Abstract
More HIV-infected women in need of services for the prevention of mother-to-child transmission of HIV (PMTCT) give birth in Nigeria than in any other nation in the world. To meet the UNAIDS/WHO goal of eliminating mother-to-child HIV transmission by 2015, multiple interventions will be required to scale up PMTCT services, especially to lower-level, rural health facilities. To address this, we are conducting a cluster-randomized controlled study to evaluate the impact and cost-effectiveness of a novel, family-focused integrated package of services for PMTCT. A systematic reassignment of patient care responsibilities coupled with the adoption of point-of-care CD4+ cell count testing could facilitate the ability of lower-cadre health providers to manage PMTCT care, including the provision and scale-up of antiretroviral therapy to pregnant women in rural settings. Additionally, as influential community members, male partners could support their partners’ uptake of and adherence to PMTCT care. We describe an innovative approach to scaling up PMTCT service provision that incorporates considerations of where and from whom women can access services (task-shifting), ease of obtaining a CD4 result (point-of-care testing), the degree of HIV service integration for HIV-infected women and their infants, and the level of family and community involvement (specifically male partner involvement). This systematic approach, if proven feasible and effective, could be scaled up in Nigeria and similar resource-limited settings as a means to accelerate progress toward eliminating mother-to-child transmission of HIV and help women with HIV infection live long, healthy lives (Trial registration: NCT01805752).
Keywords: prevention of mother-to-child HIV transmission, task shifting, male participation, point-of-care diagnostics, Nigeria, antiretroviral therapy uptake
1. Introduction
Nigeria bears the highest burden of mother-to-child HIV transmission in the world. Each year, 230,000 HIV-infected women in need of services for prevention of mother-to-child transmission of HIV (PMTCT) give birth in Nigeria, more than in any other nation [1]. In 2010, only 14% of pregnant women in Nigeria received HIV testing, and only 8% of eligible HIV-positive women were offered antiretroviral prophylaxis [1]. This shortfall is partly due to limited availability – only 39% of all facilities provide any PMTCT services, and the majority of services are concentrated in urban tertiary health centers [2].
A major barrier to effective PMTCT scale-up in Nigeria is the shortage of skilled health care providers who are trained in HIV prevention, especially in rural areas, where most primary and secondary level facilities are located [3]. However, studies elsewhere suggest that less-specialized cadres of health care workers are capable of effectively delivering HIV care with comparable or even better health outcomes as physicians [4–11]. Decentralization of PMTCT11 services to such facilities can therefore be undertaken via a systematic realignment of duties among health workers (“task-shifting”). Task shifting is feasible, beneficial, and efficacious when used to scale-up antiretroviral therapy (ART) services in sub-Saharan Africa [3–5]. Task-shifting is associated with improvement in quality of clinical care [6], reduced waiting time [7,8], decreased clinic costs [9], increased HIV service uptake [6], higher job satisfaction [10] and favorable ART adherence and survival [11]. However, task shifting could also compromise the quality of care, especially in the absence of adequate training, close mentoring and sustained health system support [12, 13].
Another bottleneck to PMTCT scale-up is the dearth of reliable and affordable laboratory infrastructure, particularly CD4+ cell count testing. WHO’s new programmatic option for PMTCT services (option B+, i.e., lifelong ART for pregnant women, regardless of CD4 levels) is yet to be widely adopted in Sub-Saharan Africa. Timely CD4+ cell count testing therefore remains an important component of management of pregnant women with HIV/AIDS. African nurses in rural primary health care centers (PHCs) can generate accurate CD4+ cell counts results using simple point-of-care (PoC) devices [12]. Several PoC CD4 testing systems are now validated and available for use in resource-limited settings, including Nigeria [15–17]. PoC devices in PHCs will make CD4+ cell count testing accessible to pregnant women, eliminating the need to travel to centralized sites for this service.
Compounding the resource barriers mentioned are cultural norms that limit a woman’s autonomy to make independent health care decisions [18,19]. In Nigeria, men substantially influence health-related decision-making in the family, yet they rarely accompany their wives to antenatal appointments [18]. Male participation in antenatal care with couple counseling and HIV testing increases PMTCT service uptake and adherence [20–22]; facilitates HIV status disclosure between couples; strengthens adoption of safe sexual behavior [23]; and reduces HIV-1 transmission in serodiscordant relationships [24–26].
The provision of comprehensive ART services to pregnant HIV-infected women in PHCs is challenging, yet feasible [27]. ART uptake is higher among women seen in antenatal clinics (ANCs) with ART services than among women who are referred out to freestanding ART clinics [28,29]. Providing same-venue services to mother-infant pairs allows HIV-infected women to be effectively managed in ANCs during the antenatal and intrapartum periods and permits HIV-infected women and their infants to be co-managed during postpartum visits to maternal and child health (MCH) clinics.
Whereas cost effectiveness studies on PMTCT exist [30–37], there is a paucity of such studies with economic data collected prospectively on actual patient cohorts [38,39]. Most studies are modeled on hypothetical cohorts and compare cost-effectiveness between various treatment regimens or between treatment regimens and no intervention.
We present the design of a parallel, cluster randomized trial in rural North Central Nigeria to evaluate the impact of a family-focused PMTCT package that includes: 1) task-shifting to lower-cadre providers at PMTCT sites; 2) PoC CD4+ cell count testing; (3) integrated mother-infant care; (4) a prominent role for male partners, working in partnership with community-based health workers/volunteers; and (5) a cost-effectiveness assessment with actual costs measured for PMTCT services.
2. Material and Methods
2.1. Setting
Friends in Global Health LLC, (FGH) a nongovernmental organization affiliated to Vanderbilt University (VU) supports the provision of comprehensive HIV/AIDS services in Abuja (Federal Capital Territory) and Niger and Kwara states of North Central Nigeria, with funding from the U.S. President’s Emergency Plan for AIDS Relief (PEPFAR). This study will be conducted in VU/FGH supported sites in Niger state (Figure 1). North Central Nigeria has the highest HIV prevalence in the country (2010 adult HIV prevalence of 7.5% vs. national HIV prevalence of 3.6%). The prevalence of HIV among adults in Niger state is estimated to be 4.0%. In Niger state, VU/FGH supports HIV treatment services, including PMTCT, in five comprehensive clinics linked to 12 PMTCT feeder sites (‘satellite’ sites) in a ‘hub-and-spoke’ model. These sites are predominantly rural primary health centers where the cultural, logistical and human resource constraints outlined earlier are especially prominent.
Figure 1.
Google™ map of North Central Nigeria showing location of Vanderbilt University/Friends in Global Health-supported study intervention clinics (green dotted icons), control/standard of care clinics (yellow plain icons) and referral sites (cross icons).
2.2. Study design
A parallel cluster randomized trial will be used to evaluate the effects of the intervention package. Twelve primary and secondary level health care facilities will be randomized to the control (standard-of-care) or intervention arms. A cluster study design was chosen because it will be impractical to limit the intervention to only a subset of clients within each facility. Additionally, a cost-effectiveness study will be conducted to assess actual costs associated with providing PMTCT services using the two service-delivery models (intervention package and standard of care).
2.3. Ethical considerations
Ethical approval was obtained from the Nigeria National Human Research Ethics Committee (Protocol # NHREC/01/01/2007-15/08/2012) and the Institutional Review Board of Vanderbilt University, USA (IRB #121257).
2.4 Specific Aims
To evaluate whether implementation of the integrated PMTCT package in primary level ANCs increases the proportion of eligible pregnant women who initiate antiretroviral medications for the purposes of PMTCT.
To determine whether implementation of the PMTCT package improves early (6 weeks) and later (12 weeks) postpartum retention of mother-infant pairs.
Conduct a cost-effectiveness analysis (CEA) of the impact of this novel PMTCT intervention compared to the existing standard-of-care referral model.
We will also measure HIV-free infant survival at 6 weeks. We are, however, unable to power the study to measure this outcome, as the number of HIV-infected pregnant women and clinics needed are not available and the amount of funding required to perform such a study would be enormous. Nevertheless, we believe that this is valuable information that needs to be collected.
In addition, evaluations of patient and clinician satisfaction with this intervention will be conducted in order to:
Assess client satisfaction with health services, comparing PMTCT services provided by lower level vs. higher level cadre health workers; and
Evaluate health care worker satisfaction with the new PMTCT service delivery model, including confidence with undertaking new responsibilities.
2.5. Study Population
2.5.1. Inclusion Criteria
HIV-infected women (and their infants) who present to ANC or delivery with unknown HIV status;
HIV-infected women (and their infants) with previous history of ARV prophylaxis or treatment, but who are not on prophylaxis or treatment at the time of presentation for antenatal care or delivery.
HIV-infected women with known HIV status who have never been on treatment at the time of presentation for antenatal care or delivery.
2.5.2. Exclusion Criteria
HIV-infected women with known status who are on ARV prophylaxis or treatment at the time of presentation to ANC
2.6. Randomization
Twelve sites were eligible for randomization. A matched randomization approach was employed, matching each site with another and then randomizing within the matched pairs. Sites were matched according to the following characteristics: total HIV patients seen during the past 6 months, monthly ANC volume, urbanization of area, and accessibility of site. To implement the matching procedure, a distance matrix was generated, describing the pairwise similarity between all sites based on the characteristics mentioned above and weighting these characteristics based on their perceived importance for the matching. The weights employed were 10, 3, 5, and 5 for total HIV patients seen, ANC volume, urbanization, and accessibility, respectively. A nonbipartite optimal matching was used to minimize the average reweighted Mahalanobis distance between pairs [40], and then randomized the 6 pairs to treatment groups A (standard of care) or B (integrated PMTCT package). The web application used to perform nonbipartite matching is available at http://data.vanderbilt.edu/rapache/matching.
The sites randomized to the intervention arm will provide an integrated package of PMTCT services in MCH settings to include: (1) PoC CD4 testing; (2) devolution of decentralized PMTCT tasks to trained midwives; (3) integrated mother-infant care; and (4) active influential family member (male partner) and community involvement. The intervention will be made available to all eligible women attending clinics that are randomized to the intervention arm. The 6 clinics randomized to the control arm will provide standard-of-care PMTCT services that include: group provision of health information; opt-out HIV testing and same-day HIV test results; infant feeding counseling; referral to hub centers for CD4 testing and treatment initiation; HBC services; infant prophylaxis and early infant diagnosis. PMTCT tasks at hub centers will be performed by licensed physicians with support from trained nurses and midwives. Table 1 lists the study sites by intervention arm and summarizes the PMTCT components that make up the intervention and standard-of-care arms of the proposed study.
Table 1.
Optimizing PMTCT Services in rural North Central Nigeria: Study sites and activities, Niger state, Nigeria
| Intervention Site | Matched SOC Site* (Control) |
|---|---|
| Sites | |
| Comprehensive Health Center, Agwara | Maternal and Child Health Clinic, Paiko |
| Rural Hospital, Kaffin Koro | Basic Health Clinic, Ijah |
| Rural Hospital, Agaie | Maternal and Child Health Clinic, Chanchaga |
| NCWS Clinic, Farin Doki | Basic Health Clinic, Garam |
| Rural Hospital, Auna | Primary Health Clinic, Gauraka |
| Basic Health Clinic, Wuse | Basic Health Clinic, Izom |
|
| |
| Activities | |
| Task-shifting to nurses, midwives, community health workers | Referral to hub centers for CD4 testing and treatment initiation |
| Point-of-care CD4 testing | |
| Enhanced male partner involvement and community engagement | |
| Integrated mother-infant care in Maternal and Child Health clinics | |
Participants in both intervention and SOC sites receive the following services: group health education; opt-out HIV testing, same-day HIV test results; infant feeding counseling; HBC services; infant prophylaxis, early infant diagnosis, and linkage to family spacing services, if desired.
SOC= Standard of Care; NCWS= National Council of Women’s Societies. Sites were matched on the basis of number of HIV patients, antenatal clinic volume, accessibility and level of urbanization.
2.7. Study Procedures
2.7.1. Standard-of-care services (SOC)
Women who test HIV-positive in control arm sites will be referred to nearby VU/FGH-supported, comprehensive clinics offering ART, based at secondary level facilities, for clinical and laboratory evaluation, and treatment initiation. The mean distance from a SOC center to the respective referral/hub site is 11 miles (range: 2 miles to 23 miles). Pregnant women with advanced WHO clinical stage disease (WHO stage 3 or 4) and/or advanced immunosuppression (CD4+ cell count <350/μL) will be started on ART for their own health, or placed on ARV prophylaxis with ART if they do not qualify for ART for their own health (“option B”). If the mother is breastfeeding, she will continue ART until 1 week after breastfeeding has ceased. The woman remains under the care of the referral center. Information on the woman’s treatment will be sent back to the PMTCT site so that her medical record can be updated accordingly.
After delivery, HIV-exposed infants will be started on daily NVP for 6 weeks, as per Nigerian guidelines. They will be asked to return to the MCH clinic at 6 weeks for follow-up, continuation of infant NVP (if mother is not on ART and is breastfeeding), immunizations, initiation of cotrimoxazole (CTX) prophylaxis and early infant diagnosis (EID) testing. Under the SOC model, women will continue their postpartum HIV care at the ART clinic, so many may choose to bring their infants to the ART clinic for evaluation, EID testing and CTX prophylaxis instead of receiving these services at the MCH clinic.
2.7.2. Intervention arm: a family-focused integrated PMTCT package
PoC CD4 testing
We will use the CyFlow® miniPOC system (Partec GmbH), a user-friendly CD4/CD4% diagnostic instrument that is well suited for use in resource-poor settings. The system is portable, robust, easy to operate, does not require cold-chain storage, can run up to 250 CD4 tests/day, requires minimal maintenance, and can operate on battery power, making it particularly suitable for remote PMTCT facilities. The FGH Lab Officer will train clinic staff in sample collection and routine internal quality control. Each intervention site will have one PoC analyzer available. All women testing HIV-positive will be offered a PoC CD4 test to determine ART eligibility on the same day they test HIV-positive. Blood samples will be sent to the referral ART site laboratory for chemistry and hematology evaluations. Baseline and follow-up labs will be conducted per Nigerian guidelines, following the same schedule as in control sites.
2.7.3. Task-shifting to lower-cadre HCWs (nurses/midwives/community health workers)
We will adopt a 3-pronged strategy (training, on-site mentoring, and continuous quality assurance) for the task-shifting component of our intervention. Lower-cadre staff at the intervention sites will undergo 5-day basic and advanced training using the Nigerian ART training curriculum and material adapted from the WHO Integrated Management of Adult Illnesses/Integrated Management of Pregnancy and Childbirth (IMAI/IMPAC) syllabus [41]. The IMAI/IMPAC material is perfect for our purposes because it was specifically developed to prepare ANC and delivery centers to provide same-site ARV prophylaxis or treatment for HIV-infected pregnant women. A minimum of three staff members will be trained at each site (two nurses/midwives, one community health worker [CHW], and, where available, one pharmacist/pharmacy technician). The CHW will assist the nurse/midwife by managing clinic flow, obtaining vital signs, and providing adherence counseling. Biannual on-site refresher training will be conducted by FGHIN staff. In intervention sites where the Nigeria Midwifery Services Scheme (MSS) is in place, we will utilize these skilled lower-cadre providers. A medical officer experienced in HIV care will provide regular on-site mentoring and consultation for complex cases. The medical officer will perform bimonthly chart reviews (QA review) to ensure that all lower-cadre health workers are providing safe and appropriate care to clients. Information obtained from the QA review will be shared with HCWs as feedback to improve service quality.
2.7.4. Integrated mother-child HIV care in MCH clinics
The task-shifting and POC CD4 testing components described will allow HIV-infected women to be effectively co-managed for pregnancy and HIV in the same site. Mother-infant pairs will be co-managed in the MCH clinic in the postpartum period and mother-infant visits aligned to reduce visit burden. This strategy will eliminate the need for the infant to receive care at the ART clinic while also receiving immunizations at the MCH clinic. Care will be provided to mother-infant pairs until the infant is at least 9 months old and has received 9-month immunizations. However, because of the 2-year duration of the study, only a small proportion of infants will attain 9 months age; primary outcomes will therefore be assessed at 6 and 12 weeks postpartum. Infants who test HIV-positive will be initiated on ART at the MCH clinic and monitored on treatment throughout the period of the study. HIV care and treatment will follow national guidelines for ART initiation, clinical care follow-up and laboratory monitoring. Women will receive counseling on healthy timing and spacing of pregnancy and will be linked to local family spacing providers, if desired for elective, safe and accessible contraception.
2.7.5. Encourage men to engage in their wives’ antenatal care
We will distribute personalized invitation letters (“love letters”) for men on behalf of health facilities, handed out to all women who test HIV positive and present for antenatal care without their partner. Personalized invitation letters have been shown to increase rates of male partner participation in counseling and testing by as much as 30% [42, 43]. The letter will request that men accompany their wives on their next antenatal care visit. During the antenatal visit, clinicians will sensitize and educate male partners on the importance of PMTCT for mother and child, test the partner, and encourage family support. This system allows health facility staff to target information about ANC services to the partners of pregnant women. The letters also create a system where men feel comfortable coming to the clinic. A request from the health facility may carry greater weight than a verbal message from their partners and reduces the onus placed on women to convince their husbands to attend antenatal care.
At facility level the project will create male-friendly health settings through capacity building of health care staff and by addressing policy and practice so that standard operational procedures include men in all pre- and post-natal appointments where both partners are willing. Male-friendly health facilities will share the following characteristics: i) health care workers with skills to attend to couples for counseling, testing and enrolment to PMTCT services; ii) physical space to allow privacy for couple counseling and testing as well as for follow-up pre- and post-natal visits; and iii) a male counselor available to attend to men who prefer to receive counseling from a male counselor.
2.7.6.Peer male mentors as ‘community mobilization champions’
FGH currently engages community volunteers to perform adherence monitoring and home based care (HBC). This study will build on this model by recruiting and training 6 male peer mentors for community mobilization per intervention clinic. Men who accompany their spouses to the health facility will be invited to become ‘champions’ of their communities so that they can educate others and share their own experience. Recruitment will be performed with the assistance of pregnant spouses and clinic staff. The expanded role of peer males will include: organizing outreach activities to raise awareness on male involvement in PMTCT; educating and encouraging men to accompany their spouses to PMTCT clinics; educating men regarding safe sex practices such as barrier methods and fidelity; distributing condoms; linking men to key referral services for couple counseling and testing, safe male circumcision, STI services and family planning; and soliciting the support of respected community leaders and influential family members, including male partners and mothers-in-law of pregnant women, through one-on-one interactions and community forums. Male champions will be paid a small stipend to support their transportation. The participation of male champions in disseminating HIV prevention education in the community addresses two critical PMTCT “prongs” i.e., the primary prevention of HIV in women, and the prevention of unintended pregnancies in HIV-infected women [44].
Mother-infant pairs who have presented for their 6-week postpartum appointment will be considered active in the program (retained in care). HBC workers will track all clients missing appointments at control and intervention sites. The HBC workers will document clients as terminated care if they: (1) discontinue services due to death or personal decision; (2) transfer their care to another clinic; or (3) are lost to follow-up (defined as being 90 days late for a clinic appointment plus 5 failed attempts at tracking the client).
The enrollment phase of the study will last 6 months. All mother-infant pairs will be followed for at least 12 weeks postpartum.
2.8. Data Collection
Routinely collected demographic, PMTCT, and HIV care and treatment data will be used in the analysis of specific aims 1 and 2. As per SOC, the WHO HIV Care Card and/or patient management and monitoring (PMM) forms will be completed for all HIV-infected women in the intervention clinics and for those enrolled into care in the SOC sites and verified in the referral centers associated with the control sites. Select clinical and laboratory data from these forms will be routinely entered into CAREWare™ (JPROG, New Orleans, LA), an electronic medical record system adapted at supported sites. All collected data will be entered by a research assistant into REDCap (Research Electronic Data Capture; Vanderbilt University, Nashville, TN, USA; http://project-redcap.org/, accessed April 3, 2013), a secure web application for building and managing online surveys and databases [45].
For specific aim 2 in the control arm, we will link PMTCT data (collected at the MCH clinic) with treatment data (collected at the referral ART clinic) because a woman and her infant may be receiving services at two different sites. This linkage process is necessary in order to determine if both mother and baby received care at 6- and 12-weeks postpartum.
Data collection for the cost effectiveness analysis will be nested within the cluster randomized trial. We will collect comprehensive data on the costs associated with providing PMTCT services (from maternal HIV testing during antenatal care to infant HIV testing at 12 weeks postpartum) in both study arms throughout the 18-month study period. The majority of costs will be obtained from the FGH business office, as VU/FGH directly supports site operations. Site personnel costs will be determined through interviews with staff and site managers.
Cost data for site personnel will be collected at two time-points from each of the 12 study clinics, and from FGH-supported referral centers where participants in the control arm receive HIV-related clinical and laboratory evaluations. In the referral centers, PMTCT services have been horizontally integrated across units (e.g., ART clinic, laboratory, pharmacy, ANC) so costs will be collected from these points of service. Our plan to gather data at two points in time reflects two considerations: (1) it is easier to identify outliers or anomalies due to measurement error and to take countermeasures to mitigate them; (2) we can measure directly the change in personnel wage rates and the prices of non-labor items, which will likely deviate from Nigeria’s sizable annual inflation rate (average 11.9% between 2008–2010). Given the cost of additional data collection and its burden on sites, gathering data at more than 2 points in time was not justifiable given funding limitations.
Program costs associated with PMTCT services include start-up and operating costs. The main categories of PMTCT start-up costs we plan to obtain, analyze, and compare between intervention and control clinics include: (1) training (e.g., staff training in care and treatment, CD4 testing procedures); (2) facilities and equipment (e.g., CD4 equipment purchases, clinic renovations); and (3) personnel (e.g., recruitment of clinic staff, project staff, or volunteers). The main categories of operating costs we will collect, analyze, and compare include: (1) personnel (e.g., compensation for clinic staff, peer mentors, volunteers); (2) training; (3) supplies and materials (e.g., consumables for HIV, CD4, and EID testing, ARVs); (4) non-personnel services (e.g., communication costs, shipping costs for EID samples); and (5) facilities and equipment (e.g., building leases, vehicles, laboratory equipment). We will exclude costs from the CEA models that were incurred to perform research-related tasks, such as data collection exclusive to the study.
For start-up costs, we will collect and report (at a minimum) the costs related to implementation of the integrated PMTCT package. Start-up costs associated with laboratory testing and treatment services at control sites will include historical data on training, equipment, and personnel. We will annualize and adjust the start-up costs from the two arms to put them on a comparable basis. Operating cost will be collected prospectively in both arms.
2.9. Primary endpoints
2.9.1. Primary outcomes
The primary outcome for Aim 1 is to compare the proportion of eligible women who initiate ARV medications for PMTCT across control and intervention arms. This will be determined through data in our electronic medical records system. Our outcome for Specific Aim 2 is retention of HIV-infected women and their infants in care at 6 and 12 weeks postpartum. Six and 12 weeks postpartum retention among women-infant pairs will be based on the proportion of mother-infant pairs enrolled in the program at the indicated time points (6 and 12 weeks, respectively). To be considered in care, the infant has to be receiving exposed infant care. We will also assess HIV-free survival at 6 and 12 weeks for all infants born to the HIV-infected pregnant women included in this study, including infant of mothers who do not come back to the clinic after initial study enrollment (described in section 2.12.2 below).
2.9.2. Secondary outcome
As a secondary outcome, we will evaluate adherence to treatment based on self-report. We will employ a modified version of the 12-item Medication Adherence Self-Report Inventory (MASRI) [46]. Optimal adherence to ART is a challenge during pregnancy and the postpartum period [47]. The MASRI has been shown to correlate well with MEMS data, pill counts and viral load assessments. It is particularly suited for our study because of its reliability and validity in assessing short term adherence, and its brevity and comprehensiveness [46]. In addition, we will encourage pregnant women to come along to their clinic visits with their most recently prescribed pill bottles. We will reconcile pill counts with refill history to determine the proportion of uningested pills [48].
2.9.3. Cost effectiveness outcomes
For the cost effectiveness analysis (Specific Aim 3), the basic outcome is the cost-effectiveness ratio (CER). The CER is the aggregate cost of the program in a given arm, divided by the total number of successful outcomes in that arm, i.e., cost per successful outcome in a trial arm. The CER will be computed for intervention and standard-of-care arms. Given the costs, outcomes, and CERs for the two arms, the evaluation uses the decision rule i.e., selects the option that has the lower CER, i.e., the lower cost per successful outcome. This variant of CEA is relatively easy to grasp and to interpret, especially when evaluating only two alternatives.
We will also measure a related outcome to the CER for the intervention arm, the incremental cost-effectiveness ratio (ICER). The ICER refers to the incremental costs of the intervention divided by the incremental change in clinical effectiveness. A separate ICER will be calculated for the two primary study outcomes (the cost per HIV-infected women who initiated treatment for PMTCT and the cost per mother-infant pair retained in care at 6 weeks postpartum) and for the secondary outcome (the cost per infant infection averted at 6 weeks of age). Calculating the ICER for our intervention complements the basic CER analysis and will permit comparisons with published studies that report ICERs for other PMTCT interventions.
For evaluation purposes, we will compare the ICERs for the intervention with a pre-specified cost-effectiveness threshold. Since no threshold is the default value used in evaluation sciences, we will compare the ICERs for the intervention to several defensible alternatives. If feasible, as discussed below, we will also conduct a sensitivity analysis of the ICERs to assess the effect of alternative estimates of clinical effectiveness and of cost on the ICER calculations and the recommendations that flow from those calculations.
2.11. Power Considerations
2.11.1. Sample size for Aim 1
A review of registers from 11 satellite clinics in Niger state estimates that 131 pregnant women tested HIV-positive during fiscal year 2011. Of these 131 HIV-infected pregnant women, 52 (40%) received ARVs. From this data, we also estimated the coefficient of variation (CV) to be just under 0.12. Sample size calculations are based on this CV and estimated prevalence of ARV receipt, matching clinics, and employing published formulas for pair-matched cluster randomized trials [49]. We anticipate having 90.1% power to detect a difference between 40% for 6 SOC clinics and 65% for 6 matched intervention clinics, recruiting approximately 25 eligible patients in each clinic (N=300, CV=0.12, P (type I error) = 0.05). We are well powered to detect various effect sizes if the trial data slightly varies from what we anticipate.
2.11.2. Sample size for Aim 2
We wish to demonstrate enhanced 6-week retention among women enrolled in the intervention arm. If only 60% of the women who initiated ART under SOC are retained at 6 weeks, then overall 6-week retention is 24.0% (=0.6 × 0.4). If 70% of those women who initiated ART under intervention are retained at 6 weeks, then overall 6-week retention is 45.5% (=0.7 × 0.65). Based on these numbers (and CV=0.12), with 12 matched sites in our trial recruiting approximately 25 patients each (N=300), we anticipate having 84.3% power to detect an increase in the proportion of HIV-infected pregnant women who return at 6 weeks post-partum from 24.0% (SOC) to 45.5% (intervention). With that said, we hope that the 6-week retention in the intervention arm will be substantially greater than 45.5%.
We wish to also demonstrate enhanced 3-month retention among women enrolled in the intervention arm. If only 50% of the women who initiated ART under SOC are retained at 12 weeks, then overall 12-week retention is 20.0%. If 65% of those women who initiated ART under intervention are retained at 12 weeks, then overall 12-week retention is 40.3%. Based on these numbers (and CV=0.12), with 12 matched sites recruiting approximately 25 patients each (N=300), we anticipate having 83.9% power to detect an increase in the proportion of HIV-infected pregnant women who return at 12 weeks post-partum from 20.0% (SOC) to 40.3% (intervention). Again, we hope that the 12-week retention in the intervention arm will be substantially greater than that used for this power calculation.
Figure 2 shows power analysis for 12 clinics (6 matched-pairs) for ARV receipt and 6- and 12- weeks retention based on various estimates of coefficient of variation derived from prior program data.
Figure 2.
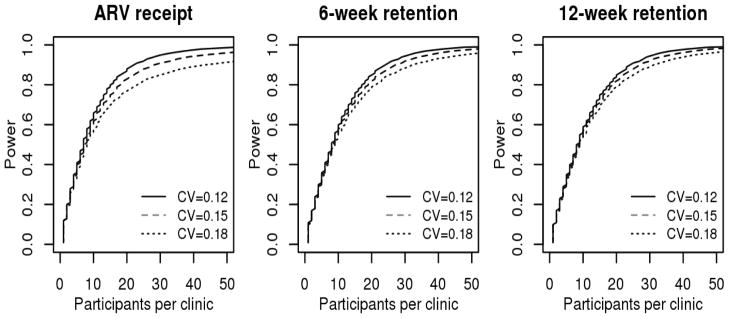
Power analysis for 12 clinics (6 matched-pairs) based on estimates of coefficient of variation (CV) derived from prior program data, North Central Nigeria
2.12. Statistical analysis
2.12.1. Statistical analysis plan for Aim 1
Our outcome of interest is the proportion of HIV-infected women eligible for treatment who initiate ART. We propose to use a generalized linear mixed effects model, with random effect for each matched clinic pair. A simple model for E(Yij), the probability of ARV receipt for the ith patient from the jth matched clinic pair is given by:
where bj is a random effect for the jth matched clinic pair which accounts for correlation of patients within matched clinics, due perhaps to similarities in prevalence of disease and/or access to care. β1 is the fixed effect of being randomized to the intervention arm, designated by Interventionj; β1 is interpreted as the difference in log-odds of ARV receipt between the intervention and control arms, conditional on the random effect. We will test the hypothesis, H0: β1 = 0. The mixed effects model will be extended to allow for covariate adjustment based on individual characteristics, such as: age, education, and distance from clinic. An intention-to-treat approach will be employed for all analyses.
2.12.2. Statistical analysis plan for Aim 2
To determine the effect of the intervention on retention of mother-infant pairs on ART at 6 weeks and again at 12 weeks, we will compare the proportion of HIV-infected women who are retained at the time period of interest using generalized linear mixed effects models, with matched clinic pair treated as a random effect. In the primary analysis, women who are lost to follow-up will be considered non-adherent to care. Secondary analysis will censor those who are lost to follow-up; inverse probability of censoring weights will be used to correct for dependent censoring.
Similar methods will be used to assess HIV-free survival among infants at 6 weeks. We will assume 3.5% HIV infection or death among infants from HIV-infected mothers who do not initiate ART, and 1.7% HIV infection or death among infants whose mothers initiate ART, but are not compliant at 6 weeks [50]. Doing so will allow for direct comparison of the two intervention arms by including all eligible participants. We will investigate the sensitivity of our HIV-free survival estimate assumptions by varying the 1.7% and 3.5% parameters. We will also present estimates of HIV-survival among infants with documented outcomes, keeping in mind that these estimates will likely be biased by the expected differences in retention and therefore may not represent the true difference among all enrolled infants.
2.12.3. Sensitivity Analysis
A decision-tree model will be used for determining infant HIV infections averted and to conduct a sensitivity analysis of cost. The critical elements used in the decision tree will include: HIV testing; maternal HIV status; CD4 testing; initiation of drugs for PMTCT; place of delivery; infant treatment; breastfeeding status; and infant HIV status. The proportion of women and infants who receive PMTCT services at each of the steps of the PMTCT cascade will be determined through prospective data collection and to a lesser extent from programmatic M&E reports. These data will serve as the base case in our analysis. We will also conduct a one-way sensitivity analysis to account for the possibility of alternative estimates of clinical effectiveness and cost.
2.13. Evaluation of Patient and Health Care Worker Satisfaction
2.13.1. Methodology for assessing patient satisfaction
We will assess patient satisfaction via a standard, closed-ended Likert-type scale validated questionnaire [51,52] measuring patient assessment of clinician effectiveness and satisfaction with the quality of care received. Surveys and semi-structured interviews will be designed using social cognitive theory [53]: questions will focus on self-efficacy, outcome expectancies, assigned importance outcomes, and intention to follow through with antenatal care services. Informed consent will be collected by research assistants. The questionnaire will be piloted at 2 randomly selected sites (1 site per study arm) in the local language (Hausa) prior to administration at 3 randomly selected intervention and 3 control sites. The survey instrument we selected has sufficient sensitivity to differentiate between different members of the health care team [51], making it an ideal tool for evaluating task-shifting. This tool will also be used to assess satisfaction with the engagement of male partners in antenatal care services. Questions will include assessments of perceived quality of examination, satisfaction with time spent with each client to assess health concerns and provide sufficient feedback to facilitate understanding, level of comfort with the provider, and willingness to follow the providers’ recommendations. The questionnaires will be administered by trained research assistants at the 6-week postpartum visit. We choose 6 weeks postpartum visit in order to minimize biased recall associated with long duration/passage of time. We will also conduct qualitative interviews with a sub-group of women (15 women each in 3 randomly selected sites per arm), after task-shifting has been completed and the love letter system is in place to learn about their experiences in greater detail. Interviews will be semi-structured, covering participants’ experience with PMTCT, reasons for accepting or refusing prophylaxis, and social and clinical barriers to PMTCT.
2.13.2. Methodology for assessing health care worker satisfaction
Health care worker satisfaction will be assessed in English using open-ended qualitative questions and scaled Likert-type questions. Based on a similar assessment given to CHWs in South Africa [54], this survey will allow for confidential written survey responses about perceived successes and challenges of task-shifting at each clinical site. The survey will contain four sections: (1) ideal responsibilities of these clinical staff; (2) training quality, length, and practicality; (3) challenges in providing successful PMTCT at their clinical site (including the neglect of other clinical duties); and (4) support and mentorship from supervisors. After informed consent is obtained by research staff, surveys will be given to clinical workers responsible for providing PMTCT services at 3 intervention and 3 control sites.
We will also conduct qualitative interviews with clinicians to learn about their experiences providing PMTCT, and to gain information about their perception of male involvement in antenatal care. Interviews will be semi-structured, with participants asked to describe their experience providing PMTCT at clinical sites, their thoughts on improving PMTCT uptake and adherence, and barriers to implementing these improvements in the clinical setting. Data will be stripped of all identifiers during analysis. Only FGH research staff and VU team members will have access to this data. Clients will not receive any remuneration for participating in the study.
2.13.3. Statistical Analysis for Evaluation of Patient and Health Care Worker Satisfaction
To describe each study population a number of demographic factors will be collected, including educational attainment, current employment, and number of previous children (if applicable). Maximum likelihood exploratory factor analysis with orthogonal rotation will be used to examine the factor structure of the newly developed instrument. If, as planned, multiple factors emerge, the internal consistency reliability of each subscale will be determined using Cronbach’s alpha. A satisfaction score will be computed as a combination of all constructs. This will yield a continuous score. To test construct validity of the patient measure, correlations between the score and uptake and adherence to PMTCT will be calculated using Wilcoxon rank-sum test for yes/no responses and Kruskal-Wallis test for categorical variables. To test construct validity of the clinician measure, correlations between the score and previous employment and educational attainment will be calculated using Spearman rank correlation for continuous variables and Kruskal-Wallis test for categorical variables.
2.13.4 Qualitative Data Analysis Plan
Audio files will be transcribed, coded and analyzed using Atlas Ti© software to assist in qualitative data analysis. The data will be analyzed using mixed methods: Framework Analysis will be used to identify responses to our questions about the drivers, core facilitators, and barriers to nurses successfully providing enhanced care for pregnant women with sub-analysis of each category done using grounded theory [55]. Two code maps will be developed to categorize data: (1) to understand the social, structural, and informational drivers, facilitators, barriers to nurses providing PMTCT care, and (2) to understand perceptions nurses have of male involvement in improving uptake of prenatal care services, including PMTCT, among women in their clinical sites. Ten percent of the data will be coded by two independent coders; only if intercoder agreement is high (as established via Cohen’s kappa) will the remaining data be coded by our expert qualitative data analyst alone.
3. Discussion
The strengths of the proposed study include: (1) A rigorous research design: The cluster randomized trial without formal study recruitment allows us to determine the real-life effectiveness of our intervention, with a triangulation of data collection methods. (2) High impact: An effective model of bridging the PMTCT coverage gap in Nigeria will have substantial impact on progress toward the global elimination of mother-to-child transmission of HIV. (3) Ideal research environment: We are supported by the strong research and service infrastructure of our institutions, familiarity with local communities, and the enthusiasm of our collaborating sites.
A source of concern with this study is the impact of new WHO PMTCT guidelines that recommend moving to option B (ART during pregnancy and breastfeeding without advanced disease; lifelong ART with advanced disease) or option B+ (lifelong ART for all pregnant/breastfeeding, HIV-infected women) [56]. With option B+, the requirement for PoC CD4 testing to determine which women are eligible to initiate ART for their own health no longer holds. Nevertheless, we believe that this model of integration, task shifting, and male partner involvement would still be important for implementation of any B+ strategy. Many PMTCT sites in Nigeria still use option A. The treatment sites in this study commenced implementation of option B in 2012. The WHO recommends that countries should prepare adequately and ensure that systems are in place (clear policy, training, logistics, etc) prior to adopting option B+ [57]. We anticipate that the government of Nigeria will provide guidance regarding transitioning to option B/B+ in the near future, but likely not before the conclusion of this study. In addition, considering the higher costs associated with viral load testing compared to CD4+ testing, we expect that CD4 cell count testing will remain a desirable choice for determining baseline immunological status and monitoring treatment response for HIV-infected women in our setting.
A limitation of this study is the possibility of overestimating the true HIV-free survival rate in the cohort, given that some infants may still be breastfeeding at the time of the 6 and 12-week EID test. Inter-clinic contamination is possible with the cluster randomized design, but should be minimized through the selection of geographically isolated clinics. The proposed cost effectiveness analysis also has limitations: (1) It will only assess program costs and vertical transmission rates through 12 weeks postpartum. Given the continued risk of transmission associated with breastfeeding infants, the analysis may overestimate the number of infections averted and the associated costs; (2) The analysis will focus only on health care expenditures associated with providing PMTCT services and averting infant infection, and will not consider the savings in medical costs that are associated with preventing an infant infection; (3) Costs incurred by clients missing work to travel to clinic appointments will not be accounted for; (4) Whereas the results of the CEA may be applicable to many settings in Nigeria, especially PEPFAR-funded programs, they may not be generalizable to other countries or to privately-funded programs.
4. Conclusion
In summary, we propose to study a plan of structural reform of health care services that can be applied by Nigerian authorities efficiently, if it proves effective in our trial. The information gained from the proposed study will provide insight into the impact of an integrated PMTCT package (task shifting, point of care CD4 testing, enhanced male partner involvement, and integrated mother-child postpartum care) on ART uptake, retention in care and 6 and 12 weeks HIV-free infant survival. These insights may enable us to identify interventions that could be implemented broadly in PMTCT programs in resource-limited settings around the world, where transmission of HIV infections to exposed infants continues to occur. This could lead to reductions in the number of HIV infections seen in infants and improvement in the overall health status of HIV positive mothers. Our reforms and our trial may provide an important path towards sustainability for PEPFAR programs, nested within the Nigerian MCH system. Current PMTCT models are not proving effective in Nigeria, and we think novel approaches need to be studied, and if effective, implemented and expanded.
Acknowledgments
We acknowledge the valuable contributions of the following persons to the successful launch of this research study: Julie Lankford, Assistant Director, Global Operations, Vanderbilt Institute for Global Health; Awwal Gambo, Research Coordinator, Friends in Global Health, Nigeria; Dr. Mukhtar Muhammad, Chief Technical Officer, Friends in Global Health, Nigeria; Ibrahim Sodangi, M&E Specialist, Friends in Global Health, Nigeria; and Dr. Saidu Ishaq, Director, Clinical Services, Friends in Global Health, Nigeria.
6. Financial support
This publication was made possible by support from the Eunice Kennedy Shriver National Institute of Child Health & Human Development of the National Institutes of Health, award number R01HD075075. The findings and conclusions are those of the authors and do not necessarily represent the official position of the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.WHO/UNAIDS/UNICEF. Progress report 2011. Geneva: World Health Organization; 2011. Global HIV/AIDS response: Epidemic update and health services progress toward universal access. [Google Scholar]
- 2.Ugo A, Hatt L, Arur ATA, Mehta-Steffen M, De Valdenebro MC, Ogungbemi K, et al. Nigeria HIV/AIDS Service Provision Assessment 2008. Bethesda, MD: Health Systems 20/20 Project, Abt Associates Inc; 2008. [Google Scholar]
- 3.Human Resources for Health: Strategic Plan 2006–2008. Kigali: Ministry of Health, Rwanda; 2006. [Google Scholar]
- 4.Bemelmans M, Van Den Akker T, Ford N, Philips M, Zachariah R, Harries A, et al. Providing universal access to antiretroviral therapy in Thyolo, Malawi through task shifting and decentralization of HIV/AIDS care. Trop Med Int Health. 2010;15:1413–1420. doi: 10.1111/j.1365-3156.2010.02649.x. [DOI] [PubMed] [Google Scholar]
- 5.Gimbel-Sherr SO, Micek MA, Gimbel-Sherr KH, Koepsell T, Hughes JP, Thomas KK, et al. Using nurses to identify HAART eligible patients in the Republic of Mozambique: results of a time series analysis. Hum Resour Health. 2007;5:7. doi: 10.1186/1478-4491-5-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Morris MB, Chapula BT, Chi BH, Mwango A, Chi HF, Mwanza J, et al. Use of task-shifting to rapidly scale-up HIV treatment services: experiences from Lusaka, Zambia. BMC Health Serv Res. 2009;9:5. doi: 10.1186/1472-6963-9-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Callaghan M, Ford N, Schneider H. A systematic review of task- shifting for HIV treatment and care in Africa. Hum Resour Health. 2010;8:8. doi: 10.1186/1478-4491-8-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Torpey KE, Kabaso ME, Mutale LN, Kamanga MK, Mwango AJ, Simpungwe J, et al. Adherence support workers: a way to address human resource constraints in antiretroviral treatment programs in the public health setting in Zambia. PLoS One. 2008;3:e2204. doi: 10.1371/journal.pone.0002204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bussmann C, Rotz P, Ndwapi N, Baxter D, Bussmann H, Wester CW, et al. Strengthening healthcare capacity through a responsive, country-specific, training standard: the KITSO AIDS training program’s support of Botswana’s national antiretroviral therapy rollout. Open AIDS J. 2008;2:10–16. doi: 10.2174/1874613600802010010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tobi P, George G, Schmidt E, Renton A. Antiretroviral treatment and the health workforce in South Africa: how have ART workers been affected by scaling up? Trop Med Int Health. 2008;13:1452–1458. doi: 10.1111/j.1365-3156.2008.02169.x. [DOI] [PubMed] [Google Scholar]
- 11.Cohen R, Lynch S, Bygrave H, Eggers E, Vlahakis N, Hilderbrand K, et al. Antiretroviral treatment outcomes from a nurse-driven, community-supported HIV/AIDS treatment programme in rural Lesotho: observational cohort assessment at two years. J Int AIDS Soc. 2009;12:23. doi: 10.1186/1758-2652-12-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brentlinger PE, Assan A, Mudender F, Ghee AE, Vallejo Torres J, Martínez P, et al. Task shifting in Mozambique: cross-sectional evaluation of non-physician clinicians’ performance in HIV/AIDS care. Hum Resour Health. 2010 Oct 12;8:23. doi: 10.1186/1478-4491-8-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stekelenburg J, Kyanamina SS, Wolffers I. Poor performance of community health workers in Kalabo District, Zambia. Health Policy. 2003 Aug;65(2):109–18. doi: 10.1016/s0168-8510(02)00207-5. [DOI] [PubMed] [Google Scholar]
- 14.Jani IV, Sitoe NE, Chongo PL, Alfai ER, Quevedo JI, Tobaiwa O, et al. Accurate CD4 T-cell enumeration and antiretroviral drug toxicity monitoring in primary healthcare clinics using point-of-care testing. AIDS. 2011;25:807–812. doi: 10.1097/QAD.0b013e328344f424. [DOI] [PubMed] [Google Scholar]
- 15.Manabe YC, Wang Y, Elbireer A, Auerbach B, Castelnuovo B. Evaluation of portable point-of-care CD4 counter with high sensitivity for detecting patients eligible for antiretroviral therapy. PLoS One. 2012;7:e34319. doi: 10.1371/journal.pone.0034319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mnyani CN, McIntyre JA, Myer L. The reliability of point-of-care CD4 testing in identifying HIV-infected pregnant women eligible for antiretroviral therapy. J Acquir Immune Defic Syndr. 2012;60:260–264. doi: 10.1097/QAI.0b013e318256b651. [DOI] [PubMed] [Google Scholar]
- 17.Diaw PA, Daneau G, Coly AA, Ndiaye BP, Wade D, Camara M, Mboup S, Kestens L, Dieye TN. Multisite evaluation of a point-of-care instrument for CD4(+) T-cell enumeration using venous and finger-prick blood: the PIMA CD4. J Acquir Immune Defic Syndr. 2011;58:e103–11. doi: 10.1097/QAI.0b013e318235b378. [DOI] [PubMed] [Google Scholar]
- 18.Iliyasu Z, Abubakar IS, Galadanci HS, Aliyu MH. Birth preparedness, complication readiness and fathers’ participation in maternity care in a northern Nigerian community. Afr J Reprod Health. 2010;14:21–32. [PubMed] [Google Scholar]
- 19.Isiugo-Abanihe UC. Sociocultural aspects of HIV/AIDS infection in Nigeria. Afr J Med Med Sci. 2006;35 (Suppl):45–55. [PubMed] [Google Scholar]
- 20.Farquhar C, Kiarie JN, Richardson BA, Kabura MN, John FN, Nduati RW, et al. Antenatal couple counseling increases uptake of interventions to prevent HIV-1 transmission. J Acquir Immune Defic Syndr. 2004;37:1620–6. doi: 10.1097/00126334-200412150-00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Painter TM. Voluntary counseling and testing for couples: a high-leverage intervention for HIV/AIDS prevention in sub-Saharan Africa. Soc Sci Med. 2001;53:1397–411. doi: 10.1016/s0277-9536(00)00427-5. [DOI] [PubMed] [Google Scholar]
- 22.Semrau K, Kuhn L, Vwalika C, Kasonde P, Sinkala M, Kankasa C, Shutes E, Aldrovandi G, Thea DM. Women in couples antenatal HIV counseling and testing are not more likely to report adverse social events. AIDS. 2005;19:603–9. doi: 10.1097/01.aids.0000163937.07026.a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mbonye AK, Hansen KS, Wamono F, Magnussen P. Barriers to prevention of mother-to-child transmission of HIV services in Uganda. J Biosoc Sci. 2010;42:271–83. doi: 10.1017/S002193200999040X. [DOI] [PubMed] [Google Scholar]
- 24.Allen S, Tice J, Van de Perre P, Serufilira A, Hudes E, Nsengumuremyi F, et al. Effect of serotesting with counselling on condom use and seroconversion among HIV discordant couples in Africa. BMJ. 1992;304:1605–9. doi: 10.1136/bmj.304.6842.1605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Allen S, Meinzen-Derr J, Kautzman M, Zulu I, Trask S, Fideli U, Musonda R, Kasolo F, Gao F, Haworth A. Sexual behavior of HIV discordant couples after HIV counseling and testing. AIDS. 2003;17:733–40. doi: 10.1097/00002030-200303280-00012. [DOI] [PubMed] [Google Scholar]
- 26.Bunnell RE, Nassozi J, Marum E, Mubangizi J, Malamba S, Dillon B, et al. Living with discordance: knowledge, challenges, and prevention strategies of HIV-discordant couples in Uganda. AIDS Care. 2005;17:999–1012. doi: 10.1080/09540120500100718. [DOI] [PubMed] [Google Scholar]
- 27.Mandala J, Torpey K, Kasonde P, Kabaso M, Dirks R, Suzuki C, et al. Prevention of mother-to-child transmission of HIV in Zambia: implementing efficacious ARV regimens in primary health centers. BMC Public Health. 2009;9:314. doi: 10.1186/1471-2458-9-314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Killam WP, Tambatamba BC, Chintu N, Rouse D, Stringer E, Bweupe M, et al. Antiretroviral therapy in antenatal care to increase treatment initiation in HIV-infected pregnant women: a stepped-wedge evaluation. AIDS. 2010;24:85–91. doi: 10.1097/QAD.0b013e32833298be. [DOI] [PubMed] [Google Scholar]
- 29.Tsague L, Tsiouris FO, Carter RJ, Mugisha V, Tene G, Nyankesha E, et al. Comparing two service delivery models for the prevention of mother-to-child transmission (PMTCT) of HIV during transition from single-dose nevirapine to multi-drug antiretroviral regimens. BMC Public Health. 2010;10:753. doi: 10.1186/1471-2458-10-753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Johri M, Ako-Arrey D. The cost-effectiveness of preventing mother-to-child transmission of HIV in low- and middle-income countries: systematic review. Cost Eff Resour Alloc. 2011;9:3. doi: 10.1186/1478-7547-9-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Maclean CC, Stringer JS. Potential cost-effectiveness of maternal and infant antiretroviral interventions to prevent mother-to-child transmission during breast-feeding. J Acquir Immune Defic Syndr. 2005;38:570–577. doi: 10.1097/01.qai.0000142919.51570.88. [DOI] [PubMed] [Google Scholar]
- 32.Mansergh G, Haddix AC, Steketee RW, Nieburg PI, Hu DJ, Simonds RJ, et al. Cost-effectiveness of short-course zidovudine to prevent perinatal HIV type 1 infection in a sub-Saharan African Developing country setting. JAMA. 1996;276:139–145. [PubMed] [Google Scholar]
- 33.Marseille E, Kahn JG, Mmiro F, Guay L, Musoke P, Fowler MG, et al. Cost effectiveness of single-dose nevirapine regimen for mothers and babies to decrease vertical HIV-1 transmission in sub-Saharan Africa. Lancet. 1999;354:803–809. doi: 10.1016/S0140-6736(99)80009-9. [DOI] [PubMed] [Google Scholar]
- 34.Marseille E, Kahn JG, Saba J. Cost-effectiveness of antiviral drug therapy to reduce mother-to-child HIV transmission in sub-Saharan Africa. AIDS. 1998;12:939–948. doi: 10.1097/00002030-199808000-00017. [DOI] [PubMed] [Google Scholar]
- 35.Shah M, Johns B, Abimiku A, Walker DG. Cost-effectiveness of new WHO recommendations for prevention of mother-to-child transmission of HIV in a resource-limited setting. AIDS. 2011;25:1093–1102. doi: 10.1097/QAD.0b013e32834670b9. [DOI] [PubMed] [Google Scholar]
- 36.Soderlund N, Zwi K, Kinghorn A, Gray G. Prevention of vertical transmission of HIV: analysis of cost effectiveness of options available in South Africa. BMJ. 1999;318:1650–1656. doi: 10.1136/bmj.318.7199.1650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stringer JS, Rouse DJ, Vermund SH, Goldenberg RL, Sinkala M, Stinnett AA. Cost-effective use of nevirapine to prevent vertical HIV transmission in sub-Saharan Africa. J Acquir Immune Defic Syndr. 2000;24:369–377. doi: 10.1097/00126334-200008010-00012. [DOI] [PubMed] [Google Scholar]
- 38.Orlando G, Meraviglia P, Valsecchi L, Mainini A, Schiavini M, Merli S, et al. cART durability and causes for treatment switching or discontinuation in HIV-positive patients older than 50 years of age. J Acquir Immune Defic Syndr. 2010;55:e12–14. doi: 10.1097/QAI.0b013e3181ef791b. [DOI] [PubMed] [Google Scholar]
- 39.Robberstad B, Evjen-Olsen B. Preventing mother to child transmission of HIV with highly active antiretroviral treatment in Tanzania--a prospective cost-effectiveness study. J Acquir Immune Defic Syndr. 2010;55:397–403. doi: 10.1097/QAI.0b013e3181eef4d3. [DOI] [PubMed] [Google Scholar]
- 40.Greevy R, Lu B, Silber JH, Rosenbaum P. Optimal multivariate matching before randomization. Biostatistics. 2004;5:263–75. doi: 10.1093/biostatistics/5.2.263. [DOI] [PubMed] [Google Scholar]
- 41.WHO, Integrated Management of Pregnancy and Childbirth (IMPAC) Pregnancy, childbirth, postpartum and newborn care: a guide for essential practice. Geneva: 2006. [PubMed] [Google Scholar]
- 42.Abdallah A, Semo B, Justman J, El-Sadr W. Increasing male participation in PMTCT. Presented at the 2006 HIV/AIDS Implementers Meeting of the President’s Emergency Plan for AIDS Relief; Durban, South Africa. 2006. p. Abstract 80. [Google Scholar]
- 43.Bolu OO, Allread V, Creek T, Stringer E, Forna F, Bulterys M, Shaffer N. Approaches for scaling up human immunodeficiency virus testing and counseling in prevention of mother-to-child human immunodeficiency virus transmission settings in resource-limited countries. Am J Obstet Gynecol. 2007;197:S83–9. doi: 10.1016/j.ajog.2007.03.006. [DOI] [PubMed] [Google Scholar]
- 44.World Health Organization. Strategic approaches to the prevention of HIV infection in infants. Report of a WHO meeting; Morges, Switzerland. 20–22 March 2002; [Accessed June 8, 2013.]. Available at: http://www.who.int/hiv/pub/mtct/en/StrategicApproachesE.pdf. [Google Scholar]
- 45.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009 Apr;42(2):377–81. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Walsh JC, Mandalia S, Gazzard BG. Responses to a 1 month self-report on adherence to antiretroviral therapy are consistent with electronic data and virological treatment outcome. AIDS. 2002;16:269–77. doi: 10.1097/00002030-200201250-00017. [DOI] [PubMed] [Google Scholar]
- 47.Nachega JB, Uthman OA, Anderson J, Peltzer K, Wampold S, Cotton MF, et al. Adherence to antiretroviral therapy during and after pregnancy in low-income, middle-income, and high-income countries: a systematic review and meta-analysis. AIDS. 2012;26:2039–52. doi: 10.1097/QAD.0b013e328359590f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Steiner JF, Prochazka AV. The assessment of refill compliance using pharmacy records: methods, validity, and applications. J Clin Epidemiol. 1997;50:105–16. doi: 10.1016/s0895-4356(96)00268-5. [DOI] [PubMed] [Google Scholar]
- 49.Hayes RJ, Bennett S. Simple sample size calculation for cluster-randomized trials. Int J Epidemiol. 1999;28:319–326. doi: 10.1093/ije/28.2.319. [DOI] [PubMed] [Google Scholar]
- 50.Cooper ER, Charurat M, Mofenson L, Hanson IC, Pitt J, Diaz C, et al. Women and Infants’ Transmission Study Group. Combination antiretroviral strategies for the treatment of pregnant HIV-1-infected women and prevention of perinatal HIV-1 transmission. J Acquir Immune Defic Syndr. 2002;29:484–94. doi: 10.1097/00126334-200204150-00009. [DOI] [PubMed] [Google Scholar]
- 51.Poulton BC. Use of the consultation satisfaction questionnaire to examine patients’ satisfaction with general practitioners and community nurses: reliability, replicability and discriminant validity. Br J Gen Pract. 1996;46:26–31. [PMC free article] [PubMed] [Google Scholar]
- 52.Baker R. Development of a questionnaire to assess patients’ satisfaction with consultations in general practice. Br J Gen Pract. 1990;40:487–490. [PMC free article] [PubMed] [Google Scholar]
- 53.Bandura A. Social cognitive theory and exercise of control over HIV infection. In: DiClemente CC, Peterson JL, editors. Preventing AIDS: Theories and methods of behavioral interventions. Plenum; New York: 1994. pp. 25–59. [Google Scholar]
- 54.Suri A, Gan K, Carpenter S. Voices from the field: perspectives from community health workers on health care delivery in rural KwaZulu-Natal, South Africa. J Infect Dis. 2007;196 (Suppl 3):S505–511. doi: 10.1086/521122. [DOI] [PubMed] [Google Scholar]
- 55.Pope C, Ziebland S, Mays N. Qualitative research in health care. Analysing qualitative data. BMJ. 2000;320:114–116. doi: 10.1136/bmj.320.7227.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.World Health Organization. [Accessed April 3, 2013.];Antiretroviral drugs for treating pregnant women and preventing HIV infection in infants: towards universal access. 2010 Available: http://whqlibdoc.who.int/publications/2010/9789241599818_eng.pdf. [PubMed]
- 57.World Health Organization. Programmatic update. Use of antiretroviral drugs for treating pregnant women and preventing HIV infection in infants. Geneva: World Health Organization; [Accessed June 8, 2013.]. http://www.who.int/hiv/PMTCT_update.pdf. [Google Scholar]


