Abstract
Bulimia nervosa (BN) is strongly familial, and additive genetic effects appear to contribute substantially to the observed familiality. In turn, behavioral components of BN, such as self-induced vomiting, are reliably measured and heritable. To identify regions of the genome harboring genetic variants conferring susceptibility to BN, we conducted a linkage analysis of multiplex families with eating disorders that were identified through a proband with BN. Linkage analysis of the entire sample of 308 families yielded a double peak, with the highest nonparametric multipoint maximum LOD score (MLS), of 2.92, on chromosome 10. Given the high heritability of self-induced vomiting and the reliability with which it can be measured, we performed linkage analysis in a subset (n=133) of families in which at least two affected relatives reported a symptom pattern that included self-induced vomiting. The highest MLS (3.39) observed was on chromosome 10, between markers D10S1430 and D10S1423. These results provide evidence of the presence of a susceptibility locus for BN on chromosome 10p. Using simulations, we demonstrate that both of these scores, 2.92 and 3.39, meet the widely accepted criterion for genomewide significance. Another region on 14q meets the criterion for genomewide suggestive linkage, with MLSs of 1.97 (full sample) and 1.75 (subset) at 62 centimorgans from p-ter.
Bulimia nervosa (BN) is a psychiatric disorder characterized by episodes of binge-eating (eating an unusually large amount of food in a discrete period of time and feeling out of control), compensatory behaviors (e.g., self-induced vomiting or laxative abuse) and over-concern with weight and shape. Although eating disorders have been considered to be largely sociocultural in origin, a substantial body of literature has now shown that BN (as well as the related eating disorders anorexia nervosa [AN] and eating disorder not otherwise specified [ED-NOS]) is strongly familial (Lilenfeld et al. 1998; Strober et al. 2000) and that this familiality is due largely to the additive effects of genes (Bulik et al. 2000). Twin studies have estimated the heritability of syndromic BN to be 54%–83% (Kendler et al. 1991; Bulik et al. 1998; Wade et al. 1999; Kortegaard et al. 2001). The diagnosis of BN in population-based samples is, however, less than optimally reliable (Wade et al. 2000), with the agreement for lifetime diagnosis of broadly defined BN assessed at intervals of five years being κ=.33. Reliability is improved when behavioral indicators, such as self-induced vomiting, are used (Wade et al. 2000). Moreover, twin studies that have dismantled the syndrome of BN into its component parts of bingeing and vomiting have shown that there is a substantial, but not perfect, genetic correlation between bingeing and vomiting (rg=0.74) and that the heritability estimate for self-induced vomiting is particularly high (72%) (Sullivan et al. 1998).
Elsewhere, we have reported a genomewide linkage screen for AN, in which we identified a region suggestive of linkage for AN on chromosome 1p (Grice et al. 2002). Moreover, after incorporating behavioral covariates into the ASP linkage analysis, we identified several areas of suggestive linkage on chromosomes 1, 2, and 13 (Devlin et al. 2002). The present study describes an entirely new nonoverlapping multicenter study, in which an allele-sharing linkage strategy was used to identify loci that may contribute to BN.
The present study was conducted under the principal direction of W.H.K. The sites of collaborative arrangements were selected on the basis of experience in the assessment of eating disorders and geographical distribution. Informed consent was obtained from all study participants, and each site obtained ethical approval from its own institutional review board.
Clinical and diagnostic information on the sample is summarized in table 1. The sample contained families with at least two affected biological relatives, all ascertained through a proband who met Diagnostic and Statistical Manual, fourth edition (DSM-IV), criteria (American Psychiatric Association 1994) for BN and who reported a family history of eating disorders in a first-to-fifth–degree relative. Assessment data were collected from 360 probands and their families. Of the probands included, 97.6% were white, 2.1% were Hispanic, and 0.3% were Asian. In the final analysis, eight probands did not meet modified DSM-IV criteria for purging BN; blood was collected from all eight, and six had at least one affected relative. Of the 352 probands who met the study diagnostic criteria, 342 supplied blood samples, and 331 had an affected relative. Thus, a total of 328 probands who provided blood samples and who had at least one affected relative were included in the study. DNA from 316 of these families was available for inclusion in the molecular analysis for the genome scan.
Table 1.
Characteristics of Sample
| Variable | EntireSample | Subgroup with Historyof Vomiting |
| Mean ± SD age (years) | 28.16 ± 9.26 | 29.04 ± 8.77 |
| % Males | 2.3 | .0 |
| Mean ± SD BMI: | ||
| Current | 21.04 ± 3.02 | 21.20 ± 3.01 |
| Minimum (past) | 17.09 ± 2.64 | 17.21 ± 2.67 |
| Maximum (past) | 23.91 ± 3.33 | 24.10 ± 3.19 |
| Number of families with: | ||
| 1 affected sibling paira | 212 | 126 |
| 3 or 4 affected siblingsa | 15 | 7 |
| Other affected relative pairsb | 81 |
0 |
| Total | 308 | 133 |
Families may have had additional affected relatives.
Families included, for example, cousin-cousin pairs, aunt-niece pairs, or half siblings.
Recruitment of the families followed standard protocols, as described elsewhere (Kaye et al. 2000). Full assessments were completed on the proband and affected relative(s) (authors' unpublished data). Eating-disorder diagnoses were confirmed by trained raters, using the Structured Interview for Anorexia Nervosa and Bulimic syndromes (SIAB) (Fichter et al. 1998).
Probands were accepted into the study if they met the following criteria: (1) a DSM-IV lifetime diagnosis of BN, purging type (purging must have included regular vomiting [with other means of purging also allowed], and bingeing and vomiting must have occurred at least twice a week for a duration of at least 6 mo) and (2) age of 13–65 years. A current or lifetime history of AN was acceptable. Exclusionary criteria for probands included mental retardation, dementia, organic brain syndromes, psychotic disorders (including schizophrenia, schizophreniform disorder, delusional disorder, and schizoaffective disorder), Turner syndrome, and any medical condition that could affect appetite, body weight, or eating (e.g., individuals with diabetes or thyroid conditions were excluded if the onset of the disease preceded the onset of the eating disorder). Individuals with bipolar 1 or bipolar 2 disorder were excluded only if symptoms of BN occurred exclusively during manic or hypomanic episodes. Probands with neurological problems were excluded, with the exception of those who had a seizure disorder that resulted from trauma that occurred after the onset of the eating disorder. Probands whose premorbid weight exceeded the body mass index (BMI) of the 95th percentile for sex and age on the Hebebrand index (Hebebrand et al. 1996) or whose high lifetime BMI was >35 were also excluded.
Affected relatives were biologically related to the proband (e.g., siblings, half siblings, cousins, etc.). Inclusion criteria for affected relatives required that they be 13–65 years of age and that they had received at least one of the following lifetime eating-disorder diagnoses: (1) DSM-IV BN, purging type or nonpurging type; (2) DSM-IV AN, restricting type or binge-eating/purging type; (3) ED-NOS-1, defined as subthreshold AN with the presence of two of three criteria A–C of DSM-IV AN and the absence of lifetime bingeing and with a lifetime ideal body weight <125% of expected for height and weight; (4) ED-NOS-2, defined as subthreshold BN with the presence of criteria A, B, D, and E of DSM-IV BN and the presence of binge eating and purging, which must have occurred “more than just experimentally” but may have occurred for <3 mo or at a lower frequency than twice a week; and (5) ED-NOS-3, defined as the presence of purging or other clearly excessive compensatory behaviors, in the absence of objective binge eating, in individuals of normal weight who have either an intense fear of gaining weight or becoming fat or an undue influence of body weight or shape on self-evaluation. Exclusion criteria for affected relatives included all exclusion criteria listed for the probands, with the following additional criteria: (1) MZ twin of the proband; (2) parent-child affected pair, in the absence of an additional affected relative; and (3) a diagnosis of binge-eating disorder as the only lifetime eating-disorder diagnosis.
The etiology of eating disorders, like all complex diseases, is surely heterogeneous. Moreover, relative to AN, the presentation and traits associated with BN are more variable. Therefore, it could be important to seek attributes of BN that minimize its heterogeneity, especially its genetic heterogeneity, and thereby amplify potential linkage signals. For the present study, we were particularly interested in the frequency of vomiting, because of the higher reliability and substantial heritability of vomiting (Sullivan et al. 1998; Wade et al. 2000). In fact, a selection criterion for probands in the present study was that they met DSM-IV criteria for BN with a minimum period of 6 mo of bingeing and vomiting at least twice a week. Purging behavior, if any, of patients with an eating disorder was assessed using the SIAB (Fichter et al. 1998). On the basis of the argument presented above, our plan for linkage analysis had two stages: first, to analyze the entire sample of families with BN and, second, to analyze families who show noteworthy elevation in vomiting behavior.
To define families exhibiting elevated vomiting behavior, we sought at least one other affected relative who reported regular vomiting behavior. Because, by design, the proband automatically met this criterion, such families would then have two or more individuals with an eating disorder that included vomiting behavior. By this criterion, 133 families (of the 316) were identified for analysis.
After a blood sample was collected from each proband, affected relative, and participating biological parent, DNA was extracted using Gentra Systems reagents, according to the manufacturer’s specifications. The Weber screening set, version 9 (Center for Medical Genetics, Marshfield Medical Research Foundation), which is a screening panel of 386 fluorescently tagged microsatellite markers dispersed at a density slightly <10 cM, was used as the basis for the genomewide scan. Twenty-six microsatellites generated incomplete data on the first round of genotyping and were excluded from further analysis. Exclusion of these markers created no gaps >25 cM in the genome scan. Mean ± SD heterozygosity of the markers was 0.77 ± 0.06. We evaluated markers and pedigrees for Mendelian errors, using the PedCheck program (O’Connell and Weeks 1998). Genotyping errors were set to missing. Nominal and imputed genetic relationships among individuals from the same family were contrasted using the Relpair (Boehnke and Cox 1997) and PREST (McPeek and Sun 2000) programs. We excluded from further analysis eight families in which relationships among affected individuals could not be resolved. Within the 308 families analyzed for linkage, the majority of the affected relative pairs (ARPs) were affected sibling pairs (ASPs). Within these 308 families there were a variety of nonindependent ARPs: 260 ASPs; 14 half siblings; 42 avuncular; 42 cousin; 6 mother-child; and 15 other. Of the families with affected siblings, 42% had participation of one or more parents. Most parents were not affected; among those that were, the family included at least one other affected relative participating in the study.
To estimate marker allele frequencies, we counted alleles while ignoring family relationships. Although population-specific estimates (Lockwood et al. 2001) could be used in the linkage analysis, we found, in a related study, that such estimates had very little impact on the linkage results (Devlin et al. 2002). Linkage analyses were implemented using GeneHunterPlus, module ASM, which assumes a linear model for risk (Kong and Cox 1997; Teng and Siegmund 1998) to obtain greater accuracy of the variance than the original GeneHunter model (Kruglyak et al. 1996).
Uncertainty in identity-by-descent status and relative sparseness of markers across the genome are expected to affect the distribution of the test statistics substantially (Teng and Siegmund 1998; Liu et al. 2001). Thus, to evaluate the results of the linkage analysis, we first performed simulations to determine the distribution of test statistics when no disease loci were present, by use of the Simulate package (Terwilliger et al. 1993). We generated 1,500 data sets for all 23 chromosomes and both samples (vomiting-enriched and total BN), and we determined two thresholds for multipoint maximum LOD scores (MLS), following Lander and Kruglyak (1995): the MLS expected to be achieved, on average, once per 20 genome scans (genomewide significant linkage) and once per single genome scan (genomewide suggestive linkage). Within the precision of our simulations, the results from the two samples were identical and were therefore combined. The 3,000 simulations yielded critical values for genomewide-significant and suggestive linkage of 2.86 and 1.56, respectively.
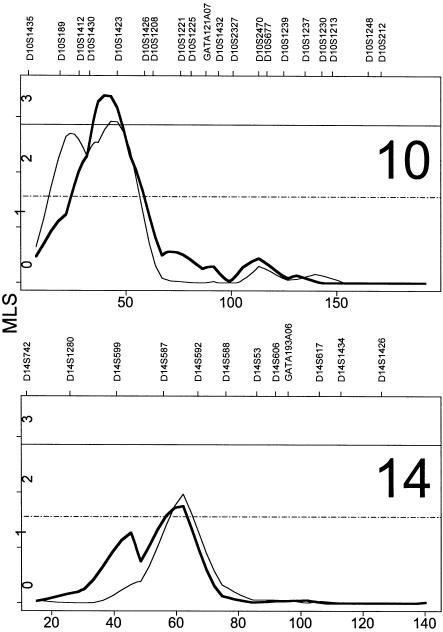
Significant linkage was achieved on chromosome 10 when the entire sample of families with BN was analyzed (fig. 1). The peak MLS score was 2.92 at 44 cM p-ter (on the basis of the asymptotic distribution of test statistic, P=.00024; by simulation, occurs at rate r=0.043 per genome scan). Interestingly, a second peak occurred further p-ter, with an MLS of 2.70 at 24 cM, which approached significance (P=.0005, r=0.074). Suggestive linkage occurred on chromosome 14 (fig. 1), with an MLS of 1.97 (P=.0023, r=0.39).
Figure 1.
Maximized LOD scores for chromosomes with significant or suggestive linkages for the sample enriched for vomiting (thick line) and the entire BN sample (thin line). Horizontal reference lines give simulation-based criteria for significant (solid line) and suggestive (dashed line) linkage for this genome scan. To see the results for the entire genome scan, please go to Computational Genetics Web site.
One instance of significant linkage occurred in the cohort enriched for vomiting (fig. 1). The peak MLS score, 3.39 (P=.00008, r=0.014), occurred on chromosome 10, at 40 cM from p-ter, between D10S1431 and D10S1423. An instance of suggestive linkage occurred on chromosome 14 (fig. 1), with an MLS of 1.75 (P=.004, r=0.65) at 62 cM from p-ter, between D14S587 and D14S592.
Camp and Farnham (2001) provide a means of adjusting for multiple testing within the context of multiple genome scans. Their approach is to first calculate the correlation between the scans (more precisely, the mean-square error [R2]), to use this correlation to determine the number of “effective” genome scans, and then to correct the critical value for significance. Application of their approach to our results is straightforward; interpretation is less so, because of the sequential nature of our tests and the fact that the first genome scan (on the full data) is significant. Nonetheless, the correlation between the scans is 0.748. Thus, R2=0.56, and the number of effective scans is 1.44. Camp and Farnham’s approach then suggests the critical threshold for the second genome scan (for the cohort enriched for vomiting) is ∼3.03. The result for the second scan (MLS=3.39) is therefore significant, even after correction.
To evaluate the robustness of these linkage results to variation in allele frequencies, we also analyzed the data from chromosomes 10 and 14, using allele frequencies estimated from our study of families with AN (Devlin et al. 2002). The use of these estimated frequencies had no material impact on the present results; in fact, the MLS scores are remarkably similar. Analysis of the genetic differentiation between these populations revealed why the linkage results are similar. By using the alleles measured on chromosomes 10 and 14 (a total of 34 markers and 358 alleles) to estimate the differentiation between the populations and by using methods of Cockerham (1969, 1972), we find an estimated Wright's Fst of 0.00077. These results are consistent with limited heterogeneity among populations of European ancestry, which is the ancestry of the vast majority of individuals in both the BN and the AN samples (Kaye et al. 2000), and with the fact that both samples are large, thereby minimizing sampling error.
This is the second multinational collaborative genomewide linkage scan of eating disorders. The first, with ARPs identified on the basis of a proband with AN, showed evidence of susceptibility genes for AN on chromosome 1 (Devlin et al. 2002; Grice et al. 2002). The present study ascertained ARPs on the basis of a proband with BN. Moreover, we reduced sample and likely genetic heterogeneity by defining a subset of ARPs that was enriched for severe vomiting behavior. This decision was based on evidence that self-induced vomiting is both reliably measured (Wade et al. 2000) and strongly heritable (Sullivan et al. 1998). This strategy proved fruitful, because the strongest linkage signal on chromosome 10p was in the sample enriched for vomiting. The full BN sample yielded a smaller, yet significant, peak in the same region, with a second peak further p-ter that approached significance. It is conceivable that this double peak represents two susceptibility loci associated with syndromal BN. One locus, between D10S1430 and D10S1423, which was elevated in the vomiting-enriched sample and in the entire BN sample, may be related to susceptibility to self-induced vomiting. The second part of the double peak may be related to another behavioral or psychological feature of BN (e.g., binge-eating) or to a personality feature (e.g., impulsivity or obsessionality) commonly associated with susceptibility to the syndrome.
It is also noteworthy that the present genome scan, based on ascertainment of probands with BN, yielded linkage results that were different from our previous scan based on ascertainment of probands with AN. AN and BN are related, but not identical, conditions. Between one-third and one-half of individuals with AN develop BN during the course of illness (Smith et al. 1993; Gillberg et al. 1994; Schork et al. 1994; van der Ham et al. 1994; Eckert et al. 1995), and family studies suggest that the disorders do not breed true (Lilenfeld et al. 1998; Strober et al. 2000). Family studies of probands with AN report an increased risk of BN among first-degree relatives, and family studies of probands with BN report elevated risk for AN among first-degree relatives (Lilenfeld et al. 1998; Strober et al. 2000). However, our study designs and careful diagnostic procedures enabled maximal differentiation between participants with AN and those with BN. One of strongest linkage signals in the AN scan was observed in a subsample of ARPs concordant for a rigorous definition of restricting subtype of AN (i.e., no binge-eating) (Grice et al. 2002). Proband status in the present study required the presence of the purging subtype of BN (although a history of AN was acceptable). Thus, our phenotypic definitions may have optimized our ability to detect differences in genetic susceptibility to AN and genetic susceptibility to BN.
Suggestive and, occasionally, significant evidence for linkage to 10p has also been observed in obesity, alcoholism, schizophrenia, bipolar disorder, and type 1 diabetes in females (Davies et al. 1994; Reed et al. 1997; Rice et al. 1997; Faraone et al. 1998; Hager et al. 1998; Mein et al. 1998; Schwab et al. 1998; Straub et al. 1998; Paterson and Petronis 1999; Hinney et al. 2000). Paterson and Petronis (1999) hypothesize that linkage signals in this region may be related to the phenomenon of transmission ratio distortion (TRD)—specifically, a biased transmission of alleles identical-by-descent to female offspring. The genetic basis for such a TRD is not apparent, and evidence in support of the hypothesis is weak (compare Paterson and Petronis [1999] with Faraone et al. [1999] and Schwab et al. [1999]). Moreover, if TRD were ubiquitous in females, then we would expect noteworthy linkage signals on this region of 10p in other female-preponderant diseases. Yet no such linkages have been observed in genome scans for non-BRCA1 and non-BRCA2 ovarian cancer (Sekine et al. 2001), female obesity (Stone et al. 2002), anorexia nervosa (Devlin et al. 2002; Grice et al. 2002), panic disorder and agoraphobia (Gelernter et al. 2001), or systemic lupus erythematosus (Gray-McGuire et al. 2001; Rao et al. 2001).
Several alternative explanations emerge for the suggestive and significant linkages observed, for complex traits, on 10p. For example, a locus could exist on chromosome 10p that generically increases susceptibility to a range of complex disorders; however, the range of traits for which linkage has been observed renders such an explanation unlikely. Alternatively, because chromosome 10p harbors a large number of genes, variants therein might contribute in unique or interactive ways to a variety of complex traits. More instances of overlap in linkage signals (whether real or spurious) are to be expected as more genome scans are performed.
It is also conceivable that some of the coincident linkages on 10p reflect shared underlying biological mechanisms. One intriguing relation is between BN and obesity.
There are several lines of evidence suggesting a relationship between these two traits. A substantial minority of women with BN report a history of obesity (Garfinkel et al. 1980; Johnson-Sabine et al. 1988); familial obesity is elevated in women with BN in comparison with AN (Garfinkel et al. 1980; Grace et al. 1985; Vieselman and Roig 1985); risk of obesity differs significantly between women with BN and healthy control women (Fairburn et al. 1997); and childhood obesity and parental obesity distinguish significantly between women with BN and control women with other psychiatric disorders (Fairburn et al. 1997). In addition, a bivariate twin study has shown a moderate genetic correlation between obesity (BMI >30) and binge-eating in females (Bulik et al., in press). Finally, Levitan et al. (2001) observed an association between a 5-HT1B polymporphism and the minimum past BMI in women with BN, suggesting an association between body weight regulation and serotonergic receptor function in these women. Although expressed obesity was a criterion for exclusion from the present study, it is possible that a tendency toward weight gain could be associated with the onset of the unhealthy eating and weight-loss behaviors characteristic of BN.
The next stages of our research will include the exploration of likely candidate genes under the observed linkage peaks. We will focus on candidates that may be related to serotonergic dysregulation (because abnormalities in serotonin function have been frequently observed in women with BN [Kaye et al. 1998, 2001]), as well as on genes that influence appetite and weight regulation. In addition, because we collected similar behavioral covariate information on both the AN ARP sample and the present BN ARP sample, we hope that the use of well-articulated diagnostic information, together with rational and common covariates for the combined sample, will provide a means to identify genes that influence risk of both AN and BN.
Acknowledgments
The authors wish to thank the Price Foundation for the support of the clinical collection of subjects and genotyping and for contribution to the support of data analysis. Data analysis was also supported by National Institutes of Health grants MH57881 (to B.D.) and K01-MH01553 (to C.B.). Genotypic markers, allele frequencies, and genetic maps were generated at the Center for Medical Genetics, Marshfield Medical Research Foundation, with support from the National Heart, Lung and Blood Institute. Michael Mullokandov, Kevin Jacobs, and Meredith Yeager helped to prepare and manage these data. The authors are indebted to the participating families for their contribution of time and effort in support of this study.
Electronic-Database Information
The URLs for data presented herein are as follows:
- Center for Medical Genetics, Marshfield Medical Research Foundation http://research.marshfieldclinic.org/genetics/ (for Weber screening set, v. 9)
- Computational Genetics Program, Western Psychiatric Institute and Clinic, http://wpicr.wpic.pitt.edu/WPICCompGen/ (for results of genome scan)
References
- American Psychiatric Association (1994) Diagnostic and statistical manual of mental disorders, 4th ed. American Psychiatric Association Press, Washington, DC [Google Scholar]
- Boehnke M, Cox NJ (1997) Accurate inference of relationships in sib-pair linkage studies. Am J Hum Genet 61:423–429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bulik CM, Sullivan PF, Kendler KS (1998) Heritability of binge-eating and broadly defined bulimia nervosa. Biol Psychiatry 44:1210–1218 [DOI] [PubMed] [Google Scholar]
- Bulik CM, Sullivan PF, Kendler KS. Genetic and environmental contributions to obesity and binge-eating. Int J Eat Disord (in press) [DOI] [PubMed] [Google Scholar]
- Bulik C, Sullivan PF, Wade T, Kendler K (2000) Twin studies of eating disorders: a review. Int J Eat Disord 27:1–20 [DOI] [PubMed] [Google Scholar]
- Camp N, Farnham J (2001) Correcting for multiple analyses in genomewide linkage studies. Ann Hum Genet 65:577–582 [DOI] [PubMed] [Google Scholar]
- Cockerham C (1969) Variance of gene frequencies. Evolution 23:72–84 [DOI] [PubMed] [Google Scholar]
- ——— (1972) Analysis of gene frequencies. Genetics 74:679–700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies JL, Kawaguchi Y, Bennett ST, Copeman JB, Cordell HJ, Pritchard LE, Reed PW, et al (1994) A genome-wide search for human type 1 diabetes susceptibility genes. Nature 371:130–136 [DOI] [PubMed] [Google Scholar]
- Devlin B, Bacanu SA, Klump KL, Bulik CM, Fichter MM, Halmi KA, Kaplan AS, Strober, M, Treasure, J, Woodside DB, Berrettini W, Kaye WH (2002) Linkage analysis of anorexia nervosa incorporating behavioral covariates. Hum Mol Genet 11:689–696 [DOI] [PubMed] [Google Scholar]
- Eckert ED, Halmi KA, Marchi P, Grove W, Crosby R (1995) Ten-year follow-up of anorexia nervosa: clinical course and outcome. Psychol Med 25:143–156 [DOI] [PubMed] [Google Scholar]
- Fairburn CG, Welch SL, Doll HA, Davies BA, O'Connor ME (1997) Risk factors for bulimia nervosa: a community-based case-control study. Arch Gen Psychiatry 54:509–517 [DOI] [PubMed] [Google Scholar]
- Faraone SV, Matise T, Svrakic D, Pepple J, Malaspina D, Suarez B, Hampe C, Chan G, Aelony A, Friedman JH, Kaufmann C, Cloninger CR, Tsuang MT (1998) Genome scan of European-American schizophrenia pedigrees: results of the NIMH Genetics Initiative and Millennium Consortium. Am J Med Genet 81:290–1295 [PubMed] [Google Scholar]
- Faraone SV, Meyer J, Matise T, Svrakic D, Pepple J, Malaspina D, Suarez B, Hampe C, Chan G, Aelony A, Friedman JH, Kaufmann C, Cloninger CR, Tsuang MT (1999) Suggestive linkage of chromosome 10p to schizophrenia is not due to transmission ratio distortion. Am J Med Genet 88:607–608 [DOI] [PubMed] [Google Scholar]
- Fichter M, Herpertz S, Quadflieg N, Herpertz-Dahlmann B (1998) Structured interview for anorexic and bulimic disorders for DSM-IV and ICD-10: updated (third) revision. Int J Eat Disord 24:227–249 [DOI] [PubMed] [Google Scholar]
- Garfinkel P, Moldofsky H, Garner D (1980) The heterogeneity of anorexia nervosa: bulimia as a distinct subgroup. Arch Gen Psychiatry 37:1036–1040 [DOI] [PubMed] [Google Scholar]
- Gelernter J, Bonvicini K, Page G, Woods S, Goddard A, Kruger S, Pauls D, Goodson S (2001) Linkage genome scan for loci predisposing to panic disorder or agoraphobia. Am J Med Genet 105:548–557 [DOI] [PubMed] [Google Scholar]
- Gillberg IC, Rastam M, Gillberg C (1994) Anorexia nervosa outcome: six-year controlled longitudinal study of 51 cases including a population cohort. J Am Acad Child Adolesc Psychiatry 33:729–739 [DOI] [PubMed] [Google Scholar]
- Grace PS, Jacobson RS, Fullager CJ (1985) A pilot comparison of purging and non-purging bulimics. J Clin Psychol 41:173–180 [DOI] [PubMed] [Google Scholar]
- Gray-McGuire C, Moser K, Gaffney P, Kelly J, Yu H, Olson J, Jedrey C, Jacobs KB, Kimberly RP, Neas BR, Rich SS, Behrens TW, Harley JB (2000) Genome scan of human systemic lupus erythematosus by regression modeling: evidence of linkage and epistasis at 4p16–15.2. Am J Hum Genet 67:1460–1469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grice DE, Halmi KA, Fichter MM, Strober M, Woodside DB, Treasure JT, Kaplan AS, Magistretti PJ, Goldman D, Bulik CM, Kaye WH, Berrettini WH (2002) Evidence for a susceptibility gene for anorexia nervosa on chromosome 1. Am J Hum Genet 70:787–792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hager J, Dina C, Francke S, Dubois S, Houari M, Vatin V, Vaillant E, Lorentz N, Basdevant A, Clement K, Guy-Grand B, Froguel P (1998) A genome-wide scan for human obesity genes reveals a major susceptibility locus on chromosome 10. Nat Genet 20:304–308 [DOI] [PubMed] [Google Scholar]
- Hebebrand J, Himmelmann GW, Heseker H, Schafer H, Remschmidt H (1996) Use of percentiles for the body mass index in anorexia nervosa: diagnostic, epidemiological, and therapeutic considerations. Int J Eat Disord 19:359–369 [DOI] [PubMed] [Google Scholar]
- Hinney A, Ziegler A, Oeffner F, Wedewardt C, Vogel M, Wulftange H, Geller F, Stubing K, Siegfried W, Goldschmidt HP, Remschmidt H, Hebebrand J (2000) Independent confirmation of a major locus for obesity on chromosome 10. J Clin Endocrinol Metab 85:2962–2965 [DOI] [PubMed] [Google Scholar]
- Johnson-Sabine E, Wood K, Patton G, Mann A, Wakeling A (1988) Abnormal eating attitudes in London schoolgirls—a prospective epidemiological study: factors associated with abnormal response on screening questionnaires. Psychol Med 18:615–622 [DOI] [PubMed] [Google Scholar]
- Kaye WH, Frank GK, Meltzer CC, Price JC, McConaha CW, Crossan PJ, Klump KL, et al (2001) Altered serotonin 2A receptor activity in women who have recovered from bulimia nervosa. Am J Psychiatry 158:1152–1155 [DOI] [PubMed] [Google Scholar]
- Kaye WH, Greeno CG, Moss H, Fernstrom J, Fernstrom M, Lilenfeld LR, Weltzin TE, Mann JJ (1998) Alterations in serotonin activity and psychiatric symptoms after recovery from bulimia nervosa. Arch Gen Psychiatry 55:927–935 [DOI] [PubMed] [Google Scholar]
- Kaye WH, Lilenfeld LR, Berrettini WH, Strober M, Devlin B, Klump KL, Goldman D, Bulik CM, Halmi KA, Fichter MM, Kaplan A, Woodside DB, Treasure J, Plotnicov KH, Pollice C, Rao R, McConaha CW (2000) A search for susceptibility loci for anorexia nervosa: methods and sample description. Biol Psychiatry 47:794–803 [DOI] [PubMed] [Google Scholar]
- Kendler KS, MacLean C, Neale MC, Kessler RC, Heath AC, Eaves LJ (1991) The genetic epidemiology of bulimia nervosa. Am J Psychiatry 148:1627–1637 [DOI] [PubMed] [Google Scholar]
- Kong A, Cox N (1997) Allele-sharing models: LOD scores and accurate linkage tests. Am J Hum Genet 61:1179–1188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kortegaard LS, Hoerder K, Joergensen J, Gillberg C, Kyvik KO (2001) A preliminary population-based twin study of self-reported eating disorder. Psychol Med 31:361–365 [DOI] [PubMed] [Google Scholar]
- Kruglyak L, Daly MJ, Reeve-Daly MP, Lander ES (1996) Parametric and nonparametric linkage analyses: a unified multipoint approach. Am J Hum Genet 58:1347–1363 [PMC free article] [PubMed] [Google Scholar]
- Lander E, Kruglyak L (1995) Genetic dissection of complex traits: guidelines for interpreting and reporting linkage results. Nat Genet 11:241–247 [DOI] [PubMed] [Google Scholar]
- Levitan R, Kaplan AS, Masellis M, Basile V, Walker M, Lipson N, Siegel G, Woodside B, Macciardi F, Kennedy SH, Kennedy J (2001) Polymorphism of the serotonin 5-HT1B receptor gene (HTR1B) associated with minimum lifetime body mass index in women with bulimia nervosa. Biol Psychiatry 50:640–643 [DOI] [PubMed] [Google Scholar]
- Lilenfeld L, Kaye W, Greeno C, Merikangas K, Plotnikov K, Pollice C, Rao R, Strober M, Bulik CM, Nagy L (1998) A controlled family study of restricting anorexia and bulimia nervosa: comorbidity in probands and disorders in first-degree relatives. Arch Gen Psychiatry 55:603–610 [DOI] [PubMed] [Google Scholar]
- Liu J, Nyholt D, Magnussen P, Parano E, Pavone P, Geschwind D, Lord C, Iversen P, Hoh J, Ott J, Gillam TC (2001) A genomewide screen for autism susceptibility loci. Am J Hum Genet 69:327–340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lockwood JR, Roeder K, Devlin B (2001) A Bayesian hierarchical model for allele frequencies. Genet Epidemiol 20:17–33 [DOI] [PubMed] [Google Scholar]
- McPeek M-S, Sun L (2000) Statistical tests for detection of misspecified relationships by use of genome-screen data. Am J Hum Genet 66:1076–1094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mein CA, Esposito L, Dunn MG, Johnson GC, Timms AE, Goy JV, Smith AN, Sebag-Montefiore L, Merriman ME, Wilson AJ, Pritchard LE, Cucca F, Barnett AH, Bain SC, Todd JA (1998) A search for type 1 diabetes susceptibility genes in families from the United Kingdom. Nat Genet 19:297–300 [DOI] [PubMed] [Google Scholar]
- O’Connell J, Weeks D (1998) PedCheck: a program for identification of genotype incompatibilities in linkage analysis. Am J Hum Genet 63:259–266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paterson AD, Petronis A (1999) Transmission ratio distortion in females on chromosome 10p11-p15. Am J Med Genet 88:657–661 [PubMed] [Google Scholar]
- Rao S, Olson J, Moser K, Gray-McGuire C, Bruner G, Kelly J, Harley J (2001) Linkage analysis of human systemic lupus erythematosus-related traits: a principal component approach. Arthritis Rheum 44:2807–2818 [DOI] [PubMed] [Google Scholar]
- Reed P, Cucca F, Jenkins S, Merriman M, Wilson A, McKinney P, Bosi E, Joner, G. Ronningen, K Thorsby, E Undlien, D Merriman, T Barnett, A Bain, S Todd, J (1997) Evidence for a type 1 diabetes susceptibility locus (IDDM10) on human chromosome 10p11-q11. Hum Mol Genet 6:1011–1016 [DOI] [PubMed] [Google Scholar]
- Rice JP, Goate A, Williams JT, Bierut L, Dorr D, Wu W, Shears S, Gopalakrishnan G, Edenberg HJ, Foroud T, Nurnberger J Jr, Gershon ES, Detera-Wadleigh SD, Goldin LR, Guroff JJ, McMahon FJ, Simpson S, MacKinnon D, McInnis M, Stine OC, DePaulo J R, Blehar MC, Reich T (1997) Initial genome scan of the NIMH genetics initiative bipolar pedigrees: chromosomes 1, 6, 8, 10, and 12. Am J Med Genet 74:247–253 [DOI] [PubMed] [Google Scholar]
- Schork EJ, Eckert ED, Halmi KA (1994) The relationship between psychopathology, eating disorder diagnosis, and clinical outcome at 10-year follow-up in anorexia nervosa. Compr Psychiatry 35:113–123 [DOI] [PubMed] [Google Scholar]
- Schwab SG, Hallmayer J, Albus M, Lerer B, Hanses C, Kanyas K, Segman R, Borrman M, Dreikorn B, Lichtermann D, Rietschel M, Trixler M, Maier W, Wildenauer DB (1998) Further evidence for a susceptibility locus on chromosome 10p14-p11 in 72 families with schizophrenia by nonparametric linkage analysis. Am J Med Genet 81:302–307 [PubMed] [Google Scholar]
- Schwab SG, Wildenauer D, Hallmayer J (1999) No evidence for segregation distortion in females in a sample of 72 families with schizophrenia with potential linkage to chromosome 10p14-p11. Am J Med Genet 88:750–751 [PubMed] [Google Scholar]
- Sekine M, Nagata H, Tsuji S, Hirai Y, Fujimoto S, Hatae M, Kobayashi I, Fujii T, Nagata I, Ushijima K, Obata K, Suzuki M, Yoshinaga M, Umesaki N, Satoh S, Enomoto T, Motoyama S, Tanaka K (2001) Localization of a novel susceptibility gene for familial ovarian cancer to chromosome 3p22-p25. Hum Mol Genet 10:1421–1429 [DOI] [PubMed] [Google Scholar]
- Smith C, Feldman S, Nasserbakht A, Steiner H (1993) Psychological characteristics and DSM-III-R diagnoses at 6-year follow-up of adolescent anorexia nervosa. J Am Acad Child Adolesc Psychiatry 32:1237–1245 [DOI] [PubMed] [Google Scholar]
- Stone S, Abkevich V, Hunt S, Gutin A, Russell D, Neff C, Riley R, Frech GC, Hensel CH, Jammulapati S, Potter J, Sexton D, Tran T, Gibbs D, Iliev D, Gress R, Bloomquist B, Amatruda J, Rae PM, Adams TD, Skolnick MH, Shattuck D (2002) A major predisposition locus for severe obesity, at 4p15-p14. Am J Hum Genet 70:1459–1468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straub RE, MacLean CJ, Martin RB, Ma Y, Myakishev MV, Harris-Kerr C, Webb BT, O'Neill FA, Walsh D, Kendler KS (1998) A schizophrenia locus may be located in region 10p15-p11. Am J Med Genet 81:296–301 [DOI] [PubMed] [Google Scholar]
- Strober M, Freeman R, Lampert C, Diamond J, Kaye W (2000) Controlled family study of anorexia nervosa and bulimia nervosa: evidence of shared liability and transmission of partial syndromes. Am J Psychiatry 157:393–401 [DOI] [PubMed] [Google Scholar]
- Sullivan PF, Bulik CM, Kendler KS (1998) The genetic epidemiology of binging and vomiting. Br J Psychiatry 173:75–79 [DOI] [PubMed] [Google Scholar]
- Teng J, Siegmund D (1998) Multipoint linkage analysis using affected relative pairs and partially informative markers. Biometrics 54:1247–1265 [PubMed] [Google Scholar]
- Terwilliger D, Speer M, Ott J (1993) Chromosome-based method for rapid computer simulation in human genetic linkage analysis. Genet Epidemiol 10:217–224 [DOI] [PubMed] [Google Scholar]
- van der Ham T, van Strien D, van Engeland H (1994) A four-year prospective follow-up study of 49 eating-disordered adolescents: differences in course of illness. Acta Psychiatr Scand 90:229–235 [DOI] [PubMed] [Google Scholar]
- Vieselman J, Roig M (1985) Depression and suicidality in eating disorders. J Clin Psychiatry 46:118–124 [PubMed] [Google Scholar]
- Wade T, Bulik C, Kendler K (2000) Reliability of bulimia nervosa and major depression. Br J Psychiatry 177:72–76 [DOI] [PubMed] [Google Scholar]
- Wade T, Neale MC, Lake RIE, Martin NG (1999) A genetic analysis of the eating and attitudes associated with bulimia nervosa: dealing with the problem of ascertainment. Behav Genet 29:1–10 [DOI] [PubMed] [Google Scholar]