Abstract
Extremely premature neonates requiring oxygen therapy develop an accumulation of reactive oxygen species (ROS), impaired alveolarization and dysmorphic pulmonary vasculature. Regulators of ROS (i.e. antioxidants), alveolarization (i.e. matrix metalloproteinases - MMPs) and microvascular maturation (i.e. vascular endothelial growth factor - VEGF) are altered in bronchopulmonary dysplasia (BPD). We tested the hypothesis that early treatment with MnTBAP, a superoxide dismutase mimetic and superoxide anion and peroxynitrite scavenger, alters lung biomarkers of angiogenesis and alveolarization during hyperoxia with intermittent hypoxia (IH) in neonatal rats. Neonatal rats were exposed to 50% O2 with brief IH episodes (12% O2) from P0 to P14, or to room air (RA). On P0, P1 & P2, the pups received a daily IP injection of 1, 5, or 10 mg/kg MnTBAP, or saline. At P14, the pups were either euthanized, or allowed to recover in RA until P21. RA littermates were similarly treated. Lung VEGF, sVEGFR-1, MMP-2, MMP-9 and TIMP-1 were determined. Low-dose MnTBAP (1 mg/kg) prevented the increase in lung VEGF induced by intermittent hypoxia noted in the control group. This dose was also effective for decreasing MMP-9 and MMP-9/TIMP-1 ratio suggesting an anti-inflammatory effect for MnTBAP. IH decreased MMP-2 with no ameliorating effect by MnTBAP. Our data demonstrate that brief, repeated intermittent hypoxia during hyperoxia can alter biomarkers responsible for normal microvascular and alveolar development. In addition to prevention of hypoxic events, the use of antioxidants needs to be explored as a possible therapeutic intervention in neonates at risk for the development of oxidative lung injury.
Keywords: Antioxidants, hyperoxia, intermittent hypoxia, matrix metalloproteinases, tissue inhibitor of metalloproteinase
Introduction
With improvements in management of extremely low gestational age neonates (ELGANs) and a rise in overall survival of this fragile population, the development of bronchopulmonary dysplasia (BPD) among these infants continues to contribute to morbidity and mortality. Supplemental oxygen and mechanical ventilation exposes the lungs of ELGANs to oxidative stress and inflammatory insults, thus contributing to the interruption of normal alveolar and pulmonary vascular growth and development, and resulting in alveolar simplification and disrupted vasculogenesis [1-4]. Exposure of the premature lungs to supraphysiologic levels of oxygen has been shown to directly damage the lung via formation of reactive oxygen species (ROS) [5-10]. In addition, ELGANs often experience frequent episodes of apnea and arterial oxygen desaturations, leading to intermittent hypoxia, which has also been shown to elicit increased ROS production and exacerbation of lung disease [11,12].
Development of the pulmonary vasculature has been found to be a necessary factor for proper alveolarization [13,14]. Vascular endothelial growth factor (VEGF) plays an essential role in regulation of pulmonary vascular growth and development, stimulating angiogenesis, vessel remodeling, and endothelial survival [15]. Studies have found decreased VEGF expression in BPD animal models, and in the lungs of premature infants who died with BPD [2,16,17]. Decreased VEGF levels in tracheal aspirates from premature infants who subsequently developed BPD have also been noted [18,19]. Conversely, it has been shown that hypoxia upregulates VEGF production in rats and human fetal lungs [14,20].
Matrix metalloproteinases (MMPs) are enzymes that degrade type IV collagen, which is the major constituent of lung basement membranes. The regulation of MMP activity is necessary for normal matrix turnover and contributes to the development of normal lung architecture [21]. MMP-2 is secreted mainly by noninflammatory cells such as fibroblasts, endothelial and epithelial cells and participates in lung development while MMP-9 is secreted mainly by inflammatory cells such as neutrophils, macrophages and is implicated in lung diseases [22,23]. Several studies have implicated MMP-2 and MMP-9 in chronic lung disease, and animal models of BPD [21,24-26]. From those studies, MMP-2 appears to decrease in chronic lung disease and hypoxia leading to arrested alveolarization and abnormal arterial remodeling which are hallmarks of BPD [22]. In contrast, MMP-2 is increased with long-term exposure to 100% O2 suggesting an association with ROS and oxidative stress [27]. Increased MMP-9 activity has been shown to correlate with severity of inflammation and oxidative stress, leading to capillary leaks and airway remodeling noted in chronic lung disease [28,29]. MMP-9 activity is increased in lung specimens from premature baboon and murine BPD models [25,30,31], and has also been found to be elevated in bronchoalveolar lavage fluid (BALF), tracheal aspirate fluid (TAF) and plasma in premature infants who developed BPD [23,24,26]. However, MMP-9-null mice demonstrated worsening lung disease, suggesting that MMP-9 may be expressed to attenuate inflammation, and may play a protective role in the lungs [32].
Fetal lung antioxidant enzymes increase dramatically towards the last 10-15% of gestation [33,34]. This period of development is not reached in premature infants, thus their pulmonary antioxidant stores may be inadequate, and their ability to upregulate antioxidant activity in response to oxidative stress may also be impaired [35,36]. Antioxidant supplementation and overexpression of endogenous antioxidants improved survival and protection in animal models [7,40] and improved pulmonary outcome at 1 year of age in preterm infants [41]. MnTBAP is a superoxide dismutase mimetic, which acts as a superoxide-scavenger, thereby reducing peroxynitrite formation [42]. We tested the hypothesis that early treatment with MnTBAP alters lung biomarkers of angiogenesis and alveolarization during hyperoxia with intermittent hypoxia (IH), simulating frequent apneas experienced by premature neonates, in neonatal rats.
Material and methods
All experiments were approved by the Memorial Health Services Institutional Animal Care and Use Committee. Animals were treated humanely, according to the guidelines outlined by the United States Department of Agriculture and the Guide for the Care and Use of Laboratory Animals. Euthanasia of the animals was conducted according to the guidelines of the American Veterinary Medical Association.
Experimental design
Certified infection-free, timed-pregnant Sprague Dawley rats were purchased from Charles River Laboratories (Wilmington, MA) at 19 days gestation. The animals were housed in an animal facility with a 12-hour-day/12-hour-night cycle and provided standard laboratory diet and water ad libitum until delivery. Within approximately 5 hours of birth, newborn rat pups delivering on the same day were pooled and randomly assigned to expanded litters of 18 pups/litter (9 males and 9 females). One dam remained with the same litter for the entire study. Each pup was weighed and measured for linear growth (crown to rump length in centimeters). The IH cycles consisted of 50% hyperoxia followed by brief episodes of hypoxia (12% O2) for 2 minutes for a total of 12 cycles per day as previously described [43]. Briefly, three episodes of hypoxia were grouped, each 10 minutes apart, followed by hyperoxia to complete 6 hours, before the cycle repeated. The oxygen concentration remained at 12% for 2 minutes. A total of 16 groups were studied. Groups 1 to 4 were exposed to IH from birth (P0) to P14 during which they received early IP injections of MnTBAP or saline from P0-P2 prior to euthanasia on P14. The treatment groups were as follows: Group 1) MnTBAP 1 mg/kg/day; Group 2) MnTBAP 5 mg/kg/day; Group 3) MnTBAP 10 mg/kg/day; and Group 4) equivalent volume saline. These groups (1-4) were euthanatized at P14 immediately following exposure to IH. Groups 5 to 8 were also exposed to IH cycling from P0 to P14, received similar MnTBAP doses from P0-P2, but were allowed to recover in room air (RA) from P14 to P21. Groups 9 to 16 served as RA controls. Groups 9 to 12 received similar MnTBAP doses or saline from P0-P2 and were euthanatized on P14. Groups 13 to 16 received similar MnTBAP doses or saline from P0-P2 and were euthanatized on P21. The doses of MnTBAP were based on previous reports which showed that IP injections of 5 mg/kg/day prolonged the lifespan of neonatal mice with nullizygous SOD2 [44,45]. The first 2 weeks of life have been shown to be most critical in ELGANs. Therefore, the pups were treated during the first 3 days of life which are equivalent to 3 weeks in human neonates.
Sample collection
Lung samples from the left lower lobe were taken from 8 male and 8 female rats in each group and rinsed in ice-cold phosphate buffered saline (pH 7.4) on ice. The specimens were placed in sterile polypropylene tubes, snap frozen in liquid nitrogen and stored at -80°C until assay.
VEGF and sVEGFR-1 assays
VEGF and sVEGFR-1 levels in the lung homogenates were assayed using commercially-available rat sandwich immunoassay kits purchased from R & D Systems, Minneapolis, MN, USA. The VEGF assay predominantly binds the monomeric VEGF164 but also detects the VEGF120 isoform. The assay recognizes the 164-amino acid splice variant of mouse VEGF (or receptor) and has a 98% and 92% affinity to the rat sequences, respectively. The assay utilizes a monoclonal anti-VEGF (or receptor) detection antibody conjugated to horseradish peroxidase and color development with tetramethylbenzidine/hydrogen peroxide (TMB solution). All assays were performed according to the manufacturer’s protocol. VEGF and sVEGFR-1 levels in the samples were determined from a linear standard curve ranging from 0 to 2000 pg/mL and 0 to 8000 pg/mL, respectively. The coefficient of variation from inter- and intra-assay precision assessment was less than 10%. VEGF and sVEGFR-1 levels in the lung homogenates were standardized using total cellular protein levels.
MMP-2 and TIMP-1 assays
MMP-2 and TIMP-1 levels in the lung homogenates were measured using commercially available rat ELISA kits purchased from Cell Applications, Inc. (San Diego, CA, USA). The assays are specific and precise quantitative methods for determination of MMP-2 or TIMP-1 in tissue homogenates. The MMP-2 assay recognizes the pro and active forms of MMP-2 and does not cross-react with other MMPs or TIMPs. Standards and samples were incubated in microtiter wells precoated with anti-MMP-2 or anti-TIMP-1 antibodies. Any MMP-2 or TIMP-1 present was bound to the wells and other components in the sample were removed by washing and aspiration. Endogenous levels of free active MMP-2 or TIMP-1 in the sample were detected. The concentrations in the sample were measured by extrapolation from a standard curve which ranged from 0 – 10,000 pg/mL for MMP-2 and 0 – 2,000 pg/mL for TIMP-1. MMP-2 or TIMP-1 levels were directly proportional to the generation of color and were represented by the rate of change of absorbance at 450 nm. Samples that were above the standard curve range was diluted and corrected for dilution. The inter- and intra-assay variability for MMP-2 and TIMP-1 were less than 10%. MMP-2 and TIMP-1 levels in the lung homogenates were standardized using total cellular protein levels.
MMP-9 assay
MMP-9 levels in the lung homogenates were analyzed using commercially- quantikine rat total MMP-9 immunoassay kits purchased from R & D Systems. The kit measures total rat MMP-9 (Pro-, active, and TIMP-complexed MMP-9). It contains NS0-expressed recombinant rat MMP-9 and antibodies raised against the recombinant factor. This immunoassay has been shown to accurately quantitate the recombinant protein. The concentration of MMP-9 in the sample was determined by extrapolation from a standard curve which ranged from 0 to 10 ng/mL. The inter- and intra-assay variabilities were <10% and the sensitivity was 0.028 ng/mL. MMP-9 levels in the lung homogenates were standardized using total cellular protein levels.
Total cellular protein levels
On the day of the assay, the lung samples were homogenized and centrifuged at 5,000 rpm at 4°C for 20 minutes, then filtered. A 10 mL portion of the filtrate was utilized for total cellular protein levels using the Bradford method (Bio-Rad, Hercules, CA) with bovine serum albumin as a standard. The standard curve was linear from 0.05 to 1.45 mg/ml of protein.
Statistical analysis
One-way and two-way analysis of variance (ANOVA) were used to determine differences among the groups for normally-distributed data, and Kruskal-Wallis test was used for non-normally- distributed data following Bartlett’s test for equality of variances. Post hoc analysis was performed using the Tukey, Bonferoni and Student-Newman-Keuls tests for significance. To compare data between RA and hyperoxia with intermittent hypoxia (IH) groups, unpaired t-test was employed for normally distributed data and Mann Whitney U tests were used for non-normal data following Levene’s test for equality of variances. Significance was set at p<0.05 and data are reported as mean±SEM. All analyses were two-tailed and performed using SPSS version 16.0 (SPSS, Inc. Chicago IL).
Results
Effect on growth
The groups treated with MnTBAP during hyperoxia/hypoxia cycling were generally heavier than RA controls. MnTBAP did not have long lasting effects on growth as it appeared to preserve body weight accretion closer to the time of administration (Data not shown).
Effect on VEGF
At P14, saline controls that underwent IH cycling showed a significant increase in VEGF production (pg/mg protein) compared to RA (277.3±24.0 vs. 203.9±21.9, p<0.05). In contrast, treament with all doses of MnTBAP prevented significant IH-induced increases of VEGF (164.7±18.0, 207.7±17.0; and 207.3± 25.0) for the 1, 5, and 10 mg/kg doses, respectively. When compared to their RA littermates, treatment with 1 mg/kg MnTBAP in IH cycling resulted in a significant reduction in VEGF (164.7±18.0 vs. 283.2±46.4, p<0.01). Interestingly, there was a bi-phasic dose response when MnTBAP was administered in RA exposed animals which resulted in higher levels of VEGF with the lowest and highest doses of MnTBAP, and lower levels with the intermediate dose. A similar finding was not noted with treatment in IH cycling (Figure 1A). At P21 during recovery in RA, VEGF continued to rise in all IH-exposed rats compared to the RA littermates. This response occurred in the Saline-control (637.1±109.9 vs. 188.9±25.2) as well as the 1 (585.5±84.7 vs. 177.7±40.3) and 5 mg/kg (559.7±63.3 vs. 370.0±42.0) treatment doses of MnTBAP, but not the highest dose of 10 mg/kg (489.5±24.7 vs. 1478.8±133.4). The higher doses of MnTBAP increased the production of VEGF in RA in a dose-dependent fashion. However, when administered in IH cycling, the response was opposite with decreased VEGF production as a function of increasing MnTBAP dose (Figure 1B).
Figure 1.
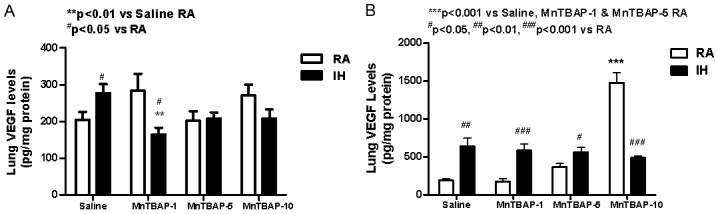
Dose response effects of MnTBAP treatment on lung VEGF levels in neonatal rats following intermittent hypoxia during hyperoxia (A, P14) and recovery in RA (B, P21). Data are presented as mean±SEM (n=18 pups per group, 9 males and 9 females). Animals were treated with IP injections of 1, 5, or 10 mg/kg MnTBAP on P0, P1 and P2 or equivalent volume saline (placebo controls).
Effect on sVEGFR-1
Soluble VEGFR-1 (measured as pg/mg protein) is an endogenous inhibitor of VEGF acting as a VEGF trap. At P14 in the saline control group, IH cycling caused a significant increase in lung sVEGFR-1 levels (4573.6±183.7, p<0.01) compared to RA (2449.9±160.8). This trend persisted to a lesser degree when treated with MnTBAP. However, during IH cycling, treatment with MnTBAP caused a significant reduction in sVEGR-1 levels (3452.6±166.4, p<0.001 and 3741.8±132.5, p<0.01) at 1 and 5 mg/kg doses compared to saline control. Treatment with MnTBAP in RA resulted in a progressive dose-response increase in sVEGFR-1 levels (3022.2±91.0, 3080.9±158.5, and 3595.8± 148.7) for the 1, 5 and 10 mg/kg doses compared to saline control (2449.9±160.8, p<0.05) (Figure 2A). Despite recovery at P21, levels of sVEGFR-1 remained elevated in IH exposed saline controls compared to RA. MnTBAP treatment did not affect sVEGR-1 levels in IH groups. On the other hand, in RA groups, MnTBAP treatment resulted in higher levels of sVEGFR-1 at the 5 (4898.0±221.1, p<0.01) and 10 (5838.4 ±292.2, p<0.001) mg/kg doses compared to saline (3807.9±144.9). Additionally, sVEGFR-1 levels were lower with MnTBAP treatment in IH cycling at the 5 (4109.0±269.8 vs. 4898.0 ±221.1, p<0.05) and 10 (4548.9±187.7 vs. 5838.4±292.2, p<0.01) compared to RA treatment groups (Figure 2B). Generally, changes in sVEGR-1 levels followed similar changes in VEGF levels across different treatment groups.
Figure 2.
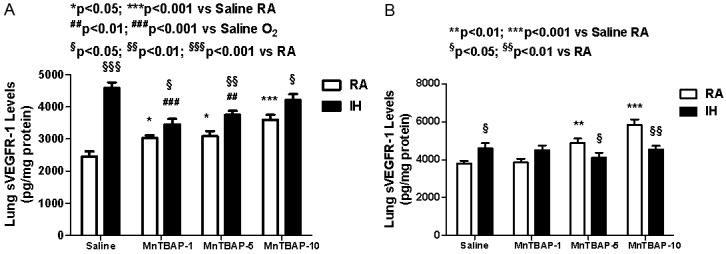
Dose response effects of MnTBAP treatment on lung sVEGFR-1 levels in neonatal rats following intermittent hypoxia during hyperoxia (A, P14) and recovery in RA (B, P21). Data are presented as mean±SEM (n=18 pups per group, 9 males and 9 females). Animals were treated with IP injections of 1, 5, or 10 mg/kg MnTBAP on P0, P1 and P2 or equivalent volume saline (placebo controls).
Effect on MMP-2
MMP-2 degrades components of the extracellular matrix and plays a key role in cell migration, tissue remodeling, and lung alveolarization. MMP-2 levels (pg/mg protein) remained unchanged with IH cycling alone or MnTBAP treatment in RA at P14 (Figure 3A). However, treatment with the lowest dose of 1 mg/kg MnTBAP in IH cycling resulted in lower MMP-2 levels (31656.0±1189.9, p<0.05) compared to the similarly treated RA littermates (35987.8± 1137.2). No effects were noted with the higher doses in RA or IH cycling. Conversely, at P21, there was a latent response of decreased MMP-2 levels in the saline control group exposed to IH cycling (24084.6±1568.0, p<0.001) compared to the RA saline control group (32860.7±1531.1). A similar but less significant reduction was noted for the lowest MnTBAP dose of 1 mg/kg (25293.4±1226.1, p<0.05) compared to RA (30110.5±1811.6). The higher dose groups remained comparable to their RA littermates. However, treatment in RA was lower with those doses (24702.0 ±1496.0, p<0.01 and 24021.0±1102.0, p<0.001, respectively) compared to saline (32860.7±1531.1) (Figure 3B).
Figure 3.
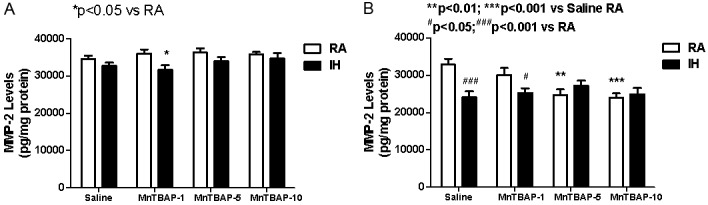
Dose response effects of MnTBAP treatment on lung MMP-2 levels in neonatal rats following intermittent hypoxia during hyperoxia (A, P14) and recovery in RA (B, P21). Data are presented as mean±SEM (n=18 pups per group, 9 males and 9 females). Animals were treated with IP injections of 1, 5, or 10 mg/kg MnTBAP on P0, P1 and P2 or equivalent volume saline (placebo controls).
Effects on MMP-9
At P14, IH cycling did not affect MMP-9 levels significantly in saline control group compared to RA (Figure 4A). However, with MnTBAP treatment, there was a significant and consistent reduction in MMP-9 (pg/mg protein) during IH-cycling with the 1 (16.9±1.7, p<0.01), 5 (17.3±1.2, p<0.01), and 10 (16.4±1.9, p<0.01) mg/kg doses compared to saline control (27.4±2.3) as well as their RA counterparts (25.7±2.3, 25.6±2.5, and 25.5±3.0, respectively). MnTBAP treatment did not affect MMP-9 levels in the RA groups (Figure 4A). At P21, after RA recovery, there was no difference in MMP-9 levels between IH and RA saline control groups (Figure 4B). MnTBAP treatment resulted in a dose-related sustained decrease of MMP-9 production in the IH-cycled groups (9.2±2.2, p<0.01, 9.2±2.2, p<0.01, and 5.9±2.1, p<0.001) for the 1, 5, and 10 mg/kg doses, respectively compared to saline treatment (19.8±1.8). Similarly, treatment in RA resulted in a significant reduction in MMP-9 levels (2.9±2.3, p<0.01) at the highest dose of MnTBAP (10 mg/kg) compared to saline (23.8±7.1) (Figure 4B).
Figure 4.
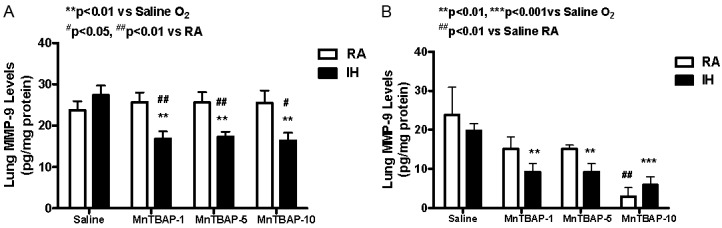
Dose response effects of MnTBAP treatment on lung MMP-9 levels in neonatal rats following intermittent hypoxia during hyperoxia (A, P14) and recovery in RA (B, P21). Data are presented as mean±SEM (n=18 pups per group, 9 males and 9 females). Animals were treated with IP injections of 1, 5, or 10 mg/kg MnTBAP on P0, P1 and P2 or equivalent volume saline (placebo controls).
Effect on TIMP-1
TIMP-1 (measured as pg/mg protein) is a 28.5 kDa glycoprotein that forms a no covalent 1:1 complex with MMPs, thereby inhibiting their proteolytic activities. At P14, IH cycling resulted in a significant increase in TIMP-1 activity in saline control group (4703.7±57.9, p<0.01) compared to RA (4084.6±159.2, Figure 5A). Treatment with MnTBAP did not affect TIMP-1 activity in IH cyling groups in all dose ranges. In contrast, only the highest dose of MnTBAP increased TIMP-1 levels (4710.0±152.5, p< 0.05) compared to saline (4080.6±159.2) in RA. A similar elevation was noted with the 5 mg/kg MnTBAP dose (4736.6±132.5, p<0.05) when administered in IH cycling compared to RA treated counterpart group (4208.0±178.4) (Figure 5A). At P21, no difference was noted between IH and RA saline controls. Only the MnTBAP dose of 1 mg/kg resulted in elevated TIMP-1 levels (3803.0±133.0, p<0.05) when administered in IH cycling compared to saline (3158.9±414.2) (Figure 5B).
Figure 5.
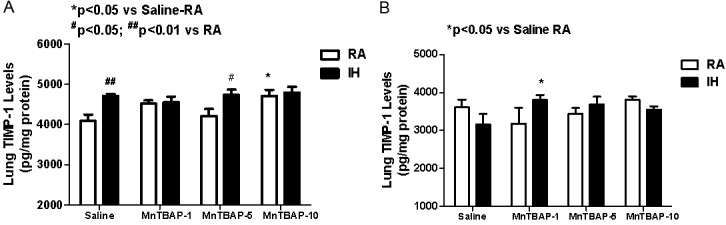
Dose response effects of MnTBAP treatment on lung TIMP-1 levels in neonatal rats following intermittent hypoxia during hyperoxia (A, P14) and recovery in RA (B, P21). Data are presented as mean±SEM (n=18 pups per group, 9 males and 9 females). Animals were treated with IP injections of 1, 5, or 10 mg/kg MnTBAP on P0, P1 and P2 or equivalent volume saline (placebo controls).
MMP/TIMP ratios
TIMP-1 inhibits the active form of many MMPs and is the most widely distributed TIMP. Therefore the ratio of MMPs to TIMPs may be more important than their actual levels. Figure 6A shows that at P14, MMP-2/TIMP-1 ratios declined in saline controls with IH cycling (6.95±0.2, p<0.01) compared to RA (8.6±0.54). This effect was also noted with MnTBAP treatment at the 1 (6.95±0.24 vs. 7.9±0.31, p<0.05) and 5 (7.2±0.13 vs. 8.7±0.5, p<0.01) mg/kg doses compared to their RA counterparts. Generally, there was no effect of MnTBAP treatment on MMP-2/TIMP-1 ratio when treatment groups were compared with their saline controls irrespective of oxygen exposure. At P21, there was no significant difference between IH cycling and RA saline controls groups (Figure 6B). However, there was a latent decline in MMP-2/TIMP-1 ratios in the IH cycled group treated with 1 mg/kg MnTBAP (6.65±0.23, p<0.001) compared to RA (9.75±0.52). With regard to MMP-9/TIMP-1 ratios, there was no significant difference in the MMP-9/TIMP-1 ratios between IH and RA saline controls. However, the ratios were most significantly affected by MnTBAP treatment both at P14 (Figure 7A, IH cycling) and P21 (Figure 7B, both groups). We noted a significant reduction of MMP-9/TIMP-1 ratio with all doses of MnTBAP treatment in IH cycling at P14 (0.0037±0.00031, 0.0037±0.0034, and 0.0035±0.0043) for the 1, 5 and 10 mg/kg doses respectively compared to saline control (0.0058±0.00049, p<0.01), as well as their RA counterparts. There was no significant change in MMP-9/TIMP-1 ratio in association with MnTBAP treatment in RA groups. At P21, there was a progressive dose response decline in MMP-9/TIMP-1 ratios both in IH cycling and RA groups. In IH cycling, all MnTBAP doses resulted in lower MMP-9/TIMP-1 ratios (0.0025±0.00063, 0.0026± 0.00061, and 0.0016±0.00057) compared to saline (0.0065±0.00057, p<0.05). However, in RA groups, significance was achieved only with the 5 (0.0044±0.00033, p<0.05) and 10 (0.00072±0.00059, p<0.001) mg/kg doses of MnTBAP treatment compared to saline (0.0071 ±0.00026).
Figure 6.
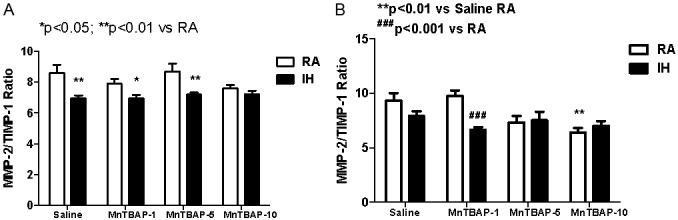
Dose response effects of MnTBAP treatment on lung MMP-2/TIMP-1 ratios levels in neonatal rats following intermittent hypoxia during hyperoxia (A, P14) and recovery in RA (B, P21). Data are presented as mean±SEM (n=18 pups per group, 9 males and 9 females). Animals were treated with IP injections of 1, 5, or 10 mg/kg MnTBAP on P0, P1 and P2 or equivalent volume saline (placebo controls).
Figure 7.
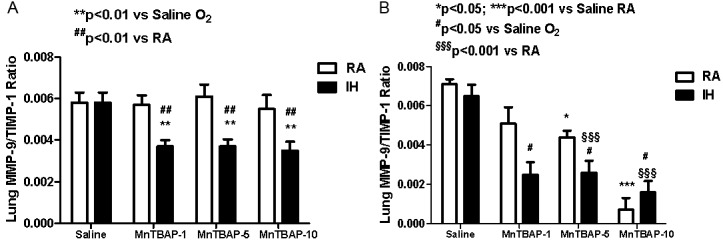
Dose response effects of MnTBAP treatment on lung MMP-9/TIMP-1 ratios in neonatal rats following intermittent hypoxia during hyperoxia (A, P14) and recovery in RA (B, P21). Data are presented as mean±SEM (n=18 pups per group, 9 males and 9 females). Animals were treated with IP injections of 1, 5, or 10 mg/kg MnTBAP on P0, P1 and P2 or equivalent volume saline (placebo controls).
Discussion
The present study demonstrates that recurrent intermittent hypoxic events during hyperoxia, occurring in the first few weeks of life, although brief, results in significant alterations in lung biomarkers of angiogenesis and alveolarization. Treatment of MnTBAP in rats for the first 3 days of life is equivalent to 3 weeks in human infants. MnTBAP does not appear to have adverse effects on growth and may even protect weight accretion. However, this protective effect on growth was not sustained when drug treatment was terminated. This finding is important since infants with the lowest early postnatal body growth are most susceptible to development of BPD [46,47]. These same infants are most susceptible to experience frequent, brief intermittent hypoxic events due to immature respiratory control, airway obstruction and ineffective ventilation [48,49]. The intermittent hypoxic events can last as little as a few seconds to as much as several minutes [50]. We used a well-established model to simulate brief intermittent hypoxia experienced by ELGANs lasting only one minute [43,51]. This model produces oxidative stress and oxidative DNA damage as evidenced by elevated levels of 8-isoprostane, a reliable biomarker for lipid peroxidation and oxidative stress and 8-hydro- xydeoxyguanosine (8-OHdG), a reliable biomarker for oxidative DNA damage, confirming previous findings by other investigators [52]. Studies using a longer period of intermittent hypoxia (10 minutes) during hyperoxia, demonstrated arrested alveolar development and oxidative stress [53]. In the present study, a shorter, one-minute clustered approach was used with therapeutic interventions specifically targeting oxidative stress and ROS. Our data demonstrated three major findings: 1) low-dose MnTBAP was sufficient to decrease elevated lung VEGF levels which had resulted from frequent, brief, intermittent hypoxic episodes; 2) using our animal model, we demonstrated that MMP-2 is suppressed by IH; and 3) MnTBAP decreased the inflammatory marker, MMP-9, as well as the MMP-9/TIMP-1 ratio, in a dose-dependent manner which may indicate an anti-inflammatory effect for MnTBAP. Together, these findings support our hypothesis and enable us to conclude that exposure of the preterm lungs to brief, intermittent hypoxia will result in long-term and possibly, permanent changes in biomarkers that regulate lung angiogenesis and alveolarization. More importantly, the use of antioxidants may have potential beneficial effects, to decrease lung inflammation.
The respiratory tract is in direct contact with inhaled O2 making it the primary target for O2-induced injury and superoxide anion release from the mitochondrial electron transport chain [54]. Under normal circumstances, superoxide anions are scavenged by endogenous SOD enzymes [42]. MnSOD participates in lung defense against ROS and is induced by hyperoxia and inflammation. Preterm infants have a limited ability to induce MnSOD in response to O2 [54]. Therefore, treatment with a manganese-based metalloporphyrin, such as MnTBAP, during the critical first few weeks of life may aid in superoxide scavenging [42,55]. It is well known that peroxynitrite and superoxide anion (which can be scavenged by MnTBAP) are ROS that induce inflammatory responses and deplete and inactivate endogenous MnSOD [42]. Consequently, it can be speculated that administration of exogenous MnTBAP may prevent alveolar and endothelial dysfunction associated with ROS and inflammation. However, this drug has not been studied extensively in animal models of BPD. Additionally, the appropriate effective dose of MnTBAP needed to achieve its potential benefits is not known. Our data showed that the lowest dose of MnTBAP (1 mg/kg) was both adequate to prevent the hypoxia-induced VEGF increases and at the same time, decrease MMP-9. We examined the lungs at P14 because in rats, bulk alveolarization ends at postnatal day 10 and the lungs enters the stage of microvascular maturation [56]. It was crucial for us to also examine the lungs at P21 following one week of reperfusion/ recovery because damage to the lungs appears to worsen during reoxygenation following hypoxia [7]. This may be due to further generation of ROS and does not appear to be neutralized by endogenous antioxidant defenses. However; it can be prevented by exogenous SOD [57].
VEGF and sVEGFR-1 levels
The first test of our hypothesis was to measure the effect of MnTBAP upon levels of VEGF and its endogenous inhibitor, sVEGFR-1. Our data showed that VEGF was increased in the saline placebo controls at both P14 and P21. At first glance, this finding appears to contrast with previous studies which demonstrated that VEGF is decreased in BPD animal models [16,17]. However, on further evaluation, it became apparent that the increase in VEGF was most likely due to the repeated, IH episodes employed in our model. Our model differs from several previously studied BPD models, which used constant hyperoxia, but did not employ IH. We contend that our model of brief intermittent hypoxia most closely resembles that experienced by ELGANs. Shima et al. [58] showed that the VEGF half-life under normoxia is 30-45 minutes in the human epithelial cell line. However, under hypoxic conditions, there was a 10-fold increase in the half-life to 6-9 hours suggesting increased stability of the VEGF molecule. Our group has previously shown that in ELGANs who developed BPD, VEGF levels in tracheal aspirate fluid were significantly elevated from as early as 3 days and remained elevated up to 8 weeks of life compared to their baseline levels [19]. Therefore, it seems reasonable to assume, that repeated hypoxic episodes may result in increased VEGF accumulation and stability. MnTBAP effectively prevented the increase in VEGF at P14, but the effect was not sustained during recovery/reperfusion (P21). Endogenous SOD response to hyperoxia in rats peaks at P10-P12 [33] and become depleted after that period. The ability for rats to upregulate oxygen-stimulated SOD activity disappears at P19-P20 [33]. This perhaps contributed to the decreased effect of MnTBAP. Alternatively, since the half-life of MnTBAP in the lungs is 9.5 hours [59], it is possible that the duration of MnTBAP administration was not sufficient and therefore led to an accumulation of ROS-induced VEGF by P21.
Another possible explanation is that MnTBAP’s conversion of superoxide anions to H2O2 leads to H2O2 accumulation, especially if catalase and/or glutathione peroxidase stores were reduced or depleted. In the absence of catalase and glutathione peroxidase to convert H2O2 to H2O and O2, the accumulating H2O2 may lead to upregulation of VEGF [60]. One interesting and unexpected finding was the increase in lung VEGF levels with the 10 mg/kg dose of MnTBAP in the RA groups at P21. This is of particular concern since it has been shown that an upregulation of VEGF increases mortality and morbidity in neonatal mice [61]. Normal cellular metabolism produces a basal level of ROS, which may be necessary to maintain a cellular “redox homeostasis” and for cellular signaling systems [55]. The high doses of MnTBAP (10 mg/kg) may be “toxic” by way of depleting these basal levels of ROS with “over-scavenging” of superoxide anion and accumulation of H2O2. It was also interesting to note that sVEGFR-1 levels increased, parallel to the VEGF levels, in the RA groups, providing further evidence for VEGF/VEGFR-1 signaling through ROS. The mirror increases in sVEGFR-1 and VEGF noted in various groups confirms the regulatory role of sVEGFR-1 as an endogenous inhibitor of VEGF.
MMPs and TIMP levels
Hypoxia decreases MMP-2 leading to arrested alveolarization and abnormal arterial remodeling which are hallmarks of BPD [22]. Even though there was no difference between RA and IH saline with regard to MMP-2, at P14, the lowest MnTBAP dose suppressed MMP-2 during IH cycling. During reperfusion/recovery, we noted a decrease in MMP-2 with IH cycling that was not due to MnTBAP treatment. Instead, high dose MnTBAP had a suppressive effect in the RA groups. MMP-2 is present in large amounts in the developing lungs and is localized in pulmonary arteries [62]. Our data provide further evidence for a hypoxic rather than a hyperoxic effect on MMP-2 and may suggest decreased microvascular maturation in response to hypoxia. Additionally, our data points to the importance of finding the fine balance between levels of ROS and antioxidants, as high dose MnTBAP caused reduced MMP-2 in RA controls, which may have deleterious effects on the lung microstructure. TIMP-1 is the specific inhibitor of MMP-9 but can also inhibit MMP-2. MMP-2/TIMP-1 ratio was suppressed at P14 but this was due to intermittent hypoxia and not MnTBAP. This apparent imbalance favoring TIMP-1 may result in abnormal alveolarization and pulmonary artery remodeling. In contrast, MnTBAP selectively suppressed MMP-9 and MMP-9/TIMP-1 ratios. Given that MMP-9 is the main gelatinase released by inflammatory cells, it is prudent to suggest that MnTBAP may be functioning in the context of inflammation. The inability to mount an early anti-inflammatory response in preterm infants may play a significant role in the development of BPD. Dik et al. [63] monitored serial bronchoalveolar lavage MMP-9 levels in premature infants diagnosed with respiratory distress syndrome (RDS) and found that an early response to inflammatory lung injury represented by an early upregulation in MMP-9 occurred within the first week of life and was associated with resolving RDS. However, infants who went on to develop CLD demonstrated decreased early MMP-9 levels and a delayed rise in MMP-9. Our findings may support the suggestion that MMP-9 is a biomarker for lung inflammation [25] and may play a role in neonatal inflammatory lung injury and BPD [32]. The most significant finding in our study and the second test of our hypothesis was the suppressive effect of MnTBAP on MMP-9 levels, which persisted during the recovery/reperfusion phase (P21). It is possible that early antioxidant supplementation with MnTBAP attenuated the early inflammatory response to intermittent hypoxia with subsequent decline in MMP-9 at P14. The lack of a late increase in MMP-9, which has been seen in CLD, along with the progression of decreasing MMP-9, may suggest an effective anti-inflammatory effect of MnTBAP. Treatment with the 10 mg/kg dose of MnTBAP resulted in a dose-dependent decrease in MMP-9. Through a superoxide-dependent mechanism, MMP-9 can be upregulated by cytokine-induced inflammation [64]. It is possible that to maintain a normal MMP-9 baseline, a basal level of ROS may be required. Again, ROS “over-scavenging” by MnTBAP could explain the reduction in MMP-9 in our RA controls.
In summary, our data showed that a low dose of MnTBAP (1 mg/kg) effectively prevented increases in lung VEGF levels that can occur in response to intermittent hypoxia. This dose was also effective for decreasing MMP-9 levels and the MMP-9/TIMP-1 ratio, which would have anti-inflammatory effects. Due to the possibility that H2O2 accumulation might occur at higher doses, MnTBAP should be studied in conjunction with other antioxidants such as catalase and glutathione peroxidase. We further demonstrated that MMP-2 is suppressed by intermittent hypoxia, findings which are in keeping with previous studies [22]. However, this MMP-2 effect was not reversed with MnTBAP and suggests that impaired pulmonary vascular remodeling might occur under these conditions. While the results of our study have significant clinical implications, there are some limitations that need consideration. TIMP-1 is a well known specific inhibitor of MMPs, but measurement of TIMP-2 might be of some value as it also binds and regulates MMP-2. Measurement of H2O2, catalase, and glutathione peroxidase would be useful to determine whether their accumulation or depletion are responsible in part for some of the biological effects that we noted, and to demonstrate whether concomitant treatment with catalase and/or glutathione peroxidase would be more effective. In conclusion, our data demonstrate that brief, repeated intermittent hypoxia during hyperoxia can alter biomarkers responsible for normal microvascular and alveolar developmental. Our findings indicate that in addition to prevention of hypoxic events, the use of antioxidants should be considered as a possible therapeutic intervention in neonates at risk for the development of oxidative lung injury.
Acknowledgements
This work was made possible through a grant from Memorial Medical Center Foundation, Long Beach, CA 90806.
References
- 1.Coalson JJ. Pathology of new bronchopulmonary dysplasia. Semin Neonatol. 2003;8:73–81. doi: 10.1016/s1084-2756(02)00193-8. [DOI] [PubMed] [Google Scholar]
- 2.De Paepe ME, Mao Q, Powell J, Rubin SE, DeKoninck P, Appel N, Dixon M, Gundogan F. Growth of pulmonary microvasculature in ventilated preterm infants. Am J Respir Crit Care Med. 2006;173:204–211. doi: 10.1164/rccm.200506-927OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ryan RM, Ahmed Q, Lakshminrusimha S. Inflammatory mediators in the immunobiology of bronchopulmonary dysplasia. Clin Rev Allergy Immunol. 2008;34:174–190. doi: 10.1007/s12016-007-8031-4. [DOI] [PubMed] [Google Scholar]
- 4.Bhatt AJ, Pryhuber GS, Huyck H, Watkins RH, Metlay LA, Maniscalco WM. Disrupted pulmonary vasculature and decreased vascular endothelial growth factor, Flt-1, and TIE-2 in human infants dying with bronchopulmonary dysplasia. Am J Respir Crit Care Med. 2001;164:1971–1980. doi: 10.1164/ajrccm.164.10.2101140. [DOI] [PubMed] [Google Scholar]
- 5.Coalson JJ, Winter V, DeLemos RA. Decreased alveolarization in baboon survivors with bronchopulmonary dysplasia. Am J Respir Crit Care Med. 1995;152:640–646. doi: 10.1164/ajrccm.152.2.7633720. [DOI] [PubMed] [Google Scholar]
- 6.Davis JM. Role of oxidant injury in the pathogenesis of neonatal lung disease. Acta Paediatr Suppl. 2002;437:23–25. doi: 10.1111/j.1651-2227.2002.tb00156.x. [DOI] [PubMed] [Google Scholar]
- 7.Saugstad OD. Bronchopulmonary dysplasia-oxidative stress and antioxidants. Semin Neonatol. 2003;8:39–49. doi: 10.1016/s1084-2756(02)00194-x. [DOI] [PubMed] [Google Scholar]
- 8.Auten RL, Davis JM. Oxygen toxicity and reactive oxygen species: the devil is in the details. Pediatr Res. 2009;66:121–127. doi: 10.1203/PDR.0b013e3181a9eafb. [DOI] [PubMed] [Google Scholar]
- 9.Rogers LK, Tipple TE, Nelin LD, Welty SE. Differential responses in the lungs of newborn mouse pups exposed to 85% or >95% oxygen. Pediatr Res. 2009;65:33–38. doi: 10.1203/PDR.0b013e31818a1d0a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jobe AH, Kallapur SG. Long term consequences of oxygen therapy in the neonatal period. Semin Fetal Neonatal Med. 2010;15:230–235. doi: 10.1016/j.siny.2010.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jackson IL, Chen L, Batinic-Haberle I, Vujaskovic Z. Superoxide dismutase mimetic reduces hypoxia-induced O2 -, TGF-β, and VEGF production by macrophages. Free Radic Res. 2007;41:8–14. doi: 10.1080/10715760600913150. [DOI] [PubMed] [Google Scholar]
- 12.Ratner V, Slinko S, Utkina-Sosunova I, Starkov A, Polin RA, Ten VS. Hypoxic stress exacerbates hyperoxia-induced lung injury in a neonatal mouse model of bronchopulmonary dysplasia. Neonatology. 2009;95:299–305. doi: 10.1159/000178798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jakkula M, Le Cras TD, Gebb S, Hirth KP, Tuder RM, Voelkel NF, Abman SH. Inhibitoin of angiogenesis decreases alveolarization in the developing rat lung. Am J Physiol Lung Cell Mol Physiol. 2000;279:L600–L607. doi: 10.1152/ajplung.2000.279.3.L600. [DOI] [PubMed] [Google Scholar]
- 14.Remesal A, Pedraz C, San Feliciano L, Ludeña D. Pulmonary expression of vascular endothelial growth factor (VEGF) and alveolar septation in a newborn rat model exposed to acute hypoxia and recovered under conditions of air or hyperoxia. Histol Histopathol. 2009;24:325–330. doi: 10.14670/HH-24.325. [DOI] [PubMed] [Google Scholar]
- 15.Abman SH. Impaired vascular endothelial growth factor signaling in the pathogenesis of neonatal pulmonary vascular disease. Adv Exp Med Biol. 2010;661:323–335. doi: 10.1007/978-1-60761-500-2_21. [DOI] [PubMed] [Google Scholar]
- 16.Maniscalco WM, Watkins RH, Pryhuber GS, Bhatt A, Shea C, Huyck H. Angiogenic factors and alveolar vasculature: development and alterations by injury in very premature baboons. Am J Physiol Lung Cell Mol Physiol. 2002;282:L811–L823. doi: 10.1152/ajplung.00325.2001. [DOI] [PubMed] [Google Scholar]
- 17.Hosford GE, Olson DM. Effects of hyperoxia on VEGF, its receptors, and HIF-2 in the newborn rat lung. Am J Physiol Lung Cell Mol Physiol. 2003;285:L161–L168. doi: 10.1152/ajplung.00285.2002. [DOI] [PubMed] [Google Scholar]
- 18.Lassus P, Turanlahti M, Heikkilä P, Andersson LC, Nupponen I, Sarnesto A, Andersson S. Pulmonary vascular endothelial growth factor and Flt-1 in fetuses, in acute and chronic lung disease, and in persistent pulmonary hypertension of the newborn. Am J Respir Crit Care Med. 2001;164:1981–1987. doi: 10.1164/ajrccm.164.10.2012036. [DOI] [PubMed] [Google Scholar]
- 19.Hasan J, Beharry KD, Valencia AM, Strauss A, Modanlou HD. Soluble vascular endothelial growth factor receptor 1 in tracheal aspirate fluid of preterm neonates at birth may be predictive of bronchopulmonary dysplasia/chronic lung disease. Pediatrics. 2009;123:1541–1547. doi: 10.1542/peds.2008-1670. [DOI] [PubMed] [Google Scholar]
- 20.Acarregui MJ, Penisten ST, Goss KL, Ramirez K, Snyder JM. Vascular endothelial growth factor gene expression in human fetal lung in vitro. Am J Respir Cell Mol Biol. 1999;20:14–23. doi: 10.1165/ajrcmb.20.1.3251. [DOI] [PubMed] [Google Scholar]
- 21.Cederqvist K, Sorsa T, Tervahartiala T, Maisi P, Reunanen K, Lassus P, Andersson S. Matrix metalloproteinases-2, -8, and -9 and TIMP-2 in tracheal aspirates from preterm infants with respiratory distress. Pediatrics. 2001;108:686–692. doi: 10.1542/peds.108.3.686. [DOI] [PubMed] [Google Scholar]
- 22.Danan C, Jarreau PH, Franco ML, Dassieu G, Grillon C, Alsamad A, LaFuma C, Harf A, Delacourt C. Gelatinase activities in the airways of premature infants and development of bronchopulmonary dysplasia. Am J Physiol Lung Cell Mol Physiol. 2002;283:L1086–L1093. doi: 10.1152/ajplung.00066.2002. [DOI] [PubMed] [Google Scholar]
- 23.Ekekezie II, Thibeault DW, Simon SD, Norberg M, Merrill JD, Ballard RA, Ballard PL, Truog WE. Low levels of tissue inhibitors of metalloproteinases with a high matrix metalloproteinase-9/tissue inhibitor of metalloproteinase-1 ratio are present in tracheal aspirate fluids of infants who develop chronic lung disease. Pediatrics. 2004;113:1709–1714. doi: 10.1542/peds.113.6.1709. [DOI] [PubMed] [Google Scholar]
- 24.Davies PL, Spiller OB, Beeton ML, Maxwell NC, Remold-O’Donnell E, Kotecha S. Relationship of proteinases and proteinase inhibitors with microbial presence in chronic lung disease of prematurity. Thorax. 2010;65:246–251. doi: 10.1136/thx.2009.116061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tambunting F, Beharry KD, Hartleroad J, Waltzman J, Stavitsky Y, Modanlou HD. Increased lung matrix metalloproteinase-9 levels in extremely premature baboons with bronchopulmonary dysplasia. Pediatr Pulmonol. 2005;39:5–14. doi: 10.1002/ppul.20135. [DOI] [PubMed] [Google Scholar]
- 26.Schulz CG, Sawicki G, Lemke RP, Roeten BM, Schulz R, Cheung P. MMP-2 and MMP-9 and their tissue inhibitors in the plasma of preterm and term neonates. Pediatr Res. 2004;55:794–801. doi: 10.1203/01.PDR.0000120683.68630.FB. [DOI] [PubMed] [Google Scholar]
- 27.Pardo A, Selman M, Ridge K, Barrios R, Sznajder JI. Increased expression of gelatinases and collagenase in rat lungs exposed to 100% oxygen. Am J Respir Crit Care Med. 1996;154:1067–1075. doi: 10.1164/ajrccm.154.4.8887609. [DOI] [PubMed] [Google Scholar]
- 28.Schock BC, Sweet DG, Ennis M, Warner JA, Young IA, Halliday HL. Oxidative stress and increased type-IV collagenase levels in bronchoalveolar lavage fluid from newborn babies. Pediatr Res. 2001;50:29–33. doi: 10.1203/00006450-200107000-00008. [DOI] [PubMed] [Google Scholar]
- 29.Fukunaga S, Ichiyama T, Maeba S, Okuda M, Nakata M, Sugino N, Furukawa S. MMP-9 and TIMP-1 in the cord blood of premature infants developing BPD. Pediatr Pulmonol. 2009;44:267–272. doi: 10.1002/ppul.20993. [DOI] [PubMed] [Google Scholar]
- 30.Pardo A, Barrios R, Maldonado V, Meléndez J, Pérez J, Ruiz V, Segura-Valdez L, Sznajder JI, Selman M. Gelatinases A and B are up-regulated in rat lungs by subacute hyperoxia. Am J Pathol. 1998;153:833–844. doi: 10.1016/S0002-9440(10)65625-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chetty A, Cao G, Severgnin M, Simon A, Warburton R, Nielsen HC. Role of matrix metalloprotease-9 in hyperoxic injury in developing lung. Am J Physiol Lung Cell Mol Physiol. 2008;295:L584–L592. doi: 10.1152/ajplung.00441.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lukkarinen H, Hogmalm A, Lappalainen U, Bry K. Matrix metalloproteinase-9 deficiency worsens lung injury in a model of bronchopulmonary dysplasia. Am J Respir Cell Mol Biol. 2009;41:59–68. doi: 10.1165/rcmb.2008-0179OC. [DOI] [PubMed] [Google Scholar]
- 33.Autor AP, Frank L, Roberts RJ. Developmental characteristics of pulmonary superoxide dismutase: relationship to idiopathic respiratory distress syndrome. Pediatr Res. 1976;10:154–158. doi: 10.1203/00006450-197603000-00002. [DOI] [PubMed] [Google Scholar]
- 34.Frank L, Sosenko IRS. Prenatal development of lung antioxidant enzymes in four species. J Pediatr. 1987;110:106–110. doi: 10.1016/s0022-3476(87)80300-1. [DOI] [PubMed] [Google Scholar]
- 35.Morton RL, Das KC, Guo X, Iklé DN, White CW. Effect of oxygen on lung superoxide dismutase activities in premature baboons with bronchopulmonary dysplasia. Am J Physiol. 1999;276:L64–L74. doi: 10.1152/ajplung.1999.276.1.L64. [DOI] [PubMed] [Google Scholar]
- 36.Asikainen TM, Heikkilä P, Kaarteenaho-Wiik R, Kinnula VL, Raivio KO. Cell-specific expression of manganese superoxide dismutase protein in the lungs of patients with respiratory distress syndrome, chronic lung disease, or persistent pulmonary hypertension. Pediatr Pulmonol. 2001;32:193–200. doi: 10.1002/ppul.1108. [DOI] [PubMed] [Google Scholar]
- 37.Wispe JR, Warner BB, Clark JC, Dey CR, Neuman J, Glasser SW, Crapo JD, Chang L, Jeffrey AW. Human Mn-superoxide dismutase in pulmonary epithelial cells of transgenic mice confers protection from oxygen injury. J Biol Chem. 1992;267:23937–23941. [PubMed] [Google Scholar]
- 38.Wilborn AM, Evers LB, Canada AT. Oxygen toxicity to the developing lung of the mouse: role of reactive oxygen species. Pediatr Res. 1996;40:225–232. doi: 10.1203/00006450-199608000-00007. [DOI] [PubMed] [Google Scholar]
- 39.Ilizarov AM, Koo H, Kazzaz JA, Mantell LL, Li Y, Bhapat R, Pollack S, Horowitz S, Davis JM. Overexpression of manganese superoxide dismutase protects lung epithelial cells against oxidant injury. Am J Respir Cell Mol Biol. 2001;24:436–441. doi: 10.1165/ajrcmb.24.4.4240. [DOI] [PubMed] [Google Scholar]
- 40.Chang LL, Subramaniam M, Yoder BA, Day BJ, Ellison MC, Sunday ME, Crapo JD. A catalytic antioxidant attenuates alveolar structural remodeling in bronchopulmonary dysplasia. Am J Respir Crit Care Med. 2003;167:57–64. doi: 10.1164/rccm.200203-232OC. [DOI] [PubMed] [Google Scholar]
- 41.Davis JM, Parad RB, Michele T, Allred E, Price A, Rosenfeld W. Pulmonary outcome at 1 year corrected age in premature infants treated at birth with recombinant human CuZn superoxide dismutase. Pediatrics. 2003;111:469–476. doi: 10.1542/peds.111.3.469. [DOI] [PubMed] [Google Scholar]
- 42.Salvemini D, Cuzzocrea S. Therapeutic potential of superoxide dismutase mimetics as therapeutic agents in critical care medicine. Crit Care Med. 2003;31:S29–S38. doi: 10.1097/00003246-200301001-00005. [DOI] [PubMed] [Google Scholar]
- 43.Coleman RJ, Beharry KD, Brock RS, Abad-Santos P, Abad-Santos M, Modanlou HD. Effects of brief, clustered versus dispersed hypoxic episodes on systemic and ocular growth factors in a rat model of oxygen-induced retinopathy. Pediatr Res. 2008;64:50–5. doi: 10.1203/PDR.0b013e31817307ac. [DOI] [PubMed] [Google Scholar]
- 44.Melov S, Ravenscroft J, Malik S, Gill MS, Walker DW, Clayton PE, Wallace DC, Malfroy B, Doctrow SR, Lithgow GJ. Extension of life-span with superoxide dismutase/catalase mimetics. Science. 2000;289:1567–1569. doi: 10.1126/science.289.5484.1567. [DOI] [PubMed] [Google Scholar]
- 45.Melov S, Doctrow SR, Schneider JA, Haberson J, Patel M, Coskun PE, Huffman K, Wallace DC, Malfroy B. Lifespan extension and rescue of spongiform encephalopathy in superoxide dismutase 2 nullizygous mice treated with superoxide dismutase–catalase mimetics. J Neurosci. 2001;21:8348–8353. doi: 10.1523/JNEUROSCI.21-21-08348.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Landry JS, Menzies D. Occurrence and severity of bronchopulmonary dysplasia and respiratory distress syndrome after a preterm birth. Paediatr Child Health. 2011;16:399–403. doi: 10.1093/pch/16.7.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Subramanian S, El-Mohandes A, Dhanireddy R, Koch MA. Association of bronchopulmonary dysplasia and hypercarbia in ventilated infants with birthweights of 500-1499 g. Matern Child Health J. 2011;15:S17–S26. doi: 10.1007/s10995-011-0863-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Martin RJ, Di Fiore JM, Macfarlane PM, Wilson CG. Physiologic basis for intermittent hypoxic episodes in preterm infants. Adv Exp Med Biol. 2012;758:351–358. doi: 10.1007/978-94-007-4584-1_47. [DOI] [PubMed] [Google Scholar]
- 49.Martin RJ, Wang K, Köroğlu Ö, Di Fiore J, Kc P. Intermittent hypoxic episodes in preterm infants: Do they Matter? Neonatology. 2011;100:303–310. doi: 10.1159/000329922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Haworth SG, Hislop AA. Lung development – the effects of chronic hypoxia. Semin Neonatol. 2003;8:1–8. doi: 10.1016/s1084-2756(02)00195-1. [DOI] [PubMed] [Google Scholar]
- 51.Brock RS, Gebrekristos BH, Kuniyoshi KM, Modanlou HD, Falcao MC, Beharry KD. Biomo lecular effects of JB1 (an IGF-I peptide analog) in a rat model of oxygen-induced retinopathy. Pediatr Res. 2011;69:35–41. doi: 10.1203/PDR.0b013e318204e6fa. [DOI] [PubMed] [Google Scholar]
- 52.Freeman BA, Topolosky MK, Crapo JD. Hyperoxia increases oxygen radical production in rat lung homogenates. Arch Biochem Biophys. 1982;216:477–484. doi: 10.1016/0003-9861(82)90236-3. [DOI] [PubMed] [Google Scholar]
- 53.Ratner V, Slinko S, Utkino-Sosunova I, Starkov A, Polin RA, Ten VS. Hypoxic Stress exacerbates hyperoxia-induced lung injury in a neonatal mouse model of Bronchopulmonary dysplasyia. Neonatology. 2009;95:299–305. doi: 10.1159/000178798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Asikainen TM, White CW. Pulmonary antioxidant defenses in the preterm newborn with respiratory distress and bronchopulmonary dysplasia in evolution: implication for antioxidant therapy. Antioxid Redox Signal. 2004;6:155–167. doi: 10.1089/152308604771978462. [DOI] [PubMed] [Google Scholar]
- 55.Valko M, Leibfritz D, Moncol J, Cronin MTD, Mazur M, Telser J. Free radicals and antioxidants in normal physiological functions and human disease. Int J Biochem Cell Biol. 2007;39:44–84. doi: 10.1016/j.biocel.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 56.Tschanz SA, Makanya AN, Haenni B, Burri PH. Effects of neonatal high-dose shrot-term glucocorticoid treatment on the lung: A morphologic and morphometric study in the rat. Pediatr Res. 2003;53:72–80. doi: 10.1203/00006450-200301000-00014. [DOI] [PubMed] [Google Scholar]
- 57.Gardner TJ, Stewart JR, Casale AS, Downey JM, Chambers DE. Reduction of myocardial ischemic injury with oxygen derived free radical scavengers. Surgery. 1983;94:423–427. [PubMed] [Google Scholar]
- 58.Shima DT, Deutsch U, D’Amore PA. Hypoxic induction of vascular endothelial growth factor (VEGF) in human epithelial cells is mediated by increases in mRNA stability. FEBS Lett. 1995;370:203–208. doi: 10.1016/0014-5793(95)00831-s. [DOI] [PubMed] [Google Scholar]
- 59.Oury TD, Thakker K, Menache M, Chang L, Crapo JD, Day BJ. Attenuation of bleomycin-induced pulmonary fibrosis by a catalytic antioxidant metalloporphyrin. Am J Respir Cell Mol Biol. 2001;25:164–169. doi: 10.1165/ajrcmb.25.2.4235. [DOI] [PubMed] [Google Scholar]
- 60.Cho M, Hunt TK, Hussain MZ. Hydrogen peroxide stimulates macrophage vascular endothelial growth factor release. Am J Physiol Heart Circ Physiol. 2001;280:H2357–H2363. doi: 10.1152/ajpheart.2001.280.5.H2357. [DOI] [PubMed] [Google Scholar]
- 61.Le Cras TD, Spitzmiller RE, Albertine KH, Greenberg JM, Whitsett JA, Akeson AL. VEGF causes pulmonary hemorrhage, hemosiderosis, and air space enlargement in neonatal mice. Am J Physiol Lung Cell Mol Physiol. 2004;287:L134–L142. doi: 10.1152/ajplung.00050.2004. [DOI] [PubMed] [Google Scholar]
- 62.Ambalavanan N, Nicola T, Li P, Bulger A, Murphy-Ullrich J, Oparil S, Chen YF. Role of matrix metalloproteinase-2 in newborn mouse lungs under hypoxic conditions. Pediatr Res. 2008;63:26–32. doi: 10.1203/PDR.0b013e31815b690d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Dik WA, van Kaam AH, Dekker T, Naber BA, Janssen DJ, Krron AA, Zimmermann LJ, Versnel MA, Lutter R. Early increased levels of matrix metalloproteinase-9 in neonates recovering from respiratory distress syndrome. Biol Neonate. 2006;89:6–14. doi: 10.1159/000088193. [DOI] [PubMed] [Google Scholar]
- 64.Gurjar MV, Deleon J, Sharma RV, Bhalla RC. Role of reactive oxygen species in IL-1 beta-stimulated sustained ERK activation and MMP-9 induction. Am J Physiol Heart Circ Physiol. 2001;281:H2568–H2574. doi: 10.1152/ajpheart.2001.281.6.H2568. [DOI] [PubMed] [Google Scholar]


