Abstract
Background
In adults, the 2 main types of myelodysplastic/myeloproliferative neoplasms (MDS/MPN) are chronic myelomonocytic leukemia (CMML) and atypical chronic myeloid leukemia (aCML). Both are associated with a poor prognosis. Allogeneic hematopoietic cell transplantation (HCT) is the only known curative treatment modality for these diseases, but data on outcomes following such treatment are limited. We analyzed the outcomes of patients with MDS/MPN after allogeneic HCT.
Methods
This retrospective study included 10 patients with MDS/MPN who received allogeneic HCT at Asan Medical Center from 2002 to 2010. Of these 10 patients, 7 had CMML, 2 had aCML, and 1 had unclassifiable MDS/MPN. Five patients received a myeloablative conditioning (MAC) regimen (busulfan-cyclophosphamide), and 5 received reduced-intensity conditioning (RIC) regimen.
Results
Neutrophil engraftment was achieved in all patients. After a median follow-up of 47.5 months among surviving patients, 4 had relapsed and 5 had died. There was only 1 treatment-related death. The 5-year rates of overall, relapse-free, and event-free survival were 42.2%, 51.9%, and 46.7%, respectively. Relapse was the leading cause of treatment failure, and all relapses were observed in patients who had received RIC and who did not develop chronic graft-versus-host disease.
Conclusion
Allogeneic HCT can induce durable remission in patients with MDS/MPN, but RIC cannot replace MAC in patients eligible for myeloablative treatments.
Keywords: MDS/MPN, Allogeneic HCT, Reduced-intensity, Relapse
INTRODUCTION
Although myelodysplastic syndrome (MDS) and myeloproliferative disorder (MPD) appear to have entirely different pathophysiological mechanisms, the existence of conditions with overlapping features is well established [1, 2]. The World Health Organization (WHO) classification of myeloid neoplasms clearly defines a MDS/MPD group, which includes chronic myelomonocytic leukemia (CMML), juvenile myelomonocytic leukemia (JMML), BCR-ABL-negative atypical chronic myeloid leukemia (aCML), and unclassifiable MDS/MPD (MDS/MPD-U) [3]. In the recently updated WHO classification system, the term MPD has been replaced by myeloproliferative neoplasm (MPN) [4]. Patients with MDS/MPN have various combinations of cytopenias and cytoses, with dysplasia of at least one lineage. Their bone marrow is characteristically hypercellular and shows dysplastic and proliferative features, as predicted from peripheral blood. By definition, the percentage of blasts in blood and bone marrow must be less than 20%.
In adults, the 2 main types of MDS/MPN are CMML and aCML, both of which are associated with poor prognosis. CMML is a progressive disease that often leads to death within months [5, 6]. Various chemotherapy regimens, including hydroxyurea, etoposide, low-dose cytarabine, topotecan, intensive chemotherapy, and hypomethylating agents, have generally been inadequate in patients with CMML owing to low response rates and short response durations [7, 8]. In approximately 25% to 40% of patients with aCML, the disease evolves into acute leukemia, while the remaining patients die of marrow failure; the median survival in patients treated with conventional chemotherapy is less than 20 months [9-11]. The only currently available therapy that is potentially curative for patients with MDS/MPN is allogeneic hematopoietic cell transplantation (HCT), but little is known about the impact of allogeneic HCT on the natural history of MDS/MPN [12-16]. We analyzed the outcomes of patients with MDS/MPN after allogeneic HCT.
MATERIALS AND METHODS
Patients
Between October 2002 and July 2010, 10 patients (9 men) with MDS/MPN underwent allogeneic HCT at the Asan Medical Center, Seoul, Korea (Table 1). Median age at the time of HCT was 43 years (range, 28 to 53 years). Seven patients had CMML, 2 had aCML, and 1 had MDS/MPN-U. MDS/MPN was diagnosed and classified using WHO criteria [3, 4]. The median interval from diagnosis to HCT was 4.1 months (range, 2.1 to 19.6 months). Five patients had not received any specific treatment for MDS/MPN, whereas the other 5 were treated with intensive chemotherapy (N=1) or hypomethylating agents (N=4) before HCT. Among the 5 previously treated patients, only 1 had responded to treatment. Eight patients had HCT-comorbidity index (HCT-CI) scores [17] of 0, with 1 each having scores of 1 and 4. Of the 7 patients with CMML, 1 had a low-risk MD Anderson Prognostic score (MDAPS) [6], 2 had intermediate-1 scores, 3 had intermediate-2 scores, and 1 had a high risk score. At the time of HCT, 7 patients had >5% bone marrow blasts and 3 had cytogenetic abnormalities. One patient had received chemotherapy and radiotherapy for non-Hodgkin's lymphoma (NHL).
Table 1.
Patient characteristics at the time of hematopoietic cell transplantation.
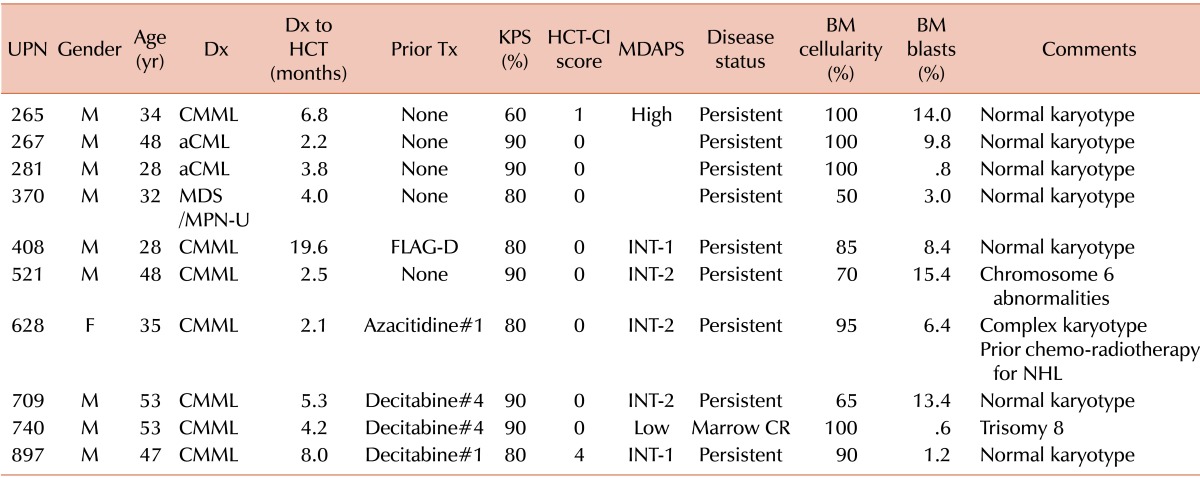
Abbreviations: UPN, unique patient number; M, male; F, female; Dx, diagnosis; CMML, chronic myelomonocytic leukemia; aCML, atypical chronic myeloid leukemia; MDS/MPN-U, myelodysplastic syndrome/myeloproliferative neoplasm-unclassifiable; HCT, hematopoietic cell transplantation; Tx, treatment; FLAG-D, fludarabine, cytarabine, granulocyte colony-stimulating factor plus daunorubicin; KPS, Karnofsky performance score; HCT-CI, hematopoietic cell transplantation-comorbidity index; MDAPS, M.D. Anderson Prognostic Score; INT, intermediate; CR, complete remission; BM, bone marrow; NHL, non-Hodgkin lymphoma.
Transplantation procedures
The 10 donors consisted of 8 men and 2 women, of median age 28.5 years (range, 25 to 48 years) (Table 2). Five donors were HLA-matched siblings, 4 were unrelated volunteers (3 HLA matched and 1 mismatched in the HLA-B-allele), and 1 was an HLA-haplo-identical familial donor. ABO incompatibilities were observed in 4 donor-recipient pairs: 2 minor and 2 major and minor incompatibilities. Graft-versus-host disease (GVHD) prophylaxis consisted of cyclosporine plus short-course methotrexate in 8 patients and cyclosporine alone in 2. Five patients received a myeloablative conditioning (MAC) regimen, consisting of intravenous busulfan (3.2 mg/kg) for 4 days and cyclophosphamide (60 mg/kg) for 2 days (BuCy). The other 5 received reduced-intensity conditioning (RIC) regimens, including 3 who received intravenous busulfan (3.2 mg/kg) for 2 days plus fludarabine (30 mg/m2) for 6 days plus anti-thymocyte globulin (Thymoglobulin; 3 mg/kg) for 3 days (BuFluATG), 1 who received intravenous busulfan (3.2 mg/kg) for 2 days plus fludarabine (30 mg/m2) for 6 days plus alemtuzumab (20 mg) for 1 day (BuFluCampath), and 1 who received melphalan (100 mg/m2) for 1 day plus fludarabine (30 mg/m2) for 5 days (MelFlu). The administration of an RIC regimen was at the discretion of the attending physician. One patient (UPN 408) received alemtuzumab owing to a shortage of anti-thymocyte globulin in Korea at that time, and 1 patient (UPN 628) received MelFlu due to the concurrent presence of active lymphoma and CMML at the time of HCT. The hematopoietic cell graft was bone marrow in 5 patients and granulocyte colony-stimulating factor (G-CSF)-mobilized peripheral blood mononuclear cells in 5.
Table 2.
Transplantation data.
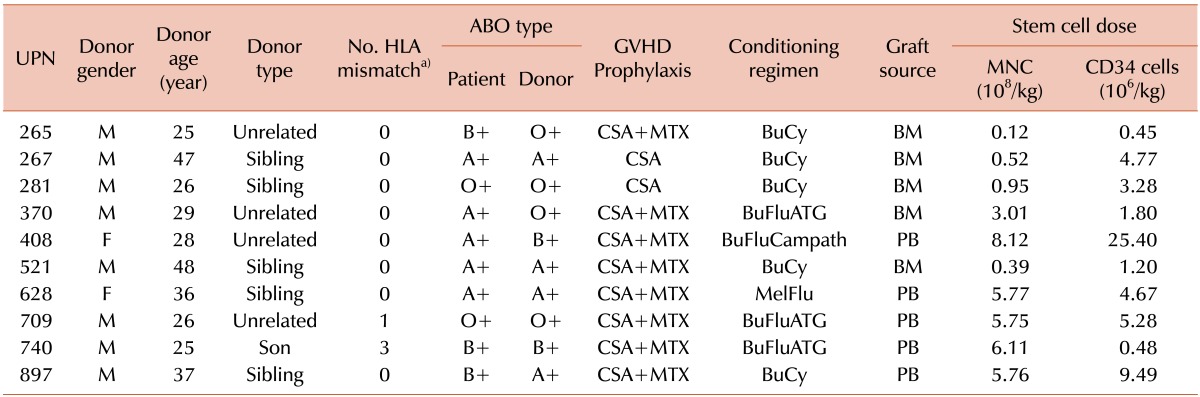
a)Number of incompatibilities among 8 ABDR loci between donor and recipient.
Abbreviations: UPN, unique patient number; HLA, human leukocyte antigen; GVHD, graft-vs-host disease; CSA, cyclosporin; MTX, methotrexate; BuCy, busulfan+cytoxan; BuFluATG, busulfan+fludarabine+thymoglobulin; BuFluCampath, busulfan+fludarabine+alemtuzumab; MelFlu, melphalan-fludarabine; BM, bone marrow; PB, peripheral blood stem cell; MNC, mononuclear cell.
Day 0 was defined as the first day of infusion of hematopoietic cell graft. Time to neutrophil engraftment was the time from day 0 until the first of 3 consecutive days at which absolute neutrophil count was ≥0.5×109/L, and time to platelet engraftment was the time from day 0 until the first of 7 consecutive days at which platelet count was ≥20×109/L without transfusion support. Hematopoietic chimerism was evaluated in bone marrow or peripheral blood cells by PCR analysis of short tandem repeats [18]. Relapse was defined according to standard morphologic criteria and/or conventional cytogenetic analysis.
Acute and chronic GVHD were diagnosed on the basis of clinical symptoms, laboratory tests, and, when possible, histopathologic assessment of the skin, oral mucosa, and gastrointestinal tract. Acute GVHD was classified according to clinical criteria [19], and chronic GVHD was graded as limited (localized to the skin or a single organ) or extensive (generalized skin or multiple organ involvement) [20].
Hepatic sinusoidal obstruction syndrome (SOS) diagnosis [21] and severity [22] was made according to previously established criteria.
Statistical analysis
Analyses were performed using the SPSS statistical package (version 18.0, IBM Corp., Armonk, NY, USA). Overall survival (OS), relapse-free survival (RFS), and event-free survival (EFS) were defined as the time from the date of HCT to the date of death, relapse, and relapse or death, respectively. Survival curves were calculated using the Kaplan-Meier method and compared using the log-rank test. Differences were considered statistically significant if the 2-tailed P value was<0.05.
RESULTS
Engraftment and chimerism
All patients achieved neutrophil engraftment (absolute neutrophil count ≥0.5×109/L) at a median of 16 days (range, 12 to 24 days), and 9 patients achieved transfusion- independent platelet engraftment (≥20×109/L) at a median of 25 days (range, 13 to 81 days). One patient died of cytomegalovirus (CMV) interstitial pneumonitis before platelet engraftment (Table 3). Nine patients attained complete chimerism, 2 to 28 weeks after HCT, but 1 showed persistent mixed chimerism and relapsed 7.8 months after HCT.
Table 3.
Post-transplant outcomes.
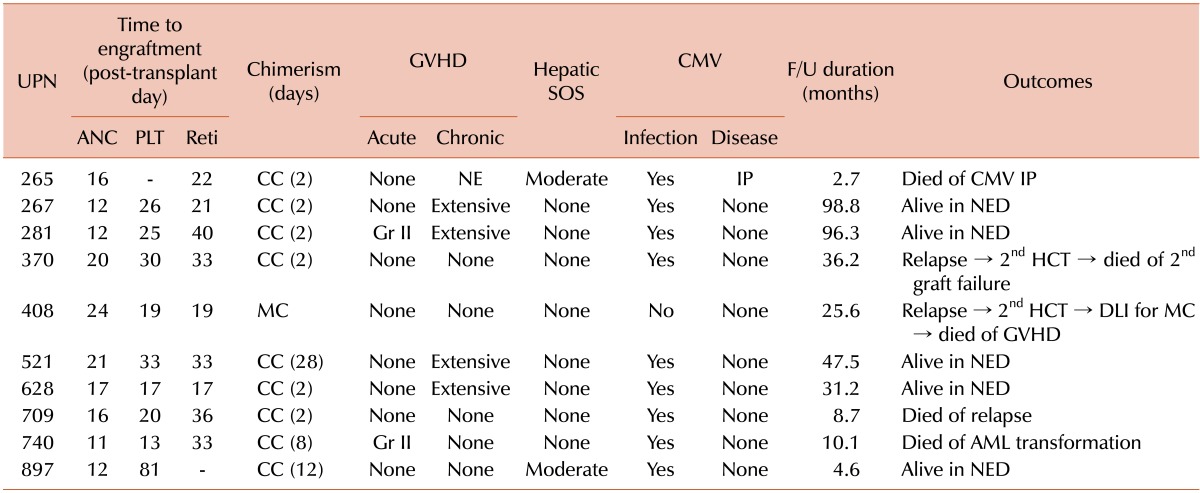
Abbreviations: UPN, unique patient number; ANC, absolute neutrophil counts ≥0.5×109/L; PLT, platelet counts ≥20×109/L; Reti, absolute reticulocyte counts ≥1.0%; CC, complete chimerism; MC, mixed chimerism; GVHD, graft-versus-host disease; Gr, grade; NE, not evaluable; SOS, sinusoidal obstruction syndrome; CMV, cytomegalovirus; IP, interstitial pneumonitis; F/U, follow-up; NED, no evidence of disease; HCT, hematopoietic cell transplantation; DLI, donor lymphocyte infusion; AML, acute myeloid leukemia.
Post-transplant complications
Acute GVHD occurred in 2 patients (20.0%); both were of grade II. Chronic GVHD occurred in 4 (44.4%) of 9 assessable patients, with all 4 being extensive. All patients experienced CMV infection, as shown by the pp65 CMV antigenemia assay, but only 1 developed CMV disease (interstitial pneumonitis) (Table 3). Two patients developed hepatic SOS of moderate severity.
Post-transplant outcomes
After a median follow-up of 47.5 months (range, 4.6 to 98.8 months) in the surviving patients, 5 patients had died. Four of these deaths were related to disease relapse, but 1 was due to CMV interstitial pneumonitis (Table 3). Four patients relapsed after allogeneic HCT. Two relapsed patients underwent a second allogeneic HCT from different donors, but both died, one of secondary engraftment failure and the other of GVHD after donor lymphocyte infusion for mixed chimerism. At the time of writing this manuscript, 5 of the 10 patients remain alive without disease relapse. The 5-year OS, RFS, and EFS rates were 42.2%, 51.9%, and 46.7%, respectively. Survival was not correlated with pretransplant HCT-CI score or MDAPS. Four of 5 patients without chronic GVHD relapsed, compared with 0 of 4 with chronic GVHD.
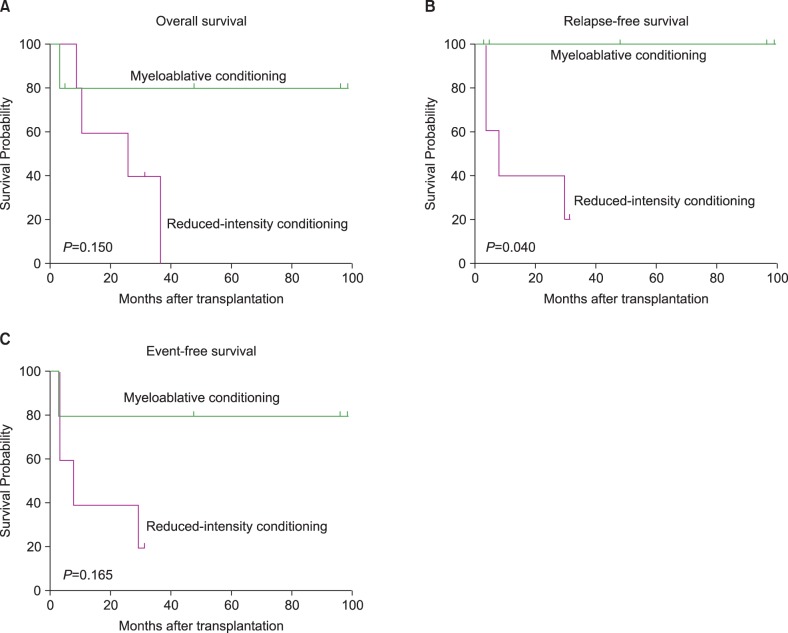
Post-transplant outcomes were compared between patients who received MAC and those who received RIC conditioning regimens. Of the 5 patients who received MAC regimens, 4 were alive without relapse and 1 had died of a non-relapse related cause. In contrast, 4 of 5 patients who received RIC regimens relapsed and died, with only 1 remaining alive without relapse. Fig. 1 shows that patients who received MAC regimens had superior OS (P =0.150), RFS (P =0.040), and EFS (P =0.165) compared with patients who received RIC regimens.
Fig. 1.
Survival differences between myeloablative conditioning and reduced-intensity conditioning; (A) overall survival, (B) relapse-free survival, (C) event-free survival.
DISCUSSION
Although allogeneic HCT has curative potential for patients with MDS/MPN, its role has not been established, primarily owing to limited data. Retrospective studies of allogeneic HCT for CMML in 12 to 50 patients have shown survival rates of 18% to 75% and day 100 treatment-related mortality (TRM) rates of 25% to 41% (Table 4) [12, 13, 16]. According to the available literature, allogeneic HCT has been performed in a total of 17 patients with aCML, with 11 remaining alive at the time of the report [14, 15, 23]. Our findings (in 7 patients with CMML, 2 with aCML, and one with MDS/MPN-U) were similar. There were 4 relapses and 1 treatment-related death. The 5-year rates of OS, RFS, and EFS were 42.2%, 51.9%, and 46.7%, respectively. At a median follow-up of 47.5 months, 4 of 7 patients with CMML, 0 of 2 with aCML, and 1 of 1 with MDS/MPN-U had died. The discrepancies in survival rates after allogeneic HCT in the literature suggest the need for guidelines related to indications and conditioning strategies. When 43 CMML patients were classified according to MDAPS and HCT-CI scores, those at higher risk, as determined by MDAPS, tended to have a higher relapse rate than those at lower risk, and those with higher comorbidity scores had reduced OS than those with lower scores [13]. The results suggest that early disease stage and low comorbidity score may be optimal indications for allogeneic HCT in patients with MDS/MPN. Our previous study of patients with MDS showed that pre-transplant comorbidity and prognostic scores were important for post-transplant outcomes [24]. Owing to the small number of patients in this study, however, we could not correlate MDAPS or HCT-CI scores with post-transplant outcomes in patients with MDS/MPN.
Table 4.
Published data for post-transplant outcomes in MDS/MPN (except juvenile myelomonocytic leukemia).
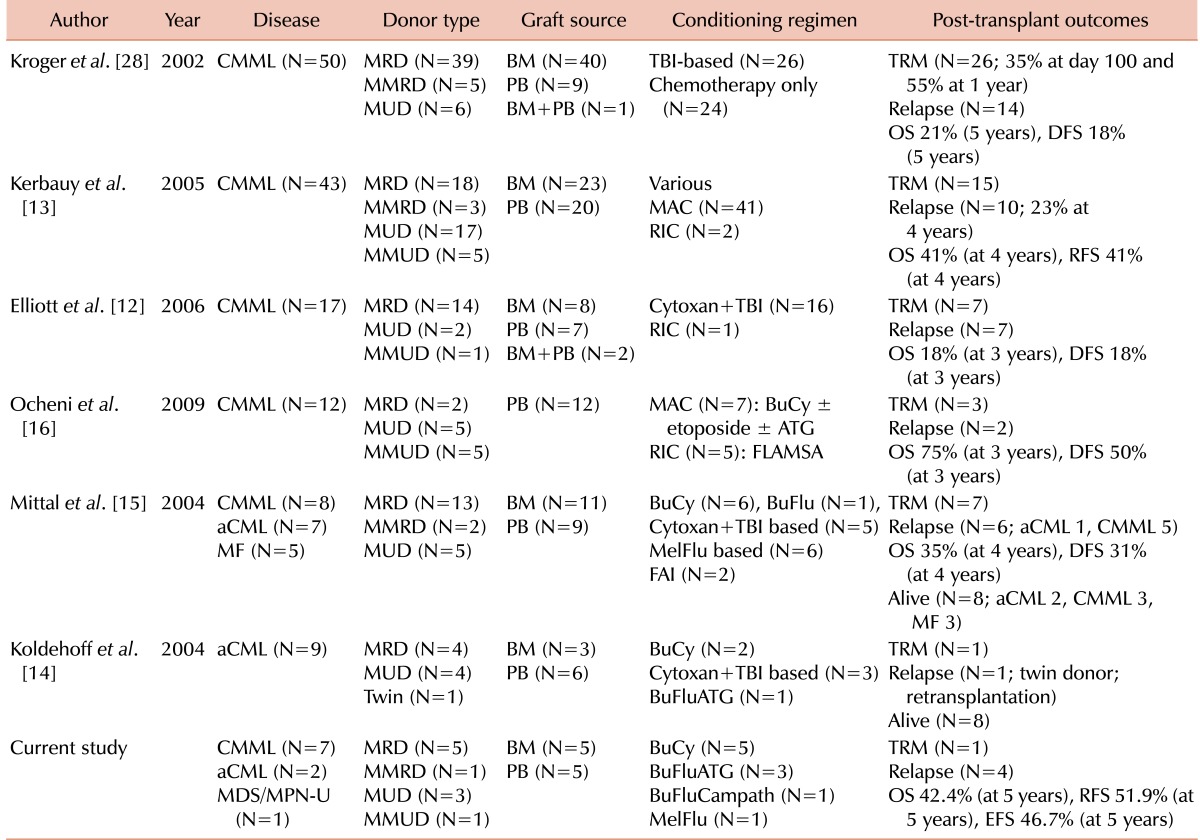
Abbreviations: MDS/MPN, myelodysplastic syndrome/myeloproliferative neoplasm; MDS/MPN-U, MDS/MPN-unclassified; CMML, chronic myelomonocytic leukemia; aCML, atypical chronic myeloid leukemia; MF, myelofibrosis; MRD, matched related donor; MMRD, mismatched related donor; MUD, matched unrelated donor; MMUD, mismatched unrelated donor; BM, bone marrow; PB, peripheral blood; TBI, total body irradiation; MAC, myeloablative conditioning; RIC, reduced-intensity conditioning; BuCy, busulfan+cytoxan; ATG, antithymocyte globulin; FLAMSA, fludarabine+amsacrine+cytarabine; BuFlu, busulfan+fludarabine; MelFlu, melphalan+fludarabine; FAI, fludarabine+cytarabine+idarubicin; BuFluATG, busulfan+fludarabine+ATG; BuFluCampath, busulfan+fludarabine+alemtuzumab; TRM, treatment-related mortality; OS, overall survival; RFS, relapse-free survival; DFS, disease-free survival; EFS, event-free survival.
One important issue related to allogeneic HCT for MDS/MPN is the choice of optimal conditioning regimen. Various intensities and combinations of chemotherapeutic agents or irradiation have been used [12-16], making it difficult to determine optimal conditioning regimens prior to allogeneic HCT for MDS/MPN. Several retrospective studies in patients with MDS showed that RIC regimens resulted in lower TRM than standard MAC regimens, but this benefit was offset by higher relapse rates [25, 26]. Of our 10 patients, 5 had received MAC and 5 had received RIC. Relapses were observed only in patients who had received RIC, and survival parameters were inferior for RIC compared with MAC. Our results suggest that RIC cannot replace MAC in patients who are eligible for myeloablative treatments.
Many patients with MDS/MPNs are elderly and frequently have comorbidities, making them ineligible for MAC regimens. RIC may enable allogeneic HCT in these patients. A study of 148 patients ineligible for conventional HCT, including 65 with MDS, 49 with acute myeloid leukemia after MDS/MPN, 27 with MPN, and 7 with CMML, assessed outcomes of allogeneic HCT following RIC (low-dose total body irradiation with or without fludarabine) [27]. In that study, the 3-year TRM for patients with CMML was 32%, and the 3-year OS and RFS rates were 43% each. Relapse was the leading cause of treatment failure. Strategies are needed to reduce relapse after HCT in patients who receive RIC. One possible strategy may be to augment alloimmunity after HCT. In a study assessing the post-transplant outcomes of 50 patients with CMML, relapse probability was found to be lower in patients with acute GVHD and higher in those with T cell-depleted grafts [28]. In addition, of 5 CMML patients who received donor lymphocyte infusions (DLIs) for relapse or mixed chimerism, 2 achieved durable remission [12]. We found an association between chronic GVHD and lower relapse rate, suggesting that graft-versus-leukemia effects, which have been well documented in various diseases [29, 30], may also occur in patients with MDS/MPN. Prophylactic DLI or early withdrawal of immunosuppressive agents following RIC HCT seems to be worth evaluating in future studies.
In summary, allogeneic HCT following MAC conditioning can induce durable remission in patients with MDS/MPN. Relapse was the leading cause of treatment failure; relapse was observed only in patients who had received RIC and who had not developed chronic GVHD. Our results suggest that RIC cannot replace MAC in patients eligible for myeloablative treatments. Future studies aiming to reduce relapse rates after RIC HCT are warranted for elderly patients and those with comorbidities, who account for most patients with MDS/MPN.
Footnotes
No potential conflicts of interest relevant to this article were reported.
References
- 1.Malcovati L, Cazzola M. Myelodysplastic/myeloproliferative disorders. Haematologica. 2008;93:4–6. doi: 10.3324/haematol.11374. [DOI] [PubMed] [Google Scholar]
- 2.Orazi A, Germing U. The myelodysplastic/myeloproliferative neoplasms: myeloproliferative diseases with dysplastic features. Leukemia. 2008;22:1308–1319. doi: 10.1038/leu.2008.119. [DOI] [PubMed] [Google Scholar]
- 3.Vardiman JW, Harris NL, Brunning RD. The World Health Organization (WHO) classification of the myeloid neoplasms. Blood. 2002;100:2292–2302. doi: 10.1182/blood-2002-04-1199. [DOI] [PubMed] [Google Scholar]
- 4.Vardiman JW, Thiele J, Arber DA, et al. The 2008 revision of the World Health Organization (WHO) classification of myeloid neoplasms and acute leukemia: rationale and important changes. Blood. 2009;114:937–951. doi: 10.1182/blood-2009-03-209262. [DOI] [PubMed] [Google Scholar]
- 5.Germing U, Kundgen A, Gattermann N. Risk assessment in chronic myelomonocytic leukemia (CMML) Leuk Lymphoma. 2004;45:1311–1318. doi: 10.1080/1042819042000207271. [DOI] [PubMed] [Google Scholar]
- 6.Onida F, Kantarjian HM, Smith TL, et al. Prognostic factors and scoring systems in chronic myelomonocytic leukemia: a retrospective analysis of 213 patients. Blood. 2002;99:840–849. doi: 10.1182/blood.v99.3.840. [DOI] [PubMed] [Google Scholar]
- 7.Beran M, Kantarjian H, O'Brien S, et al. Topotecan, a topoisomerase I inhibitor, is active in the treatment of myelodysplastic syndrome and chronic myelomonocytic leukemia. Blood. 1996;88:2473–2479. [PubMed] [Google Scholar]
- 8.Oki Y, Jelinek J, Shen L, Kantarjian HM, Issa JP. Induction of hypomethylation and molecular response after decitabine therapy in patients with chronic myelomonocytic leukemia. Blood. 2008;111:2382–2384. doi: 10.1182/blood-2007-07-103960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Breccia M, Biondo F, Latagliata R, Carmosino I, Mandelli F, Alimena G. Identification of risk factors in atypical chronic myeloid leukemia. Haematologica. 2006;91:1566–1568. [PubMed] [Google Scholar]
- 10.Kurzrock R, Kantarjian HM, Shtalrid M, Gutterman JU, Talpaz M. Philadelphia chromosome-negative chronic myelogenous leukemia without breakpoint cluster region rearrangement: a chronic myeloid leukemia with a distinct clinical course. Blood. 1990;75:445–452. [PubMed] [Google Scholar]
- 11.Onida F, Ball G, Kantarjian HM, et al. Characteristics and outcome of patients with Philadelphia chromosome negative, bcr/abl negative chronic myelogenous leukemia. Cancer. 2002;95:1673–1684. doi: 10.1002/cncr.10832. [DOI] [PubMed] [Google Scholar]
- 12.Elliott MA, Tefferi A, Hogan WJ, et al. Allogeneic stem cell transplantation and donor lymphocyte infusions for chronic myelomonocytic leukemia. Bone Marrow Transplant. 2006;37:1003–1008. doi: 10.1038/sj.bmt.1705369. [DOI] [PubMed] [Google Scholar]
- 13.Kerbauy DM, Chyou F, Gooley T, et al. Allogeneic hematopoietic cell transplantation for chronic myelomonocytic leukemia. Biol Blood Marrow Transplant. 2005;11:713–720. doi: 10.1016/j.bbmt.2005.05.008. [DOI] [PubMed] [Google Scholar]
- 14.Koldehoff M, Beelen DW, Trenschel R, et al. Outcome of hematopoietic stem cell transplantation in patients with atypical chronic myeloid leukemia. Bone Marrow Transplant. 2004;34:1047–1050. doi: 10.1038/sj.bmt.1704686. [DOI] [PubMed] [Google Scholar]
- 15.Mittal P, Saliba RM, Giralt SA, et al. Allogeneic transplantation: a therapeutic option for myelofibrosis, chronic myelomonocytic leukemia and Philadelphia-negative/BCR-ABL-negative chronic myelogenous leukemia. Bone Marrow Transplant. 2004;33:1005–1009. doi: 10.1038/sj.bmt.1704472. [DOI] [PubMed] [Google Scholar]
- 16.Ocheni S, Kroger N, Zabelina T, Zander AR, Bacher U. Outcome of allo-SCT for chronic myelomonocytic leukemia. Bone Marrow Transplant. 2009;43:659–661. doi: 10.1038/bmt.2008.366. [DOI] [PubMed] [Google Scholar]
- 17.Sorror ML, Maris MB, Storb R, et al. Hematopoietic cell transplantation (HCT)-specific comorbidity index: a new tool for risk assessment before allogeneic HCT. Blood. 2005;106:2912–2919. doi: 10.1182/blood-2005-05-2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Antin JH, Childs R, Filipovich AH, et al. Establishment of complete and mixed donor chimerism after allogeneic lymphohematopoietic transplantation: recommendations from a workshop at the 2001 Tandem Meetings of the International Bone Marrow Transplant Registry and the American Society of Blood and Marrow Transplantation. Biol Blood Marrow Transplant. 2001;7:473–485. doi: 10.1053/bbmt.2001.v7.pm11669214. [DOI] [PubMed] [Google Scholar]
- 19.Przepiorka D, Weisdorf D, Martin P, et al. 1994 Consensus Conference on Acute GVHD Grading. Bone Marrow Transplant. 1995;15:825–828. [PubMed] [Google Scholar]
- 20.Przepiorka D, Anderlini P, Saliba R, et al. Chronic graft-versus-host disease after allogeneic blood stem cell transplantation. Blood. 2001;98:1695–1700. doi: 10.1182/blood.v98.6.1695. [DOI] [PubMed] [Google Scholar]
- 21.McDonald GB, Hinds MS, Fisher LD, et al. Veno-occlusive disease of the liver and multiorgan failure after bone marrow transplantation: a cohort study of 355 patients. Ann Intern Med. 1993;118:255–267. doi: 10.7326/0003-4819-118-4-199302150-00003. [DOI] [PubMed] [Google Scholar]
- 22.Batsis I, Yannaki E, Kaloyannidis P, et al. Veno-occlusive disease prophylaxis with fresh frozen plasma and heparin in bone marrow transplantation. Thromb Res. 2006;118:611–618. doi: 10.1016/j.thromres.2005.10.010. [DOI] [PubMed] [Google Scholar]
- 23.Kapaun P, Kabisch H, Held KR, Walter TA, Hegewisch S, Zander AR. Atypical chronic myelogenous leukemia in a patient with trisomy 8 mosaicism syndrome. Ann Hematol. 1993;66:57–58. doi: 10.1007/BF01737691. [DOI] [PubMed] [Google Scholar]
- 24.Lee JH, Lee JH, Lim SN, et al. Allogeneic hematopoietic cell transplantation for myelodysplastic syndrome: prognostic significance of pre-transplant IPSS score and comorbidity. Bone Marrow Transplant. 2010;45:450–457. doi: 10.1038/bmt.2009.190. [DOI] [PubMed] [Google Scholar]
- 25.Alyea EP, Kim HT, Ho V, et al. Impact of conditioning regimen intensity on outcome of allogeneic hematopoietic cell transplantation for advanced acute myelogenous leukemia and myelodysplastic syndrome. Biol Blood Marrow Transplant. 2006;12:1047–1055. doi: 10.1016/j.bbmt.2006.06.003. [DOI] [PubMed] [Google Scholar]
- 26.Martino R, Iacobelli S, Brand R, et al. Retrospective comparison of reduced-intensity conditioning and conventional high-dose conditioning for allogeneic hematopoietic stem cell transplantation using HLA-identical sibling donors in myelodysplastic syndromes. Blood. 2006;108:836–846. doi: 10.1182/blood-2005-11-4503. [DOI] [PubMed] [Google Scholar]
- 27.Laport GG, Sandmaier BM, Storer BE, et al. Reduced-intensity conditioning followed by allogeneic hematopoietic cell transplantation for adult patients with myelodysplastic syndrome and myeloproliferative disorders. Biol Blood Marrow Transplant. 2008;14:246–255. doi: 10.1016/j.bbmt.2007.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kroger N, Zabelina T, Guardiola P, et al. Allogeneic stem cell transplantation of adult chronic myelomonocytic leukaemia. A report on behalf of the Chronic Leukaemia Working Party of the European Group for Blood and Marrow Transplantation (EBMT) Br J Haematol. 2002;118:67–73. doi: 10.1046/j.1365-2141.2002.03552.x. [DOI] [PubMed] [Google Scholar]
- 29.Lee JH, Choi SJ, Lee JH, et al. Anti-leukemic effect of graft-versus-host disease on bone marrow and extramedullary relapses in acute leukemia. Haematologica. 2005;90:1380–1388. [PubMed] [Google Scholar]
- 30.Weiden PL, Flournoy N, Thomas ED, et al. Antileukemic effect of graft-versus-host disease in human recipients of allogeneic-marrow grafts. N Engl J Med. 1979;300:1068–1073. doi: 10.1056/NEJM197905103001902. [DOI] [PubMed] [Google Scholar]