Abstract
Accumulation of amyloid-β (Aβ) peptides (predominantly Aβ40, 42) and their aggregation into plaques in the brain are thought to be the one of the major causes of Alzheimer’s disease (AD). Originally discovered in our Chinese hamster ovary (CHO) cell line stably expressing human wild-type amyloid precursor protein (APP) (CHO/APPwt) cultures devoid of Aβ production, we found that Mycoplasma selectively degrades soluble Aβ in a time and dose (colony forming unit) dependent manner. Moreover, we fully characterized the Mycoplasma species as Mycoplasma hyorhinis (M. hyorhinis) by genetic and colony morphological analyses by light microscopy. Most interestingly, we attenuated the pathogenicity of M. hyorhinis by γ irradiation (3.5 Gy), and found that its ability to degrade Aβ was retained. On the other hand, heated and sonicated M. hyorhinis failed to retain this ability to degrade Aβ, suggesting that this degradation requires viable cells and likely a biologically active signaling pathway. In addition, we found that M. hyorhinis can degrade Aβ produced in AD model mice (PSAPP mice) ex vivo. Finally, we found that irradiated (non-pathogenic) M. hyorhinis also can degrade Aβ produced in PSAPP mice in vivo. These studies suggest that irradiated (non-pathogenic) M. hyorhinis can be a novel and alternative biological strategy for AD treatment.
Keywords: Mycoplasma, Alzheimer’s disease, amyloid-β peptide, amyloid precursor protein
Introduction
Alzheimer’s disease (AD) is one of the most common neurodegenerative disorders worldwide, manifested by memory loss followed by progressive dementia. Characteristic AD pathology includes the presence of “senile” plaques, composed of the particularly highly neurotoxic 42 amino acid form of Aβ (Aβ42), as well as hyper-phosphorylated forms of tau protein in various regions of the brain [1]. An array of cellular, animal, and clinical studies have provided extensive characterization of how these toxic structures form deep inside the brain, but their precise etiology remains unclear. Moreover, a number of clinical trials, including anti-amyloid therapy such as Aβ vaccination [2,3], are ongoing worldwide for better prognosis and treatment of this devastating disease [4]. However, none of these trials have resulted in any meaningful translatable treatment that improves cognition to any significant degree.
One approach to enhance removal of Aβ pathology might involve a typical part of the human microbiome, specifically involving parasites known as Mycoplasma. Recently, Zhao and colleagues reported that Mycoplasma from a cell culture contamination efficiently and rapidly degrades extracellular Aβ released into the conditioned media by HEK293 cells stably transfected with the Swedish mutant form of human APP695 [5]. Notably, abolition of the Mycoplasma contaminant by quinolone-based antibiotics restored extracellular Aβ accumulation in these cells. Further studies indicate that Mycoplasma can reduce Aβ-mediated cellular toxicity by enhancing the calpain inhibitor calpastatin, reducing calpain activity as well as calpain-mediated cellular apoptosis [6,7].
Mycoplasmas are among the simplest self-replicating bacteria and the consequences of an infection for the host cell are variable, ranging from no apparent effect to induction of apoptosis [8]. They are characterized phenotypically from other bacteria by their minute size, with a diameter of 0.2-0.4 μm, minute genome, and lack of a cell wall. Since they are widespread in nature, including as parasites of humans [8], it is important to further characterize its role in the pathogenesis of AD. The present study was undertaken to assess the role of Mycoplasma in Aβ degradation both in vitro and in vivo. Further studies were undertaken to assess whether or not its pathogenicity and ability to break down Aβ could be differentiated by treatment with γ irradiation. These studies could be the basis for development of future Mycoplasma-based AD treatment.
Materials and methods
Reagents and antibodies
Aβ40, 42 and sAPPα ELISA kits and synthetic Aβ40, 42 peptides were purchased from Invitrogen (Carlsbad, CA). For immunoblotting (IB), we used mouse monoclonal anti-N-terminal Aβ1-17 antibody (6E10, Covance Research Products, Emeryville, CA) and β-actin antibody (Sigma-Aldrich, St Louis, MO). M. hyorhinis DNA was extracted by DNeasy Blood and Tissue kit (Qiagen, Valencia, CA) and Real Time PCR (RT-PCR) was performed using the MycoSensor QPCR Assay Kit (Stratagene, LaJolla, CA). The restriction endonucleases HpaII, PfIFI, XbaI, HaeIII, and BstBI were purchased from New England Biolabs (Ipswich, MA, UK). Penicillin and streptomycin were purchased from Invitrogen and Mycoplasma Removal Agent (MRA) was purchased from MP Biomedicals, Inc. (Solon, OH).
Cell culture
Mycoplasma-free Chinese hamster ovary (CHO) cell line stably expressing human wild-type APP (CHO/APPwt) was kindly provided by Dr. Stefanie Hahn (University of Heinrich Heine, Düsseldorf, Germany) and cultured in Dulbecco’s modified Eagle’s medium (DMEM) with 5% fetal bovine serum (FBS), 1 mM sodium pyruvate, and 100 units/ml of penicillin and streptomycin for 24 h. Aβ40, 42 peptides and sAPPα protein concentrations (200 pg/ml, 500 pg/ml, and 2,800 pg/ml, respectively) in CHO/APPwt-media were then determined by ELISA kits and the media was stored at -80°C for future study. For dose and time dependent analyses regarding degradation of Aβ40, 42 peptides by M. hyorhinis, CHO/APPwt-media, containing natural Aβ40, 42 peptides, was incubated with M. hyorhinisat 0, 0.8, 1.7, 3.25, and 7.5 × 106 CFU for 120 min. Moreover, CHO/APPwt-media was incubated with 3.25 × 106 CFU M. hyorhinis over 0, 30, 60, 120, and 240 min at 37°C.
Mycoplasma-infected (3.25 × 106 colony forming units (CFU) of M. hyorhinis) murine neuroblastoma N2a cells were cultured in DMEM containing 5% FBS in the absence or presence of MRA. After 24 h incubation at 37°C and 5% CO2, the media was collected, centrifuged at 20,000 x g and the pellet was resuspended in 200 μl PBS per dish. These samples were used for Mycoplasma-infected media (Myco-media) and MRA containing Mycoplasma infected media (Myco-media/MRA), respectively. In addition, some of Myco-media were filtered through 0.2 μm filters (Myco-media/Filtered). All of these conditioned media were aliquoted and kept at -80°C until the further study. All experiments using Mycoplasma were conducted in compliance with protocols approved by the University of South Florida (USF) Institutional BioSafety Committee (IBC).
PCR
For Mycoplasma species identification, infected N2a cells were cultured for one week without antibiotic and supernatant was collected after centrifugation at 20,000 g for 3 min. DNA extraction (DNeasy Blood and Tissue Kit, Qiagen) and PCR analysis (MycoSensor PCR Assay Kit, Stratagene) were both performed according to the protocol described by Uphoff and Drexler [9]. To further confirm our identification, Mycoplasma colonies were seeded on solid agar plates, incubated for 9 d at 37°C and colonies were identified by light microscopy based on their typical and characteristic appearance on the agar media, as described previously [10,11].
M. hyorhinis growth and titration
M. hyorhinis (ATCC 17981-TTR) was obtained from the American Type Culture Collection (ATCC) and grown statically at 37°C with 5% CO2 in Mycoplasma medium (ATCC® Medium 243) as described by Edward and Freundt [12] for 1 w. The media was then centrifuged at 20,000 g for 3 min followed by separation of the cell supernatant. Agar plates were prepared with the same medium containing 1% purified agar (Sigma-Aldrich). Ten microliters of the M. hyorhinis containing supernatant were serially diluted with Mycoplasma medium, seeded in the agar plates and incubated for 9 d at 37°C [10]. M. hyorhinis colonies were observed and counted by light microscopy.
Irradiation of M. hyorhinis
To irradiate M. hyorhinis, we utilized the modified protocol of Bender and colleagues [13] at the H. Lee Moffitt Cancer Center irradiation facility (Tampa, FL). M. hyorhinis was exposed to 3.5 Gy γ-rays for 19 h. Its DNA was then extracted and analyzed by RT-PCR, to determine whether the irradiation procedure was successful in eliminating the bacteria pathogenicity via DNA damage.
Immunoblotting analysis
Cells in culture were washed three times with ice-cold PBS and lysed with cell lysis buffer (Cell Signaling Technology Inc., Danvers, MA). Both supernatant and cell lysates were cryopreserved at -80°C for IB analysis until testing. Aβ40, 42 peptides secreted from cells or present in brain homogenates were analyzed by IB using 6E10 antibody according to our previous methods [14].
Mice
Four and Six-month-old female doubly transgenic PSAPP mice, bearing mutant human APP and mutant human presenilin 1 (PS1) transgenes, were purchased from the Jackson Laboratory (Bar Harbor, ME). Intracerebroventricular (i.c.v.) injection of M. hyorhinis was performed as described previously [15,16]. All mice were kept and maintained in the Morsani College of Medicine Animal Facility at USF (Tampa, FL) and all animal model experiments were conducted in compliance with protocols approved by the USF Institutional Animal Care and Use Committee.
Tissue preparation
Mice were euthanized with isoflurane anesthesia followed by transcardial perfusion with ice-cold PBS. Brain tissues were isolated rapidly, divided into left and right hemispheres at the level of the longitudinal fissure of the cerebrum and then coronally sliced in 3 mm thickness using a mouse brain slicer (Muromachi Kikai, Tokyo, Japan). The left hemisphere was incubated with 18 × 107 CFU of M. hyorhinis (Myco-media) and right hemisphere was incubated with clean media free of M. hyorhinis over 8 h at 37°C and 5% CO2. Soluble Aβ40, 42 levels were measured in homogenates of each sample by ELISA and protein expression was examined by IB according to our previous studies [15-17].
Statistical analysis
All data were normally distributed; therefore, in instances of single mean comparisons, Levene’s test for equality of variances followed by t test for independent samples was used to assess significance. In instances of multiple mean comparisons, one-way analysis of variance (ANOVA) was used, followed by post hoc comparison using Bonferonni’s method. Levels were set at 0.05 for all analyses. The Statistical Package for the Social Sciences, release IBM 10.0.5 SPSS (IBM, Armonk, NY) was used for all data analyses.
Results and discussion
Mycoplasma degrades natural Aβ40, 42 peptides produced by CHO/APPwt cells
To assess the effect of Mycoplasma on Aβ40, 42 degradation, conditioned media from CHO/APPwt cells (CHO/APPwt-media), was mixed with (1) media from clean N2a cells (Clean media), (2) media from N2a cells infected with Mycoplasma (Myco-media), (3) filtered Myco-media (Myco-media/Filtered), or (4) Myco-media containing MRA (Myco-media/MRA), each respectively in four different ratios (1:0, 1:1/4, 1:1/2, and 1:1). The resulting media was then analyzed after 2 h incubation by ELISA and IB for Aβ40, 42 and sAPPα. Aβ40, 42 levels were significantly decreased in CHO/APPwt-media treated with Myco-media at 1:1/4, 1:1/2, and 1:1 compared with 1:0 dilutions (Figure 1A). By contrast, Aβ40, 42 levels were unchanged in CHO/APPwt-media treated with clean, filtered, or MRA containing Myco-media. These results were confirmed with IB analysis using 6E10 antibody (Figure 1B). Mycoplasma did not alter sAPPα levels irrespective of the treatment condition used, suggesting that Mycoplasma selectively degrade Aβ peptides. In addition, Mycoplasma did not alter either amyloidogenic or non-amyloidogenic APP processing as evidenced by IB analysis of C-terminal fragment of APP (data not shown). Furthermore, we incubated Myco-media with the conditioned media from murine splenocytes challenged with concanavalin A (ConA, 5 μg/ml) and containing IL-2, IL-6, or IFNγ and assessed cytokine levels by ELISA. Mycoplasma did not degrade other peptides, such as IL-2, IL-6, or IFNγ (data not shown).
Figure 1.
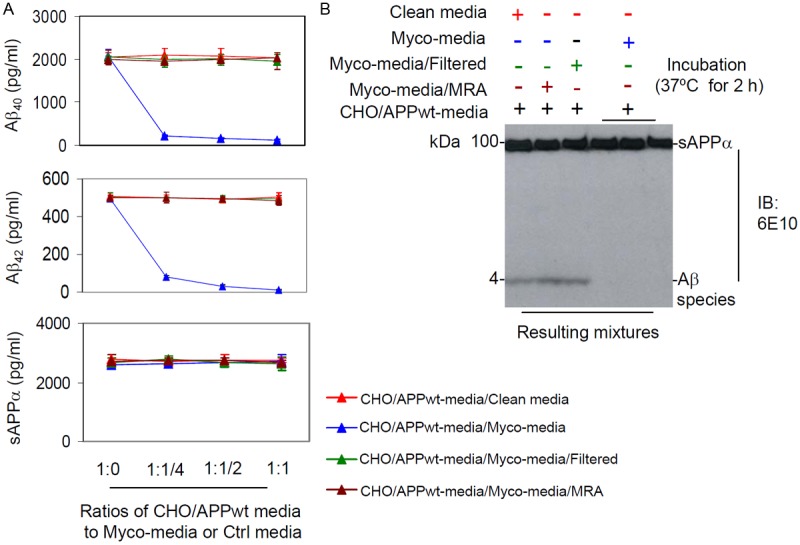
Mycoplasma selectively degrades naturally produced Aβ peptides. Conditioned media collected after 24 h culture of CHO/APPwt cells were incubated with (1) media from clean N2a cells (Clean media), (2) media from N2a cells infected with Mycoplasma (Myco-media), (3) filtered Myco-media (Myco-media/Filtered), or (4) Myco-media containing MRA (Myco-media/MRA) each respectively in four different ratios (1:0, 1:1/4, 1:1/2, and 1:1) at 37°C for 2 h. Aβ40, 42 and sAPPα levels in the media were then determined by (A) ELISA (presented as mean ± SD), and (B) IB using an anti-N-terminal Aβ1-17 antibody (6E10). These results are representative of four independent experiments with three replicates for each condition.
Dose and time dependent degradation of Aβ40, 42 peptides by M. hyorhinis
By restriction pattern digestion, we determined the responsible organism as M. hyorhinis for Aβ40, 42 peptide degradation (Figure 2A). To further confirm this result, M. hyorhinis colonies were detected on microbiological media by light microscopy (Figure 2B). To assess the minimum dose of M. hyorhinis that can completely degrade Aβ40, 42 peptides, CHO/APPwt-media, containing natural Aβ40, 42 peptides, was incubated with M. hyorhinis at 0, 0.8, 1.7, 3.25, and 7.5 × 106 CFU for 120 min. Near complete degradation of Aβ40, 42 occurred with M. hyorhinis at 3.25 × 106 CFU (Figure 2C). To define the minimum time required for Aβ40, 42 peptide degradation, CHO/APPwt-media was incubated with 3.25 × 106 CFU M. hyorhinis over 0, 30, 60, 120, and 240 min at 37°C. ELISA results indicate that 120 min is the minimum time period required for complete degradation of Aβ40, 42 peptides (Figure 2D).
Figure 2.
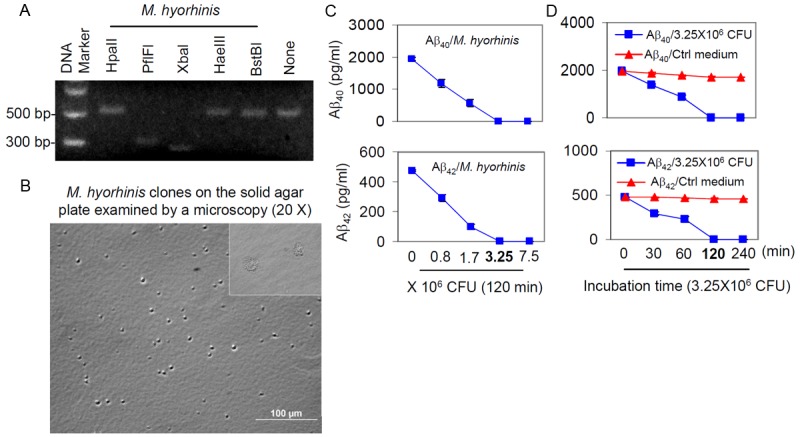
Identification of M. hyorhinis and characterization of Aβ40, 42 degradation. A: Mycoplasma genomic DNA from infected N2a cell cultures was extracted, amplified and analyzed by restriction fragment length polymorphism (RFLP) using five different restriction endonucleases. The digestion pattern observed identified the Mycoplasma as M. hyorhinis. B: M. hyorhinis colonies were observed on solid agar plate by light microscopy (20X). C: CHO/APPwt media was incubated with 0, 0.8, 1.7, 3.25, or 7.5 × 106 colony forming units (CFU) of M. hyorhinis at 37°C for 120 min. D: CHO/APPwt media was incubated with 3.25 × 106 CFU of M. hyorhinis or clean Mycoplasma media (Ctrl Medium) for 0, 30, 60, 120 and 240 min at 37°C. Aβ40 and Aβ42 were then determined in the media by ELISA and presented as mean ± SD. These results are representative of three independent experiments with three replicates for each condition.
Aβ40, 42 peptides in both natural and synthetic forms are degraded by M. hyorhinis in a similar manner
To further assess the ability of M. hyorhinis to degrade Aβ40, 42 peptides, we compared the ability of M. hyorhinis to degrade both natural and synthetic Aβ40, 42 peptides, contained in CHO/APPwt media, and synthetic Aβ40, 42 peptides by IB using 6E10 antibody. M. hyorhinis at 0, 1.7, and 3.25 × 106 CFU decreased both natural (Figure 3A) and synthetic Aβ40, 42 peptides significantly in a similar dose dependent pattern (Figure 3B). As expected, sAPPα bands (100 kDa) were absent in Figure 3B, as we used synthetic Aβ40, 42 peptides.
Figure 3.
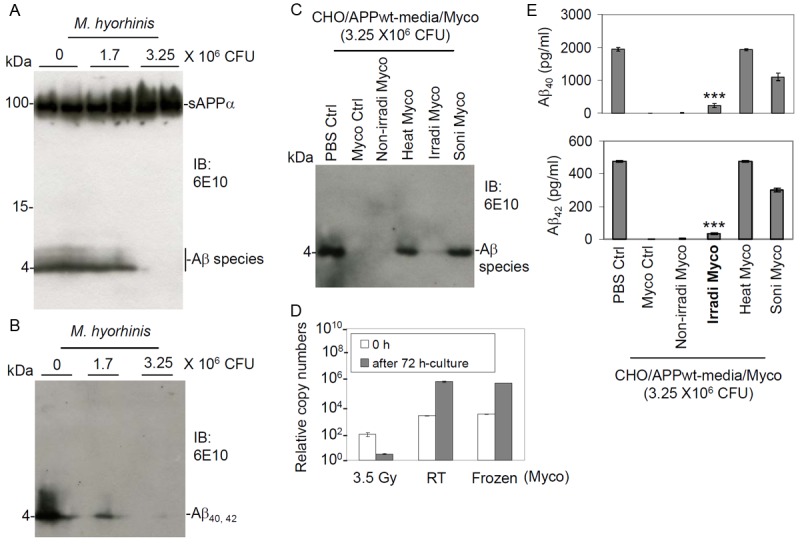
Degradation of Aβ40, 42 peptides using irradiated M. hyorhinis. M. hyorhinis at 0, 1.7, and 3.25 × 106 CFU were incubated with (A) CHO/APPwt-media or (B) synthetic human Aβ40, 42 peptides (each peptide at 500 ng/ml) at 37°C for 120 min. Aβ and sAPPα levels were then determined by IB using 6E10 antibody. These results are representative of two independent experiments. In addition, CHO/APPwt-media were incubated with PBS or 3.25 × 106 CFU of (1) cryopreserved (Myco Ctrl), (2) fresh, non-irradiated (Non-irradi Myco), (3) irradiated (Irradi Myco), (4) heated (56°C for 10 min, Heat Myco), or (5) sonicated M. hyorhinis (Soni Myco) at 37°C for 120 min. The resulting mixtures were then subjected Aβ40 and Aβ42 analysis by (C) IB analysis and (E) ELISA (presented as mean ± SD). Both Aβ40 and Aβ42 levels were significantly decreased by Myco Ctrl, Non-irradi Myco, and Irradi Myco (***p < 0.001). (D) RT-PCR results of irradiated (3.5 Gy), fresh (kept in room temperature, RT) and cryopreserved M. hyorhinis (Frozen, -80°C) at 0 and 72 h incubation. These results are representative of two independent experiments with three replicates for each condition.
Degradation of Aβ40, 42 peptides by irradiated M. hyorhinis
Since M. hyorhinis is considered to be a pathogenic parasite, we sought to determine whether irradiated (non-pathogenic) M. hyorhinis could retain its ability to degrade Aβ40, 42 peptides. CHO/APPwt-media were incubated at 37°C for 120 min with PBS or 3.25 × 106 CFU of (1) cryopreserved (Myco Ctrl), (2) fresh, non-irradiated (Non-irradi Myco), (3) irradiated (3.5 Gy, Irradi Myco), (4) heated (56°C × 10 min; Heat Myco), or (5) sonicated M. hyorhinis. (Soni Myco). The resulting mixtures were then analyzed for Aβ40, 42 peptides by ELISA and IB using 6E10 antibody. The irradiated M. hyorhinis was able to significantly degrade Aβ40, 42 peptides, similar to that observed with the cryopreserved (Myco Ctrl) or fresh, nonirradiated (Non-irradi Myco) M. hyorhinis (Figure 3C and 3E). As expected, we found that heated or sonicated M. hyorhinis failed to degrade Aβ40, 42 peptides. In addition, RT-PCR analysis of DNA extracted from M. hyorhinis after irradiation showed that this treatment almost completly eliminated the proliferative capacity of M. hyorhinis (Figure 3D).
Degradation of brain tissue-derived Aβ40, 42 peptides of PSAPP mice by M. hyorhinis
We have shown that M. hyorhinis can degrade both natural and synthetic Aβ40, 42 peptides. Furthermore, we wished to determine whether this organism could degrade Aβ40, 42 peptides ex vivo. We removed brain tissue of six-month-old female PSAPP transgenic mice and divided them into left and right hemispheres. Coronal slices (3 mm in thickness) from the left and right hemispheres were incubated with 1.8 × 107 CFU of M. hyorhinis or clean media for 8 h respectively. The levels of Aβ40, 42 were significantly reduced in samples containing M. hyorhinis compared with those in clean media as determined by ELISA (Figure 4A) and confirmed by IB analysis using 6E10 antibody (Figure 4B). Finally, we attempted to determine whether this organism could degrade Aβ40, 42 peptides in vivo. We analyzed soluble Aβ40, 42 peptides in four-month-old female PSAPP transgenic mice following i.c.v. injection of irradiated M. hyorhinis. The levels of Aβ40 and Aβ42 were significantly reduced in PSAPP transgenic mice i.c.v. injected with irradiated M. hyorhinis compared to those injected with PBS as determined by ELISA (Figure 4C) and confirmed by IB analysis using 6E10 antibody (Figure 4D).
Figure 4.
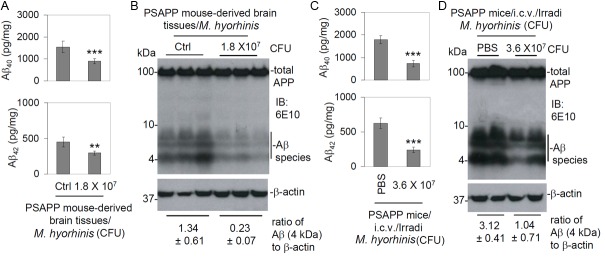
Degradation of Aβ40, 42 peptides of PSAPP mice ex vivo and in vivo by M. hyorhinis. Six-month-old transgenic PSAPP mice were euthanized and brain tissue was removed from each mouse and divided into left and right hemispheres. The left hemisphere of each mouse was incubated with 1.8 × 107 CFU M. hyorhinis and the right hemisphere was incubated with clean media (Ctrl, without M. hyorhinis) for 8 h respectively. After incubation, brain tissue was homogenized and soluble Aβ40, 42 peptides were measured individually by (A) ELISA (presented as mean ± SD, n = 3) and confirmed by (B) IB analysis using 6E10 antibody. Mycoplasma significantly decreased both Aβ40 and Aβ42 peptides. Furthermore, PSAPP mice at 4 months of age were intracerebroventricular (i.c.v.) injected with irradiated M. hyorhinis at 3.6 × 107 CFU/mouse (n = 5) and euthanized 48 h after the injection. Mouse brain homogenates were prepared (the right half of brain tissues (the non-injection side)) and subjected to soluble Aβ40, 42 peptide ELISA (C) and IB analysis (D). The ratio of Aβ to β-actin for brain tissues incubated with M. hyorhinis or clean media, as well as that prepared from PSAPP mice i.c.v. injected with M. hyorhinis or PBS, are shown below IB, indicating that M. hyorhinis indeed degraded Aβ (**p < 0.01, ***p < 0.001).
Discussion
The present study explored a new approach for removing Aβ from the brain involving Mycoplasma. We show that Mycoplasma can degrade Aβ, both naturally produced by CHO/APPwt cells and synthetic, in a dose and time dependent fashion and that the Mycoplasma species mediating this effect is M. hyorhinis. This degradation appears to be selective, since Mycoplasma did not alter the levels of sAPPα or degrade other peptides such as IL-2, IL-6, or IFNγ. In addition, M. hyorhinis retained its ability to reduce Aβ after γ-ray irradiation, a treatment which substantially reduced its pathogenicity. However, its ability to reduce Aβ was eliminated by filtration, sonication, heat or treatment with MRA, indicating that this effect is not mediated by a direct molecular interaction of Aβ with released or cytoplasmic constituents but required intact viable cells and possibly a signal transduction process. Finally, we showed that M. hyorhinis can reduce Aβ ex vivo in cortical brain slices isolated from PSAPP mice, an AD mouse model, as well as in vivo in PSAPP transgenic mice.
These results confirm and extend those previously reported by Zhao et al. [8], who showed that M. hyorhinis can reduce Aβ naturally produced by SH-SY5Y cells overly expressing the Swedish mutant form of APP and that this effect was eradicated by treatment of the Mycoplasma with a quinolone-based antibiotic. Subsequent studies showed that M. hyorhinis can reduce Aβ-mediated cellular toxicity by enhancing levels of the calpain inhibitor, calpastatin, thereby reducing calpain activity and calpain-mediated apoptosis [6]. This effect was further shown to be due to a membrane lipoprotein in M. hyorhinis which induces nuclear translocation of the transcription factor, nuclear factor-kappa B( NF-κB) [7].
Taken together, these results implicate M. hyorhinis, a natural part of the human microbiome, as a potentially effective and novel treatment for AD. Another potential known benefit of Mycoplasma is their propensity to consume fatty acids and cholesterol for their own membrane synthesis [18]. However, the major disadvantage with using Mycoplasma as a potential therapeutic agent is its disease causing properties. Mycoplasma hyorhinis was first isolated from the respiratory tract of young pigs, and has been implicated in pleuritis, peritonitis, pericarditis, arthritis, and otitis media of swine [19,20]. The interest in M. hyorhinis has recently increased after the detection of this organism in human beings [21], where it can affect membrane properties and cellular functions related to the immune system [22]. Additional studies implicate M. hyorhinis in the pathogenesis of cancer, such as melanoma [23,24]. However, by treatment such as irradiation, it may be possible to reduce its pathogenicity while retaining its ability to reduce Aβ. Given the simple genome of Mycoplasma, it may also be possible to harness the natural properties of this bacterium by genome modification, thereby reducing its pathogenicity while enhancing its beneficial effects for the treatment of AD and other neurological diseases. As there is yet no effective treatment for neurological diseases such as AD, this possibility merits further investigation.
Acknowledgements
This work was supported by the NIH/NIA (R01AG032432) and the Silver Endowment. We would like to thank David Kang for his helpful discussion. Ahsan Habib and Juan Deng equally contribute to this work.
Disclosure of conflict of interest
None.
References
- 1.Selkoe DJ. Alzheimer‘s disease: genes, proteins, and therapy. Physiol Rev. 2001;81:741–766. doi: 10.1152/physrev.2001.81.2.741. [DOI] [PubMed] [Google Scholar]
- 2.Morgan D. Immunotherapy for Alzheimer‘s disease. J Intern Med. 2011;269:54–63. doi: 10.1111/j.1365-2796.2010.02315.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liu Y, Giunta B, Zhou H, Tan J, Wang Y. Immunotherapy for Alzheimer disease: the challenge of adverse effects. Nat Rev Neurol. 2012;8:465–469. doi: 10.1038/nrneurol.2012.118. [DOI] [PubMed] [Google Scholar]
- 4.Thies W, Bleiler L Alzheimer‘s Association. 2013 Alzheimer‘s disease facts and figures. Alzheimers Dement. 2013;9:208–245. doi: 10.1016/j.jalz.2013.02.003. [DOI] [PubMed] [Google Scholar]
- 5.Zhao H, Dreses-Werringloer U, Davies P, Marambaud P. Amyloid-β peptide degradation in cell cultures by mycoplasma contaminants. BMC Res Notes. 2008;1:38. doi: 10.1186/1756-0500-1-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Elkind E, Vaisid T, Kornspan JD, Barnoy S, Rottem S, Kosower NS. Neuroprotective effects of Mycoplasma hyorhinis against amyloid-β-peptide toxicity in SH-SY5Y human neuroblastoma cells are mediated by calpastatin upregulation in the mycoplasma-infected cells. Neurochem Int. 2011;58:497–503. doi: 10.1016/j.neuint.2011.01.005. [DOI] [PubMed] [Google Scholar]
- 7.Elkind E, Vaisid T, Kornspan JD, Barnoy S, Rottem S, Kosower NS. Calpastatin upregulation in Mycoplasma hyorhinis-infected cells is promoted by the mycoplasma lipoproteins via the NF-kB pathway. Cell Microbiol. 2012;14:840–851. doi: 10.1111/j.1462-5822.2012.01760.x. [DOI] [PubMed] [Google Scholar]
- 8.Razin S, Yogev D, Naot Y. Molecular biology and pathogenicity of mycoplasmas. Microbiol Mol Biol Rev. 1998;62:1094–156. doi: 10.1128/mmbr.62.4.1094-1156.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Uphoff CC, Drexler HG. Detecting mycoplasma contamination in cell cultures by polymerase chain reaction. Methods Mol Biol. 2011;731:93–103. doi: 10.1007/978-1-61779-080-5_8. [DOI] [PubMed] [Google Scholar]
- 10.Uphoff CC, Drexler HG. Detection of mycoplasma in leukemia–lymphoma cell lines using polymerase chain reaction. Leukemia. 2002;16:289–293. doi: 10.1038/sj.leu.2402365. [DOI] [PubMed] [Google Scholar]
- 11.Hayflick L, Koprowski H. Direct agar isolation of mycoplasmas from human leukaemic bone marrow. Nature. 1965;205:713–714. doi: 10.1038/205713b0. [DOI] [PubMed] [Google Scholar]
- 12.Edward D, Freundt E. Type strains of species of the order Mycoplasmatales, including designation of neotypes for Mycoplasma mycoides subsp. mycoides, Mycoplasma agalactiae subsp. agalactiae, and Mycoplasma arthritidis . Int J Syst Bacteriol. 1973;23:55–61. [Google Scholar]
- 13.Bender E, Freitzshce J, Bar M, Nordheim W. The inactivation of mycoplasmas and bacteria in calf serum by 60Co γ rays. Arch Exp Veterinarmed. 1989;43:783–788. [PubMed] [Google Scholar]
- 14.Zhu Y, Hou H, Rezai-Zadeh K, Giunta B, Ruscin A, Gemma C, Jin J, Dragicevic N, Bradshaw P, Rasool S, Glabe CG, Ehrhart J, Bickford P, Mori T, Obregon D, Town T, Tan J. CD45 deficiency drives amyloid-β peptide oligomers and neuronal loss in Alzheimer's disease mice. J Neurosci. 2011;31:1355–1365. doi: 10.1523/JNEUROSCI.3268-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Obregon D, Hou H, Deng J, Giunta B, Tian J, Darlington D, Shahaduzzaman M, Zhu Y, Mori T, Mattson MP, Tan J. Soluble amyloid precursor protein-α modulates β-secretase activity and amyloid-β generation. Nat Commun. 2012;3:777. doi: 10.1038/ncomms1781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Deng J, Hou H, Giunta B, Mori T, Wang YJ, Fernandez F, Weggen S, Araki W, Obregon D, Tan J. Autoreactive-Aβ antibodies promote APP β-secretase processing. J Neurochem. 2012;120:732–740. doi: 10.1111/j.1471-4159.2011.07629.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Giunta B, Hou H, Zhu Y, Salemi J, Ruscin A, Shytle RD, Tan J. Fish oil enhances anti-amyloidogenic properties of green tea EGCG in Tg2576 mice. Neursci Lett. 2010;471:134–138. doi: 10.1016/j.neulet.2010.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Razin S, Efrati H, Kutner S, Rottem S. Cholesterol and phospholipid uptake by mycoplasmas. Rev Infec Dis. 1982;4:S85–S92. doi: 10.1093/clinids/4.supplement_1.s85. [DOI] [PubMed] [Google Scholar]
- 19.Friis NF, Feenstra AA. Mycoplasma hyorhinis in the etiology of serositis among piglets. Acta Vet Scand. 1994;35:93–98. doi: 10.1186/BF03548359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Morita T, Fukuda H, Awakura T, Shimada A, Umemura T, Kazama S, Yagihashi T. Demonstration of Mycoplasma hyorhinis as a possible primary pathogen for porcine otitis media. Vet Pathol. 1995;32:107–111. doi: 10.1177/030098589503200202. [DOI] [PubMed] [Google Scholar]
- 21.Huang S, Li JY, Wu J, Meng L, Shou CC. Mycoplasma infections and different human carcinomas. World J Gastroenterol. 2001;7:266–269. doi: 10.3748/wjg.v7.i2.266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rottem S. Interaction of mycoplasmas with host cells. Physiol Rev. 2003;83:417–432. doi: 10.1152/physrev.00030.2002. [DOI] [PubMed] [Google Scholar]
- 23.Gazit R, Rechnitzer H, Achdout H, Katzenell A, Katz G, Markel G, Arnon TI, Gonen-Gross T, Mizrahi S, Gruda R, Rottem S, Mandelboim O. Recognition of Mycoplasma hyorhinis by CD99-Fc molecule. Eur J Immunol. 2004;34:2032–2040. doi: 10.1002/eji.200324845. [DOI] [PubMed] [Google Scholar]
- 24.Gong M, Meng L, Jiang B, Zhang J, Yang H, Wu J, Shou C. p37 from Mycoplasma hyorhinis promotes cancer cell invasiveness and metastasis through activation of MMP-2 and followed by phosphorylation of EGFR. Mol Cancer Ther. 2008;7:530–537. doi: 10.1158/1535-7163.MCT-07-2191. [DOI] [PubMed] [Google Scholar]


