Abstract
Object
Recent studies indicate that M13 bacteriophage, a very large nanoparticle, binds to β-amyloid and α-synuclein proteins, leading to plaque disaggregation in models of Alzheimer and Parkinson disease. To determine the feasibility, safety, and characteristics of convection-enhanced delivery (CED) of M13 bacteriophage to the brain, the authors perfused primate brains with bacteriophage.
Methods
Four nonhuman primates underwent CED of M13 bacteriophage (900 nm) to thalamic gray matter (4 infusions) and frontal white matter (3 infusions). Bacteriophage was coinfused with Gd-DTPA (1 mM), and serial MRI studies were performed during infusion. Animals were monitored for neurological deficits and were killed 3 days after infusion. Tissues were analyzed for bacteriophage distribution.
Results
Real-time T1-weighted MRI studies of coinfused Gd-DTPA during infusion demonstrated a discrete region of perfusion in both thalamic gray and frontal white matter. An MRI-volumetric analysis revealed that the mean volume of distribution (Vd) to volume of infusion (Vi) ratio of M13 bacteriophage was 2.3 ± 0.2 in gray matter and 1.9 ± 0.3 in white matter. The mean values are expressed ± SD. Immunohistochemical analysis demonstrated mean Vd:Vi ratios of 2.9 ± 0.2 in gray matter and 2.1 ± 0.3 in white matter. The Gd-DTPA accurately tracked M13 bacteriophage distribution (the mean difference between imaging and actual bacteriophage Vd was insignificant [p > 0.05], and was −2.2% ± 9.9% in thalamic gray matter and 9.1% ± 9.5% in frontal white matter). Immunohistochemical analysis revealed evidence of additional spread from the initial delivery site in white matter (mean Vd:Vi, 16.1 ± 9.1). All animals remained neurologically intact after infusion during the observation period, and histological studies revealed no evidence of toxicity.
Conclusions
The CED method can be used successfully and safely to distribute M13 bacteriophage in the brain. Furthermore, additional white matter spread after infusion cessation enhances distribution of this large nanoparticle. Real-time MRI studies of coinfused Gd-DTPA (1 mM) can be used for accurate tracking of distribution during infusion of M13 bacteriophage.
Keywords: bacteriophage, brain, convection-enhanced delivery, white matter, gray matter, oncology, Macaca mulatta
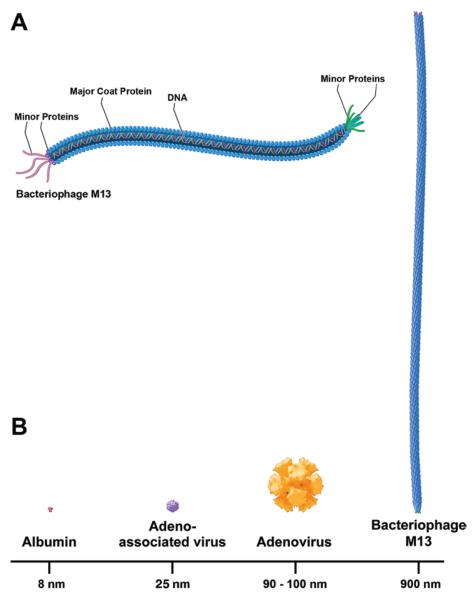
The M13 bacteriophage is a filamentous nanometer-sized biological particle (900 nm in length and 6-to 10-nm wide; molecular weight 12 MDa).21 It is composed of a coat with approximately 3000 copies of major protein and several copies of minor protein. The coat surrounds a single-stranded, closed circular DNA (Fig. 1A). Recent data indicate that the M13 bacteriophage may have therapeutic potential for the treatment of AD1,11 and PD8,9,13 that is related to its ability to facilitate plaque disaggregation via binding to β-amyloid and α-synuclein plaques. Due to its exceptionally large size, a major limitation to the therapeutic use of M13 bacteriophage in the nervous system is achieving adequate brain distribution across the blood-brain barrier.
Fig. 1.
A: Schematic illustration showing the shape and exterior coat proteins of M13 bacteriophage. The M13 bacteriophage's coat is composed of approximately 3000 copies of major and several copies of minor proteins. This coat surrounds a single-stranded, closed circular DNA. B: Size comparison of M13 bacteriophage (900 nm) relative to albumin (8 nm), AAV (25 nm), and adenovirus (90 to 100 nm).
Previous studies of CED have described the distribution of small molecules, proteins, and viral-sized particles.5,18,26,27 Recently, studies have shown adequate distribution of nanoparticles, including AAV (25 nm)5 and adenovirus (85 nm).5 Convective distribution characteristics associated with significantly larger nanoparticles have not been established. We characterized distribution properties in thalamic gray matter and frontal white matter, while monitoring infusate distribution by using coinfusion of Gd-DTPA and MRI.
Methods
Experimental Animals
Four adult nonhuman primates (Macaca mulatta) underwent bilateral thalamic gray matter or frontal white matter infusions. All procedures were performed in accordance with the National Research Council's Guide for the Care and Use of Laboratory Animals, and were approved by the Animal Care and Use Committee of the National Institute of Neurological Disorders and Stroke.
Preparation of M13 Bacteriophage and Gd-DTPA Infusate
The M13 bacteriophage (NeuroPhage Pharmaceuticals, Inc.) was diluted with PBS to a final concentration of 1013 viral particles/ml. Clinical-grade Gd-DTPA (Bayer Pharmaceuticals) was also diluted with bacteriophage in fusate to a final concentration of 1 mM. The dilutions were prepared 1 hour before infusion.
Surgical Placement of Infusion Pedestal
We used MRI-compatible infusion pedestals as previously described by Rosenbluth et al.22 Approximately 1–14 days before infusion, general endotracheal anesthesia (0.5%–3% isoflurane) was induced in each primate, and each was intubated for the stereotactic placement of the infusion pedestal. Using sterile surgical technique, a midline incision was made, exposing the underlying muscle and calvaria. A 12.5-mm bur hole was made directly over the target site, exposing the underlying dura mater. The infusion pedestal was placed at an appropriate angle (as determined by preoperative MRI) and secured using nylon screws and fixative cement. Covering caps were placed over the pedestals.
Convective Coinfusion of M13 Bacteriophage and Gd-DTPA
Coinfusion of M13 bacteriophage and Gd-DTPA was performed as previously described.14 Briefly, bilateral thalamic gray matter (4 infusions) or frontal white matter (3 infusions) was coinfused (Vi 65–70 μl and 25–30 μl M13 bacteriophage and Gd-DTPA, respectively) (Table 1). Infusion times ranged from approximately 70 minutes in white matter to 155 minutes in gray matter. During infusion, animals were anesthetized and placed into a stereotactic frame (Crist Instrument Co., Inc.). A fused silica step cannula,22 which was made by inserting a 28-gauge (0.3-mm inner diameter) into a 22-gauge (0.6-mm outer diameter) infusion cannula, was stereotactically placed through the previously implanted pedestal into the target structure. Infusions were initially started at 0.1 μl/minute and then increased to 0.5 μl/minute for the duration of the infusion until the desired infusion volume was attained. After recovery from anesthesia, the animals were monitored for neurological or behavioral changes resulting from the infusions into the targeted areas. Three days after infusion the animals were killed. The brain was perfused and removed for sectioning and histological studies.
TABLE 1.
Distribution of M13 bacteriophage by CED in 4 nonhuman primates*
| Animal No. | Site of Infusion | Vi (ml) | Vd on Imaging (ml) | Vd on Histology (ml) | Difference (%) |
|---|---|---|---|---|---|
| 1 | gray matter, rt | NA | NA | NA | NA |
| gray matter, lt | 66.6 | 185 | 212 | −14 | |
| 2 | gray matter, rt | 66.8 | 193 | 187 | 3 |
| gray matter, lt | 66.5 | 190 | 183 | 4 | |
| 3 | white matter, rt (infusion) | 26.5 | 62 | 63 | −1 |
| white matter, rt (infusion & tracking) | 26.5 | NA | 596 | NA | |
| white matter, lt | 26.1 | 56 | 48 | 17 | |
| 4 | white matter, rt | 30 | 74 | 66 | 12 |
| white matter, rt (infusion & tracking) | 30 | NA | 286 | NA |
NA = not analyzable.
Tracking of Infusion Using MRI
Immediately before infusion, MRI coordinates (T1-weighted, coronal images) were obtained to confirm accurate cannula placement at the target site of interest (thalamic gray matter or frontal white matter). During the infusions, coronal T1-weighted MRI studies (slice thickness 1 mm without spacing) were obtained on a 3-T machine constantly at 8-minute intervals until infusion completion.
Analysis of MRI Findings for Gd-DTPA Distribution
As previously described,14 the Vd of Gd-DTPA on the MRI studies was analyzed using a Sun Microsystems, Inc. workstation running MEDx 3.4 software (Sensor Systems, Inc.). A 10% threshold segmentation analysis was performed to calculate the Vd of imaged Gd-DTPA. This Vd was compared with the Vi to obtain the Vd:Vi ratio.
Analysis of M13 Bacteriophage Distribution and Perfused Tissue
Three days after infusion, primates were killed with administration of intravenous sodium pentobarbital (90 mg/kg). Transcardiac perfusion was performed with 0.5 L of 10% heparinized saline and 1 L of 4% paraformaldehyde. Brains were removed after perfusion and fixed in 4% paraformaldehyde. Brains were then washed with PBS, placed in a 30% sucrose solution in 0.01 M PBS for cryopreservation, and then sectioned through the infusion site at 40 μm on a freezing sliding microtome.
Immunostaining for M13 bacteriophage was performed on every sixth serial section (40-μm thickness). Each section was washed 3 times in PBS and blocked in 1% hydrogen peroxide. Sections were washed again in PBS and blocked using background sniper (Biocare Medical). The sections were incubated with primary monoclonal mouse anti-M13 antibody (GE Healthcare, Inc.) overnight. After washing, the sections were incubated with secondary MACH 2 mouse HRP-polymer antibody (Biocare Medical) for 1 hour. The reaction product was visualized using diaminobenzidine and hydrogen peroxide, and tissue was mounted on glass slides, dehydrated, and coverslipped with mounting media.
Fixed and coverslipped slides were digitized on low-power microscopy using AxioVision software (version 4.8; Carl Zeiss, Inc.), and the distribution of M13 bacteriophage was determined using ImageJ software (NIH ImageJ, version 1.44). The Cavalieri method was used to calculate the Vd from serial sections immunostained for M13 bacteriophage.
We performed H & E and Nissl staining on adjacent representative sections extending from the cannula and infusion sites for a qualitative assessment of changes in tissue architecture and toxicity.
Statistical Analysis
Excel (Microsoft Corp.) and Prism (Graphpad Software) software programs were used for statistical analysis and graphical representation of the results. A Student t-test was performed, with a p value set at 0.05.
Results
Imaging of Coinfused M13 Bacteriophage and Gd-DTPA
The T1-weighted MRI studies revealed a clear distinction between the perfused region and surrounding tissue, which was defined by a hyperintensity that increased in Vd with increasing Vi (Figs. 2 right and 3A). Similar patterns of distribution were obtained in both gray matter (Fig. 2 right) and white matter (Fig. 3A). Initially, the infusate formed an anulus around the tip of the infusion cannula. A greater Vi was associated with an increase in the infusate's radial distribution appearing in a semicircular pattern. The MRI-volumetric analysis of serial imaged Gd-DTPA distribution revealed a linear relationship between increasing Vd and Vi in gray matter (R2 = 0.98) and white matter (R2 = 0.98) (Fig. 4). The mean Vd:Vi ratio of Gd-DTPA was 2.3 ± 0.2 in gray matter and 1.9 ± 0.3 in white matter (Tables 1 and 2).
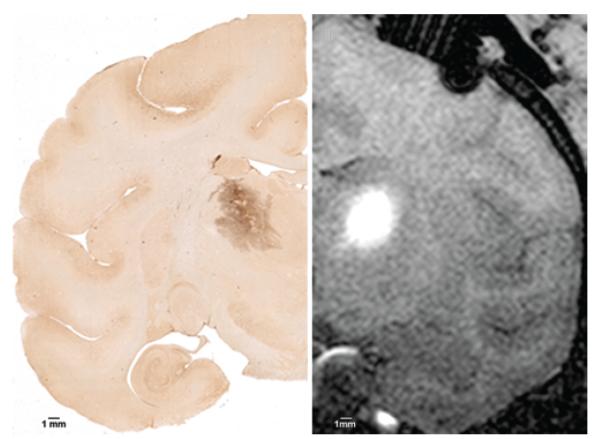
Fig. 2.
Immunohistochemical and MRI studies of thalamic infusion. Left: Representative 40-μm brain section showing immunostaining for M13 bacteriophage. This section shows a distinct dark-staining region in the thalamic gray matter without evidence of white matter tracking. The Vd is calculated by adding areas of the stained region in serial sections. Right: Coronal T1-weighted MRI study obtained with contrast agent showing CED to thalamic gray matter of bacteriophage M13 and Gd-DTPA. The distinct hyperintense region shows Gd-DTPA distribution (Vd) and corresponds to the M13 staining seen on immunohistochemical studies.
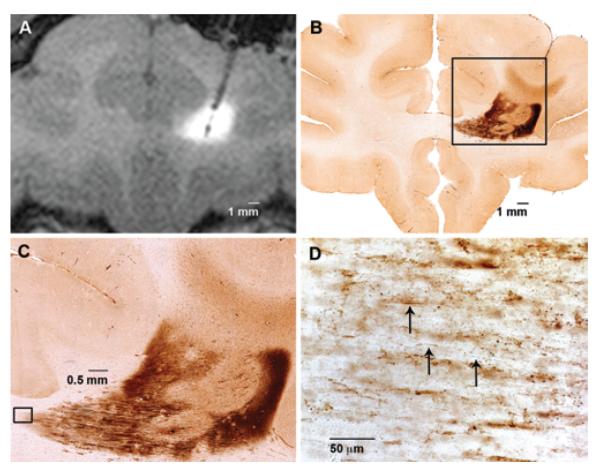
Fig. 3.
Magnetic resonance imaging and immunohistochemical studies of the frontal white matter infusion showing M13 bacteriophage distribution and axonal transport. A: Coronal T1-weighted MRI study obtained with contrast agent showing CED to frontal white matter of bacteriophage M13 and Gd-DTPA. The distinct hyperintense region shows Gd-DTPA distribution (Vd) and corresponds only to the dark-staining region seen on immunohistochemical studies. B: Representative 40-μm section showing immunohistochemical staining for bacteriophage M13 in frontal white matter. This section shows a distinct darker-staining region representing the initial infusion, with a lighter-stained region extending through the corpus callosum and to the cortex, representing axonal transport. C: Immunohistochemical staining for bacteriophage M13 at higher magnification (× 10) of inset in panel B, further showing lighter-stained M13 bacteriophage extension emanating from the initial infusion site. D: Immunohistochemical staining for bacteriophage M13 at high magnification (× 40) of inset in panel C, showing fibers (arrows). This is histological evidence for axonal transport.
Fig. 4.
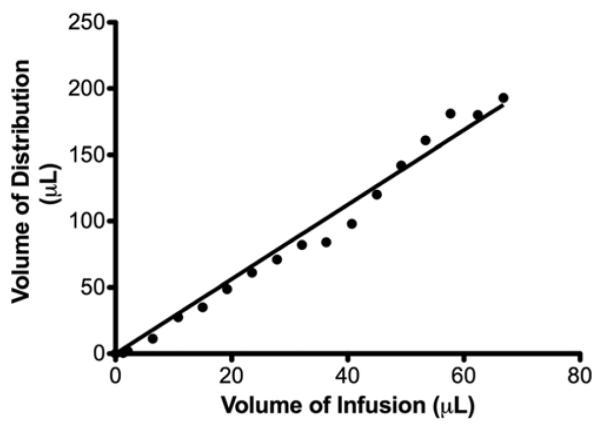
Graph showing a linear relationship between MRI-confirmed Vd and Vi. There was a linear relationship (R2 = 0.98) between Vi and Vd.
TABLE 2.
The mean Vd:Vi ratios and differences in CED of M13 bacteriophage in nonhuman primates*
| Mean Vd:Vi |
||||
|---|---|---|---|---|
| Site of Infusion | Imaging | Histology | Difference (%) | p Value |
| gray matter | 2.3 ± 0.15 | 2.9 ± 0.24 | −2.2 ± 9.9 | 0.65 |
| white matter | 1.9 ± 0.26 | 2.1 ± 0.28 | 9.1 ± 9.5 | 0.55 |
| white matter (infusion & tracking) | NA | 16.1 ± 9.1 | NA | NA |
Values are expressed as the mean ± SD.
Distribution Characteristics of M13 Bacteriophage
Tissue analysis demonstrated discrete staining of M13 bacteriophage (Figs. 2 left and 3B). Although distribution of phage was clearly identifiable in the region perfused, there were differences in distribution between gray and white matter infusions.
Gray Matter
Although 4 thalami were perfused in primates, only 3 were available for analysis (Table 1); 1 thalamic infusion was not analyzable due to tissue damage during processing. According to immunohistochemical studies, distribution of phage in the thalamic gray matter was contained in the discrete region of perfusion, as predicted by MRI studies of coinfused Gd-DTPA (Fig. 2). The Vd:Vi ratio for gray matter was 2.9 ± 0.2 (Table 2). Distribution of the bacteriophage was restricted to the thalamic gray matter.
White Matter
Immunohistochemical distribution of phage in the frontal white matter was most prominent in the region perfused, as determined by MRI (Fig. 3). In 2 of the 3 perfused hemispheres, there was also evidence of additional bacteriophage distribution emanating from the site of initial infusion to the immediately adjacent fiber tracts and through the corpus callosum (Fig. 3B and C). High-magnification analysis of this lighter-staining distribution area revealed a fibril-staining pattern that was consistent with axonal transport (Fig. 3C and D). Lighter-staining regions of axonal distribution were clearly distinguished from the darker-staining region by the density and character of the stain. The lighter-staining area had a fibril-staining pattern, and was less densely stained than the darker region, which was visualized using high-magnification analysis. On preinfusion MRI, one hemisphere showed evidence of stroke in the white matter lateral and superficial to, but not within, the infusion site. This white matter infusion did not show evidence of transport, and thus was not included in the transport region's Vd:Vi calculations. The calculated Vd:Vi ratio in the white matter was 2.1 ± 0.3 in the discrete, densely staining region (Table 2), and 16.1 ± 9.1 when including additional spread in the lighter-staining adjacent white matter regions.
Safety of M13 Bacteriophage Infusion
All animals tolerated the procedure without evidence of neurological deficits or clinical impairment during the 3-day postinfusion period. The H & E and Nissl staining showed minor gliosis, with few neurons demonstrating loss of normal architecture in the region immediately surrounding the cannula track. The lack of clinical evidence of toxicity was further substantiated histologically by the lack of architectural damage within the phage distribution area. In one primate, there was radiological evidence of an infarct in the left frontal lobe at the site of pedestal placement due to cortical vessel coagulation during the pedestal placement procedure.
Discussion
Convective Delivery of Nano-Sized Particles
Previous studies have examined the use of CED of nanomolecules into rodent and primate brain.5,12,17,23,26 Chen and colleagues5 examined the effect of molecular size, infusate osmolarity, and surface coating on the distribution properties of nanoparticles, including viruses and virus-sized particles. Their study showed that AAV (25 nm), adenovirus (80–100 nm), and large polystyrene spheres (20–200 nm) were distributed within the extracellular space of brain parenchyma when CED was used. Furthermore, Chen and colleagues found that infusate surface properties can play a larger role than size in limiting distribution. Similarly, Szerlip and colleagues26 demonstrated that virus (AAV) and virus-sized particles (iron oxide nanoparticles; 24 nm) could be successfully distributed using convection in white and gray matter. Here, we extended these previous findings by studying the convective properties of a significantly larger (Fig. 1B) nanometer-sized molecule, the M13 bacteriophage (Fig. 1A).
Current Study Findings
Similar to previous findings with nanometer-sized particles, our study demonstrates that CED of M13 bacteriophage is feasible. Nevertheless, there are critical differences between the convective properties of bacteriophage compared with small molecules, proteins, and other nanometer-sized particles (Fig. 1B).
Volumetric Analysis of Bacteriophage Distribution
The M13 bacteriophage was successfully distributed in gray and white matter. Immunohistochemical analysis of bacteriophage distribution in white matter revealed that the mean Vd:Vi ratio was 2.1 ± 0.3 (Table 2). This is similar to distribution associated with AAV, as described by Szerlip and colleagues, but smaller than that expected for small molecules and proteins (Vd:Vi ratio of 4:1 or 5:1).26 Alternatively, the Vd:Vi ratio of bacteriophage in gray matter was 2.9 ± 0.2, which is less than the distribution seen with AAV (Vd:Vi ratio of approximately 4:1).3,26 These differences are probably due to the larger size of the bacteriophage and its long, linear shape (Fig. 1B), which necessitates the distribution of the particles parallel to one another in the extracellular space. Taken together, these results suggest that distribution patterns within differing tissues depend not only on infusate size and tissue characteristics but also on the infused molecule's shape, surface properties, and interaction with surrounding tissue.5,19
Evidence of Bacteriophage Axonal Transport
Infusion of bacteriophage in frontal white matter was associated with additional spread beyond the site of immediate infusion that expanded the overall Vd:Vi ratio to 16. The frontal white matter infusion without evidence of transport had a cortical infarct with extension just beyond the infusion site. This suggested that M13 bacteriophage transport was an active process, similar to axonal transport. The axonal transport of viral particles has recently been described, with the convection of AAV vectors12,15,16 into mouse12,15 and primate brains.16 Kells and colleagues demonstrated axonal transport and cortical transgene expression after CED of AAV type 2 vector into primate thalamus. Similarly, high-magnification analysis of white matter infusion in the current study revealed histological evidence of M13 bacteriophage axonal transport (Fig. 3C and D). Unlike AAV, infusion into gray matter did not show signs of axonal transport to interconnected cortical thalamic projection sites. The mechanism of additional distribution of M13 bacteriophage in white matter is unknown, but may occur through either nonspecific axonal endocytosis,7 axon-specific receptor-mediated internalization,20 or extraaxonal transport.
Accuracy of Gd-DTPA as a Surrogate Imaging Agent to Track Convective Bacteriophage Distribution
The Gd-DTPA (1 mM) was an accurate MRI surrogate tracer that predicted infused distribution of the M13 bacteriophage. Based on work by Asthagiri and colleagues,2 a 1-mM concentration of Gd-DTPA was used (rather than a higher concentration [5 mM]), because it should provide a Vd most closely consistent with nanometer-sized particles. Serial MRI-volumetric analysis of Gd-DTPA revealed a mean Vd:Vi of 2.3 ± 0.2 in gray matter and 1.9 ± 0.3 in white matter. The percent differences between MRI-predicted distribution and immunohistochemical distribution were not significant in the cerebral white and gray matter; they were 9.1% (p = 0.6) and −2.2% (p = 0.7), respectively (Table 2).
It is not likely that Gd-DTPA affects the distribution or interacts with M13 bacteriophage. Several studies describe the accurate tracking of other nanometer-sized particles and viral capsids for which Gd-DTPA was used, without reporting interactions between the infusate and surrogate tracer.10,17,23,26 Furthermore, there is currently no evidence suggesting an interaction between Gd-DTPA and M13 bacteriophage or its predominant coat proteins. Given the large size of M13 bacteriophage and data from large protein studies,4 it is unlikely that diffusion played a role in distribution within brain parenchyma at the 3-day time point. This lack of diffusion permits the accurate tracking of M13 bacteriophage with 1 mM Gd-DTPA, while also allowing for the assessment of short-term safety. Together, these findings suggest that 1 mM Gd-DTPA effectively tracks M13 bacteriophage over the infusion volumes described here.6
Safety
Similar to previous studies examining the use of CED for nanometer-sized molecules,18,24–26 in our study convective infusion of M13 bacteriophage was safe. All animals were neurologically and clinically intact after infusion, without any signs of distress or complications. Histological analysis of tissue sections in the perfused and surrounding regions revealed minimal cytoarchitectural damage along the cannula track, without evidence of tissue damage within the phage distribution area. Given that the animals were monitored for 3 days after infusion, this safety profile accounts for short-term clinical and histological changes. Future studies are necessary to assess the long-term safety of CED of the M13 bacteriophage into primate brain.
Clinical Applications
Emerging evidence indicates that the linear structure of the M13 bacteriophage permits it to bind to Aβ, tau, and α-synuclein fibrils, causing plaque disaggregation in AD1,11 and PD,8,9 respectively. Specifically, prior studies have shown that the M13 bacteriophage can reduce α-synuclein in vitro8,9 and Lewy bodies in vivo in mouse models of PD.14 Similarly, binding assays show that M13 bacteriophage binds strongly to Aβ peptide, with subsequent plaque dissociation.1,11 In an in vivo study, M13 bacteriophage–treated AD mice had signifi-cant Aβ-plaque reduction and improvement in cognitive behavioral tasks.1 In mouse models of both AD and PD, M13 bacteriophage was shown to distribute via intrathecal and intraventricular delivery routes, with some limitation (Fisher R, Kimberley S, Gannon S, Krishnan R, Tsubery H, et al: NPT001: A novel therapeutic approach for clearing of both beta-amyloid plaques and neurofibrillary tangles in Alzheimer's disease, presented at the 4th Edition of Clinical Trials on Alzheimer's Disease, San Diego, CA, November 3–5, 2011). Intraparenchymal infusion using CED is advantageous in that it achieves safe, specific targeting within gray matter and a widespread distribution within white matter. The current findings indicate that the use of CED could be a feasible delivery route for M13 bacteriophage. Specifically, the characterized M13 bacteriophage distribution within the gray matter can be applied for use in PD, targeting the substantia nigra. Evidence of axonal transport seen in white matter infusions reveals the possibility that M13 bacteriophage can be distributed in white matter.
Conclusions
The CED method can be used successfully and safely to distribute M13 bacteriophage in the brain. Furthermore, axonal transport may enhance distribution of this large nanoparticle. Real-time MRI of coinfused Gd-DTPA (1 mM) can be used for accurate tracking of the distribution of this bacteriophage.
Acknowledgments
The authors thank Elliot Mufson, Ph.D., at Rush University Department of Neurological Sciences, for his assistance with immunohistochemistry and his review/edit of the manuscript. The authors thank Muhammad Nadeem, M.D., at Rush University Department of Neurological Sciences, for his assistance with immunohistochemistry. The authors thank Kim Gannon, Ph.D., at NeuroPhage Pharmaceuticals, for her assistance with the phage dosing and study development.
This research was supported by the Intramural Research Program of the National Institute of Neurological Disorders and Stroke at the NIH. This research was performed under a Materials Trans fer Agreement with NeuroPhage Pharmaceuticals, Inc., Cambridge, Massachusetts. Dr. Lonser holds a patent with the NIH. The authors report no conflict of interest concerning the materials or methods used in this study or the findings specified in this paper.
Abbreviations used in this paper
- AAV
adenoma-associated virus
- AD
Alzheimer disease
- CED
convection-enhanced delivery
- PBS
phosphate-buffered saline
- PD
Parkinson disease
- Vd
volume of distribution
- Vi
volume of infusion
Footnotes
Disclosure Author contributions to the study and manuscript preparation include the following. Conception and design: Lonser, Ksendzovsky, Walbridge. Acquisition of data: Lonser, Ksendzovsky, Walbridge, Heiss. Analysis and interpretation of data: Lonser, Ksendzovsky, Walbridge, Saunders, Asthagiri. Drafting the article: Lonser, Ksendzovsky. Critically revising the article: all authors. Reviewed submitted version of manuscript: all authors. Approved the final version of the manuscript on behalf of all authors: Lonser. Statistical analysis: Lonser, Ksendzovsky. Administrative/technical/material support: Ksendzovsky, Walbridge. Study supervision: Lonser, Ksendzovsky, Walbridge.
References
- 1.Alzheimer's and Parkinson's diseases: advances, concepts and new challenges. Proceedings of the 9th International Conference on Alzheimer's and Parkinson's Diseases. Prague, Czech Republic; March 11–15, 2009; Neurodegener Dis 7:5–215, 2010. [DOI] [PubMed] [Google Scholar]
- 2.Asthagiri AR, Walbridge S, Heiss JD, Lonser RR. Effect of concentration on the accuracy of convective imaging distribution of a gadolinium-based surrogate tracer. Laboratory investigation. J Neurosurg. 2011;115:467–473. doi: 10.3171/2011.3.JNS101381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bankiewicz KS, Eberling JL, Kohutnicka M, Jagust W, Pivirotto P, Bringas J, et al. Convection-enhanced delivery of AAV vector in parkinsonian monkeys; in vivo detection of gene expression and restoration of dopaminergic function using pro-drug approach. Exp Neurol. 2000;164:2–14. doi: 10.1006/exnr.2000.7408. [DOI] [PubMed] [Google Scholar]
- 4.Bobo RH, Laske DW, Akbasak A, Morrison PF, Dedrick RL, Oldfield EH. Convection-enhanced delivery of macromolecules in the brain. Proc Natl Acad Sci USA. 1994;91:2076–2080. doi: 10.1073/pnas.91.6.2076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen MY, Hoffer A, Morrison PF, Hamilton JF, Hughes J, Schlageter KS, et al. Surface properties, more than size, limiting convective distribution of virus-sized particles and viruses in the central nervous system. J Neurosurg. 2005;103:311–319. doi: 10.3171/jns.2005.103.2.0311. [DOI] [PubMed] [Google Scholar]
- 6.Croteau D, Walbridge S, Morrison PF, Butman JA, Vortmeyer AO, Johnson D, et al. Real-time in vivo imaging of the convective distribution of a low-molecular-weight tracer. J Neurosurg. 2005;102:90–97. doi: 10.3171/jns.2005.102.1.0090. [DOI] [PubMed] [Google Scholar]
- 7.Deinhardt K, Schiavo G. Endocytosis and retrograde axonal traffic in motor neurons. Biochem Soc Symp. 2005;(72):139–150. doi: 10.1042/bss0720139. [DOI] [PubMed] [Google Scholar]
- 8.Dimant H, Sharon N, Solomon B. Modulation effect of filamentous phage on alpha-synuclein aggregation. Biochem Biophys Res Commun. 2009;383:491–496. doi: 10.1016/j.bbrc.2009.04.048. [DOI] [PubMed] [Google Scholar]
- 9.Dimant H, Solomon B. Filamentous phages reduce alpha-synuclein oligomerization in the membrane fraction of SH-SY5Y cells. Neurodegener Dis. 2010;7:203–205. doi: 10.1159/000295664. [DOI] [PubMed] [Google Scholar]
- 10.Fiandaca MS, Varenika V, Eberling J, McKnight T, Bringas J, Pivirotto P, et al. Real-time MR imaging of adeno-associated viral vector delivery to the primate brain. Neuroimage. 2009;47(Suppl 2):T27–T35. doi: 10.1016/j.neuroimage.2008.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Frenkel D, Solomon B. Filamentous phage as vector-mediated antibody delivery to the brain. Proc Natl Acad Sci USA. 2002;99:5675–5679. doi: 10.1073/pnas.072027199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hadaczek P, Kohutnicka M, Krauze MT, Bringas J, Pivirotto P, Cunningham J, et al. Convection-enhanced delivery of adeno-associated virus type 2 (AAV2) into the striatum and trans port of AAV2 within monkey brain. Hum Gene Ther. 2006;17:291–302. doi: 10.1089/hum.2006.17.291. [DOI] [PubMed] [Google Scholar]
- 13.Havas D, Rauter G, Prokesch M, Hutter-Paier B, Windisch M, Solomon B. Effects of filamentous phage treatment on brain pathology of alpha-synuclein tg mice. Neurodegenerative Dis. 2007;4(Suppl 1):169. Abstract. [Google Scholar]
- 14.Heiss JD, Walbridge S, Asthagiri AR, Lonser RR. Image-guided convection-enhanced delivery of muscimol to the primate brain. Laboratory investigation. J Neurosurg. 2010;112:790–795. doi: 10.3171/2009.7.JNS09652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kaspar BK, Lladó J, Sherkat N, Rothstein JD, Gage FH. Retrograde viral delivery of IGF-1 prolongs survival in a mouse ALS model. Science. 2003;301:839–842. doi: 10.1126/science.1086137. [DOI] [PubMed] [Google Scholar]
- 16.Kells AP, Hadaczek P, Yin D, Bringas J, Varenika V, Forsayeth J, et al. Efficient gene therapy-based method for the delivery of therapeutics to primate cortex. Proc Natl Acad Sci USA. 2009;106:2407–2411. doi: 10.1073/pnas.0810682106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Krauze MT, Forsayeth J, Yin D, Bankiewicz KS. Convection-enhanced delivery of liposomes to primate brain. Methods Enzymol. 2009;465:349–362. doi: 10.1016/S0076-6879(09)65018-7. [DOI] [PubMed] [Google Scholar]
- 18.Lieberman DM, Laske DW, Morrison PF, Bankiewicz KS, Oldfield EH. Convection-enhanced distribution of large molecules in gray matter during interstitial drug infusion. J Neurosurg. 1995;82:1021–1029. doi: 10.3171/jns.1995.82.6.1021. [DOI] [PubMed] [Google Scholar]
- 19.MacKay JA, Deen DF, Szoka FC., Jr Distribution in brain of liposomes after convection enhanced delivery; modulation by particle charge, particle diameter, and presence of steric coating. Brain Res. 2005;1035:139–153. doi: 10.1016/j.brainres.2004.12.007. [DOI] [PubMed] [Google Scholar]
- 20.Matteoli M, Verderio C, Rossetto O, Iezzi N, Coco S, Schiavo G, et al. Synaptic vesicle endocytosis mediates the entry of tetanus neurotoxin into hippocampal neurons. Proc Natl Acad Sci USA. 1996;93:13310–13315. doi: 10.1073/pnas.93.23.13310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rasched I, Oberer E. Ff coliphages: structural and functional relationships. Microbiol Rev. 1986;50:401–427. doi: 10.1128/mr.50.4.401-427.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rosenbluth KH, Luz M, Mohr E, Mittermeyer S, Bringas J, Bankiewicz KS. Design of an in-dwelling cannula for convection-enhanced delivery. J Neurosci Methods. 2011;196:118–123. doi: 10.1016/j.jneumeth.2010.12.022. [DOI] [PubMed] [Google Scholar]
- 23.Saito R, Krauze MT, Bringas JR, Noble C, McKnight TR, Jackson P, et al. Gadolinium-loaded liposomes allow for real-time magnetic resonance imaging of convection-enhanced delivery in the primate brain. Exp Neurol. 2005;196:381–389. doi: 10.1016/j.expneurol.2005.08.016. [DOI] [PubMed] [Google Scholar]
- 24.Sanchez CE, Tierney TS, Gale JT, Alavian KN, Sahin A, Lee JS, et al. Recombinant adeno-associated virus type 2 pseudotypes: comparing safety, specificity, and transduction efficiency in the primate striatum. Laboratory investigation. J Neurosurg. 2011;114:672–680. doi: 10.3171/2010.8.JNS091583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Su X, Kells AP, Huang EJ, Lee HS, Hadaczek P, Beyer J, et al. Safety evaluation of AAV2-GDNF gene transfer into the dopaminergic nigrostriatal pathway in aged and parkinsonian rhesus monkeys. Hum Gene Ther. 2009;20:1627–1640. doi: 10.1089/hum.2009.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Szerlip NJ, Walbridge S, Yang L, Morrison PF, Degen JW, Jarrell ST, et al. Real-time imaging of convection-enhanced delivery of viruses and virus-sized particles. J Neurosurg. 2007;107:560–567. doi: 10.3171/JNS-07/09/0560. [DOI] [PubMed] [Google Scholar]
- 27.Varenika V, Kells AP, Valles F, Hadaczek P, Forsayeth J, Bankiewicz KS. Controlled dissemination of AAV vectors in the primate brain. Prog Brain Res. 2009;175:163–172. doi: 10.1016/S0079-6123(09)17511-8. [DOI] [PubMed] [Google Scholar]