To the Editor:
Recent studies have shown that most Dutch families with atypical multiple-mole melanoma (FAMMM) have a 19-bp germline deletion (p16-Leiden) in exon 2 of the p16 gene (p16 [MIM 600160]) (Gruis et al. 1995; van der Velden et al. 1999, 2001). Incomplete penetrance and variable clinical expression in p16-Leiden carriers point to the fact that other genetic mechanisms can compensate for the p16 loss of function (Gruis et al. 1995). Indeed, van der Velden et al. (2001) reported in the Journal that variants in the melanocortin-1 receptor modify the risk of melanoma in p16-Leiden carriers. It is interesting that, apart from reports on simultaneous pancreatic tumors, other cancer types have never been found in such families with p16-Leiden (Gruis et al. 1995).
Recently, we found a hereditary heterozygous p16-Leiden mutation in a man who neither smoked more than five cigarettes daily nor abused alcohol, initially diagnosed at age 54 years, who simultaneously developed three carcinomas of the pharynx and oral cavity. The patient showed a familial accumulation of tumor diseases. Both of his parents and his only sister died of cancer very early (the mother of gynecologic cancer, the father of liver carcinoma, and the sister of leukemia). Dutch relatives are not known.
DNA from tumors and blood was extracted according to a standard protocol (Sambrook et al. 1989). Mutation analysis of exon 1 and exon 2 of the p16 gene was done by PCR-SSCP sequencing as described by Schneider-Stock et al. (1998).
The p16-Leiden mutation was found in the heterozygous constitution in all three tumors and in the blood of the patient. We investigated all DNA samples for p16 promotor methylation to check the status of the retained wild-type allele and, thus, to assess the real functional significance of this finding. The methylation status of the p16 promotor was determined by methylation-specific PCR (Herman et al. 1996). The primer sequences for the unmethylated reactions were (sense) 5′-TTA TTA GAG GGT GGG GTG GAT TGT-3′ and (antisense) 5′-CAA CCC CAA ACC ACA ACC ATA A-3′, which amplify a 151-bp product. The primer sequences for the methylated reaction were (sense) 5′-TTA TTA GAG GGT GCG GAT CGC-3′ and (antisense) 5′-GAC CCC GAA CCG CGA CCG TAA-3′, which amplify a 150-bp product. The two forward primers were labeled with FAM dye. PCR was done using 1/10 of bisulfite reaction and a MasterAmp Optimization Kit (Biozym) in an automated thermocycler (PTC 200) according to the manufacturer's instructions. PCR products were analyzed using an ABI310 sequencer (injection time between 20–30 s, Pop 4, dRhodamine Matrix standard ABI Prism [Perkin Elmer]). The p16 promotor was methylated in all three tumor samples but not in the blood of the patient (fig. 1).
Figure 1.
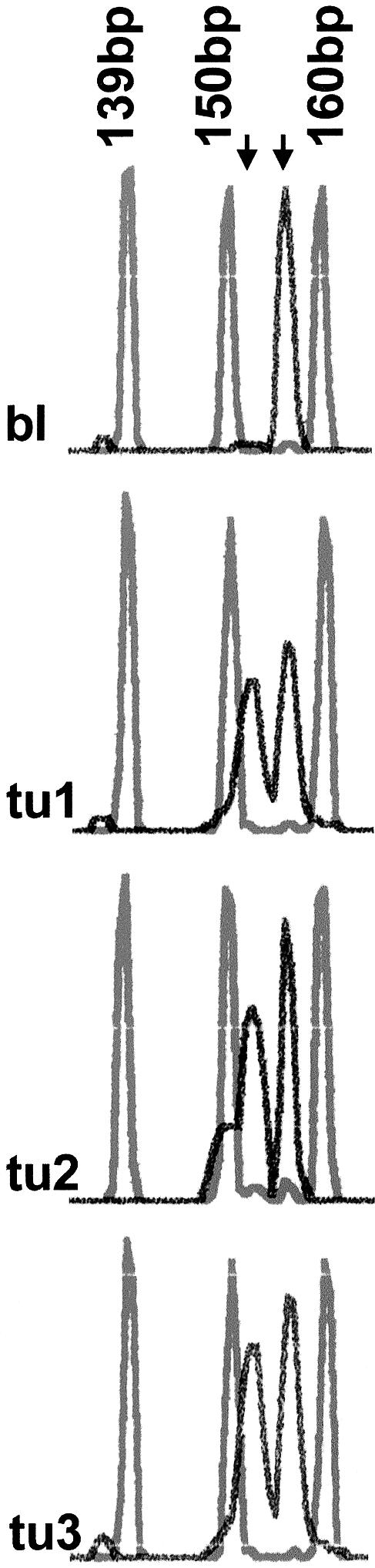
Analysis of p16INK4 promotor methylation on ABI310 Prism. Methylated (left arrow) and unmethylated (right arrow) signals can be detected between the 150-bp and the 160-bp ROX matrix standards. There is a near-equal amplification of unmethylated and methylated DNA in the three head and neck tumors but no methylation in the blood DNA of the patient; bl = blood DNA; tu1–tu3 = tumors 1–3, respectively.
This result led us to suggest a loss of p16 protein expression and, thus, a complete loss of p16 tumor suppressor function. For p16 immunohistochemistry, a monoclonal mouse antibody to p16 (1:100 dilution, Quartett) was used according to the manufacturer's instructions. Indeed, all tumor sections were immunohistochemically p16-negative. The third tumor showed an additional gross rearrangement in the p53 gene (75-bp insertion in exon 4).
This is the first report on p16-Leiden mutation in orolaryngeal carcinomas, although p16 alterations are very common in this tumor type (50% p16 promotor methylation [Bittencourt Rosas et al. 2001], 30% p16 mutations [Esteller et al. 2001a; Poi et al. 2001]). Because of the heterozygous p16-Leiden constitution, our proband was a suitable model for studying the type of p16 inactivation in the three metachronous carcinomas. To date, the methylation status of p16-Leiden carriers has never been checked. To the best of our knowledge, this is one of the only a few clear examples that aberrant promotor methylation might function as the “second genetic hit” in a familial cancer syndrome. Whereas no methylation could be recognized in the blood of the patient's DNA, all three tumors showed inactivation of the retained wild-type allele, with the somatic event being aberrant promotor methylation. There are only a few reports demonstrating the role of promotor methylation for biallelic gene inactivation (Grady et al. 2000; Esteller et al. 2001b). Furthermore, it has to be mentioned that the p16-Leiden mutation should also affect the p14ARF transcript, because alternative splicing of the first exon and common downstream exons permit this locus to encode two different products that regulate the cell cycle via two distinct pathways.
It is noteworthy that contact with the patient began five months before the actual date of examination when the patient attended a talk. His presence was not because of symptoms of a tumor but because of genetic findings. This early meeting led to discovery of the tumor of the tongue while the tumor was still in a less-advanced stage. Therefore, the tumor could be easily resected, leaving the tongue almost unaffected. Consequently, the patient is now in good physical condition (Karnofsky 70%–80%). The patient was informed about the hereditary mutation and was included in the risk program for upper-abdominal screening because p16-Leiden mutation has been reported to be a risk factor for developing pancreatic carcinoma (Lal et al. 2000; Vasen et al. 2000; Lynch et al. 2002).
We think that regarding head and neck cancer, our data is novel and may have consequences for further studies of families with FAMMM.
Acknowledgments
We thank the patient who consented to the study and gave his blood sample. A.G. is a Ph.D. student supported by the Graduierten-Kollege of the Medical Faculty of the Otto-von-Guericke University, Magdeburg.
Electronic-Database Information
The accession number and URL for data presented herein are as follows:
- Online Mendelian Inheritance in Man (OMIM), http://www.ncbi.nlm.nih.gov/Omim/ (for p16 and p14 [MIM 600160])
References
- Bittencourt Rosas SL, Koch W, da Costa Carvalho MG, Wu L, Califano J, Westra W, Jen J, Sidransky D (2001) Promotor hypermethylation pattern of p16, O6-methylguanine-DNA-methyltransferase, and death-associated protein kinase in tumors and saliva of head and neck cancer patients. Cancer Res 61:939–942 [PubMed] [Google Scholar]
- Esteller M, Corn PG, Maylin SB, Herman JG (2001a) A gene hypermethylation profile of human cancer. Cancer Res 61:3225–3229 [PubMed] [Google Scholar]
- Esteller M, Fraga M, Guo M, Gacia-Foncillas J, Hedenfalk I, Godwin AK, Trojan J, Vaurs-Barriere C, Bignon YJ, Ramus S, Benitez J, Caldes T, Akiyama Y, Yuasa Y, Launonen V, Canal MJ, Rodriguez R, Capella G, Peinado MA, Borg A, Aaltonen LA, Ponder BA, Baylin SB, Herman JG (2001b) DNA methylation patterns in hereditary human cancers mimic sporadic tumorigenesis. Hum Mol Genet 10:3001–3007 [DOI] [PubMed] [Google Scholar]
- Grady WM, Willis J, Guilford PJ, Dunbier AK, Toro TT, Lynch H, Wiesner G, Ferguson K, Eng C, Park JG, Kim SJ, Markowitz S (2000) Methylation of the CDH1 promotor as the second genetic hit in hereditary diffuse gastric cancer. Nat Genet 26:16–17 [DOI] [PubMed] [Google Scholar]
- Gruis NA, van der Velden P, Sandkuijl LA, Prins DE, Weaver-Feldhaus J, Kamb A, Bergman W, Frants RR (1995) Homozygotes for CDKN2 (p16) germline mutation in Dutch familial melanoma kindreds. Nat Genet 10:351–353 [DOI] [PubMed] [Google Scholar]
- Herman JG, Graff JR, Myohänen S, Nelkin BD, Maylin SB (1996) Methylation-specific PCR: a novel PCR assay for methylation status of CpG islands. Proc Natl Acad Sci USA 93:9821–9826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lal G, Liu L, Hogg D, Lassam NJ, Redston MS, Gallinger S (2000) Patients with both pancreatic adenocarcinoma and melanoma may harbor germline CDKN2A mutations. Genes Chrom Cancer 27:358–361 [PubMed] [Google Scholar]
- Lynch HT, Brand RE, Hogg D, Deters CA, Fusaro RM, Lynch JF, Li L, Knezetic J, Lassam NJ, Goggins M, Kern S (2002) Phenotypic variation in eight extended CDKN2A germline mutation familial atypical multiple mole melanoma-pancreatic carcinoma-prone families: the familial atypical mole melanoma-pancreatic carcinoma syndrome. Cancer 94:84–96 [DOI] [PubMed] [Google Scholar]
- Poi MJ, Yen T, Li J, Song H, Lang JC, Schuller DE, Pearl DK, Casto B, Tsai MD, Weghorst CM (2001) Somatic INK4a-ARF locus mutations: a significant mechanism of gene inactivation in squamous cell carcinomas of the head and neck. Mol Carcinog 30:26–36 [DOI] [PubMed] [Google Scholar]
- Sambrook J, Fritsch EF, Maniatis T (2nd ed) (1989) Molecular cloning—a laboratory manual. Cold Spring Harbor Laboratory, Cold Spring Harbor, NY [Google Scholar]
- Schneider-Stock R, Walter H, Haeckel C, Radig K, Rys J, Roessner A (1998) Gene alterations at the CDKN2A (p16/MTS1) locus in soft tissue tumors. Int J Oncol 13:325–329 [DOI] [PubMed] [Google Scholar]
- van der Velden PA, Sandkuijl LA, Bergman W, Hille ETM, Frants RR, Gruis NA (1999) A locus linked to p16 modifies melanoma risk in Dutch familial atypical multiple mole melanoma (FAMMM) syndrome families. Genome Res 9:575–580 [PMC free article] [PubMed] [Google Scholar]
- van der Velden PA, Sandkuijl LA, Bergman W, Pavel S, van Mourik L, Frants RR, Gruis NA (2001) Melanocortin-1 receptor variant R151C modifies melanoma risk in Dutch families with melanoma. Am J Hum Genet 69:774–779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasen HFA, Gruis NA, Frants RR, van der Velden PA, Hille ETM, Bergman W (2000) Risk of developing pancreatic cancer in families with familial atypical multiple mole melanoma associated with a specific 19 deletion of p16 (p16-Leiden). Int J Cancer 87:809–811 [PubMed] [Google Scholar]


