein levels from closed eyes. Plot of the first principle component of normalized protein levels. The first eigenvector was multiplied by the data matrix to generate a score value for each sample. Green points are controls, red points are cases. Black points are mean values and black lines represent 95% SE regions for each group.
Redesigned IL8 Detection Protocol
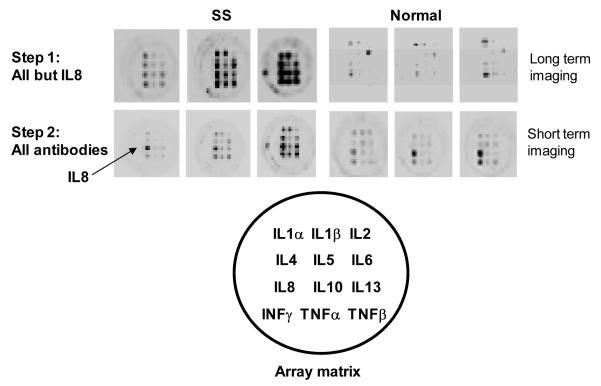
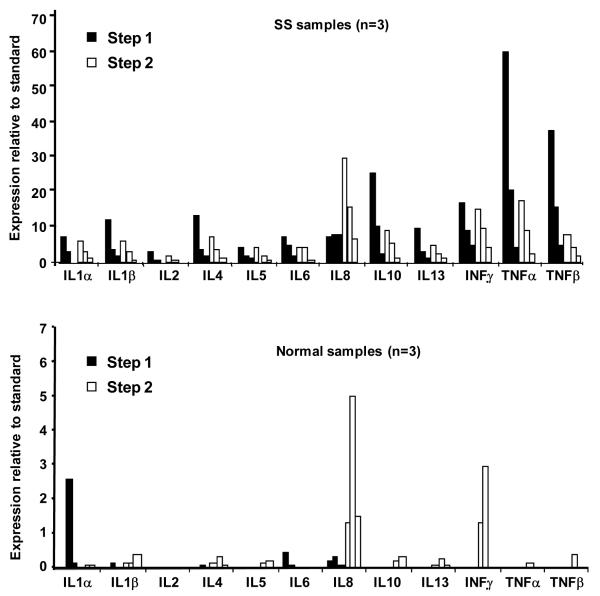
The two-step protocol was adopted using arrays manufactured upon request. OTF samples taken from three SS patients and three normal controls were assayed using the step one and step two antibody cocktails. In this protocol imaging of the array without secondary antibodies to IL8 is carried out for a very long time in order to obtain data for relatively weak signals. In the second step since the signal for IL8 is much stronger imaging is carried out for a very short time. Results are shown in Figures 3 and 4. Overall levels of cytokine expression varied considerably among SS patients (Figure 3, left panel and Figure 4, top panel) and were low to zero in normals (Figure 3, right panel and Figure 4, bottom panel). For the SS patients, a consistent pattern of relative expression levels was observed when step one or step two assays were considered individually. However, the same cytokine measured in the same patient could vary considerably between the step one and step two assays. For example, the same “high-medium-low” pattern of expression is seen for the three SS levels of TNFα in step one and step two, but the magnitude of the values in step one is two to three times higher than those reported in step two. In contrast, the SS levels for IFNγ, IL1α, and IL5 are consistent in relative and absolute values between step one and two assays (see Figure 4, top panel).
Figure 3.
Micro well plate arrays of OTF Sjögren’s syndrome and normal tear samples. Step one arrays did not have the IL8 specific antibody in the secondary antibody cocktail. Step two is a re-probe of the same membrane with the IL8 antibody included in the cocktail. Raw images of cytokines expression levels are shown.
Figure 4.
Top Panel: Normalized expression levels of SS samples (n=3) using step one and step two assays shown with black and white bars, respectively. Bottom Panel: Normalized expression levels of normal samples (n=3) using step one and step two assays, shown with black and white bars, respectively.
As expected, the protein standard for IL8 was measured at a much lower level in the step one probes than in step two probes. However, we were surprised to see that all of the SS tear samples gave a positive reading for IL8 in the absence of secondary antibody (Figure 4, top panel). IL8 levels in SS step one were reported to be very similar in all three patients. They were not, however, below background level. We hypothesize this “pseudo IL8” to be IL8 in a complex with other cytokines in tears that are then detected by the antibody specific for that cytokine. In normal samples, IL8 levels were almost absent in step one (little or no “pseudo-IL8) and detectable in step two (Figure 4, bottom panel). If the “pseudo IL8” result stems from IL8 binding to one or more other cytokines, this result may reflect the lower availability of binding partners for IL8 in control eyes.
DISCUSSION
Taking advantage of the screening capacity of micro well antibody protein arrays, we probed the human tear fluid for the presence and distribution of several cytokines that directly or indirectly modulate inflammatory and immune-mediated processes. Our findings on a small set of tear samples from a sub-class of aqueous-deficient dry eye patients with a defined auto-immune disease demonstrate an overall increase in inflammatory mediators in the tears of SS patients. Additional study is necessary to confidently describe and quantify the distribution of inflammatory cytokines in this disease and to determine whether similar changes are observed in the cytokine profile of tears in other dry eye conditions. Moreover, the techniques developed and presented here require further optimization in order to allow one to screen large numbers of trace proteins in a single tear sample and thereby identify new biomarkers related to specific ocular surface disease. With further work we feel this type of analysis will eventually prove useful for both diagnostic and therapeutic purposes.3, 4
Although protein array technology has been marketed for several years as a sensitive and reliable method of quantifying proteins in several different biological fluids, the application of this technology to human tears has required significant modification of manufacturer-derived protocols. Specifically, the validation of commercially available micro well plate arrays (designed for the study of serum and other biological fluids) required substantial modification to the suggested blocking protocol in order to reduce tear-induced matrix effects and increase sensitivity. In this study, tears eluted from Schirmer strips provided a significant reduction in nonspecific background luminance, suggesting that nonspecific binding of tear proteins to the micro well surface could be effectively “absorbed” by the tear strip. Tears eluted from the strips also exhibited far less nonspecific blocking activity when used to spike standard controls (not shown). Thus, a simple change in the collection technique helped to eliminate persistent tear-specific matrix effects and thereby improve overall sensitivity. As a result, however, the array profile of our samples includes components of the ocular surface and we can not state to what degree this profile is reflective of changes in the tear film or the ocular surface. Because Schirmer strips provide a practical method of sampling in individuals with severe dry eye conditions, we believe the distinction of protein origin (i.e., tear film vs. ocular surface) is less vital than developing a clinically applicable technology that can be used to probe for dozens of bioactive proteins that may contribute to ocular surface pathology. While the arrays we used were designed to probe for inflammatory cytokines, it is possible to develop and manufacture arrays targeting other proteins once biomarkers of the diesase processes are identified. These could include a range of proteins such as aquaporin, small proline rich protein and lactrin which have been tentatively identified as biomarkers of dry eye syndromes.13, 14 Following additional optimization, careful study with a larger sample size may ultimately reveal a specific “protein fingerprint” characteristic of SS that could be of significant diagnostic value. Such technology may ultimately be used as a diagnostic tool for evaluating the efficacy of therapeutic intervention.
An additional dilemma in our studies was the high constitutive levels of IL8. Tears collected from control patients with a normal ocular surface consistently revealed a signal for IL8 and the blooming effect induced by a strong IL8 signal obstructed surrounding wells in both SS and controls. This blooming effect can be avoided by shifting to a fluorescent or an infrared detection system, which has the added advantage of increasing the linear range of the assays ten fold. In theory infrared detection can be carried out using a high end digital camera making the analysis accessible to even the most modest laboratory facilities. These types of protocols are well established and are currently being considered for future studies.
Further exploration of the intense signal for IL8 suggested extraordinarily high levels of IL8 in tear fluid, far beyond that reported in many bead array assays.7,15,16 Using a two phase detection system we showed a heterogeneous signal for IL8, with a much lower level of signal detected for IL8 using a secondary antibody cocktail devoid of the probe for IL8. In additional studies, not detailed here, we have gone on to show that this moiety in tears, referred to as “pseudo-IL8”, most likely is a complex of IL8 with several other cytokines. This complexing agent could be alpha 2-macroglobulin, which is present in tears and is known to complex with a wide range of inflammatory cytokines.17 Interestingly, the “pseudo-IL8” was present in all the SS samples and was nearly absent in normal controls. This may reflect the overall greater availability of binding partners for IL8 in SS samples where cytokine expression was generally increased.
The presence of protein complexes combined with the potential impact of tear specific matrix effects reveals at least two important factors that may contribute to the wide range of discrepancies in dot sandwich ELISA results reported in the literature3, 4 These results clearly demonstrate the inherent difficulties one can face when interpreting the results of micro well arrays with human tears. Despite finding distinct differences between disease and control tears, the relative distribution of cytokines varied considerably. Further optimization of array technology is also necessary to more precisely define the concentration of individual cytokines in a single tear sample. Although cytokine standards are available for quantification with currently available commercial products, definitive validation requires independent verification using quantitative techniques such as western blotting. Taken together, our results emphasize the necessity to verify the specificity, linearity, and validity of each array since blooming effects, matrix effects, image capture methods and cross-talk between capture and probe antibodies can greatly affect the signal-to-noise ratio and limit the ability to obtain meaningful results.
In summary, protein array technology is a powerful screening tool with broader applicability than classic methods. The techniques developed and presented here require further optimization in order to allow one to screen large numbers of trace proteins in a single tear sample and thereby identify new biomarkers related to specific ocular surface diseases. In addition to studying the distribution of proteins in human tears, this technology can be applied to the analysis of other ocular fluids including aqueous and vitreous humor. Preliminary studies have identified several novel proteins in the vitreous of diseased eyes and some of these proteins may ultimately provide targets for intervention to block degenerative and/or vascular diseases of the retina. If adequately developed and optimized, one may envision the availability of protein antibody arrays for routine diagnostic applications and for monitoring treatment efficacy. 3, 4 The routine use of this technology, however, will ultimately depend on ongoing development to increase sensitivity and specificity, reduce cost and optimize for use with small sample volumes.
ACKNOWLEDGMENTS
Grant Information: Supported by the National Institutes of Health, National Eye Institute Grants R01EY 016203 (NAM) and ALTA California (NAM).
REFERENCES
- 1.Mrugacz M, Zelazowska B, Bakunowicz-Lazarczyk A, Kaczmarski M, Wysocka J. Elevated tear fluid levels of MIP-1alpha in patients with cystic fibrosis. J Interferon Cytokine Res. 2007;27:491–5. doi: 10.1089/jir.2007.0149. [DOI] [PubMed] [Google Scholar]
- 2.Yoon KC, Jeong IY, Park YG, Yang SY. Interleukin-6 and tumor necrosis factor-alpha levels in tears of patients with dry eye syndrome. Cornea. 2007;26:431–7. doi: 10.1097/ICO.0b013e31803dcda2. [DOI] [PubMed] [Google Scholar]
- 3.Sack R, Conradi L, Beaton A, Sathe S, McNamara N, Leonardi A. Antibody array characterization of inflammatory mediators in allergic and normal tears in the open and closed eye environments. Exp Eye Res. 2007;85:528–38. doi: 10.1016/j.exer.2007.07.004. [DOI] [PubMed] [Google Scholar]
- 4.Sack RA, Conradi L, Krumholz D, Beaton A, Sathe S, Morris C. Membrane array characterization of 80 chemokines, cytokines, and growth factors in open- and closed-eye tears: angiogenin and other defense system constituents. Invest Ophthalmol Vis Sci. 2005;46:1228–38. doi: 10.1167/iovs.04-0760. [DOI] [PubMed] [Google Scholar]
- 5.Shoji J, Inada N, Sawa M. Antibody array-generated cytokine profiles of tears of patients with vernal keratoconjunctivitis or giant papillary conjunctivitis. Jpn J Ophthalmol. 2006;50:195–204. doi: 10.1007/s10384-005-0319-4. [DOI] [PubMed] [Google Scholar]
- 6.Cook EB, Stahl JL, Lowe L, Chen R, Morgan E, Wilson J, Varro R, Chan A, Graziano FM, Barney NP. Simultaneous measurement of six cytokines in a single sample of human tears using microparticle-based flow cytometry: allergics vs. non-allergics. J Immunol Methods. 2001;254:109–18. doi: 10.1016/s0022-1759(01)00407-0. [DOI] [PubMed] [Google Scholar]
- 7.Uchino E, Sonoda S, Nakao K, Sakamoto T. Alteration of tear cytokine balance by eye closure: analysis by multicytokine assay. Graefes Arch Clin Exp Ophthalmol. 2006;244:747–9. doi: 10.1007/s00417-005-0127-z. [DOI] [PubMed] [Google Scholar]
- 8.Waterhouse JP. Focal Adenitis in Salivary and Lacrimal Glands. Proc R Soc Med. 1963;56:911–8. doi: 10.1177/003591576305601031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.van Bijsterveld OP, Mackor AJ. Sjogren’s syndrome and tear function parameters. Clin Exp Rheumatol. 1989;7:151–4. [PubMed] [Google Scholar]
- 10.Stern ME, Pflugfelder SC. Inflammation in dry eye. Ocul Surf. 2004;2:124–30. doi: 10.1016/s1542-0124(12)70148-9. [DOI] [PubMed] [Google Scholar]
- 11.Sack RA, Bogart BI, Beaton A, Sathe S, Lew G. Diurnal variations in tear glycoproteins: evidence for an epithelial origin for the major non-reducible > or = 450 kDa sialoglycoprotein(s) Curr Eye Res. 1997;16:577–88. doi: 10.1076/ceyr.16.6.577.5069. [DOI] [PubMed] [Google Scholar]
- 12.Saeed AI, Sharov V, White J, Li J, Liang W, Bhagabati N, Braisted J, Klapa M, Currier T, Thiagarajan M, Sturn A, Snuffin M, Rezantsev A, Popov D, Ryltsov A, Kostukovich E, Borisovsky I, Liu Z, Vinsavich A, Trush V, Quackenbush J. TM4: a free, open-source system for microarray data management and analysis. Biotechniques. 2003;34:374–8. doi: 10.2144/03342mt01. [DOI] [PubMed] [Google Scholar]
- 13.Swamynathan SK, Katz JP, Kaestner KH, Ashery-Padan R, Crawford MA, Piatigorsky J. Conditional deletion of the mouse Klf4 gene results in corneal epithelial fragility, stromal edema, and loss of conjunctival goblet cells. Mol Cell Biol. 2007;27:182–94. doi: 10.1128/MCB.00846-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li S, Nikulina K, DeVoss J, Wu AJ, Strauss EC, Anderson MS, McNamara NA. Small proline-rich protein 1B (SPRR1B) is a biomarker for squamous metaplasia in dry eye disease. Invest Ophthalmol Vis Sci. 2008;49:34–41. doi: 10.1167/iovs.07-0685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Malvitte L, Montange T, Vejux A, Baudouin C, Bron AM, Creuzot-Garcher C, Lizard G. Measurement of inflammatory cytokines by multicytokine assay in tears of patients with glaucoma topically treated with chronic drugs. Br J Ophthalmol. 2007;91:29–32. doi: 10.1136/bjo.2006.101485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Leonardi A, Fregona IA, Plebani M, Secchi AG, Calder VL. Th1- and Th2-type cytokines in chronic ocular allergy. Graefes Arch Clin Exp Ophthalmol. 2006;244:1240–5. doi: 10.1007/s00417-006-0285-7. [DOI] [PubMed] [Google Scholar]
- 17.Kendrick BS, Cleland JL, Lam X, Nguyen T, Randolph TW, Manning MC, Carpenter JF. Aggregation of recombinant human interferon gamma: kinetics and structural transitions. J Pharm Sci. 1998;87:1069–76. doi: 10.1021/js9801384. [DOI] [PubMed] [Google Scholar]