Abstract
Aim
Despite its increasing popularity, little is known about the health effects of waterpipe smoking (WPS), particularly on the cardiovascular system. To investigate the role of WPS as a risk factor for vascular disease, we evaluated its effect on endothelial cell function, which is an early event in vascular disease pathogenesis. We assessed the changes in cell viability, ROS generation, inflammatory and vasodilatory markers and in vitro angiogenesis of human aortic endothelial cells in response to waterpipe smoke condensate exposure.
Methods and results
Mainstream waterpipe smoke condensate (WSC) was generated using a standard laboratory machine protocol. Compared to control, WSC induced cell cycle arrest, apoptosis, and oxidative stress in human primary endothelial cells. In addition, we assayed for impaired endothelium-dependent vasodilation and induced inflammation by studying the effect of WPS on the content and activity of AMPK, eNOS proteins and NF-κB p65 ser536 phosphorylation, respectively. WSC inhibited AMPK/eNOS phosphorylation and induced phosphorylation of p65. Moreover, we evaluated endothelial cells repair mechanism related properties that include migration/invasion and in vitro tube formation upon treatment with WSC. WSC reduced the motility and inhibited angiogenic potential of HAEC cells.
Conclusions
WPS induced endothelial cell dysfunction as evident by exerting oxidative stress, inflammation, and impaired endothelial vasodilatory function and repair mechanisms. All together these data provide evidence for the potential contribution of WPS to endothelial dysfunction and thus to vascular disease.
Keywords: Waterpipe smoking, Endothelial dysfunction, Oxidative stress, Vascular disease
1. Introduction
Waterpipe tobacco smoking (WPS) is increasing in popularity and prevalence worldwide (Maziak, 2011; Taha et al., 2010). Little is known, however, about its health implications and there is a perception that it is less dangerous than cigarette smoking (Smith-Simone et al., 2008). This perception is being challenged by converging lines of evidence; waterpipe smoke analysis, toxicant content, and health effects research have hinted at the dangers of WPS (AKl et al., 2010; Eissenberg and Shihadeh, 2009; Khabour et al., 2012; Schubert et al., 2011).
Studies on the chemistry of waterpipe smoke demonstrate that tobacco smoke content and toxicant exposure associated with waterpipe is at least comparable to that of cigarettes (Eissenberg and Shihadeh, 2009; Schubert et al., 2011). Comparing the mainstream waterpipe smoke generated using a popular type of ma’ssel tobacco mixture with that of a single cigarette, Shihadeh showed that waterpipe smokers are exposed to substantial amounts of nicotine, CO, tar, polycyclic aromatic hydrocarbons and heavy metals such as arsenic, cobalt, chromium, and lead (Al Rashidi et al., 2008; Shihadeh and Saleh, 2005). The data suggest that, relative to a single cigarette, a single waterpipe smoking session is associated with 1.7 times the nicotine, 6.5 times the CO, and 46.4 times the tar (Djordjevic et al., 2000; Shihadeh and Saleh, 2005). These results indicate that waterpipe smoke condensate (WSC) contains alarming levels of toxicants know as causal factors in the initiation and elevated incidence of cardiovascular disease (CVD) in cigarette smokers (Ambrose and Barua, 2004). Since the adverse effects of cigarette smoke have been thoroughly documented in relation to CVD and because many toxic constituents of WSC overlap with or exceed that of cigarette smoke, therefore, more research is needed to clarify waterpipe-induced risk of tobacco-caused diseases, particularly CVD.
A few studies (AKl et al., 2010; Neergaard et al., 2007) addressing the adverse health consequences of WPS show an association with a variety of health risks similar to those associated with cigarette smoking. Of particular interest is the finding that WPS is as important risk factor as cigarette smoking for CVD with WPS associated with increased risk of atherosclerosis (Israel et al., 2003) and coronary heart disease (Jabbour et al., 2003). Recent studies by Wolfram et al. also demonstrated that WPS exerts a significant pro-oxidant atherogenic stimulus (Wolfram et al., 2003). By measuring 8-epi-PGF2a levels, a marker for in vivo oxidation injury, Wolfram et al. showed significant increase in 8-epi-PGF2a levels in waterpipe smokers that was also elevated in cigarette smokers. Furthermore, two studies assessing the acute effects of WPS on some parameters of the cardio-respiratory system detected acute biologic changes that might result in health problems (Hakim et al., 2011; Shaikh et al., 2008). Recent studies on human subjects demonstrated significant elevation of blood pressure and heart rate observed among waterpipe smokers (Al-Kubati et al., 2006; Al-Safi, 2005; Shafagoj and Mohammed, 2002).
The endothelium is the second site to encounter the products of tobacco combustion (Blann and McCollum, 1993) and its dysfunction is an early feature of atherogenesis in vitro (Celermajer et al., 1996). The endothelium is an active, dynamic tissue that controls many important functions. It plays a vital role in vascular homeostasis, vascular tone regulation, thrombosis, angiogenesis, and inflammation (Félétou, 2011). In response to various stimuli, endothelial cells produce and release a large number of vasoactive substances, growth modulators and other factors that mediate these functions. However, cardiovascular risk factors, like smoking, alter many of the normal endothelial functions which precede the development of pathological changes and subsequent clinical complications (Reriani et al., 2010).
In this study, we investigate the effect of mainstream WSC on endothelial cell function in vitro and discuss the implication of these cellular responses in the pathophysiology of vascular disease.
2. Materials and methods
2.1. Smoking machine protocol and WSC preparation
A standard smoking protocol (Beirut Method) was used as described by Shihadeh and Saleh (2005), which consists of a total of 171 puffs of 0.53 l volume, a puff duration of 2.6 s, and an inter puff interval of 17 s. The waterpipe was prepared by filling the head with 10 g of “Nakhla” brand tobacco mixture known as “Two Apples”, covering it with aluminum foil and perforating the foil to allow air passage. A charcoal, “Three Kings” brand quick-light briquette was ignited and placed on the top of the head at the beginning of the smoking session. Another half charcoal briquette was added at puff number 105. Water in the water bowl was changed at the beginning of every smoking session. The condensate was collected using Pall Type A/E glass fiber filters as described by Shihadeh and Saleh (2005). Filters were stored in airtight containers and at −20 °C. WSC preparation was performed as described by Rammah et al. Briefly, cell culture media was added to each filter to yield a concentration of 40 mg/ml, the filter was then pressed in a syringe to ensure recovery of media added. All recovered media was then mixed together and sterilized using 0.22 μm filters (Costar, USA) (Rammah et al., 2012).
2.2. Cell culture and proliferation assays
Human aortic endothelial cells (HAEC) were grown in Endothelial Cell Basal Medium (EBM) supplemented with FBS, BBE, hEGF, and GA-1000 (EGM BulletKit, Lonza, Belgium, CC-3121 and CC-4133). Cells were seeded at a density of 104 cells/cm2. Treatment started 24 h post-seeding. For the proliferation assay, the cells were treated with a final concentration of 4 mg/ml. This treatment was either repeated daily for 3 consecutive days (Repeated Exposure, RE), or was performed only once and was replaced with fresh complete media 24 h later (Single Exposure, SE). Cell viability was assessed every 24 h using cell counting of live and dead cells with trypan blue staining.
2.3. Cytotoxicity assay by real-time cell impedance analysis
Real-Time Cell Analyzer (RTCA) xCELLigence System (Roche Applied Science, Mannheim, Germany) was used to dynamically monitor cell proliferation rates upon different doses of WSC. The RTCA system monitors cellular events at set intervals via measuring electrical impedance across microelectrodes on the bottom of tissue culture E-plates®. The impedance measurement provides information about cell number, viability, morphology and adhesion. The impedance was expressed as cell index (CI), an arbitrary unit. The RTCA Software supplied by the manufacturer analyzes these measurements and calculates the doubling time of the cells based on cell index. The experiments were performed as described by Rammah et al. The results are presented as plotted by the RTCA software. Standard deviations of duplicates of wells with different treatments were calculated with the RTCA software.
2.4. Cell cycle analysis
Analysis of the cell cycle was carried out as described previously by Rammah et al. (2012). Briefly cells were plated at a density of 104 cells/cm2 and either treated once or repeatedly for 3 consecutive days. DNA content was conducted using propidium iodide stain and analyzed by flow cytometry (Beckton Dickensson, USA, FacSort). Data acquisition was performed using Cell Quest. Data analysis and percentage of cells in phases of the cell cycle were determined using Flow Jo, applying the Watson Pragmatic Model for fit analysis.
2.5. Annexin V/PI staining by flow cytometric analysis
Detection of early apoptotic cells was performed with the Annexin V-FITC/PI staining as described by Rammah et al. (2012). Briefly, cells were plated at a density of 104 cells/cm2 and were either treated once or repeatedly for 3 consecutive days. Treated and untreated cells were then assayed for Annexin V-FITC and PI staining. Data analysis was performed using Flow Jo software. Samples stained with Annexin V-FITC and PI, are represented by dot plots of PI versus Annexin V intensity.
2.6. Western blot analysis
Expression of proteins regulating some of the endothelial cell functions was analyzed by western blot as previously described by our group (Rammah et al., 2012). Primary antibodies against actin (Sigma, Germany), phospho-eNOS (Ser 1177) (cell signaling, USA), eNOS (cell signaling, USA), phospho-AMPK (Thr 172) (cell signaling, USA), AMPKa (cell signaling, USA) and Phospho-NF-kB p65 (Ser 536) antibody (cell signaling, USA) were used with HRP conjugated secondary antibodies. Films, exposed in the linear range, were then analyzed using Image J®. Protein bands were normalized using actin as reference.
2.7. Measurement of intracellular ROS
Intracellular ROS generation was assessed by using the probe 5-(and-6)-chloromethyl-2′,7′-dichlorodihydrofluorescein diacetate, acetyl ester (CM-H2DCFDA; Molecular Probes, USA) according to the manufacturer’s protocol.
Cells adhering to confocal dishes (Matek®, USA) at 80% confluence were washed with PBS and then incubated with 5 μM H2DCFDA at 37 °C for 15 min. Cell were then washed with PBS and incubated with phenol-free media. Immediately, cells were live imaged using a laser scanning confocal microscope (LSM710 Confocal Microscope Carl Zeiss, Germany) equipped for live-cell imaging. Fluorescence intensity was assessed, after 10 min WSC was added to the cells, in clusters of cells identified as regions of interest, and background as an area without cells or with minimal cellular fluorescence. Intensity values are reported before and after adding the WSC along with the background intensity.
2.8. RTCA migration and invasion assays
Invasion or migration assays were measured using xCELLigence RTCA instrument according to the manufacturer’s protocol. Cells were seeded on a cellular invasion/migration plate (CIM-plate 16®). As cells migrate or invade (when coated with Matrigel) from the upper chamber into the bottom chamber, they interact and adhere to the electronic sensors, thus causing an increase in electrical impedance. Changes in the impedance correlate with number of migrated or invaded cells, therefore allowing automatic and continuous measurement of migration. Studies have shown that data generated using the RTCA system is in agreement with data collected using conventional techniques (Jurmeister et al., 2012).
For invasion assays, the upper surface of the membrane was pre-coated with 30 μl of growth factor-reduced Matrigel (Becton Dickinson, San Jose, CA) diluted in serum-free medium at a ratio of 1:20, incubated at 37 °C/5% CO2 for 4 h, then washed with PBS. For all migration and invasion assays, 160 μl of EBM Full growth medium was added to the lower chamber of each well (used as a chemo-attractant) and 30 μl to the upper chamber and the plate pre-incubated for 1 h at 37 °C/5% CO2. HAEC cells were seeded at a density of 20,000 cells/well into the upper chambers with an additional 120 μl in serum-free medium. Migration and invasion was monitored by recording cell impedance every 15 min for a minimum of 18 h.
2.9. Matrigel-induced capillary tube formation
The Matrigel®-based assay has been widely used as an in vitro measure of angiogenic potential and was performed as described by El-Sabban et al. (2002). Briefly, a 24-well plate was coated with 200 μl/well growth factor-reduced Matrigel (Becton Dickinson, San Jose, CA). HAEC cells (4 × 104 cells/well) were then seeded into each well. After 30 min of incubation, the cells were treated with WSC. Induction of tube formation was assessed after incubation with VEGF (20 ng/ml; R&D system, Minneapolis, MN, USA) at 37 °C for 48 h. The capillary-like network formation was observed using an inverted phase contrast microscope (Carl Zeiss, Germany) and images were captured at 24 h at 40× magnification with a digital output camera (Axiocam HRc Zeiss, Germany) attached to the microscope.
3. Results
3.1. WSC induced endothelial cells growth inhibition
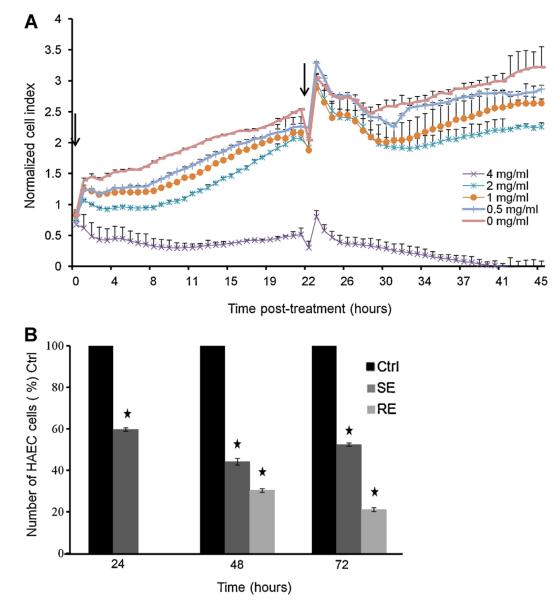
Using a Real-Time Cell Analyzer (RTCA) based assay, we tested the cytotoxicity of WSC on HAEC cells. This technique allows us to dynamically monitor changes in the properties of HAEC cells after addition of different concentrations of WSC. Concentrations of 6–8 mg/ml were acutely cytotoxic with cell death occurred within 1 h of exposure with no recovery as the normalized cell index was below zero (data not shown). Concentration of 4 mg/ml was cytostatic, with rapid inhibition of cell growth 24 h post-exposure, however, the cells sustained their viability as indicated by the constancy of cell index (4 mg/ml increase the doubling time by 2-fold) (Fig. 1A). Based on these results, a 4 mg/ml dose (cytostatic dose) was used for further studies of the effect of WSC on HAECs. WSC at concentrations ranging from 0.5 to 2 mg/ml resulted in an increase of doubling time (0.5, 1 and 2 mg/ml increased the doubling time by 10% ± 0.5, 26% ± 0.2 and 67% ± 0.2, respectively).
Fig. 1.
WSC induced growth inhibition. (A) Effect of different concentrations of WSC on HAEC cells viability 48 h post-treatment using xCELLigence system. Arrowheads represent the time when 4 mg/ml of WSC were added to HAEC cells. (B) Effect of WSC (4 mg/ml) on HAEC cells proliferation (single and repeated exposure) 24, 48 and 72 h post-treatment using trypan blue exclusion assay. The graphs represent average of duplicates of a single representative experiment ± S.D. of three independent experiments.
We then investigated the effect of 4 mg/ml of WSC on HAEC cell proliferation for 24, 48 and 72 h as described in a previous section. At each time point, cells were compared to the control set at 100%. WSC at the dose of 4 mg/ml interfered with the growth of HAEC cells. When treated repeatedly (RE), the increase in cell number was significantly lower than the control. WSC induced a decrease in cell proliferation by 40% ± 0.75, 70% ± 0.5 and 80% ± 1 after 24, 48 and 72 h of treatment, respectively. The decrease in cells number was not due to an increase in cell death, as evident by the ability of the cells to exclude the cell-membrane impermeable trypan blue dye (data not shown). Moreover, when treated only once (SE), the cells regained their proliferative capacity after treatment cessation (Fig. 1B).
3.2. WSC induced endothelial cells apoptosis and cell cycle arrest
Whether WSC-induced inhibition of cell proliferation was due to an actual inhibition of cell cycle or to an increase of cellular apoptosis was assessed. Apoptosis was analyzed by Annexin V-FITC binding after 24, 48 and 72 h of exposure to WSC. An increase in fluorescent Annexin V binding to cells indicates early apoptosis induction. Following WSC-treatment, apoptosis was undetectable at 24 h, while early apoptotic populations were observed at 48 and 72 h of treatment (lower right quadrant, where 22% and 16.5% of HAEC were Annexin V positive cells after 48 and 72 h of treatment, respectively). Relative to the basal level of apoptosis (the percentage of the untreated cells that are positive for apoptosis), HAEC cells, upon single exposure or repeated exposure, have a high percentage of apoptotic cells (Fig. 2A).
Fig. 2.
WSC induced cell cycle arrest and apoptosis of HAEC cells. (A) Representative diagrams of FITC-Annexin V/PI staining by flow cytometry of HAEC cells 24, 48, and 72 h post-treatment with WSC. (B) Effect of WSC on cell cycle distribution of HAEC cells. (Upper panel) The percentage of cells in G0/G1 and S phase of control cells and cells treated once (single exposure) for 24, 48 and 72 h (lower panel) the percentage of cells in G0/G1 and S phase of control cells and cells treated repeatedly (repeated exposure) for 24, 48 and 72 h as a percentage of the control. The results are shown as percentage of the negative control value and represent means ± S.E.M. of three independent experiments. *Represents statistical significance (p < 0.05) using Student’s t-test.
Furthermore, we studied the effect of WSC on HAEC cell cycle distribution. WSC resulted in the accumulation of the cells in G0/G1 and a decrease in the percentage of cells in the S phase, suggesting therefore a cell cycle arrest at G0/G1 (Fig. 2B). This effect was sustained as long as the treatment continued. WSC repeated treatment resulted in an increase of the S phase cell population by 20%, 50% and 30% upon 24, 48 and 72 h of treatment, respectively (Fig. 2B, upper panel). This effect was abolished upon removal of the treatment and replacement with fresh complete media as evident by the increase in the percentage of cells in the S phase (Fig. 2B, lower panel).
3.3. WSC increased reactive oxygen species (ROS) production in HAEC cells
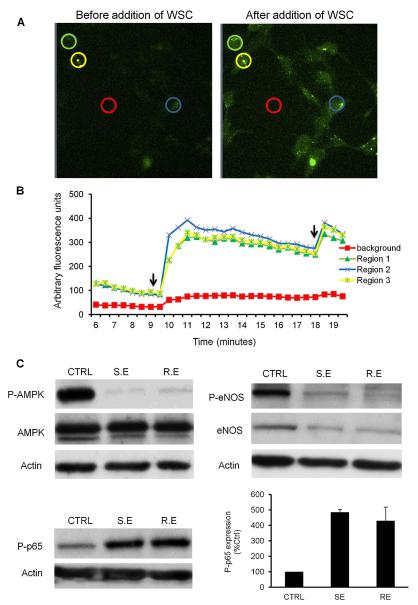
Generation of high levels of intracellular ROS plays an important role in stress-induced signaling and has been shown to stimulate related processes like apoptosis, cell cycle arrest and inflammation (Kregel and Zhang, 2007). We considered a potential role for ROS in stimulating cellular apoptosis and inducing endothelial cell injury. To test for and quantify the WSC-induced intracellular oxidative stress, we determined the potential of WSC to oxidize the oxidation-sensitive plasma membrane-permeable dye carboxy-H2DCF-DA (Fig. 3). WSC induced an increase in the fluorescent intensity by 4-fold relative to the basal levels of fluorescence (Fig. 3B). Thus, ROS production was significantly increased in WSC-treated cells compared to the basal level of ROS produced. Furthermore, ROS production was significantly increased when the cells were treated with additional dose of WSC suggesting that WSC ROS generation is re-inducible in HAEC. These data showed that WSC has a considerably high potential to induce intracellular oxidative stress in endothelial cells.
Fig. 3.
WSC induced oxidative stress, impaired AMPK-induced eNOS activation and impaired AMPK-suppressed inflammation in HAEC cells. WSC increased ROS production in HAEC. (A) ROS production in HAEC cells untreated and treated with 4 mg/ml of WSC was assessed using florescent dye carboxy-H2DCF-DA as described in Section 2. (B) The intracellular oxidative stress was analyzed where the fluorescence intensity plotted on the y-axis correlates with the magnitude of intracellular oxidative stress of the selected regions. 0% WSC represents the base line. The arrows represent the time when 4 mg/ml of WSC were added to HAEC cells and oxidative stress was measured over a period of 10 min. Colored circles represent areas chosen for fluorescent analysis. The results shown are representative of three independent experiments. (C) WSC down-regulated phospho-AMPK, eNOS and phospho-eNOS protein expression thus impairing vasodilatory function and mediated p65 phosphorylation as an early step of inflammation in HAEC cells. AMPK/phospho-AMPK, e-NOS/phospho-eNOS and phospho-p65 protein expression in HAEC cells untreated and treated (SE and RE) with 4 mg/ml of WSC for 48 h using western blot. Equal loading was determined through re-probing with β-actin. Western blotting data are representative of three independent experiments (CTRL: control, SE: single exposure, RE: repeated exposure).
3.4. WSC impaired AMPK-induced eNOS activation and impaired AMPK-suppressed inflammation in HAEC cells
Oxidative stress results in endothelial cell dysfunction through modulating adenosine monophosphate-activated protein kinase (AMPK) or AMPK-dependent pathways (Xu and Zou, 2009). AMPK is known to be a sensor of cellular energy and cellular redox status. In addition, it is essential for maintaining endothelial homeostasis by increasing NO bioactivity and mitochondrial biogenesis and by suppressing inflammation and the production of ROS in endothelial cells (Colombo and Moncada, 2009; Wang et al., 2009). Due to the various stated functions of AMPK in endothelial cell function, the effect of WSC on AMPK protein expression level and AMPK phosphorylation was assessed in HAECs.
Our data showed that after 48 h of WSC treatment, AMPK protein levels were not affected. However, AMPK phosphorylation was significantly decreased in WSC-treated cells compared to the control (Fig. 3C). These data revealed that WSC induced AMPK Thr 172 dephosphorylation, thereby inactivating the AMPK enzyme.
Moreover, there is abundant data showing the critical role of AMPK in endothelium-dependent vasodilatory function through modulating endothelial cell nitric oxide synthase (eNOS) activation and thus nitric oxide (NO) bioactivity (Schulz et al., 2005). AMPK and eNOS are essential mediators of NO-induced endothelial function and thus the effect of WSC on eNOS protein levels in HAEC cells was determined. Incubation of HAEC with WSC for 48 h caused a decrease in eNOS protein content and phosphorylation of eNOS was completely abolished upon WSC treatment. Moreover, as with eNOS phosphorylation, the decrease in eNOS protein content was not reversed after culturing HAEC in control media for 24 h (Fig. 3C). These results suggest that WSC-impaired eNOS activation and decreased expression might contribute to a decline in NO availability. NO is primarily responsible for the vasodilatory function of the endothelium. Hence, a specific defect in the endothelium-derived NO system might be responsible for the impairment of endothelial vasodilatory function (Cardillo and Panza, 1998).
Another feature of endothelial dysfunction is inflammation (Libby et al., 2002). NF-κB is a major player in the development of endothelial inflammation (Csiszar et al., 2008). Cytokine-mediated NF-κB activation in endothelial cells is inhibited by AMPK activation. Therefore, AMPK appears to be a natural suppressor of NF-κB and inflammation in endothelial cells (Xu and Zou, 2009). However, it was recently suggested that NF-κB activation by phosphorylation of p65 is a critical step in the development of endothelial inflammation (Schubert et al., 2002). This correlates with its binding to the DNA thus augmenting the expression of inflammatory genes (Viatour et al., 2005). To determine the NF-κB phosphorylation status, western blots were performed using a specific antibody that recognizes Ser 536 phosphorylated-p65. We demonstrated that HAEC cells treated with WSC for 48 h increased phosphorylated p65 levels relative to untreated cells (Fig. 3C). These findings indicated that WSC mediates p65 Ser 536 phosphorylation as an early step of inflammation.
3.5. WSC impaired cell migration/invasion and angiogenesis of HAEC cells
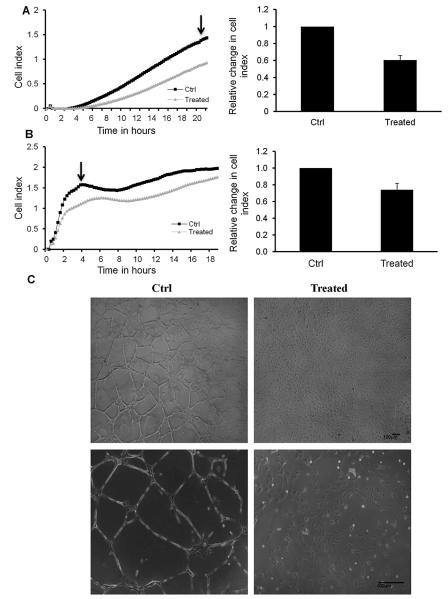
NO is a vaso-regulatory molecule and an essential mediator of endothelial cell migration and VEGF-induced angiogenesis (Papapetropoulos et al., 1997). Angiogenesis is considered as a repair mechanism that involves endothelial cell migration, proliferation, and differentiation, as well as tube formation (Lamalice et al., 2007). In this study, the effect of WSC on the ability of endothelial cells to replicate and replace the lost and damaged cells, thus maintaining vascular integrity was evaluated. Different aspects of angiogenesis were assessed independently. The proliferation assay assesses cell cycle changes and DNA synthesis, which was mentioned early in this study. The cell migration assay assesses endothelial mobility and related to cytoskeletal reorganization (Fig. 4A). The migratory and invasive function of endothelial cells in response to WSC was evaluated using RTCA. As shown in Fig. 4A and B treatment with WSC resulted in a significant decrease in migration and invasion potential of HAEC cells, which is reflected by a significantly lower cell index relative to the control.
Fig. 4.
WSC impaired migration/invasion and VEGF-induced capillary tube formation of endothelial cells. (A) The effect of WSC (4 mg/ml) on HAEC cell migration. (B) The effect of WSC (4 mg/ml) on HAEC cell invasion using xCELLigence system. Arrows indicate the time point at which changes in cell index were calculated to generate the bar graphs. The bar graphs represent changes in the cell index upon exposure relative to the control. Results are percentage of negative control and represent means of 3 independent experiments ± S.D. (C) Representative photographs of tube formation of untreated (right panel) and treated (left panel) HAEC cells with 4 mg/ml of WSC. The lower panels are light microscopy images of higher magnification of the same field. Photographs are representative of three independent experiments.
The tube formation assay measures endothelial differentiation associated with cytoskeletal reorganization and intracellular organelle trafficking (Fig. 4C). No tube formation was observed in WSC-treated cells compared to control cells. These results indicate that WSC altered the ability of endothelial cells to proliferate, migrate and differentiate and thus impaired repair mechanism.
4. Discussion
In this study we demonstrated that the highest non-cytotoxic concentration of WSC interfered with endothelial cell proliferation. This effect on endothelial cells was due to both inhibition of cell cycle progression and cellular apoptosis, as evidenced by increased PS externalization and decreased percentage of S phase cells in WSC-treated cells. Also our results showed that WSC resulted in increased production of ROS. Increased ROS impairs the cellular redox balance, induces apoptosis and triggers inflammation and injury. Moreover, WSC interfered with the capacity of endothelial cells for repair manifested by decreased motility and impaired tube formation. These processes are implicated in the disturbance of the endothelial integrity (Wong and Gotlieb, 1986) and interfere with the balance of injury and repair mechanisms (Dimmeler and Zeiher, 2004). It has been shown that loss of endothelial integrity is one of the important early events for the genesis of vascular disease (Dimmeler and Zeiher, 2004). Such results indicate that WPS disturb the endothelial structural integrity in vitro thus supporting the associations of elevated incidence of vascular diseases in waterpipe smokers.
In addition to endothelium structural integrity, functional activity is required to maintain barrier function and plays a crucial role in the prevention of CVD (Galley and Webster, 2004). In this study, we also showed that WSC contributes to the dysfunction of endothelial cells that includes modulating the vasomotor tone and altering the anti-inflammatory properties of the endothelium. Endothelium-dependent vasodilation is, to a great extent, maintained by NO, the endothelium-derived relaxing factor (EDRF). NO is produced in endothelial cells by eNOS enzyme. A substantial body of evidence suggests that derangements in the eNOS/NO pathway represent the most important cause for reduced NO bioavailability (Kawashima and Yokoyama, 2004). Therefore, reduced eNOS-derived NO bioactivity impair endothelial function and is a critical step for genesis of vascular disease. WSC down-regulated eNOS expression and impaired AMPK-mediated eNOS phosphorylation by inhibiting the phosphorylation of AMPK. Thereby WSC is implicated in the endothelial cell dysfunction represented by impaired endothelium-dependent vasodilation.
Another mechanism that interferes with NO availability is its degradation by superoxides and other ROS. This is supported by studies demonstrating that oxidant stress alters many functions of the endothelium, including modulation of vasomotor tone. Elevated levels of ROS deplete bioavailable NO and exacerbate local oxidant stress by reacting directly with NO to form peroxynitrite, which, in turn, imparts further oxidative injury to the endothelium (Cai and Harrison, 2000). In our study, WSC elevates the level of ROS produced by endothelial cells. Our results indicated that WSC alters endothelium-dependent vascular relaxation through reduced eNOS-derived NO bioactivity and ROS enhanced degradation of NO. This, thereby, represents further evidence for the involvement of WPS in endothelial cell dysfunction.
Previous studies indicated that vascular inflammation is a feature of endothelial dysfunction and documented the contribution of inflammation to atherogenesis. Emerging results describe AMPK signaling as natural suppressor of endothelium inflammation and NF-κB activation as major player in the development of vascular inflammation (Csiszar et al., 2008; Libby et al., 2002; Schubert et al., 2002; Xu and Zou, 2009). In addition, endothelium-derived NO has been recognized to be an anti-inflammatory molecule by inhibiting NF-κB activation. We showed that WSC induces an inflammatory response through activation of NF-κB mediated pro-inflammatory signaling associated with inhibition of AMPK and NO synthesis. These findings provide a potential mechanism for the association of waterpipe smoking with atherosclerotic disease.
Angiogenesis is critical in many physiological processes as well as in restoring endothelial function in some pathological conditions. Endothelial angiogenesis or re-endothelization plays an essential role in the repair process of lung tissue and is implicated in a number of processes in COPD (Siafakas et al., 2007). Accumulating evidence implicate NO in physiological and pathological angiogenesis (Matsunaga et al., 2002). Such data indicate that NO significantly contributes to the pro-survival/pro-angiogenic program of capillary endothelium by triggering cell growth and differentiation via eNOS activation, cyclic GMP (cGMP) elevation, mitogen activated kinase (MAPK) activation and fibroblast growth factor-2 (FGF-2) expression (Ziche and Morbidelli, 2000). We have observed that WSC interfered with endothelial cell survival, proliferation, motility and tube formation accompanied with decreased eNOS activity. These observations indicate that WPS hinders endothelial cell properties related to the repair mechanism. In addition, we have described the effect of WSC on alveolar type II derived cells and demonstrated that WPS as a risk factor in the pathogenesis of COPD (Rammah et al., 2012). Along with our present findings we infer that waterpipe smoking not only causes direct injury to lung cells but also inhibits compensatory angiogenesis therefore impairing repair mechanism.
In conclusion, concurrent with other studies (Al-Safi, 2005; Ghasemi et al., 2010; Hakim et al., 2011; Jabbour et al., 2003; Wolfram et al., 2003), our data suggests a strong association between WPS and endothelial cell dysfunction in vitro (Fig. 5). It is presumed that WPS components enter the bloodstream and interact with endothelial cells. Upon exposure to WSC, the endothelial cells initiate a cascade of signals that lead to the disruption of the structural and functional integrity of the endothelium along with its repair mechanism; thereby proposing a potential mechanism for involvement of WPS in the genesis of vascular disease.
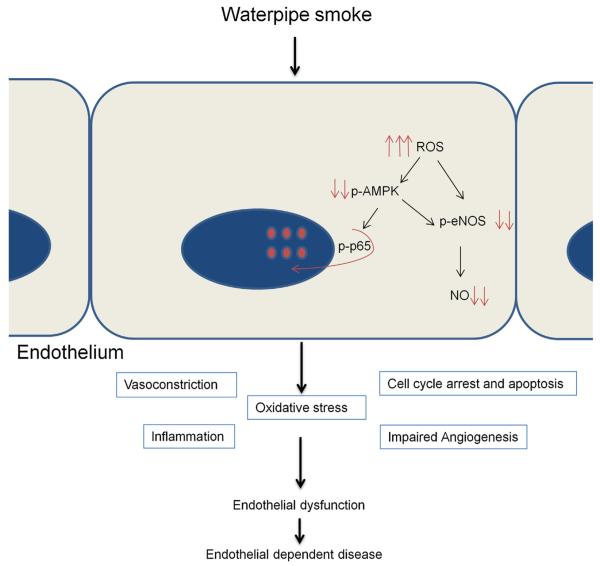
Fig. 5.
Proposed model of WPS as a contributing factor for endothelial cell dysfunction and as a risk factor for endothelial-dependent disease. After exposure to WSC, endothelial cells increase their ROS production, which in turn induces cell cycle arrest, cellular apoptosis and inflammation. These processes are responsible for the disturbance of the endothelial integrity and interfere with the balance of injury and repair mechanisms. WSC down-regulates eNOS expression, impairs AMPK-mediated eNOS phosphorylation, and impairs AMPK-suppressed inflammation. Thus, WPS is implicated in the endothelial dysfunction represented by impaired endothelium-dependent vasodilation and altered anti-inflammatory properties of the endothelium. Moreover, WSC interferes with the repair mechanism of HAEC cells through inhibited angiogenesis. WPS initiates a cascade of signals that lead to the disruption of the structural and functional integrity of the endothelium facilitating the progression of endothelial-dependent disease.
WSC contains, in addition to tobacco, additives that include sweeteners (ma’ssel), artificial flavors and is consumed with charcoal continuously burning on top of the aluminum foil covering the tobacco mix. So a direct comparison with cigarette smoke is not very easy to achieve. Through comparing the effects of waterpipe and cigarette smoke extract on endothelial cell function, our findings showed similar effect of WSC as that induced by CSE on endothelial cell activity and function in vitro (Bernhard et al., 2003; Michaud et al., 2006; Noronha-Dutra et al., 1993; Su et al., 1998). However, this type of comparison cannot be extrapolated to actual effects in cigarette and waterpipe smokers due to various limitations.
This present study is limited by its focus on the effect of WPS on human endothelial cells function in vitro. Previous studies investigating the health consequences of WPS on human subjects support the notion that WPS is associated with increased risk of vascular disease, but none of these studies identify potential cellular mechanism for such association and could only detect changes in some biological parameters of waterpipe smokers. In view of this limitation, our study provided the first in vitro demonstration of the effect of WPS on endothelial cell function. Additional in vivo studies and clinical observations are necessary to determine whether WPS alters the function of endothelial cells in animal models or humans thus describing WPS as a risk factor for vascular disease.
HIGHLIGHTS.
▶ The role of WPS as a risk factor for vascular disease is poorly studied.
▶ WSC induced cell cycle arrest, apoptosis, oxidative stress and inflammation in HAEC cells.
▶ WSC reduced the motility and inhibited angiogenic potential of HAEC cells.
▶ WPS impaired endothelial vasodilatory function and repair mechanisms of HAEC cells.
▶ WPS induced endothelial cell dysfunction.
Acknowledgments
The authors would like to thank Najeeb Halabi for critically reviewing this manuscript.
Funding This work was supported by the National Institutes of Health [grant number R01CA120142 and R01DA025659].
Footnotes
Conflict of interest statement None declared.
References
- Akl EA, Gaddam S, Gunukula SK, Honeine R, Jaoude PA, Irani J. The effects of waterpipe tobacco smoking on health outcomes: a systematic review. International Journal of Epidemiology. 2010;39:834–857. doi: 10.1093/ije/dyq002. [DOI] [PubMed] [Google Scholar]
- Al Rashidi M, Shihadeh A, Saliba NA. Volatile aldehydes in the mainstream smoke of the narghile waterpipe. Food and Chemical Toxicology. 2008;46:3546–3549. doi: 10.1016/j.fct.2008.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Kubati M, Al-Kubati AS, Al’ Absi M, Fiser B. The short-term effect of water-pipe smoking on the baroreflex control of heart rate in normotensives. Autonomic Neuroscience. 2006;126–127:146–149. doi: 10.1016/j.autneu.2006.03.007. [DOI] [PubMed] [Google Scholar]
- Al-Safi SA. Does smoking affect blood pressure and heart rate? European Journal of Cardiovascular Nursing. 2005;4:286–289. doi: 10.1016/j.ejcnurse.2005.03.004. [DOI] [PubMed] [Google Scholar]
- Ambrose JA, Barua RS. The pathophysiology of cigarette smoking and cardiovascular disease: an update. Journal of the American College of Cardiology. 2004;43:1731–1737. doi: 10.1016/j.jacc.2003.12.047. [DOI] [PubMed] [Google Scholar]
- Bernhard D, Pfister G, Huck CW, Kind M, Salvenmoser W, Bonn GK, Wick G. Disruption of vascular endothelial homeostasis by tobacco smoke: impact on atherosclerosis. FASEB Journal. 2003;17:2302–2304. doi: 10.1096/fj.03-0312fje. [DOI] [PubMed] [Google Scholar]
- Blann AD, McCollum CN. Adverse influence of cigarette smoking on the endothelium. Thrombosis and Haemostasis. 1993;70:707–711. [PubMed] [Google Scholar]
- Cai H, Harrison DG. Endothelial dysfunction in cardiovascular diseases: the role of oxidant stress. Circulation Research. 2000;87:840–844. doi: 10.1161/01.res.87.10.840. [DOI] [PubMed] [Google Scholar]
- Cardillo C, Panza JA. Impaired endothelial regulation of vascular tone in patients with systemic arterial hypertension. Vascular Medicine. 1998;3:138–144. doi: 10.1177/1358836X9800300208. [DOI] [PubMed] [Google Scholar]
- Celermajer DS, Adams MR, Clarkson P, Robinson J, McCredie R, Donald A, Deanfield JE. Passive smoking and impaired endothelium-dependent arterial dilatation in healthy young adults. New England Journal of Medicine. 1996;334:150–154. doi: 10.1056/NEJM199601183340303. [DOI] [PubMed] [Google Scholar]
- Colombo SL, Moncada S. AMPKα1 regulates the antioxidant status of vascular endothelial cells. Biochemical Journal. 2009;421:163–169. doi: 10.1042/BJ20090613. [DOI] [PubMed] [Google Scholar]
- Csiszar A, Wang M, Lakatta EG, Ungvari Z. Inflammation and endothelial dysfunction during aging: role of NF-κB. Journal of Applied Physiology. 2008;105:1333–1341. doi: 10.1152/japplphysiol.90470.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimmeler S, Zeiher AM. Vascular repair by circulating endothelial progenitor cells: the missing link in atherosclerosis? Journal of Molecular Medicine. 2004;82:671–677. doi: 10.1007/s00109-004-0580-x. [DOI] [PubMed] [Google Scholar]
- Djordjevic MV, Stellman SD, Zang E. Doses of nicotine and lung carcinogens delivered to cigarette smokers. Journal of the National Cancer Institute. 2000;92:106–111. doi: 10.1093/jnci/92.2.106. [DOI] [PubMed] [Google Scholar]
- Eissenberg T, Shihadeh A. Waterpipe tobacco and cigarette smoking direct comparison of toxicant exposure. American Journal of Preventive Medicine. 2009;37:518–523. doi: 10.1016/j.amepre.2009.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Sabban ME, Merhi RA, Haidar HA, Arnulf B, Khoury H, Basbous J, Nijmeh J, De Thé H, Hermine O, Bazarbachi A. Human T-cell lymphotropic virus type 1-transformed cells induce angiogenesis and establish functional gap junctions with endothelial cells. Blood. 2002;99:3383–3389. doi: 10.1182/blood.v99.9.3383. [DOI] [PubMed] [Google Scholar]
- Félétou M. The endothelium. Part I: Multiple functions of the endothelial cells – focus on endothelium-derived vasoactive mediators. Colloquium Series on Integrated Systems Physiology: From Molecule to Function. 2011;3:1–306. [Google Scholar]
- Galley HF, Webster NR. Physiology of the endothelium. British Journal of Anaesthesia. 2004;93:105–113. doi: 10.1093/bja/aeh163. [DOI] [PubMed] [Google Scholar]
- Ghasemi A, Syedmoradi L, Momenan AA, Zahediasl S, Azizi F. The influence of cigarette and qalyan (hookah) smoking on serum nitric oxide metabolite concentration. Scandinavian Journal of Clinical & Laboratory Investigation. 2010;70:116–121. doi: 10.3109/00365511003611282. [DOI] [PubMed] [Google Scholar]
- Hakim F, Hellou E, Goldbart A, Katz R, Bentur Y, Bentur L. The acute effects of water-pipe smoking on the cardiorespiratory system. Chest. 2011;139:775–781. doi: 10.1378/chest.10-1833. [DOI] [PubMed] [Google Scholar]
- Israel E, El-Setouhy M, Gadalla S, Aoun ESA, Mikhail N, Mohamed MK. Water pipe (Sisha) smoking in cafes in Egypt. Journal of the Egyptian Society of Parasitology. 2003;33:1073–1085. [PubMed] [Google Scholar]
- Jabbour S, El-Roueiheb Z, Sibai AM. Nargileh (water-pipe) smoking and incident coronary heart disease: a case-control study. Annals of Epidemiology. 2003;13:570. [Google Scholar]
- Jurmeister S, Baumann M, Balwierz A, Keklikoglou I, Ward A, Uhlmann S, Zhang JD, Wiemann S, Sahin Ö. MicroRNA-200c represses migration and invasion of breast cancer cells by targeting actin-regulatory proteins FHOD1 and PPM1F. Molecular and Cellular Biology. 2012;32:633–651. doi: 10.1128/MCB.06212-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawashima S, Yokoyama M. Dysfunction of endothelial nitric oxide synthase and atherosclerosis. Arteriosclerosis, Thrombosis, and Vascular Biology. 2004;24:998–1005. doi: 10.1161/01.ATV.0000125114.88079.96. [DOI] [PubMed] [Google Scholar]
- Khabour OF, Alzoubi KH, Bani-Ahmad M, Dodin A, Eissenberg T, Shihadeh A. Acute exposure to waterpipe tobacco smoke induces changes in the oxidative and inflammatory markers in mouse lung. Inhalation Toxicology. 2012;24:667–675. doi: 10.3109/08958378.2012.710918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kregel KC, Zhang HJ. An integrated view of oxidative stress in aging: basic mechanisms, functional effects, and pathological considerations. American Journal of Physiology: Regulatory Integrative and Comparative Physiology. 2007;292:R18–R36. doi: 10.1152/ajpregu.00327.2006. [DOI] [PubMed] [Google Scholar]
- Lamalice L, Boeuf FL, Huot J. Endothelial cell migration during angiogenesis. Circulation Research. 2007;100:782–794. doi: 10.1161/01.RES.0000259593.07661.1e. [DOI] [PubMed] [Google Scholar]
- Libby P, Ridker PM, Maseri A. Inflammation and atherosclerosis. Circulation. 2002;105:1135–1143. doi: 10.1161/hc0902.104353. [DOI] [PubMed] [Google Scholar]
- Matsunaga T, Weihrauch DW, Moniz MC, Tessmer J, Warltier DC, Chilian WM. Angiostatin inhibits coronary angiogenesis during impaired production of nitric oxide. Circulation. 2002;105:2185–2191. doi: 10.1161/01.cir.0000015856.84385.e9. [DOI] [PubMed] [Google Scholar]
- Maziak W. The global epidemic of waterpipe smoking. Addictive Behaviors. 2011;36:1–5. doi: 10.1016/j.addbeh.2010.08.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michaud SE, Dussault S, Groleau J, Haddad P, Rivard A. Cigarette smoke exposure impairs VEGF-induced endothelial cell migration: role of NO and reactive oxygen species. Journal of Molecular Cell Cardiology. 2006;41:275–284. doi: 10.1016/j.yjmcc.2006.05.004. [DOI] [PubMed] [Google Scholar]
- Neergaard J, Singh P, Job J, Montgomery S. Waterpipe smoking and nicotine exposure: a review of the current evidence. Nicotine and Tobacco Research. 2007;9:987–994. doi: 10.1080/14622200701591591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noronha-Dutra AA, Epperlein MM, Woolf N. Effect of cigarette smoking on cultured human endothelial cells. Cardiovascular Research. 1993;27:774–778. doi: 10.1093/cvr/27.5.774. [DOI] [PubMed] [Google Scholar]
- Papapetropoulos A, García-Cardeña G, Madri JA, Sessa WC. Nitric oxide production contributes to the angiogenic properties of vascular endothelial growth factor in human endothelial cells. Journal of Clinical Investigation. 1997;100:3131–3139. doi: 10.1172/JCI119868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rammah M, Dandachi F, Salman R, Shihadeh A, El-Sabban M. In vitro cytotoxicity and mutagenicity of mainstream waterpipe smoke and its functional consequences on alveolar type II derived cells. Toxicology Letters. 2012;211:220–231. doi: 10.1016/j.toxlet.2012.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reriani MK, Lerman LO, Lerman A. Endothelial function as a functional expression of cardiovascular risk factors. Biomarkers in Medicine. 2010;4:351–360. doi: 10.2217/bmm.10.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schubert J, Hahn J, Dettbarn G, Seidel A, Luch A, Schulz TG. Mainstream smoke of the waterpipe: does this environmental matrix reveal as significant source of toxic compounds? Toxicology Letters. 2011;205:279–284. doi: 10.1016/j.toxlet.2011.06.017. [DOI] [PubMed] [Google Scholar]
- Schubert SY, Neeman I, Resnick N. A novel mechanism for the inhibition of NF-κB activation in vascular endothelial cells by natural antioxidants. FASEB Journal. 2002;16:1931–1933. doi: 10.1096/fj.02-0147fje. [DOI] [PubMed] [Google Scholar]
- Schulz E, Anter E, Zou M-H, Keaney JF. Estradiol-mediated endothelial nitric oxide synthase association with heat shock protein 90 requires adenosine monophosphate-dependent protein kinase. Circulation. 2005;111:3473–3480. doi: 10.1161/CIRCULATIONAHA.105.546812. [DOI] [PubMed] [Google Scholar]
- Shafagoj YA, Mohammed FI. Levels of maximum end-expiratory carbon monoxide and certain cardiovascular parameters following hubble-bubble smoking. Saudi Medical Journal. 2002;23:953–958. [PubMed] [Google Scholar]
- Shaikh RB, Vijayaraghavan N, Sulaiman AS, Kazi S, Shafi MSM. The acute effects of waterpipe smoking on the cardiovascular and respiratory systems. Journal of Preventive Medicine and Hygiene. 2008;49:101–107. [PubMed] [Google Scholar]
- Shihadeh A, Saleh R. Polycyclic aromatic hydrocarbons, carbon monoxide, tar, and nicotine in the mainstream smoke aerosol of the narghile water pipe. Food and Chemical Toxicology. 2005;43:655–661. doi: 10.1016/j.fct.2004.12.013. [DOI] [PubMed] [Google Scholar]
- Siafakas NM, Antoniou KM, Tzortzaki EG. Role of angiogenesis and vascular remodeling in chronic obstructive pulmonary disease. International Journal of Chronic Obstructive Pulmonary Disease. 2007;2:453–462. [PMC free article] [PubMed] [Google Scholar]
- Smith-Simone S, Maziak W, Ward KD, Eissenberg T. Waterpipe tobacco smoking: knowledge, attitudes, beliefs, and behavior in two U.S. samples. Nicotine and Tobacco Research. 2008;10:393–398. doi: 10.1080/14622200701825023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su Y, Han W, Giraldo C, De Li Y, Block ER. Effect of cigarette smoke extract on nitric oxide synthase in pulmonary artery endothelial cells. American Journal of Respiratory Cell and Molecular Biology. 1998;19:819–825. doi: 10.1165/ajrcmb.19.5.3091. [DOI] [PubMed] [Google Scholar]
- Taha AZ, Sabra AA, Al-Mustafa ZZ, Al-Awami HR, Al-Khalaf MA, Al-Momen MM. Water pipe (shisha) smoking among male students of medical colleges in the eastern region of Saudi Arabia. Annals of Saudi Medicine. 2010;30:222–226. doi: 10.4103/0256-4947.62838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viatour P, Merville M-P, Bours V, Chariot A. Phosphorylation of NF-κB and IκB proteins: implications in cancer and inflammation. Trends in Biochemical Sciences. 2005;30:43–52. doi: 10.1016/j.tibs.2004.11.009. [DOI] [PubMed] [Google Scholar]
- Wang S, Xu J, Song P, Viollet B, Zou M-H. In vivo activation of AMP-activated protein kinase attenuates diabetes-enhanced degradation of GTP cyclohydrolase I. Diabetes. 2009;58:1893–1901. doi: 10.2337/db09-0267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfram RM, Chehne F, Oguogho A, Sinzinger H. Narghile (water pipe) smoking influences platelet function and (iso-)eicosanoids. Life Sciences. 2003;74:47–53. doi: 10.1016/j.lfs.2003.06.020. [DOI] [PubMed] [Google Scholar]
- Wong MK, Gotlieb AI. Endothelial cell monolayer integrity. I. Characterization of dense peripheral band of microfilaments. Arteriosclerosis. 1986;6:212–219. doi: 10.1161/01.atv.6.2.212. [DOI] [PubMed] [Google Scholar]
- Xu J, Zou M-H. Molecular insights and therapeutic targets for diabetic endothelial dysfunction. Circulation. 2009;120:1266–1286. doi: 10.1161/CIRCULATIONAHA.108.835223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziche M, Morbidelli L. Nitric oxide and angiogenesis. Journal of Neuro-Oncology. 2000;50:139–148. doi: 10.1023/a:1006431309841. [DOI] [PubMed] [Google Scholar]