Abstract
CD133 (prominin-1) is a member of the transmembrane glycoprotein family and was initially described as a specific marker to select human hematopoietic progenitor cells. Later it was recognised as an important marker to identify and isolate the specific cell subpopulation termed “cancer stem cells” (CSCs). Many studies showed that CD133+ cells have stemness properties such as self-renewal, differentiation ability, high proliferation and they are also able to form tumors in xenografts. Moreover it has been demonstrated that CD133+ cells are more resistant to radiation and standard chemotherapy than CD133- cells. Despite this other investigations demonstrated that also CD133- cells can show the same characteristics as those positive for CD133+. Hence, some inconsistencies among published data on CD133 function can be ascribed to different causes questioning the main role as specific marker of cancer stem cells. In fact, many authors indicate that CD133 is expressed both in differentiated and undifferentiated cells, and CD133- cancer cells can also initiate tumors. Indeed, it is still a matter of debate whether CD133+ cells truly represent the ultimate tumorigenic population. However, the belief that CD133 may act as a universal marker of CSCs has been met with a high degree of controversy in the research community. In this review there is an attempt to highlight: i) the role and function of CD133, with an overview of the current stage of knowledge regarding this molecule, ii) the difficulty often encountered in its identification iii) the utility of CD133 expression as a prognostic marker.
Keywords: Prominin-1 (CD133), cancer stem cells, epithelial-mesenchymal transition, glycosylation, epigenetic regulation, circulating tumor cells
Introduction
Prominin-1 (CD133) was the first protein identified as “Prominin”; it originates from the Latin word “prominere” meaning to protrude. It is a member of the pentaspan transmembrane (5-TM) glycoprotein family. In humans, the gene of Prominin-1 is placed on chromosome 4p15 and encodes a transmembrane glycoprotein of 120 kD [1,2].
A structural model of CD133 proposed by Miraglia et al. showed that this protein is characterized by: an extracellular N-terminus, a cytoplasmic C-terminus, 2 small cysteine rich cytoplasmic loops and 2 very large extracellular loops each containing 4 potential sites for N-linked glycosylation [1,2] (Figure 1).
Figure 1.
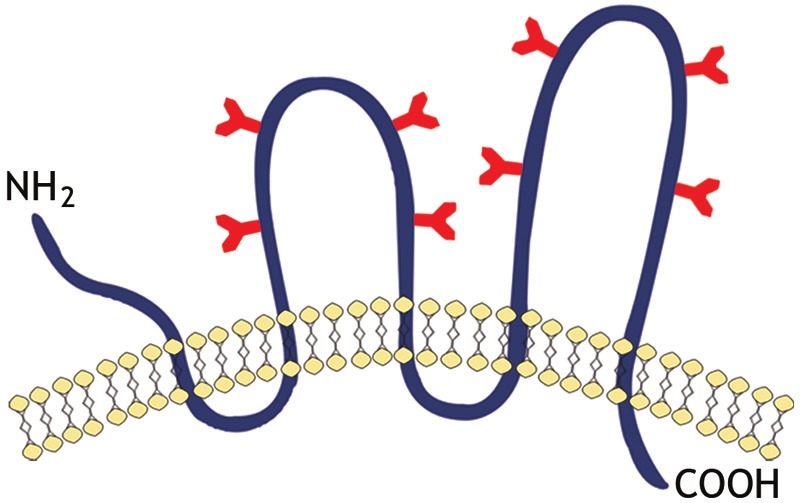
Schematic representation of transmembrane glycoprotein, CD133. A structure model of CD133 proposed by Miraglia et al. This protein has an extracellular N-terminus, a cytoplasmic C-terminus, 2 small cysteine rich cytoplasmic loops and 2 very large extracellular loops each containing 4 potential sites for N-linked glycosylation.
It is regulated by five alternative promoters (P1-P5) during embryonic development, and also presents numerous splice variants [3-5].
Unfortunately at the moment neither ligands nor the functions of CD133 are yet fully known but they have a distinct and restricted expression in the context of production of the plasma membrane, such as in the epithelial microvilli. In fact as recently reported by Marzesco in the book by D. Corbeil “Biochemically, the prominin-1 directly interacts with plasma membrane cholesterol within a distinct cholesterol-based membrane microdomain. It is specifically concentrated in plasma membrane protrusion as at the apical plasma membrane of neural cancer stem cells (NSCs) and other epithelial cell types.” [6].
As a consequence many authors attribute a functional role to CD133 as an “organizer” of the plasma membrane topology [7-9]. In addition, the interaction with cholesterol in a specific new micro-domain suggests that CD133 may also be important in maintaining an appropriate lipid composition within the plasma membrane. Initially, it has been described as a surface antigen specific expressed by human hematopoietic stem cells [1,10] and as a marker expressed by murine neuroepithelial cells and several other embryonic epithelia [11].
Yin et al. in 1997, [10] succeeded in obtaining a new monoclonal antibody that was able to recognize the AC133 epitope of CD133. This epitope had a restricted expression in populations of CD34+ progenitors, in bone marrow and in the adult blood and fetal liver cells. It was for this reason that CD133 was proposed as marker of progenitor hematopoietic cells [9,12]. Clearly identifying the antigen AC133 does not mean identifying the CD133, as AC133 monoclonal antibody binds only to glycosylated epitope of CD133.
Subsequently FloreK et al. [13] defined an antibody that recognized human CD133 independently of glycosylation (αhE2); it permitted the detection of CD133 on the apical membrane of the proximal tubules of the adult kidney and mammary gland.
In the meantime Fargeas et al. [14] cloned prominin-2, a second member of the prominin family. Prominin-2 shared many similarities with prominin-1 including similar structural topology and restricted expression within plasmalemmal protrusions. Prominin-2 mRNA was found in human kidney, digestive tract, prostate, trachea, salivary gland, thyroid gland, mammary gland, and placenta, and in tumours of the human lung and nervous system [14].
Furthermore the molecule prominin-1, as reported by many authors, was found on endothelial [15], lymphangiogenic [16] and myoangiogenic [17] progenitors.
Indeed, CD133 alone or in a combination with other markers is currently used for the isolation of normal stem cells from several tissues, such as bone marrow [1,10], brain [18,19], kidney [20] prostate [21], liver [22], pancreas [23,24], sarcoma [25,26] and skin [27].
It is also used for the identification and isolation of a putative cancer stem cell population from malignant tumors of brain [28,29], prostate [30], liver [31,32], pancreas [33], lung [34,35], colon [36-38], ovary [39] as indicated in Table 1.
Table 1.
CD133 identification in human solid cancer
| Tumor Type | References |
|---|---|
| Brain | Singh, 2003; Liu, 2006 |
| Melanoma | Fang, 2005 |
| Sarcoma | Tirino, 2008, 2011 |
| Prostate | Collins, 2005; Miki, 2007 |
| Lung | Eramo, 2007; Tirino, 2009 |
| Colon | Ricci-Vitiani, 2007 |
| Skin | Monzani, 2007 |
| Pancreas | Olempska, 2007 |
| Liver | Ma, 2002; Yin, 2007 |
| Ovary | Ferrandina, 2007; Baba, 2009 |
Several studies indicated the difficulty in isolating CSC pure population [40] and in addition there is profound controversy and debate regarding antibody for use of CSC identification; especially for CD133 that nowadays is the main marker used to identify this rare subpopulation of cells. In this context, different questions could be taken into consideration.
Most probably, the difference in the recognition by the different antibodies resides in the differential affinity to various glycosylated forms of CD133. In fact as affirmed by Kemper et al. “CD133 could be probably differentially folded as a result of differential glycosylation that masks specific epitopes” [4].
Another cause of different expression of CD133 could be attributed to a change in both promoter activity and splice variant expression of gene. In fact as recently reported by Grosse-Gehling et al. “the two large extracellular domains of human CD133 may be affected by alternative splicing, with possible consequences on the presence of specific epitopes” [5].
It is for all these reasons that many questions are still present about the real role of CD133, in fact nowadays many researchers believe that the fraction CD133 acts like CD133+ but it is rather likely that the CD133 is present and cannot be seen because it is masked by glycosylation.
In this scenario, the purpose of this review is to focus on and debate with a critical eye everything that has been reported about the role of CD133 that remains still enigmatic.
CD133 as marker of normal and cancer stem cells
Stem cells are unspecialized primitive cells and have the capacity to develop into different cell types of the body through a process called cell differentiation. They are characterized by their ability to self-renew and undergo multilineage differentiation.
Given that CD133 was originally discovered to identify to CD34+ population of hematopoietic stem cells [10], interest has been directed towards the potential role of CD133 as cell surface marker of adult stem cells.
In human haematopoietic lines, CD133 antigen expression is restricted to CD34+ cells, while it was observed in many other human cell lines and differentiated cells. In this context it is interesting to note that the cells AC133+/CD34+ cells have a high clonogenic capacity compared to those AC133-/CD34+ [8-13]. AC133 clone being expressed only in stem and progenitor cells, while CD133 is also expressed in differentiated cells it is for this reason that AC133 is the most promising marker of stem cells.
In this context, this epitope appears to be useful to better characterize samples in allogenic hematopoietic stem cell transplantation, using CD133+ and CD34+.
CD133+ cells were isolated by some human organs, for example the kidney, and characterized for their potential of self-renewal and differentiation in vitro and in vivo and found to be able to contribute to tissue regeneration and differentiation both in epithelial and endothelial lines.
As already mentioned in the introduction CD133 alone or in a combination with other markers is actually used for the isolation of normal stem cells from several tissues such as bone marrow [1,10], brain [18,19], kidney [20] prostate [21], liver [22], pancreas [23,24], sarcoma [25,26] and skin [27].
In conclusion, the data in the literature show a significant role of this marker in the processes involving normal activity of adult stem cell [10,41]. In particular CD133/AC133 clone seems to be a marker of stem cells with the capacity of addressing mature lines and differentiate to form functional non-haematopoietic adult lineages and this could be used in tissue regeneration and in the therapy of many pathologies [9] as Duchenne’s dystrophy [42].
Moreover the interest for CD133 has grown significantly in reference models of carcinogenesis that, with different variations, propose that tumors are formed in normal adult stem cells or in early progenitor cells preserving the properties of self-renewal and differentiation even when they form tumor heterogeneous masses, perpetuating their presence, as a fraction minority, capable of producing recurrences and metastases.
Nowadays tumors are viewed as heterogeneous aberrant tissues containing a hierarchy of cells that originate from a single cancer stem cell (CSC). One of the major problems related to tumor progression is the formation of metastases that are the leading cause of death by cancer; in fact they are responsible for more than 90% of cancer associated mortality.
The CSC hypothesis postulates that a small subpopulation of cancer cells drives tumor growth and metastasis (Figure 2). In fact accumulating evidence suggests that a subpopulation of tumor cells with distinct stem-like properties is responsible for tumor initiation, invasive growth, and possibly dissemination to distant organ sites [43].
Figure 2.
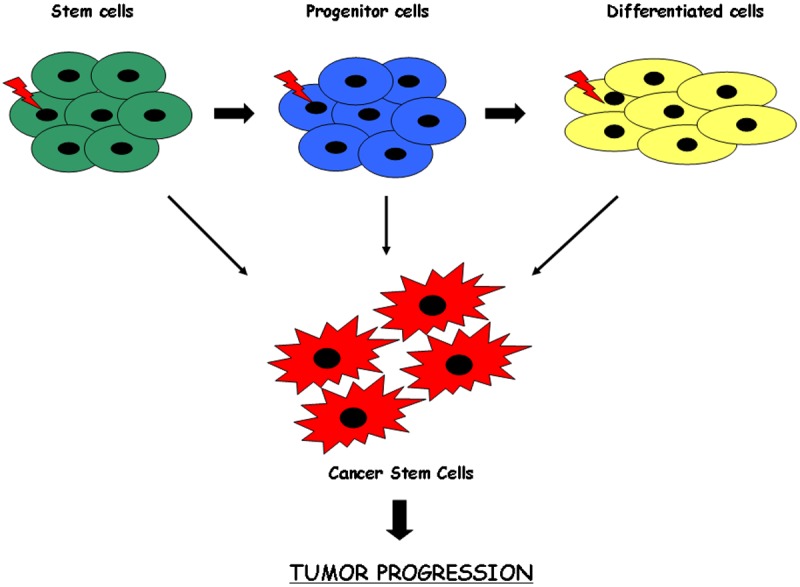
A model of the origin of the cancer stem cells. Mutations in stem cells, progenitors cells or differentiated cells might give rise to cancer stem cells. The resultant cancer stem cell has lost the ability to regulate its own cell division. These cells represent a rare population responsible for tumor initiation, invasive growth and possibly dissemination in distant organs.
Two basic topics underlie the hypothesis that CSCs may originate from normal tissue stem cells. First, CSCs share many features with normal stem cells, including self-renewal, differentiation, drug resistance, and migration capacity. Second, the longevity of stem cells makes them susceptible to accumulation of genetic and epigenetic damage in such a way as to make them good candidates for the emergence of the neoplastic transformation.
Considerable evidence correlated the acquisition of CSC traits with the epithelial to mesenchymal transition (EMT) transdifferentiation program (Figure 3). EMT is an embryonic key developmental physiological program that is often activated during cancer invasion and metastasis [44]. It is a process by which transformed epithelial cells can acquire the mesenchymal phenotype as well as the ability to migrate, to invade, to resist apoptosis, and to disseminate [45]. A set of pleiotropic transcriptional factors, including Snail, Slug, Twist, and Zeb1/2, orchestrates the EMT and related migratory processes during embryogenesis. These transcriptional regulators are expressed in various combinations in some malignant tumor types and have been shown to be causally important for programming invasion in experimental models of carcinoma formation; some of them have been found to elicit metastasis when ectopically over-expressed [46]. Induction of this program in certain model systems can induce many features of stem cells, including self-renewal ability and the antigenic phenotypes associated with both normal cells and CSCs.
Figure 3.
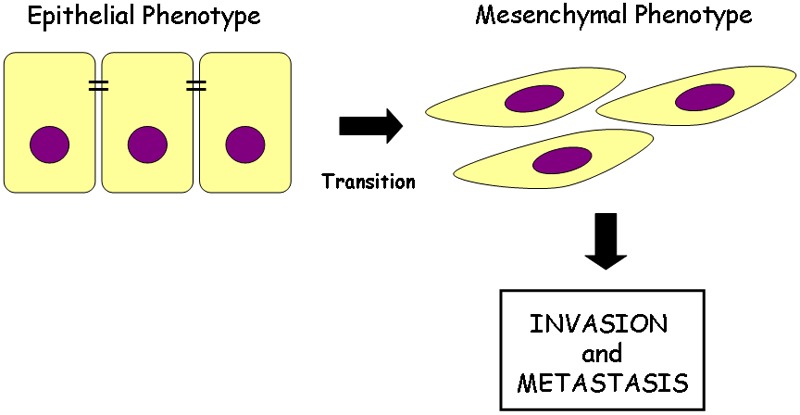
Epithelial cell plasticity. In response to extracellular signals, epithelial cells undergo epithelial-mesenchymal transition (EMT) are characterized by loss of epithelial marker and acquisition of fibroblast-like phenotype. EMT has been closely associated with the acquisition of aggressive traits by carcinoma cells; infect epithelial cells loose their polarity and cell-cell adhesion and gain migratory and invasive properties to become mesenchymal cells.
In particular, EMT is characterized by altered cell surface marker expression, increased tumor formation and is thought to endow cancer cells with migratory and invasive properties. A wide variety of solid tumours that express stem/progenitor cell marker such as CD133 have been reported to have more aggressive biological behaviour, poor prognosis and high recurrence [26,35]. At the same time EMT, closely related invasion, has been suggested to generate CSCs [44]. In this scenario it is possible to consider the CD133 expression as a prognostic factor for high grade tumors [47].
The most widely used methods for the isolation of CSC from the tumor is based on the distinction of this population according to 1) the expression of markers of stem cell such as: CD24 [48,49], CD29 [49], CD44 [50,51], CD133 [37,52], ALDH1 [53,54]; 2) the expression of nuclear proteins that regulate the efflux of some dyes from the core, the Hoechst 33342 [55,56] identifies a subpopulation chemoresistant (the so-called side population) and 3) the ability to grow in anchorage independence, defined in vitro by the formation of “spheroids” in serum-free and enriched with growth factors [57-59]. This method, described by some recent studies, is based on the existence of a minority subpopulation of cells, both isolated from biopsies of patients and from cell lines with the property of “self-renewal” and capable of forming spherical colonies that grow in suspension [60,61]. However, the appropriateness of these markers is an ongoing discussion.
Numerous studies have clearly pointed to the possible role of CD133 as a marker of these cells, also in combination with other markers.
In fact, cells that express this marker retain the ability to self-renewal, proliferation and differentiation both in vitro and in vivo with a process that seems to unite and rebuild the tumor original phenotype. Experimental evidence supports the growing importance of CD133 as a marker in the central phenotype of CSCs and it can be used for target therapy. Currently CD133 is used for detection of CSCs in several malignant tumors as reported in Table 1.
In this context, it is important to keep in mind that in most of the studies cited CD133 was revealed through the glycosylated epitope, AC133, which turns out to be the most reliable marker of CSCs. In fact currently the most commonly used antibodies to identify CD133 are: AC133 (CD133/1) and 293C/AC141 (CD133/2), which recognizes distinct epitopes [62]. AC133 is frequently used to isolate CSCs and is suggested to recognize a glycosylated epitope on CD133 [1], which contains eight putative N-linked glycosylation sites. AC141 recognizes the same antigen as AC133 but a different epitope [1].
As reported by Kemper et al. [4] the use of CD133 as a marker to identify, isolate and characterize CSCs is controversial because its expression pattern is debated. In fact different groups showed that AC133+ cells sorted from primary carcinomas can form tumors in immunodeficient mice (compared to AC133-) with same morphology of the original tumour [36-38].
CD133+ and CD133- cell fractions display similar stemness and differentiation capabilities
To elucidate the molecular function of CD133, many investigators have analyzed CD133 positive and negative subpopulations of different cell lines.
One of the most common methods used to analyze the CD133+ cell fraction is to perform a cell sorting to separate the negative fraction from that enriched with CD133. For example Tirino et al. isolated CD133+ from CD133- cells in A549 cell line. For CD133 staining, cells were stained with mouse anti-human CD133 PE (Miltenyi Biotec, Calderara di Reno, Bologna, Italy). The antibody was incubated for 30 min at 4°C in the dark. After incubation, the samples were washed with PBS and analysed by FACSAriaII (Becton Dickinson, Franklin Lakes, NJ, USA). They found that the mean expression level of CD133 were about 4%. The enrichment of CD133+ cells was obtained after cell sorting with an increase from 4% to 40% (Figure 4) [63].
Figure 4.
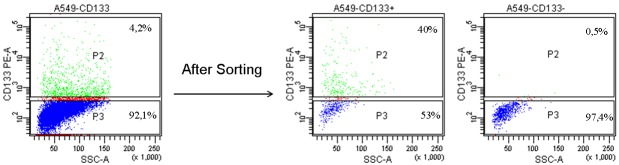
Cytometric analyses for CD133. Expression level of CD133 was about 4%. The enrichment of CD133+ cells was obtained after cell sorting with an increase from 4% to 40%.
Although for the most part in the scientific literature the idea is referred to that the CD133+ cells are those capable of giving greater tumorigenicity, many researchers contemplate the hypothesis that is the fraction of CD133- to have greater invasive or similar capacity.
These contradictory data have generated confusion regarding the precise expression pattern of CD133 in the adult tissues and the hierarchy of tumor-initiating cells. However, the enigmatic role of CD133 opens a series of debates that culminated in the thought whether: is CD133 the true marker to identify CSCs?
The following are among the various hypotheses that different research groups have advanced about the role of CD133 in various solid tumors.
In 2007 two distinctive groups O’Brien et al. [36] et Ricci Vitiani et al. [38] found that in colon cancer CD133+ cell was able to initiate tumor growth compared to CD133- that did not show ability to form tumors. In this way they supported the idea that CD133+ cells could produce tumours with preserved self-renewal and differentiation capabilities.
This data was also confirmed in 2008 by Shmelkov et al. [64]; in fact the authors showed that in colon cancer CD133+ tumor cells might give rise to the more aggressive CD133- subset, which is also capable of tumor initiation in NOD/SCID mice. Also Chao et al. [65], a few years later, have indicated that in colorectal cancer CD133+ cells enhanced tumorigenic potential compared with CD133-.
Regarding lung cancer in 2009 Tirino et al. [35] analysed the presence of CD133 antigen both in fresh human NSCLC specimens and in stabilised cell line. The authors isolated a population of CD133+ cells from NSCLC that was able to give rise to spheres that can act as tumour-initiating cells and represent the cancer-initiating cells capable of giving rise to primary tumour growth, invasion and spread as distant metastases.
In the same year, in contrast with Tirino, Meng et al. [66] isolated CD133+ and CD133- cells from two different lung cancer cell lines (A549 and H446); they found that the two subpopulations of CD133 displayed similar abilities of colony formation, self-renewal, proliferation, differentiation, and invasion, as well as resistance to chemotherapy drugs. In this way they concluded that CD133 could not be used as a stem cell marker.
Concerning sarcoma in 2011 Tirino et al. [25] selected a CD133+ subpopulation from sarcoma stabilized cell lines that displayed the capacity to grow as sarcospheres. Their findings showed the existence of cancer stem cells in bone sarcomas and highlight CD133 as a pivotal marker for identification of these cells. In 2012, Kimura et al. [67] analyzed CD133+ and CD133- subpopulations of synovial sarcoma (SS) cell lines and found that CD133- subpopulation exhibited high cell proliferation and tumorigenicity associated with AKT hyperphosphorylation. Their results suggested that CD133 has negative effect on the growth of cells through AKT-dependent signalling pathway.
Rocco et al. [68] in 2012 evaluated the expression of CD133 and CD44 in primary gastric cancer (GC). Although CD133+ and CD44+ were detectable in human primary GCs, they did not express stem-like properties and they did not exhibit tumor-initiating properties by xenograft transplantation experiments. In contrast with the study of Rocco et al., in 2013 the group of Cai [69] analyzed the expression of CD133 in gastric cancer using KATO III cell line. They found that CD133+ cells in primary lesion of GC correlated with higher invasion ability which may promote the metastasis of gastric cancer via up-regulation of EMT related factors.
Tirino el al. [63] demonstrated, starting from non small lung cancer cell line, that CD133+ cells (identified by us as CSCs) showed higher motility and tumorigenicity compared with CD133- (identified by us as non-CSCs).
In this context and in the light of all the works cited, it seems that not only the fraction of CD133+ cells (identified as CSCs) posses tumorigenic and invasive capacity, but also CD133- cells may have these potentialities. Often the CD133 molecule is masked and this can distort the effect of CD133- that appear to show the same effect of CD133+. This difficulty in its identification could be due to: i) differential glycosylation, ii) epigenetic modifications iii) tumor microenvironment.
Differential glycosylation of CD133
The glycosylation is an enzymatic process by which glycans bind to proteins, lipids, or other organic molecules and is a form of co/post-translational modification.
Recent evidence showed that differences of CD133 expression detected using antibodies against CD133 could to be due to differences in recognition of levels of CD133 glycosylation or masking of glycosylated epitope [4,70-72].
Currently, the use of CD133 as stemness marker in solid tumors is the subject of great debate because it is its same expression pattern to be controversial. In fact, many research groups have demonstrated that CD133 positive and not negative cells were able to regenerate new tumors in immunocompromised mice resembling the histological characteristics of original tumor [28,36] but, at the same time and on the contrary, CD133 mRNA expression was found also in cells not defined as stem cells [64,73,74]. Therefore, during differentiation, it was possible to observe a decrease of levels of AC133, without altering the CD133 mRNA levels. These studies highlight clearly that there is a contradiction among AC133 as stemness marker and the broad CD133 mRNA and protein expression. Consequently, it seems that CD133 expression is not restricted to stem cell fraction but it is also expressed by differentiated cells [4,75]. In parallel, it is also important to understand how differential glycosylation can affect the differentiation, and the mechanisms involved in tumor progression.
As resulted from several studies [5,76-79], sialylation and alterations in post-translational modification of CD133 seems to be significantly involved in cancer progression playing a specific role in invasiveness and metastasis. In fact, they may influence antibody binding, both in terms of the nature of epitope and the epitope’s accessibility. Miraglia and colleagues [1] showed that human AC133 epitope was susceptible to glycosylation modification. This consideration arose from fact that AC133 antibody binding was abolished after tunicamycin treatment affecting both the CD133 stability and its transport to cell membrane [80]. Other studies have demonstrated that glycosylation and conformational modifications of CD133 may lead to the loss of the AC133 epitope recognition in tissue samples and that CD133 glycosylation may change on the basis of the tissue considered and stage of differentiation [13,81].
In this context, Kemper et al. [4] showed that AC133 epitope decreased during differentiation of CSCs correlating with the loss of clonogenicity. But this did not correlate with modifications of CD133 promoter activity, mRNA level, splicing, protein expression and finally CD133 surface expression. On the other hand, they observed that CD133 glycosylation changed during CSCs differentiation. In particular, a reduction of glycosylation that correlated with a decrease of AC133 was found. But this reduction was not due to decrease or loss of glycosylated epitopes as evidenced both by immunoblotting assay and using unglycosylated bacterially expressed CD133 protein, but may be due to masking of CD133 epitopes. In fact, the authors demonstrated also that differentially glycosylated CD133 may be detected on differentiated tumor cells. They concluded that CD133 is detectable both on stem and differentiated cells. The differences of CD133 expression derived from epitope masking that may be due to differential folding of the protein as a result of differential glycosylation. Moreover, they suggested that AC133 can be used to identify CSCs, but prudence and caution are necessary in interpreting the data obtained. Another important contribution in studying CD133 glycosylation derived from work of Taïeb et al. and Donovan et al.
Taïeb et al. [71] demonstrated that the extracellular N-terminal domain of the CD133 protein may undergo conformational modifications due to membranous gangliosides, that could mask the AC133 epitope. The studies above mentioned highlight that the different expression of CD133 detected during differentiation is not attributable to transcriptional or translational changes, but post-translational changes and depends on the structure assumed by the CD133 with the change of glycosylation that very probably hides the epitope to antibody.
Already Donovan et al. [72] demonstrated that in IN699 pediatric glioblastoma cells, the ganglioside GD3 did not affect the biological behaviour of CD133, being GD3 overexpressed both in CD133+ and CD133- cell subpopulations. But, in this context, the authors hypothesized that glycosylation of CD133 may be modulated by GD3. They suggested that glycosylated AC133 may sequester the GD3 antigen to the N-terminal of the protein, causing de-N-glycosylation of the CD133.
Epigenetic regulation of CD133
Another important topic regards “epigenetic modifications” that may influence the transcription of CD133 gene. This is controlled in a tissue-specific manner by five alternative promoters (P1-P5) leading to at least 16 alternative splicing patterns of the 5’-UTR of CD133 transcripts as widely reported by Shmelkov et al. [82] and Tabu et al. [83].
In particular, promoters P1-P3 are situated in a CpG island. Therefore, DNA methylation may occur in these promoters leading to a possible epigenetic regulation of CD133. It is well known that hypermethylation of CpG islands represses gene expression blocking its transcription, while hypomethylation leads to increase of gene expression [84-86]. The methylation status of the PROM1 promoters and the effect of demethylating agents on CD133 expression have been widely studied. Gregory CA et al. [87] as well as Meregalli et al. [88] showed that CD133 mRNA levels were inversely correlated to the DNA methylation status of the CpG sites in various cancer cell lines. An interesting study has been conducted by Baba et al. [89] that showed CD133 expression and its role in ovarian cancer. They identified a cell subpopulation expressing CD133 marker starting from ovarian stabilized and primary cancer cell lines, and ascetic fluid of ovarian cancer patients. They sorted CD133 positive and negative cells and characterized them. CD133+ ovarian cancer cells were able to generate CD133+ and CD133- daughter cells, and to form tumours greater than CD133- cells when implanted in immunocompromised mice. Moreover, CD133+ ovarian cancer cells resulted be more resistance to cisplatin treatment than CD133- cells. Having defined the characteristics of CD133+ cells, Baba et al. [89] focused their attention on epigenetic modifications occurring at level of CD133 gene. They found that both histone modifications and promoter methylation were associated to CD133 transcription. In particular, they observed that, when CD133- ovarian cancer cells were treated with DNA methyltransferase and histone deacetylase inhibitors, a synergistic increase in cell surface CD133 expression was obtained. In addition, DNA methylation of active P2 promoter led to a inhibition of CD133 transcription. They showed also that promoter methylation increased in CD133- progeny of CD133+ cells, with CD133+ cells maintaining a loss of methylated or unmethylated state. The study of Baba et al. [89] highlights that CD133 promoter methylation may explain the loss of surface expression of CD133 in differentiated cells being cell differentiation affected also by epigenetic changes [90-92] and may be generally representative of epigenetic repression. Consequently, CD133 promoter methylation could be used as potential biomarker providing important and relevant information for prognosis and treatment. Baba et al. [89] concluded hypothesizing that the biological behaviour of ovarian cancer cells may be epigenetically detected and such cells might be used as potential chemotherapeutic targets.
Another epigenetic regulation studied is histone modification such as histone acetylation and deacetylation. In particular, acetylation is associated with transcriptional activation, whereas deacetylation leads to gene repression [93].
Histone modifications may be affected also by DNA hypermethylation and by various inhibitors that are used to epigenetically regulate the PROM1 gene [94]. In this perspective, Yi et al. [95] studied the role both of Histone H3 specifically di-methylated at position Lys 4 (H3K4me2) associated usually con active genes and Histone H3 specifically tri-methylated at position Lys 27 (H3K27me3) placed in low expression genes [96] in the promoter region of CD133 in colorectal cancer cell lines. They found that many genes associated with DNA methylation showed a bivalent chromatin with the presence of mark H3K27me3 but, in parallel, balanced by the contemporary presence of the mark H3K4me. The same profile has been evidenced for CD133 expression with a bivalent chromatin in which a balance between H3K27me3 and H3K4me was observed. Therefore, Yi et al. [95] demonstrated that histone modification may explain the discordance of DNA methylation status with CD133 expression in these cells. What mechanisms are involved in generating different 5’-UTRs that may affect coding sequence and CD133 expression are also unknown.
Expression of CD133 in hypoxic oxygen condition
Due to poor and abnormal vascular development, the majority of solid tumors presents median levels of pO2 lower than those of the tissue of origin; in addition, the hypoxic areas are characterized by low levels of pH and glucose.
Has been demonstrated the ability of the stem cell to live in a state of hypoxia, these cells are able to create the energy they require making the most of anaerobic glycolysis. Hypoxia is needed for stem cells to maintain their isolated “niche” and to force the differentiated cell daughters to walk away if they do not want to die and then allowing them to go on to colonize the tissue. This ability to resist hypoxia has been demonstrated also for neoplastic stemness. A stem cell under hypoxic conditions remains in a quiescent state (G0 phase) to maintain the highest integrity of the enclosure (only a few undergo cell division), and this also applies to cancer stem cells.
Furthermore, through stabilization of the transcription factors HIF-1α (hypoxia inducible factor 1α), hypoxia results in an increase in the expression of proteins related to angiogenesis, such as VEGF (vascular endothelial growth factor) and its receptor VEGFR, the glycolytic metabolism and adaptation to oxidative stress [97]. In this way HIFs are crucial for the adaptive response to oxygen tension (Figure 5).
Figure 5.
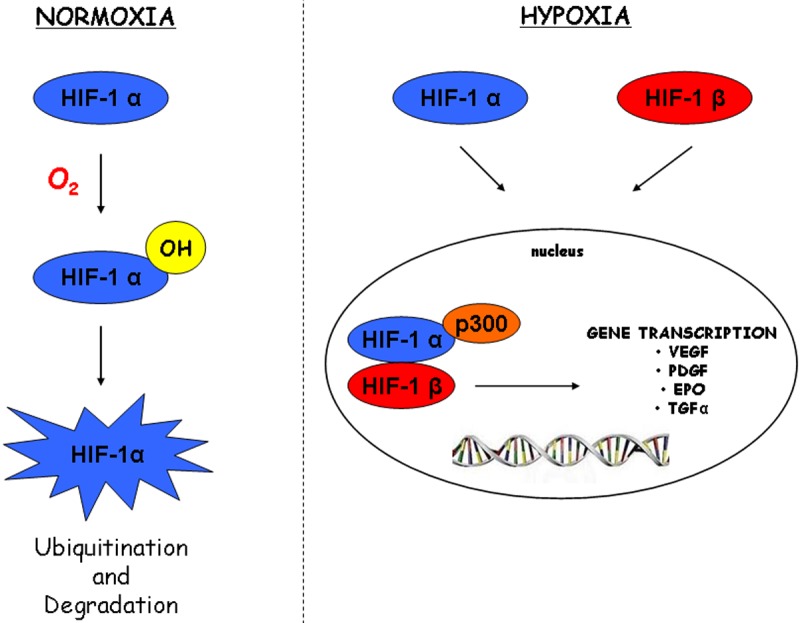
Schematic representation of the hypoxia inducible factor pathway. In normoxia HIFα subunit is hydroxylated by proline hydroxylase (PHD). The hydroxylated (OH) HIFα is recognised by a product of the von Hippel-Lindau tumour suppressor gene (VHL) which tags the HIFα-OH with polyubiquitin for subsequent degradation by the ubiquitin-proteosome pathway. In hypoxia HIFα and HIFβ subunits dimerize, then translocate in the nucleus where subunits interact with hypoxia regulatory elements (HREs), recruiting the transcriptional co-activator p300, activating the full transcription of downstream genes which regulate cell survival, motility, metabolism and angiogenesis.
In 2009 Soeda et al. [98] analyzed the correlation between CD133 and HIF-1α in glioma stem cells.
The authors showed that hypoxia promoted self-renewal capacity of CD133+ glioma stem cells, preceded by upregulation of HIF-1α. The effect was abolished when HIF-1α was knockdown. Their findings suggested that response to hypoxia by CSCs involves the activation of HIF-1α to enhance the self-renewal activity of CD133-positive cells and to inhibit the induction of CSC differentiation.
Also Iida et al in 2011 [99] analyzed the correlation between CD133 and hypoxia inducible factors. The authors found that in three different lung cancer cell lines the CD133 expression level was increased under hypoxic cultivation. Given that P1 promoter was associated with hypoxia-induced promoter activity of CD133; they found a direct connection between P1 promoter with OCT4 and SOX2. In hypoxic condition, the hypoxia inducible factors increased the expression of OCT4 and SOX2 and through their connection with P1 they increased the expression of CD133. The effect was abolished with knockdown of both OCT4 and SOX2.
A very interesting study is that of Donovan et al. [72]. In the manuscript the authors, starting from pediatric glioblastoma, hypothesized not only that the presence of CD133 could be the source of tumor resistance but also that maintenance of this molecule by hypoxia dictates cellular and molecular behaviour. The authors demonstrated that with decreasing oxygen tension there was an increase of CD133 expression; so they found a direct link between decreasing oxygen tension and CD133 expression. Moreover HIF-2α has been seen to increase in hypoxic conditions along with the increasing expression of CD133. This may be implicated in the regulation of the stem cell phenotype with decreasing oxygen tension. Their results also suggested plasticity in CD133 expression. “Spontaneous” formation of the CD133 phenotype and microenvironmental influence suggest that CD133 is not a definitive brain tumor CSC marker. Its presence in pediatric GB may, however, significantly define biologic behaviour and subsequent clinical outcome.
Because of the apparent complexity of the microenvironment, plus additional factors that can seemingly affect the expression of the CD133 epitope, it is not surprising that there are contradictory reports in the literature regarding the functional properties and expression of CD133.
To conclude, diverse microenvironmental factors have been shown to have an impact on CD133 expression, but it remains a matter of debate.
Relation between CD133 and circulating tumor cells
As reported by Franco et al “Recently, circulating tumour cells (CTCs) have aroused much interest in cancer research, representing potential prognostic biomarkers and a reliable mean to predict metastasis development. The presence of CTCs are a pre-requisite to develop distant metastasis” [100]. In fact circulating tumor cells (CTCs) are a potential aggressive population, able to generate a metastasis. Therefore, identifying this population may be determinant in order to address therapy.
Always according Franco et al. “In several clinical studies, CTCs enumeration has been used as biomarker in the prognostic stratification and in the evaluation of disease response during therapy. In addition, CTCs number could be useful for prognostication in early stage of disease, for identification of patients requiring adjuvant therapy, or during follow-up in order to detect relapses” [100]. In fact the presence of CTCs in peripheral blood of patients affected by some types of tumors, such as breast cancer could be used to address or control the therapy, being CTCs enumeration being important also in early stage of disease because it may identify patients needing an appropriate adjuvant therapy.
Two concepts could be linked to CTCs: CSCs and EMT-program. EMT is a process by which the cells undergo a switch from epithelial to mesenchymal phenotype. Consequently, EMT cells acquire mobility and are able to move towards other sites to metastasize. Therefore, CTCs could undergo EMT program. In fact, according to recent findings, more invasive CTCs may lose their epithelial antigens by the EMT process, acquiring stemness characteristics, thus suggesting that their identification cannot be based only on the expression of epithelial-specific transcripts, such as cytokeratins. It has been also proposed that CTCs represent a highly heterogeneous population, since most of them die during migration, and very few are able to grow into clinically evident metastases [101]. It has been also suggested that more aggressive CTCs may share similar genotypic and phenotypic characteristics with the CSCs [102]. This could potentially explain the eventual relapse of disease in patients previously considered to be cured by primary therapy. Unfortunately, few reports have described correlation between CTCs and CD133 CSCs. Three recent papers have investigated the putative correlation between CD133 and CTCs.
First, Giordano et al. [103] focused their attention on HER2+ metastatic breast cancer and demonstrated that CD326-CD45- cell fraction over-expressing SNAIL and ZEB1 showed a higher percentage of cells positive for CD133 than those with normal level of SNAIL and ZEB1. The authors concluded that the patients affected by HER2+ metastatic breast cancer have EMT-CTCs.
Shimada and colleagues [104] studied CTCs in patients affected by colorectal cancer. They demonstrated that CEA/CK/CD133 mRNA detection may be considered as biomarker to identify patients affect by colon cancer at high risk with Dukes’ stage B and C.
Nadal et al. [105] showed that CTCs derived from non metastatic breast cancer patients expressed CD133. In particular, when the authors performed a stratification on basis of therapy, they found that 65% of patients had CTCs identified as cells positive for CD133 and CK at baseline, whereas after systemic therapy only 47.8% of patients had CD133+CK+ CTCs. Before any treatment, CD133+CK+ CTCs were detected, in particular, in patients affected by luminal breast cancer. Nadal et al. [105] concluded their research stating that CD133 could be used as marker of chemoresistance.
Summarizing, CTCs could be identified as cells that expressed also CD133 or EMT markers. Therefore, the open question remains the enumeration of CTCs with current protocols that usually involve only the use of epithelial markers and not stem or EMT markers. In fact, CTC analysis is generally based on numeration, which is considered to have a prognostic value, and the Cell Search System (Veridex, Raritan, NJ, USA) has been cleared by the U.S. Food and Drug Administration as an aid to monitor patients with metastatic breast, prostate, and colon cancer [106,107]. This analytical method is able to predict free disease and overall survival, but cells arising from EMT often escape detection [108]. These findings may imply that current CTCs detection methods underestimate the most important subpopulation of CTCs involved in cancer dissemination, which often share both EMT and stemness features. This could explain why, through currently used methods, CTCs are undetected in 40% of patients with metastatic breast cancer. Therefore, there is an urgent need for optimizing CTC detection methods through the combination of EMT/CSC markers (such as CD133) with CTC phenotype.
CD133 as a prognostic value
CSCs as mentioned above are responsible for tumor iniation, invasive growth, dissemination to distant organs, radiotherapy and hemotherapy resistance and given that CD133 is the main marker used to identify, it was thought that this molecule could have a prognostic value.
Some studies have correlated the expression of CD133 either survival, recurrence, metastasis and therapy resistance. Currently, many researchers are also trying to correlate the levels of expression of CD133 with clinical and histopathological parameters of patients such as age, tumor size, tumor stages, grading, preoperatory chemotherapy conditions.
In this context, one of the important aspects is definitely related to the technique used for the identification of CD133. Some studies performed on lung cancer cell line showed that no significant expression of CD133 by either flow cytometry, polymerase chain reaction (PCR), immunohistochemistry (IHC) or immunoblotting was detectable. In this context Di Bonito and colleagues [109] found that the expression of CD133 was strikingly hyper expressed in tubular variant of breast cancer using three different techniques with the same results (flow cytometry, IHC, RT-PCR).
However currently many authors relate the expression of CD133 with poor prognosis in cancer of the colon [110-113], brain [114,115], liver [116], stomach [117,118], endometrium [119], ovary [120] and lung [121,122].
Regarding colon cancer Horst et colleagues [113] supported the idea that CD133 expression is a marker with high prognostic impact in fact its levels strongly correlated with liver metastasis formation. Other authors like Choi et al. [123] and Kojima et al. [124] assessed that survival was not correlated to the expression of CD133.
Lei Wen et al. [118] evaluated the expression of CD133 in patients with primary gastric cancer. Although not with a high series they found that CD133 overexpression was associated with common clinopathological poor prognostic factor.
Ha Shin et al. [115] analyzed 67 patients with glioblastoma. The authors found a direct link between the overexpression of CD133 and poor survival. They concluded that high level of CD133 was a prognostic indicator of tumor recurrence and shorter survival.
Recently Pirozzi and colleagues [122] correlated the expression of CD133 with EMT-markers (CD90 and CD326) in patients with non small cell lung cancer. They found that CD133 was very closely associated with metastatic spread, in fact CD133 expression demonstrated a strong significant association with patients exhibiting progressive disease when compared to CD90/CD326 expression.
Future perspective
Researchers around the world are constantly scrambling to understand the biological and molecular mechanisms that lead to tumor formation and subsequent metastasis.
In fact one of the major problems related to tumor progression is the formation of metastases. Metastasis is the leading cause of death by cancer and it is responsible for more than 90% of cancer associated mortality. The process of metastatic dissemination remains poorly understood due to its complexity.
In this scenario, the “cancer stem cells” hypothesis might explain some aspects of tumor progression.
A matter of controversy remains the identification of CSCs. The CD133 today is used as the main marker for the identification of cancer stem cells, often in combination with other markers. Unfortunately, the information available today about the CD133 have not clarified yet the biological functions of this molecule and it is for this reason that its role is still enigmatic and controversial.
Although for the most part in the literature the idea is referred to that the CD133+ cells are those capable of giving greater tumorigenicity, many researchers contemplate the hypothesis that it is the fraction of CD133- to have greater invasive or similar capacity.
Moreover as reported by Bidlingmaier “the anti-CD133 antibodies typically used (AC133 and AC141 mAbs) recognize undefined glycosylated epitopes, the possibility remains that the glycosylation status of CD133, rather than expression of the CD133 protein itself, can act as an indirect marker of the CSC phenotype. So the question that remains unanswered is whether CD133 or its glycosylation status could play a direct role in regulating the CSC phenotype” [125].
Some researchers relate the CD133 to various clinical parameters and some evidence shows that CD133+ cells are equipped with resistance to radio-chemotherapy. This suggests a prognostic role for this molecule.
Obviously further studies are required: to reveal the biological functions of CD133, to assess its correct use in the identification of CSCs and to assign definitively its prognostic role.
This may be of primary importance in the development of new therapeutic strategies and new prognostic procedures against highly aggressive and metastatic tumors.
Acknowledgements
We thank: Dr. Ciro Coletta for his strong encouragement in writing the manuscript; Dr. Alessandra Trocino for providing excellent bibliographic services and assistance; Dr. Antonio Sorbo for producing images. This work was supported by grants from the Current Research of 2012/13 of the Italian Department of Health to G. Pirozzi.
Disclosure of conflict of interest
None.
References
- 1.Miraglia S, Godfrey W, Yin AH, Atkins K, Warnke R, Holden JT, Bray RA, Waller EK, Buck DW. A novel five-transmembrane hematopoietic stem cell antigen: isolation, characterization, and molecular cloning. Blood. 1997;90:5013–21. [PubMed] [Google Scholar]
- 2.Corbeil D, Fargeas CA, Huttner WB. Rat prominin, like its mouse and human orthologues, is a pentaspan membrane glycoprotein. Biochem Biophys Res Commun. 2001;285:939–44. doi: 10.1006/bbrc.2001.5271. [DOI] [PubMed] [Google Scholar]
- 3.Tabu K, Kimura T, Sasai K, Wang L, Bizen N, Nishihara H, Taga T, Tanaka S. Analysis of an alternative human CD133 promoter reveals the implication of Ras/ERK pathway in tumor stem-like hallmarks. Mol Cancer. 2010;9:39. doi: 10.1186/1476-4598-9-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kemper K, Sprick MR, de Bree M, Scopelliti A, Vermeulen L, Hoek M, Zeilstra J, Pals ST, Mehmet H, Stassi G, Medema JP. The AC133 epitope, but not the CD133 protein, is lost upon cancer stem cell differentiation. Cancer Res. 2010;70:719–29. doi: 10.1158/0008-5472.CAN-09-1820. [DOI] [PubMed] [Google Scholar]
- 5.Grosse-Gehling P, Fargeas CA, Dittfeld C, Garbe Y, Alison MR, Corbeil D, Kunz-Schughart LA. CD133 as a biomarker for putative cancer stem cells in solid tumours: limitations, problems and challenges. J Pathol. 2013;229:355–78. doi: 10.1002/path.4086. [DOI] [PubMed] [Google Scholar]
- 6.Marzesco AM. Prominin-1 containing Membrane Vescicles: origins, formation and utility. In: Corbeil D, editor. Prominin-1 (CD133): New insight on Stem & Cancer Stem Cell Biology. Springer; 2013. p. 42. [DOI] [PubMed] [Google Scholar]
- 7.Röper K, Corbeil D, Huttner WB. Retention of prominin in microvilli reveals distinct cholesterol-based lipid micro-domains in the apical plasma membrane. Nat Cell Biol. 2000;2:582–92. doi: 10.1038/35023524. [DOI] [PubMed] [Google Scholar]
- 8.Corbeil D, Roper K, Fargeas CA, Joester A, Huttner WB. Prominin: a story of cholesterol, plasma membrane protrusions and human pathology. Traffic. 2001;2:82–91. doi: 10.1034/j.1600-0854.2001.020202.x. [DOI] [PubMed] [Google Scholar]
- 9.Mizrak D, Brittan M, Alison M. CD133: molecule of the moment. J Pathol. 2008;214:3–9. doi: 10.1002/path.2283. [DOI] [PubMed] [Google Scholar]
- 10.Yin AH, Miraglia S, Zanjani ED, Almeida-Porada G, Ogawa M, Leary AG, Olweus J, Kearney J, Buck DW. AC133, a novel marker for human hematopoietic stem and progenitor cells. Blood. 1997;90:5002–5012. [PubMed] [Google Scholar]
- 11.Weigmann A, Corbeil D, Hellwig A, Huttner WB. Prominin, a novel microvillispecific polytopic membrane protein of the apical surface of epithelial cells, is targeted to plasmalemmal protrusions of non-epithelial cells. Proc Natl Acad Sci U S A. 1997;94:12425–12430. doi: 10.1073/pnas.94.23.12425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Donovan LK, Pilkington GJ. CD133: holy of grail of neuro-oncology or promiscuous red-herring? Cell Prolif. 2012;45:527–37. doi: 10.1111/j.1365-2184.2012.00842.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Florek M, Haase M, Marzesco AM, Freund D, Ehninger G, Huttner WB, Corbeil D. Prominin-1/CD133, a neural and hematopoietic stem cell marker, is expressed in adult human differentiated cells and certain types of kidney cancer. Cell Tissue Res. 2005;319:15–26. doi: 10.1007/s00441-004-1018-z. [DOI] [PubMed] [Google Scholar]
- 14.Fargeas CA, Florek M, Huttner WB, Corbeil D. Characterization of prominin-2, a new member of the prominin family of pentaspan membrane glycoproteins. J Biol Chem. 2003;278:8586–96. doi: 10.1074/jbc.M210640200. [DOI] [PubMed] [Google Scholar]
- 15.Peichev M, Naiyer AJ, Pereira D, Zhu Z, Lane WJ, Williams M, Oz MC, Hicklin DJ, Witte L, Moore MA, Rafii S. Expression of VEGFR-2 and AC133 by circulating human CD34(+) cells identifies a population of functional endothelial precursors. Blood. 2000;95:952–958. [PubMed] [Google Scholar]
- 16.Salven P, Mustjoki S, Alitalo R, Alitalo K, Rafii S. VEGFR-3 and CD133 identify a population of CD34+ lymphatic/vascular endothelial precursor cells. Blood. 2003;101:168–172. doi: 10.1182/blood-2002-03-0755. [DOI] [PubMed] [Google Scholar]
- 17.Shmelkov SV, Meeus S, Moussazadeh N, Kermani P, Rashbaum WK, Rabbany SY, Hanson MA, Lane WJ, St Clair R, Walsh KA, Dias S, Jacobson JT, Hempstead BL, Edelberg JM, Rafii S. Cytokine preconditioning promotes codifferentiation of human fetal liver CD133+ stem cells into angiomyogenic tissue. Circulation. 2005;111:1175–1183. doi: 10.1161/01.CIR.0000157155.44008.0F. [DOI] [PubMed] [Google Scholar]
- 18.Uchida N, Buck DW, He D, Reitsma MJ, Masek M, Phan TV, Tsukamoto AS, Gage FH, Weissman IL. Direct isolation of human central nervous system stem cells. Proc Natl Acad Sci U S A. 2000;97:14720–14725. doi: 10.1073/pnas.97.26.14720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee A, Kessler JD, Read TA, Kaiser C, Corbeil D, Huttner WB, Johnson JE, Wechsler-Reya RJ. Isolation of neural stem cells from the postnatal cerebellum. Nat Neurosci. 2005;8:723–729. doi: 10.1038/nn1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sagrinati C, Netti GS, Mazzinghi B, Lazzeri E, Liotta F, Frosali F, Ronconi E, Meini C, Gacci M, Squecco R, Carini M, Gesualdo L, Francini F, Maggi E, Annunziato F, Lasagni L, Serio M, Romagnani S, Romagnani P. Isolation and characterization of multipotent progenitor cells from the Bowman’s capsule of adult human kidneys. J Am Soc Nephrol. 2006;17:2443–2456. doi: 10.1681/ASN.2006010089. [DOI] [PubMed] [Google Scholar]
- 21.Richardson GD, Robson CN, Lang SH, Neal DE, Maitland NJ, Collins AT. CD133, a novel marker for human prostatic epithelial stem cells. J Cell Sci. 2004;117:3539–3545. doi: 10.1242/jcs.01222. [DOI] [PubMed] [Google Scholar]
- 22.Kordes C, Sawitza I, Müller-Marbach A, Ale-Agha N, Keitel V, Klonowski-Stumpe H, Häussinger D. CD133+ hepatic stellate cells are progenitor cells. Biochem Biophys Res Commun. 2007;352:410–417. doi: 10.1016/j.bbrc.2006.11.029. [DOI] [PubMed] [Google Scholar]
- 23.Oshima Y, Suzuki A, Kawashimo K, Ishikawa M, Ohkohchi N, Taniguchi H. Isolation of mouse pancreatic ductal progenitor cells expressing CD133 and c-Met by flow cytometric cell sorting. Gastroenterology. 2007;132:720–732. doi: 10.1053/j.gastro.2006.11.027. [DOI] [PubMed] [Google Scholar]
- 24.Sugiyama T, Rodriguez RT, McLean GW, Kim SK. Conserved markers of fetal pancreatic epithelium permit prospective isolation of islet progenitor cells by FACS. Proc Natl Acad Sci U S A. 2007;104:175–180. doi: 10.1073/pnas.0609490104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tirino V, Desiderio V, Paino F, De Rosa A, Papaccio F, Fazioli F, Pirozzi G, Papaccio G. Human primary bone sarcomas contain CD133+ cancer stem cells displaying high tumorigenicity in vivo. FASEB J. 2012;25:2022–30. doi: 10.1096/fj.10-179036. [DOI] [PubMed] [Google Scholar]
- 26.Tirino V, Desiderio V, d’Aquino R, De Francesco F, Pirozzi G, Graziano A, Galderisi U, Cavaliere C, De Rosa A, Papaccio G, Giordano A. Detection and characterization of CD133+ cancer stem cells in human solid tumours. PLoS One. 2008;3:e3469. doi: 10.1371/journal.pone.0003469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ito Y, Hamazaki TS, Ohnuma K, Tamaki K, Asashima M, Okochi H. Isolation of murine hair-inducing cells using the cell surface marker prominin-1/CD133. J Invest Dermatol. 2006;127:1052–1060. doi: 10.1038/sj.jid.5700665. [DOI] [PubMed] [Google Scholar]
- 28.Singh SK, Clarke ID, Terasaki M, Bonn VE, Hawkins C, Squire J, Dirks PB. Identification of a cancer stem cell in human brain tumors. Cancer Res. 2003;63:5821–5828. [PubMed] [Google Scholar]
- 29.Singh SK, Hawkins C, Clarke ID, Squire JA, Bayani J, Hide T, Henkelman RM, Cusimano MD, Dirks PB. Identification of human brain tumor initiating cells. Nature. 2004;432:396–401. doi: 10.1038/nature03128. [DOI] [PubMed] [Google Scholar]
- 30.Collins AT, Berry PA, Hyde C, Stower MJ, Maitland NJ. Prospective identification of tumorigenic prostate cancer stem cells. Cancer Res. 2005;65:10946–10951. doi: 10.1158/0008-5472.CAN-05-2018. [DOI] [PubMed] [Google Scholar]
- 31.Suetsugu A, Nagaki M, Aoki H, Motohashi T, Kunisada T, Moriwaki H. Characterization of CD133+ hepatocellular carcinoma cells as cancer stem/progenitor cells. Biochem Biophys Res Commun. 2006;351:820–824. doi: 10.1016/j.bbrc.2006.10.128. [DOI] [PubMed] [Google Scholar]
- 32.Yin S, Li J, Hu C, Chen X, Yao M, Yan M, Jiang G, Ge C, Xie H, Wan D, Yang S, Zheng S, Gu J. CD133 positive hepatocellular carcinoma cells possess high capacity for tumorigenicity. Int J Cancer. 2007;120:1444–1450. doi: 10.1002/ijc.22476. [DOI] [PubMed] [Google Scholar]
- 33.Hermann PC, Huber SL, Herrler T, Aicher A, Ellwart JW, Guba M, Bruns CJ, Heeschen C. Distinct populations of cancer stem cells determine tumor growth and metastatic activity in human pancreatic cancer. Cell Stem Cell. 2007;1:313–323. doi: 10.1016/j.stem.2007.06.002. [DOI] [PubMed] [Google Scholar]
- 34.Eramo A, Lotti F, Sette G, Pilozzi E, Biffoni M, Di Virgilio A, Conticello C, Ruco L, Peschle C, De Maria R. Identification and expansion of the tumorigenic lung cancer stem cell population. Cell Death Differ. 2008;15:504–514. doi: 10.1038/sj.cdd.4402283. [DOI] [PubMed] [Google Scholar]
- 35.Tirino V, Camerlengo R, Franco R, Malanga D, La Rocca A, Viglietto G, Rocco G, Pirozzi G. The role of CD133 in the identifcation and characterisation of tumor-initiating cells in non small cell lung cancer. Eur J Cardiothorac Surg. 2009;36:446–453. doi: 10.1016/j.ejcts.2009.03.063. [DOI] [PubMed] [Google Scholar]
- 36.O’Brien CA, Pollett A, Gallinger S, Dick JE. A human colon cancer cell capable of initiating tumor growth in immunodeficient mice. Nature. 2007;445:106–110. doi: 10.1038/nature05372. [DOI] [PubMed] [Google Scholar]
- 37.Todaro M, Alea MP, Di Stefano AB, Cammareri P, Vermeulen L, Iovino F, Tripodo C, Russo A, Gulotta G, Medema JP, Stassi G. Colon cancer stem cells dictate tumor growth and resist cell death by production of interleukin-4. Cell Stem Cell. 2007;1:389–402. doi: 10.1016/j.stem.2007.08.001. [DOI] [PubMed] [Google Scholar]
- 38.Ricci-Vitiani L, Lombardi DG, Pilozzi E, Biffoni M, Todaro M, Peschle C, De Maria R. Identification and expansion of human colon-cancer-initiating cells. Nature. 2007;445:111–5. doi: 10.1038/nature05384. [DOI] [PubMed] [Google Scholar]
- 39.Ferrandina G, Bonanno G, Pierelli L, Perillo A, Procoli A, Mariotti A, Corallo M, Martinelli E, Rutella S, Paglia A, Zannoni G, Mancuso S, Scambia G. Expression of CD133-1 and CD133-2 in ovarian cancer. Int J Gynecol Cancer. 2008;18:506–14. doi: 10.1111/j.1525-1438.2007.01056.x. [DOI] [PubMed] [Google Scholar]
- 40.Wang J, Sakariassen PO, Tsinkalovsky O, Immervoll H, Boe SO, Svendsen A, Prestegarden L, Rosland G, Thorsen F, Stuhr L, Molven A, Bjerkvig R, Enger PO. CD133 negative glioma cells form tumors in nude rats and give rise to CD133 positive cells. Int J Cancer. 2008;122:761–768. doi: 10.1002/ijc.23130. [DOI] [PubMed] [Google Scholar]
- 41.Corbeil D, Röper K, Hellwig A, Tavian M, Miraglia S, Watt SM, Simmons PJ, Peault B, Buck DW, Huttner WB. The human AC133 haematopoietic stem cell antigen is also expressed in epithelial cells and targeted to plasma membrane protrusions. J Biol Chem. 2000;275:5512–5520. doi: 10.1074/jbc.275.8.5512. [DOI] [PubMed] [Google Scholar]
- 42.Torrente Y, Bellicchi M, Sampaolesi M, Pisati F, Meregalli M, D’Antona G, Tonlorenzi R, Poretti L, Gavina M, Machaoui K, Pellegrino MA, Furling D, Mouly V, Butler-Browne GS, Bottinelli R, Cossu G, Bresolin N. Human circulating AC133(+) stem cells restore dystrophin expression and ameliorate function in dystrophic skeletal muscle. J Clin Invest. 2004;114:182–195. doi: 10.1172/JCI20325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Reya T, Morrison SJ, Clarke MF, Weissman IL. Stem cells, cancer, and cancer stem cells. Nature. 2001;414:105–111. doi: 10.1038/35102167. [DOI] [PubMed] [Google Scholar]
- 44.Pirozzi G, Camerlingo R, Franco R, La Rocca A, Liguori E, Martucci N, Paino F, Normanno N, Rocco G. Epithelial to mesenchymal transitino by TGF-β1 induction increases stemness characteristics in primary non small cell lung cancer cell line. PLoS One. 2011;6:e21548. doi: 10.1371/journal.pone.0021548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Thiery JP, Acloque H, Huang RY, Nieto MA. Epithelial–mesenchymal transitions in development and disease. Cell. 2009;139:871–890. doi: 10.1016/j.cell.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 46.Micalizzi DS, Farabaugh SM, Ford HL. Epithelial-mesenchymal transition in cancer: parallels between normal development and tumor progression. J Mammary Gland Biol Neoplasia. 2010;15:117–134. doi: 10.1007/s10911-010-9178-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pirozzi G, Tirino V, Camerlingo R, La Rocca A, Martucci N, Scognamiglio G, Franco R, Cantile M, Normanno N, Rocco G. Prognostic value of cancer stem cells, epithelial-mesenchymal transitino and circulating tumor cells in lung cancer. Oncology Reports. 2013;29:1763–8. doi: 10.3892/or.2013.2294. [DOI] [PubMed] [Google Scholar]
- 48.Vassilopoulos A, Wang RH, Petrovas C, Ambrozak D, Koup R, Deng CX. Identification and characterization of cancer initiating cells from BRCA1 related mammary tumors using markers for normal mammary stem cells. Int J Biol Sci. 2008;4:133–42. doi: 10.7150/ijbs.4.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Vermeulen L, Todaro M, de Sousa Mello F, Sprick MR, Kemper K, Perez Alea M, Richel DJ, Stassi G, Medema JP. Single-cell cloning of colon cancer stem cells reveals a multi-lineage differentiation capacity. Proc Natl Acad Sci U S A. 2008;105:13427–32. doi: 10.1073/pnas.0805706105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Al Hajj M, Wicha MS, Benito-Hernandez A, Morrison SJ, Clarke MF. Prospective identification of tumorigenic breast cancer cells. Proc Natl Acad Sci U S A. 2003;100:3983–8. doi: 10.1073/pnas.0530291100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Prince ME, Sivanandan R, Kaczorowski A, Wolf GT, Kaplan MJ, Dalerba P, Weissman IL, Clarke MF, Ailles LE. Identification of a subpopulation of cells with cancer stem cell properties in head and neck squamous cell carcinoma. Proc Natl Acad Sci U S A. 2007;104:973–8. doi: 10.1073/pnas.0610117104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Monzani E, Facchetti F, Galmozzi E, Corsini E, Benetti A, Cavazzin C, Gritti A, Piccinini A, Porro D, Santinami M, Invernici G, Parati E, Alessandri G, La Porta CA. Melanoma contains CD133 and ABCG2 positive cells with enhanced tumourigenic potential. Eur J Cancer. 2007;43:935–46. doi: 10.1016/j.ejca.2007.01.017. [DOI] [PubMed] [Google Scholar]
- 53.Charafe-Jauffret E, Ginestier C, Iovino F, Wicinski J, Cervera N, Finetti P, Hur MH, Diebel ME, Monville F, Dutcher J, Brown M, Viens P, Xerri L, Bertucci F, Stassi G, Dontu G, Birnbaum D, Wicha MS. Breast cancer cell lines contain functional cancer stem cells with metastatic capacity and a distinct molecular signature. Cancer Res. 2009;69:1302. doi: 10.1158/0008-5472.CAN-08-2741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ginestier C, Hur MH, Charafe-Jauffret E, Monville F, Dutcher J, Brown M, Jacquemier J, Viens P, Kleer CG, Liu S, Schott A, Hayes D, Birnbaum D, Wicha MS, Dontu G. ALDH1 is a marker of normal and malignant human mammary stem cells and a predictor of poor clinical outcome. Cell Stem Cell. 2007;1:555–67. doi: 10.1016/j.stem.2007.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Addla SK, Brown MD, Hart CA, Ramani VAC, Clarke NW. Characterization of the Hoechst 33342 side population from normal and malignant human renal epithelial cells. Am J Physiol Renal Physiol. 2008;295:F680–7. doi: 10.1152/ajprenal.90286.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ho MM, Ng AV, Lam S, Hung JY. Side population in human lung cancer cell lines and tumors is enriched with stem-like cancer cells. Cancer Res. 2007;67:4827–33. doi: 10.1158/0008-5472.CAN-06-3557. [DOI] [PubMed] [Google Scholar]
- 57.Jordan CT, Guzman ML, Noble M. Cancer stem cells. New Engl J Med. 2006;355:1253–61. doi: 10.1056/NEJMra061808. [DOI] [PubMed] [Google Scholar]
- 58.Dean M. Cancer stem cells: redefining the paradigm of cancer treatment strategies. Mol Interv. 2006;6:140–8. doi: 10.1124/mi.6.3.5. [DOI] [PubMed] [Google Scholar]
- 59.Colleen W, Alman BA. Side population cells in human cancers. Cancer Lett. 2008;268:1–9. doi: 10.1016/j.canlet.2008.03.048. [DOI] [PubMed] [Google Scholar]
- 60.Wilson H, Huelsmeyer M, Chun R, Young KM, Friedrichs K, Argyle DJ. Isolation and characterisation of cancer stem cells from canine osteosarcoma. Vet J. 2008;175:69–75. doi: 10.1016/j.tvjl.2007.07.025. [DOI] [PubMed] [Google Scholar]
- 61.Gibbs CP, Kukekov VG, Reith JD, Tchigrinova O, Suslov ON, Scott EW, Ghivizzani SC, Ignatova TN, Steindler DA. Stem-like cells in bone sarcomas: implications for tumorigenesis. Neoplasia. 2005;7:967–76. doi: 10.1593/neo.05394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Green CL, Loken M, Buck D, Deeg HJ. Discordant expression of AC133 and AC141 in patients with myelodysplastic syndrome (MDS) and acute myelogeneous leukemia (AML) Leukemia. 2000;14:770–2. doi: 10.1038/sj.leu.2401736. [DOI] [PubMed] [Google Scholar]
- 63.Tirino V, Camerlingo R, Bifulco K, Irollo E, Montella R, Paino F, Sessa G, Carriero MV, Normanno N, Rocco G, Pirozzi G. TGF-β1 exposure induces epithelial to mesenchymal transition both in CSCs and non-CSCs of the A549 cell line, leading to an increase of migration ability in the CD133(+) A549 cell fraction. Cell Death Dis. 2013;4:e620. doi: 10.1038/cddis.2013.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Shmelkov SV, Butler JM, Hooper AT, Hormigo A, Kushner J, Milde T, St Clair R, Baljevic M, White I, Jin DK, Chadburn A, Murphy AJ, Valenzuela DM, Gale NW, Thurston G, Yancopoulos GD, D’Angelica M, Kemeny N, Lyden D, Rafii S. CD133 expression is not restricted to stem cells, and both CD133+ and CD133- metastatic colon cancer cells initiate tumors. J Clin Invest. 2008;118:2111–20. doi: 10.1172/JCI34401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Chao C, Carmical JR, Ives KL, Wood TG, Aronson JF, Gomez GA, Djukom CD, Hellmich MR. CD133+ colon cancer cells are more interactive with the tumor microenvironment than CD133- cells. Lab Invest. 2011;92:420–36. doi: 10.1038/labinvest.2011.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Meng X, Li M, Wang X, Wang Y, Ma D. Both CD133+ and CD133 subpopulations of A549 and H446 cells contain cancer-initiating cells. Cancer Sci. 2009;100:1040–6. doi: 10.1111/j.1349-7006.2009.01144.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kimura T, Wang L, Tabu K, Nishihara H, Mashita Y, Kikuchi N, Tanino M, Hiraga H, Tanaka S. CD133 Negatively Regulates Tumorigenicity via AKT Pathway in Synovial Sarcoma. Cancer Invest. 2012;30:390–397. doi: 10.3109/07357907.2012.672607. [DOI] [PubMed] [Google Scholar]
- 68.Rocco A, Liguori E, Pirozzi G, Tirino V, Compare D, Franco R, Tatangelo F, Palaia R, D’Armiento FP, Pollastrone G, Affuso A, Bottazzi EC, Masone S, Persico G, Nardone G. CD133 and CD44 cell surface markers do not identify cancer stem cells in primary human gastric tumors. J Cell Physiol. 2012;227:2686–93. doi: 10.1002/jcp.23013. [DOI] [PubMed] [Google Scholar]
- 69.Cai C, Yu JW, Wu JG, Lu RQ, Ni XC, Wang SL, Jiang BJ. CD133 promotes the invasion and metastasis of gastric cancer via epithelial-mesenchymal transition. Zhonghua Wei Chang Wai Ke Za Zhi. 2013;16:662–7. [PubMed] [Google Scholar]
- 70.Lee J, Kotliarova S, Kotliarov Y, Li A, Su Q, Donin NM, Pastorino S, Purow BW, Christopher N, Zhang W, Park JK, Fine HA. Tumor stem cells derived from glioblastomas cultured in bFGF and EGF more closely mirror the phenotype and genotype of primary tumors than do serum-cultured cell lines. Cancer Cell. 2006;9:391–403. doi: 10.1016/j.ccr.2006.03.030. [DOI] [PubMed] [Google Scholar]
- 71.Taïeb N, Maresca M, Guo XJ, Garmy N, Fantini J, Yahi N. The first extracellular domain of the tumour stem cell marker CD133 contains an antigenic ganglioside-binding motif. Cancer Lett. 2009;278:164–73. doi: 10.1016/j.canlet.2009.01.013. [DOI] [PubMed] [Google Scholar]
- 72.Donovan LK, Potter NE, Warr T, Pilkington GJ. A Prominin-1-Rich Pediatric Glioblastoma: Biologic Behavior Is Determined by Oxygen Tension-Modulated CD133 Expression but Not Accompanied by Underlying Molecular Profiles. Transl Oncol. 2012;5:141–54. doi: 10.1593/tlo.11337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zhu L, Gibson P, Currle DS, Tong Y, Richardson RJ, Bayazitov IT, Poppleton H, Zakharenko S, Ellison DW, Gilbertson RJ. Prominin 1 marks intestinal stem cells that are susceptible to neoplastic transformation. Nature. 2009;457:603–607. doi: 10.1038/nature07589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Immervoll H, Hoem D, Sakariassen PØ, Steffensen OJ, Molven A. Expression of the “stem cell marker” CD133 in pancreas and pancreatic ductal adenocarcinomas. BMC Cancer. 2008;8:48. doi: 10.1186/1471-2407-8-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lardon J, Corbeil D, Huttner WB, Ling Z, Bouwens L. Stem cell marker prominin-1/AC133 is expressed in duct cells of the adult human pancreas. Pancreas. 2008;36:e1–e6. doi: 10.1097/mpa.0b013e318149f2dc. [DOI] [PubMed] [Google Scholar]
- 76.Gregoriadis G, Fernandes A, Mital M, McCormack B. Polysialic acids: potential in improving the stability and pharmacokinetics of proteins and other therapeutics. Cell Mol Life Sci. 2000;57:1964–1969. doi: 10.1007/PL00000676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Miyagi T, Wada T, Yamaguchi K, Hata K. Sialidase and malignancy: a minireview. Glycoconj J. 2004;20:189–198. doi: 10.1023/B:GLYC.0000024250.48506.bf. [DOI] [PubMed] [Google Scholar]
- 78.Li M, Song L, Qin X. Glycan changes: cancer metastasis and anti-cancer vaccines. J Biosci. 2010;35:665–673. doi: 10.1007/s12038-010-0073-8. [DOI] [PubMed] [Google Scholar]
- 79.Sgambato A, Puglisi MA, Errico F, Rafanelli F, Boninsegna A, Rettino A, Genovese G, Coco C, Gasbarrini A, Cittadini A. Post-translational modulation of CD133 expression during sodium butyrate-induced differentiation of HT29 human colon cancer cells: implications for its detection. J Cell Physiol. 2010;224:234–241. doi: 10.1002/jcp.22124. [DOI] [PubMed] [Google Scholar]
- 80.Mak AB, Blakely KM, Williams RA, Penttilä PA, Shukalyuk AI, Osman KT, Kasimer D, Ketela T, Moffat J. CD133 Nglycosylation processing contributes to cell-surface recognition of the primitive cell marker AC133. J Biol Chem. 2011;286:41046–41056. doi: 10.1074/jbc.M111.261545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hemmoranta H, Satomaa T, Blomqvist M, Heiskanen A, Aitio O, Saarinen J, Natunen J, Partanen J, Laine J, Jaatinen T. N-glycan structures and associated gene expression reflect the characteristic N-glycosylation pattern of human hematopoietic stem and progenitor cells. Exp Hematol. 2007;35:1279–1292. doi: 10.1016/j.exphem.2007.05.006. [DOI] [PubMed] [Google Scholar]
- 82.Shmelkov SV, Jun L, St Clair R, McGarrigle D, Derderian CA, Usenko JK, Costa C, Zhang F, Guo X, Rafii S. Alternative promoters regulate transcription of the gene that encodes stem cell surface protein AC133. Blood. 2004;103:2055–2061. doi: 10.1182/blood-2003-06-1881. [DOI] [PubMed] [Google Scholar]
- 83.Tabu K, Sasai K, Kimura T, Wang L, Aoyanagi E, Kohsaka S, Tanino M, Nishihara H, Tanaka S. Promoter hypomethylation regulates CD133 expression in human gliomas. Cell Res. 2008;18:1037–1046. doi: 10.1038/cr.2008.270. [DOI] [PubMed] [Google Scholar]
- 84.Laird PW, Jaenisch R. The role of DNA methylation in cancer genetic and epigenetics. Annu Rev Genet. 1996;30:441–464. doi: 10.1146/annurev.genet.30.1.441. [DOI] [PubMed] [Google Scholar]
- 85.Iacobuzio-Donahue CA. Epigenetic changes in cancer. Annu Rev Pathol. 2009;4:229–249. doi: 10.1146/annurev.pathol.3.121806.151442. [DOI] [PubMed] [Google Scholar]
- 86.Gibney ER, Nolan CM. Epigenetics and gene expression. Heredity. 2010;105:4–13. doi: 10.1038/hdy.2010.54. [DOI] [PubMed] [Google Scholar]
- 87.Gregory CA, Prockop DJ, Spees JL. Non-hematopoietic bone marrow stem cells: molecular control of expansion and differentiation. Exp Cell Res. 2005;306:330–335. doi: 10.1016/j.yexcr.2005.03.018. [DOI] [PubMed] [Google Scholar]
- 88.Meregalli M, Farini A, Belicchi M, Torrente Y. CD133(+) cells isolated from various sources and their role in future clinical perspectives. Expert Opin Biol Ther. 2010;10:1521–1528. doi: 10.1517/14712598.2010.528386. [DOI] [PubMed] [Google Scholar]
- 89.Baba T, Convery PA, Matsumura N, Whitaker RS, Kondoh E, Perry T, Huang Z, Bentley RC, Mori S, Fujii S, Marks JR, Berchuck A, Murphy SK. Epigenetic regulation of CD133 and tumorigenicity of CD133+ ovarian cancer cells. Oncogene. 2009;28:209–218. doi: 10.1038/onc.2008.374. [DOI] [PubMed] [Google Scholar]
- 90.Gan Q, Yoshida T, McDonald OG, Owens GK. Concise review: epigenetic mechanisms contribute to pluripotency and cell lineage determination of embryonic stem cells. Stem Cells. 2007;25:2–9. doi: 10.1634/stemcells.2006-0383. [DOI] [PubMed] [Google Scholar]
- 91.Balch C, Nephew KP, Huang TH, Bapat SA. Epigenetic ‘bivalently marked’ process of cancer stem cell-driven tumorigenesis. Bioessays. 2007;29:842–845. doi: 10.1002/bies.20619. [DOI] [PubMed] [Google Scholar]
- 92.Ohm JE, McGarvey KM, Yu X, Cheng L, Schuebel KE, Cope L, Mohammad HP, Chen W, Daniel VC, Yu W, Berman DM, Jenuwein T, Pruitt K, Sharkis SJ, Watkins DN, Herman JG, Baylin SB. A stem cell-like chromatin pattern may predispose tumor suppressor genes to DNA hypermethylation and heritable silencing. Nat Genet. 2007;39:237–242. doi: 10.1038/ng1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Ruau D, Ensenat-Waser R, Dinger TC, Vallabhapurapu DS, Rolletschek A, Hacker C, Hieronymus T, Wobus AM, Müller AM, Zenke M. Pluripotency associated genes are reactivated by chromatin modifying agents in neurosphere cells. Stem Cells. 2008;26:920–6. doi: 10.1634/stemcells.2007-0649. [DOI] [PubMed] [Google Scholar]
- 94.Fahrner JA, Eguchi S, Herman JG, Baylin SB. Dependence of histone modifications and gene expression on DNA hypermethylation in cancer. Cancer Res. 2002;62:7213–7218. [PubMed] [Google Scholar]
- 95.Yi JM, Tsai HC, Glöckner SC, Lin S, Ohm JE, Easwaran H, James CD, Costello JF, Riggins G, Eberhart CG, Laterra J, Vescovi AL, Ahuja N, Herman JG, Schuebel KE, Baylin SB. Abnormal DNA methylation of CD133 in colorectal and glioblastoma tumors. Cancer Res. 2008;68:8094–8103. doi: 10.1158/0008-5472.CAN-07-6208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Lee TI, Jenner RG, Boyer LA, Guenther MG, Levine SS, Kumar RM, Chevalier B, Johnstone SE, Cole MF, Isono K, Koseki H, Fuchikami T, Abe K, Murray HL, Zucker JP, Yuan B, Bell GW, Herbolsheimer E, Hannett NM, Sun K, Odom DT, Otte AP, Volkert TL, Bartel DP, Melton DA, Gifford DK, Jaenisch R, Young RA. Control of developmental regulators by polycomb in human embryonic stem cells. Cell. 2006;125:301–13. doi: 10.1016/j.cell.2006.02.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Ohno H, Shirato K, Sakurai T, Ogasawara J, Sumitani Y, Sato S, Imaizumi K, Ishida H, Kizaki T. Effect of exercise on HIF-1 and VEGF signalling. J Phys Fitness Sports Med. 2012;1:5–16. [Google Scholar]
- 98.Soeda A, Park M, Lee D, Mintz A, Androutsellis-Theotokis A, McKay RD, Engh J, Iwama T, Kunisada T, Kassam AB, Pollack IF, Park DM. Hypoxia promotes expansion of the CD133-positive glioma stem cells through activation of HIF-1alpha. Oncogene. 2009;28:3949–59. doi: 10.1038/onc.2009.252. [DOI] [PubMed] [Google Scholar]
- 99.Iida H, Suzuki M, Goitsuka R, Ueno H. Hypoxia induces CD133 expression in human lung cancer cells by up-regulation of OCT3/4 and SOX2. Int J Oncol. 2012;40:71–9. doi: 10.3892/ijo.2011.1207. [DOI] [PubMed] [Google Scholar]
- 100.Franco R, Cantile M, Zito Marino F, Pirozzi G. Circulating tumor cells as emergin tumor biomarkers in lung cancer. J Thorac Dis. 2012;4:438–439. doi: 10.3978/j.issn.2072-1439.2012.08.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Gage T, Fan SL. What goes around, comes around: a review of circulating tumor cells. MLO Med Lab Obs. 2010;42:32, 34–36. [PubMed] [Google Scholar]
- 102.Maheswaran S, Haber DA. Circulating tumor cells: a window into cancer biology and metastasis. Curr Opin Genet Dev. 2010;20:96–99. doi: 10.1016/j.gde.2009.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Giordano A, Gao H, Anfossi S, Cohen E, Mego M, Lee BN, Tin S, De Laurentiis M, Parker CA, Alvarez RH, Valero V, Ueno NT, De Placido S, Mani SA, Esteva FJ, Cristofanilli M, Reuben JM. Epithelial-mesenchymal transition and stem cell markers in patients with HER2-positive metastatic breast cancer. Mol Cancer Ther. 2012;11:2526–34. doi: 10.1158/1535-7163.MCT-12-0460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Shimada R, Iinuma H, Tamura J, Horiuchi A, Nozawa K, Ishihara S, Matsuda K, Watanabe T, Hashiguchi Y. Usefulness of CTCs in tumor drainage vein blood as a biomarker for prognosis in colorectal cancer patients with Dukes’ stage B and C. Gan To Kagaku Ryoho. 2012;39:1763–5. [PubMed] [Google Scholar]
- 105.Nadal R, Ortega FG, Salido M, Lorente JA, Rodríguez-Rivera M, Delgado-Rodríguez M, Macià M, Fernández A, Corominas JM, García-Puche JL, Sánchez-Rovira P, Solé F, Serrano MJ. CD133 expression in circulating tumor cells from breast cancer patients: Potential role in resistance to chemotherapy. Int J Cancer. 2013 Nov 15;133:2398–407. doi: 10.1002/ijc.28263. [DOI] [PubMed] [Google Scholar]
- 106.Ross JS, Slodkowska EA. Circulating and disseminated tumor cells in the management of breast cancer. Am J Clin Pathol. 2009;132:237–245. doi: 10.1309/AJCPJI7DEOLKCS6F. [DOI] [PubMed] [Google Scholar]
- 107.Liu MC, Shields PG, Warren RD, Cohen P, Wilkinson M, Ottaviano YL, Rao SB, Eng-Wong J, Seillier-Moiseiwitsch F, Noone AM, Isaacs C. Circulating tumor cells: a useful predictor of treatment efficacy in metastatic breast cancer. J. Clin. Oncol. 2009;27:5153–5159. doi: 10.1200/JCO.2008.20.6664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Mego M, De Giorgi U, Dawood S, Wang X, Valero V, Andreopoulou E, Handy B, Ueno NT, Reuben JM, Cristofanilli M. Characterization of metastatic breast cancer patients with non-detectable circulating tumor cells. Int J Cancer. 2011;129:417–423. doi: 10.1002/ijc.25690. [DOI] [PubMed] [Google Scholar]
- 109.Di Bonito M, Collina F, Cantile M, Camerlingo R, Cerrone M, Marra L, Liguori G, Pirozzi G, Botti G. Aberrant Expression of Cancer Stem Cells Marker Prominin-1 in Low-Grade Tubulobular Breast Carcinoma: A Correlative Study between qRT-PCR, Flow-Cytometric and Immunohistochemistry Analysis. J Breast Cancer. 2012;15:15–23. doi: 10.4048/jbc.2012.15.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Li CY, Li BX, Liang Y, Peng RQ, Ding Y, Xu DZ, Zhang X, Pan Z, Wan DS, Zeng YX, Zhu XF, Zhang XS. Higher percentage of CD133+ cells is associated with poor prognosis in colon carcinoma patients with stage IIIB. J Transl Med. 2009;7:56. doi: 10.1186/1479-5876-7-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Artells R, Moreno I, Diaz T, Martinez F, Gel B, Navarro A, Ibeas R. Tumour CD133 mRNA expression and clinical outcome in surgically resected colorectal cancer patients. Eur J Cancer. 2010;46:642–649. doi: 10.1016/j.ejca.2009.11.003. [DOI] [PubMed] [Google Scholar]
- 112.Horst D, Kriegl L, Engel J, Kirchner T, Jung A. CD133 expression is an independent prognostic marker for low survival in colorectal cancer. Br J Cancer. 2008;99:1285–1289. doi: 10.1038/sj.bjc.6604664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Horst D, Scheel SK, Liebmann S, Neumann J, Maatz S, Kirchner T. The cancer stem cell marker CD133 has high prognostic impact but unknown functional relevance for the metastasis of human colon cancer. J Pathol. 2009;219:427–434. doi: 10.1002/path.2597. [DOI] [PubMed] [Google Scholar]
- 114.Metellus P, Nanni-Metellus I, Delfino C, Colin C, Tchogandjian A, Coulibaly B, Fina F, Loundou A, Barrie M, Chinot O, Ouafiz LH, Figarella-Branger D. Prognostic impact of CD133 mRNA expression in 48 glioblastoma patients treated with concomitant radiochemotherapy: a prospective patient cohort at a single institution. Ann Surg Oncol. 2011;18:2937–2945. doi: 10.1245/s10434-011-1703-6. [DOI] [PubMed] [Google Scholar]
- 115.Shin JH, Lee YS, Hong YK, Kang CS. Correlation between the prognostic value and the expression of the stem cell marker CD133 and isocitrate dehydrogenase1 in glioblastomas. J Neurooncol. 2013 doi: 10.1007/s11060-013-1234-z. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 116.Sasaki A, Kamiyama T, Yokoo H, nakanishi K, Kubota K, Haga H, Matsushita M, Ozaki M, Matsuno Y, Todo S. Cytoplasmic expression of CD133 is an important risk factor for overall survival in hepatocellular carcinoma. Oncol Rep. 2010;24:537–546. doi: 10.3892/or_00000890. [DOI] [PubMed] [Google Scholar]
- 117.Ishigami S, Ueno S, Arigami T, Uchikado Y, Setoyama T, Arima H, Kita Y, Kurahara H, Okumura H, Matsumoto M, Kijima Y, Natsugoe S. Prognostic impact of CD133 expression in gastric carcinoma. Anticancer Res. 2010;30:2453–2457. [PubMed] [Google Scholar]
- 118.Wen L, Chen XZ, Yang K, Chen ZX, Zhang B, Chen JP, Zhou ZG, Mo XM, Jian-Kun Hu JK. Prognostic Value of Cancer Stem Cell Marker CD133 Expression in Gastric Cancer: A Systematic Review. PLoS One. 2013;8:e59154. doi: 10.1371/journal.pone.0059154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Nakamura M, Kyo S, Zhang B, Zhang X, Mizumoto Y, Takakura M, Maida Y, Mori N, Hashimoto M, Ohno S, Inoue M. Prognostic impact of CD133 expression as a tumor-initiating cell marker in endometrial cancer. Hum Pathol. 2010;41:1516–1529. doi: 10.1016/j.humpath.2010.05.006. [DOI] [PubMed] [Google Scholar]
- 120.Silva IA, Bai S, McLean K, Yang K, Griffth K, Thomas D, Ginestier C, Johnston C, Kueck A, Reynolds RK, Wicha MS, Buckanovich RJ. Aldehyde dehydrogenase in combination with CD133 defines angiogenic ovarian cancer stem cells that portend poor patient survival. Cancer Res. 2011;71:3991–4001. doi: 10.1158/0008-5472.CAN-10-3175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Woo T, Okudela K, Mitsui H, Yazawa T, Ogawa N, Tajiri M, Yamamoto T, Rino Y, Kitamura H, Masuda M. Prognostic value of CD133 expression in stage I lung adenocarcinomas. Int J Clin Exp Pathol. 2010;4:32–42. [PMC free article] [PubMed] [Google Scholar]
- 122.Pirozzi G, Tirino V, Camerlingo R, La Rocca A, Martucci N, Scognamiglio G, Franco R, Cantile M, Normanno N, Rocco G. Prognostic value of cancer stem cells, epithelial-mesenchymal transition and circulating tumor cells in lung cancer. Oncol Rep. 2013;29:1763–8. doi: 10.3892/or.2013.2294. [DOI] [PubMed] [Google Scholar]
- 123.Choi D, Lee HW, Hur KY, Kim JJ, Park GS, Jang SH, Song YS, Jang KS, Paik SS. Cancer stem cell markers CD133 and CD24 correlate with invasiveness and differentiation in colorectal adenocarcinoma. World J Gastroenterol. 2009;15:2258–2264. doi: 10.3748/wjg.15.2258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Kojima M, Ishii G, Atsumi N, Fujii S, Saito N, Ochiai A. Immunohistochemical detection of CD133 expression in colorectal cancer: a clinicopathological study. Cancer Sci. 2008;99:1578–1583. doi: 10.1111/j.1349-7006.2008.00849.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Bidlingmaier S, Zhu X, Liu B. The utility and limitations of glycosylated human CD133 epitopes in defing cancer stem cells. J Mol Med (Berl) 2008;86:1025–1032. doi: 10.1007/s00109-008-0357-8. [DOI] [PMC free article] [PubMed] [Google Scholar]


