Abstract
Ossification of Ligamentum Flavum (OLF) is associated with serious neurologic symptoms including thoracic myelopathy and spinal stenosis. The pathogenesis of thoracic OLF is mainly due to the localized mechanical stress on the ligament induced enchondral ossification. However, despite numerous epidemiological and basic science studies, the mechanism of this process remains unclear. Studies have suggested that inflammatory cytokines, such as IL-6, TNF-α, seem to play a crucial role in OLF. In this review, we summarise the mechanistic information on the roles of inflammation cytokines in OLF and discuss about several therapeutic methods for OLF. Further studies on the role of cytokines in OLF should provide important insights into the designation of therapeutic strategies in preventing human spinal stenosis caused by OLF.
Keywords: Ossification of ligamentum flavum (OLF), cytokine, pathology
Introduction
Ossification of the ligamentum flavum (OLF) is one of the important causes of thoracic myelopathy (TM) that leads to the narrowing of the spinal canal. OLF can significantly contribute to a spatial reduction of the thoracic spinal canal, resulting in slowly progressive paraphrases or acute paraplegia after trauma to the back. OLF usually exists with myelopathy in the setting of thoracic spinal stenosis, but its aetiology is not well defined. However, OLF has been associated with skeletal fluorosis, trauma, diffuse skeletal hyperostosis (DISH), ankylosing spondylitis, diabetes, hemochromatosis hyperthyroidism, and deposition of calcium pyrophosphate crystals [1]. OLF may present as distinct syndromes. It involves chronic spinal cord compression over a long period of time and presents with unsteady gait, difficulty with balance and climbing stairs [2-4]. Because symptoms vary and are subjective, diagnosis of TM caused by OLF is sometimes difficult when based on symptoms and physical examination. Since this disease is the ectopic bone formation within the ligamentum flavum, it may results in mass effect and neurological compromise [5]. The low thoracic region is the most common region of occurrence, and followed by the cervical and lumber spine.
Etiology of OLF
The etiologic causes for most of the OLF are unknown. Diffuse idiopathic skeletal hyperostoses (DISH), Ankylosing spondylosis, Hemochromatosis, fluorosis, calcium pyrophosphate dehydrate deposition disease and traumas are the possible initial causes [6,7]. Pathological study of the ligamentum flavum suggested that the ligaments will get hypertrophied, thickened, and calcified before it turns ossified [8]. The process of ossification starts at the base of the ligament with enchondral ossification of the vascularised fibro cartilaginous tissues. It starts from capsular sides and gradually spread anteriorly and medially compressing the spinal card from posterior-lateral sides. Several studies have indicated the possible roles of mechanical, genetic, metabolic, and cell biological factors in the development and progression of OLF [9]. However, the pathogenic role of cytokines for OLF is not fully elucidated. There are two types of OLF. The first one is more common, as it presents as gradual onset of myelopathy with slow deterioration in neurological status. Another proposed aetiology is the elevation of fibronectin concentration in plasma, as it promotes the formation of fibroblasts in ligaments; this will further results in endochondral ossification [10].
Risk factors
Previous reports have shown that this disease has a genetic predilection with a majority of documented cases are Japanese population [10]. Recent studies suggested that genetic modifications on numerous growth factors including BMP and TGF-β revealed their roles in regulating the development, growth, maintenance as well as the formation of new cartilage and bone tissues. While in human study, ligamentum flavum cells were susceptible to adenovirus-mediated markers [11].
A case study conducted recently by Mobbs had demonstrated dietary habits may also be another independent risk factor. A patient from a low risk genetic background developed multilevel thoracic ossification as he followed standard Japanese dietary habits since young. Furthermore, higher incidents of obesity, disturbance in glucose metabolism, higher prevalence of diabetes mellitus and hyperinsulinism were observed in patients with OLF [10].
Inflammatory cytokines and OLF
Cytokines are small secreted proteins that serve as messengers between cells. They are largely involved in the regulation of reproduction, growth and development, normal homeostatic regulation, response to injury and self-repairing of human immunity. Inflammatory cytokines, such as interleukins, tumor necrosis factor (TNF-α), T cell growth factor (TGF) and chemokine, are part of immune response to injuries and infections, and effects on either to increase or to decrease inflammation. Pro-inflammatory cytokines are responsible for early responses, such as IL-1-alpha, IL-1-beta IL-6, and TNF-α [12]. Other more pro-inflammatory mediators include members of the IL-20 family, IFN-gamma, TGF-β, IL-17, IL-18 and a variety of other chemokines. These cytokines act as endogenous pyrogens (IL-1, Il-6, TNF-alpha), to up-regulate the synthesis of secondary mediators [13]. Pro-inflammatory cytokines stimulate the production of acute phase proteins to attract inflammatory cells. In contrast, anti-inflammatory cytokines such as IL-4, IL-10, IL-16 and TGF-beta, contribute to the control of the magnitude to the inflammatory responses in vivo by the inhibition of the production of the pro-inflammatory cytokines or by counteracting many biological effects of pro-inflammatory mediators.
Studies have found that degenerated intervertebral disk spontaneously produces inflammatory cytokines, which might affect the adjacent LF through local milieu of the spinal canal [14]. Histopathologically, the progress of calcification and ossification was closely associated with the degeneration elastic fibers and with significant expression of inflammatory cytokines in the ossification front, such as BMP-2, TGF-beta, and VEGF [4]. Degenerated, herniated intervertebral disks and facet arthrosis may also influence LF through the secreted inflammatory cytokines and cause hypertrophy and ossification of LF.
Previous study by Park et al have showed that under resting conditions, the mRNA levels of β-catenin, Runx2, Sox9, and osteopontin in cultured OLF cells were significantly higher than in the control non-OLF cells. Application of cyclic tensile strain to OLF cells resulted in significant increases of mRNA expression levels of β-catenin, Runx2, Sox9, and osteopontin. Hypertrophic chondrocytes elevated around the calcification front were immunopositive for Runx2 and osteopontin. Immunoreactivity of β-catenin and Sox9 was strongly present in premature chondrocytes in the fibrocartilage area. They also found that accumulation of hypertrophic chondrocytes was evident around the calcified area at the ossification front, and this suggests that the differentiation of these cells seems to be related with the ossification process. Under resting conditions (no tensile strain), the mRNA levels of β-catenin, Runx2, Sox9, and osteopontin in cultured OLF cells were significantly higher than in the control non-OLF cells. Application of cyclic tensile strain to OLF cells resulted in significant increases in mRNA expression levels of β-catenin, Runx2, Sox9, and osteopontin at 24 hours. Hypertrophic chondrocytes present around the calcification front were immunopositive for Runx2 and osteopontin. Immunoreactivity of β-catenin and Sox9 was strongly elevated in premature chondrocytes in the fibrocartilage area. These results indicated that cyclic tensile strain applied to OLF cells activated their ossification through a process mediated by the β-catenin signaling pathway. The induced inflammatory cytokines also up-regulate collagen synthesis during the pathogenesis of LF hypertrophy and ossification [14]. An in vitro study carried out by Park et al found that there were significant increases in DNA and mRNA synthesis of type I, V, XI collagen and osteocalcin in human LF cultures with inflammatory cytokines IL-1α, IL-6, PGE2, TNF-α.
Figure 1 showed a schematic representation of cytokine and cellular interaction in the tumour-necrosis factor (TNF)-dependent cytokine cascade illustrating the role of TNF in the cytokine network of OLF. The degenerated intervertebral disk spontaneously produces inflammatory cytokines, such as IL-6, IL-12, IL-18 and TNF. These pro-inflammatory cytokines further stimulate the key interacting cells, CD4+ T cells and macrophage. These interacting cells further affect the adjacent LF through local milieu of the spinal canal.
Figure 1.
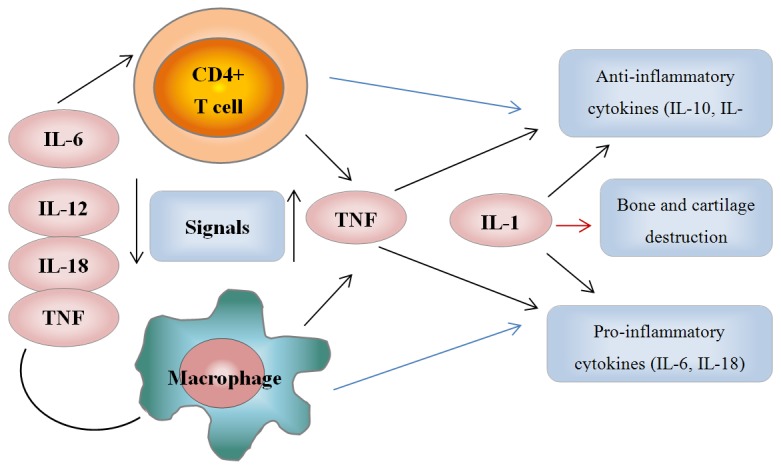
A schematic representation of cytokine and cellular interaction in the tumour-necrosis factor (TNF)-dependent cytokine cascade illustrating the role of TNF in the cytokine network of OLF.
Diagnosis and treatments
OLF should be included in causes of thoracic paraplegia. X-rays are not very classical. MRI-T2 weighted sagittal plane shows indentation on the thoracic card from behind. CT- Axial plane scanning and MRI shows typical ossification of OLF [15,16].
Patients with thoracic myelopathy due to OLF usually underwent decompressive laminectomy and excision of the ligamentum flavum. The pathogenesis of thoracic OLF is mainly due to the localized mechanical stress on the ligament [17]. Wang et al. suggested that the Laminectomy combined with lateral fusion is the most effective treatment for thoracic OLF [3]. Furthermore, in terms of the configuration of the ossified lesions, en bloc laminectomy is suitable for the treatment of lateral-type and diffuse-type OLF, and the separating laminectomy is suitable for the thickened, nodular-type OLF. Decompressive Laminectomy, Laminopl-asty and retaining posterior elements are the common options for the treatment of OLF. Laminectomy and fusion can prevent increases in kyphotic deformity. Complications are present when it is associated with ossification of dura. Commonest complication is CSF leak due to associated dural calcification; this will cause meningitis, delayed wound healing, incomplete neurological recovery or deterioration in neurological status. Other approaches include surgically removal of boney lesions using high-speed drill and curettes and/or reconstruction with a dural graft substitute.
In summary, OLF is an important cause of thoracic myeloradiculopathy. Dietary habits, cytokines and other mediators, such as bone morphogenetic proteins are important in the ossification process of human thoracic ligamentum flavum. In order to design therapeutic strategies in preventing human spinal stenosis caused by OLF, the effects of cytokines on OLF are required to be investigated in further details.
References
- 1.Kruse JJ, Awasthi D, Harris M, Waguespack A. Ossification of the ligamentum flavum as a cause of myelopathy in North America: report of three cases. J Spinal Disord. 2000;13:22–25. doi: 10.1097/00002517-200002000-00004. [DOI] [PubMed] [Google Scholar]
- 2.Fong SY, Wong HK. Thoracic myelopathy secondary to ligamentum flavum ossification. Ann Acad Med Singapore. 2004;33:340–346. [PubMed] [Google Scholar]
- 3.Wang W, Kong L. Ossification of ligamentum. J Neurosurg Spine. 2007;6:96. doi: 10.3171/spi.2007.6.1.96. author reply 96-7. [DOI] [PubMed] [Google Scholar]
- 4.Yayama T, Uchida K, Kobayashi S, Kokubo Y, Sato R, Nakajima H, Takamura T, Bangirana A, Itoh H, Baba H. Thoracic ossification of the human ligamentum flavum: histopathological and immunohistochemical findings around the ossified lesion. J Neurosurg Spine. 2007;7:184–193. doi: 10.3171/SPI-07/08/184. [DOI] [PubMed] [Google Scholar]
- 5.Kawaguchi Y, Yasuda T, Seki S, Nakano M, Kanamori M, Sumi S, Kimura T. Variables affecting postsurgical prognosis of thoracic myelopathy caused by ossification of the ligamentum flavum. Spine J. 2013;13:1095–107. doi: 10.1016/j.spinee.2013.03.001. [DOI] [PubMed] [Google Scholar]
- 6.Fujimura M, Takeuchi T, Kitajima H, Nakayama M. Chorioamnionitis and serum IgM in Wilson-Mikity syndrome. Arch Dis Child. 1989;64:1379–1383. doi: 10.1136/adc.64.10_spec_no.1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Inamo Y, Ayusawa M, Yamashita T, Sasaki T, Takeuchi S, Okuni M. Serum content of zinc and vitamin C in severely handicapped children. Tohoku J Exp Med. 1989;158:301–307. doi: 10.1620/tjem.158.301. [DOI] [PubMed] [Google Scholar]
- 8.Okuda T, Baba I, Fujimoto Y, Tanaka N, Sumida T, Manabe H, Hayashi Y, Ochi M. The pathology of ligamentum flavum in degenerative lumbar disease. Spine (Phila Pa 1976) 2004;29:1689–1697. doi: 10.1097/01.brs.0000132510.25378.8c. [DOI] [PubMed] [Google Scholar]
- 9.Uchida K, Yayama T, Cai HX, Nakajima H, Sugita D, Guerrero AR, Kobayashi S, Yoshida A, Chen KB, Baba H. Ossification process involving the human thoracic ligamentum flavum: role of transcription factors. Arthritis Res Ther. 2011;13:R144. doi: 10.1186/ar3458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mobbs RJ, Dvorak M. Ossification of the ligamentum flavum: diet and genetics. J Clin Neurosci. 2007;14:703–705. doi: 10.1016/j.jocn.2006.01.010. [DOI] [PubMed] [Google Scholar]
- 11.Kunimoto S, Nosaka C, Xu CZ, Takeuchi T. Serum effect on cellular uptake of spermidine, spergualin, 15-deoxyspergualin, and their metabolites by L5178Y cells. J Antibiot (Tokyo) 1989;42:116–122. doi: 10.7164/antibiotics.42.116. [DOI] [PubMed] [Google Scholar]
- 12.Podichetty VK. The aging spine: the role of inflammatory mediators in intervertebral disc degeneration. Cell Mol Biol (Noisy-le-grand) 2007;53:4–18. [PubMed] [Google Scholar]
- 13.Feldmann M. Development of anti-TNF therapy for rheumatoid arthritis. Nat Rev Immunol. 2002;2:364–371. doi: 10.1038/nri802. [DOI] [PubMed] [Google Scholar]
- 14.Park JO, Lee BH, Kang YM, Kim TH, Yoon JY, Kim H, Kwon UH, Lee KI, Lee HM, Moon SH. Inflammatory cytokines induce fibrosis and ossification of human ligamentum flavum cells. J Spinal Disord Tech. 2013;26:E6–12. doi: 10.1097/BSD.0b013e3182698501. [DOI] [PubMed] [Google Scholar]
- 15.Guo JJ, Yang HL, Cheung KM, Tang TS, Luk KD. Classification and management of the tandem ossification of the posterior longitudinal ligament and flaval ligament. Chin Med J (Engl) 2009;122:219–224. [PubMed] [Google Scholar]
- 16.Guo JJ, Luk KD, Karppinen J, Yang H, Cheung KM. Prevalence, distribution, and morphology of ossification of the ligamentum flavum: a population study of one thousand seven hundred thirty-six magnetic resonance imaging scans. Spine (Phila Pa 1976) 2010;35:51–56. doi: 10.1097/BRS.0b013e3181b3f779. [DOI] [PubMed] [Google Scholar]
- 17.Cai HX, Yayama T, Uchida K, Nakajima H, Sugita D, Guerrero AR, Yoshida A, Baba H. Cyclic tensile strain facilitates the ossification of ligamentum flavum through beta-catenin signaling pathway: in vitro analysis. Spine (Phila Pa 1976) 2012;37:E639–46. doi: 10.1097/BRS.0b013e318242a132. [DOI] [PubMed] [Google Scholar]


