Summary
Background
Artemisinin-resistant Plasmodium falciparum has been reported in Pailin, western Cambodia, detected as a slow parasite clearance rate in vivo. Emergence of this phenotype in western Thailand and possibly elsewhere threatens to compromise the effectiveness of all artemisinin-based combination therapies. Parasite genetics is associated with parasite clearance rate but does not account for all variation. We investigated contributions of both parasite genetics and host factors to the artemisinin-resistance phenotype in Pursat, western Cambodia.
Methods
Between June 19 and Nov 28, 2009, and June 26 and Dec 6, 2010, we enrolled patients aged 10 years or older with uncomplicated falciparum malaria, a density of asexual parasites of at least 10 000 per μL of whole blood, no symptoms or signs of severe malaria, no other cause of febrile illness, and no chronic illness. We gave participants 4 mg/kg artesunate at 0, 24, and 48 h, 15 mg/kg mefloquine at 72 h, and 10 mg/kg mefloquine at 96 h. We assessed parasite density on thick blood films every 6 h until undetectable. The parasite clearance half-life was calculated from the parasite clearance curve. We genotyped parasites with 18 microsatellite markers and patients for haemoglobin E, α-thalassaemia, and a mutation of G6PD, which encodes glucose-6-phosphate dehydrogenase. To account for the possible effects of acquired immunity on half-life, we used three surrogates for increased likelihood of exposure to P falciparum: age, sex, and place of residence. This study is registered with ClinicalTrials.gov, number NCT00341003.
Findings
We assessed 3504 individuals from all six districts of Pursat province seeking treatment for malaria symptoms. We enrolled 168 patients with falciparum malaria who met inclusion criteria. The geometric mean half-life was 5.85 h (95% CI 5.54–6.18) in Pursat, similar to that reported in Pailin (p=0.109). We identified two genetically different parasite clone groups: parasite group 1 (PG1) and parasite group 2 (PG2). Non-significant increases in parasite clearance half-life were seen in patients with haemoglobin E (0.55 h; p=0.078), those of male sex (0.96 h; p=0.064), and in 2010 (0.68 h; p=0.068); PG1 was associated with a significant increase (0.79 h; p=0.033). The mean parasite heritability of half-life was 0.40 (SD 0.17).
Interpretation
Heritable artemisinin resistance is established in a second Cambodian province. To accurately identify parasites that are intrinsically susceptible or resistant to artemisinins, future studies should explore the effect of erythrocyte polymorphisms and specific immune responses on half-life variation.
Funding
Division of Intramural Research, National Institute of Allergy and Infectious Diseases, National Institutes of Health.
Introduction
Artemisinin resistance in Plasmodium falciparum has not yet been fully defined. In Pailin, western Cambodia, it has been described as a slower parasite clearance rate after artesunate treatment than in Wang Pha, western Thailand.1 A 2012 report2 showed that artemisinin resistance is emerging in western Thailand, and predicts that in 2–6 years it will reach levels reported in Pailin. In Cambodia, the provinces of Pailin, Battambang, Pursat, and Kampot constitute zone 1—a region where the global plan for artemisinin resistance containment focuses its parasite elimination efforts because persistent parasitaemia has been reported more than 72 h after artemisinin-based combination therapy.3 However, parasite clearance rates in zone 1 provinces other than Pailin (population 70482 in 2008;4 area 803 km2) have not been reported.
In Pailin and western Thailand, the finding that genetically related P falciparum clones are cleared at similar rates in different patients suggests that parasite genetics has a role.2,5 However, much variation in parasite clearance rates remains unexplained, implying that host factors affect this phenotype. Erythrocyte polymorphisms are common in western Cambodia, where greater than 40% of individuals carry at least the haemoglobin E (HbE) polymorphism.6,7
Erythrocyte polymorphisms and acquired immunity could account for some variation in parasite clearance rates, thus affecting association analyses between these rates and parasite genotypes. One possibility is that oxidative stress exerted by HbE,8 α-thalassaemia,9 and erythrocytes deficient in glucose-6-phosphate dehydrogenase (G6PD)10 stimulates ring-stage parasites to upregulate their antioxidant defences, thereby antagonising the pro-oxidant activity of artemisinins11 and delaying parasite clearance by the spleen.12–14 Another possibility is that effectors of acquired immunity (eg, immune IgG) remove parasites from the circulation,15 thereby accelerating parasite clearance. To assess the effects of parasite genetics and host factors on the artemisinin-resistance phenotype in western Cambodia, we studied parasite clearance rates in response to artesunate in Pursat province (population 397107 in 2008;4 area 12692 km2; 146 km south of Pailin).
Methods
Study design and participants
During the 2009 and 2010 malaria seasons (June 19 to Nov 28, 2009; June 26 to Dec 6, 2010) we did a study at the Sampov Meas Referral Hospital in Pursat, Cambodia. We enrolled patients from all six districts of Pursat province. Patients were referred to our study clinic from Sampov Meas, Krakor, Bakan, and Kandieng districts. Our study team recruited patients from Kravanh and Veal Veng districts by travelling daily through these westernmost regions to assess patients presenting at the side of the road. Residents of Pursat typically acquire malaria in the forested regions of Veal Veng along Cambodia's western border with Thailand (unpublished). There, individuals engaged in activities such as hunting, mining, and logging are exposed to forest-dwelling Anopheles spp vectors.
Eligible patients were aged 10 years or older and had uncomplicated falciparum malaria with a density of P falciparum asexual parasites of at least 10000 per μL of whole blood, no symptoms or signs of severe malaria,16 no other cause of febrile illness, and no chronic illness. To be eligible, women aged 15–45 years had to have a negative pregnancy test. Patients had to be willing to remain in hospital until parasitaemia became undetectable. To quantify parasite density, an expert microscopist (SSr) counted the number of asexual parasites per 200 leucocytes in Giemsa-stained thick blood films. This number was multiplied by 40, assuming the patient had 8000 leucocytes per μL of whole blood.
All adults or the parents or guardians of children aged 10–17 years provided written informed consent. The study protocol was approved by the Cambodian National Ethics Committee for Health Research and the National Institute of Allergy and Infectious Diseases Institutional Review Board.
Procedures
Immediately before the first dose of artesunate, initial parasite density was counted and a venous blood sample was obtained for research purposes. Patients were given directly observed oral doses of 4 mg/kg artesunate (50 mg per tablet; Cipla, Mumbai, India) at 0, 24, and 48 h, and subsequently 15 mg/kg mefloquine (250 mg per tablet; provided by UNITAID and UNICEF) at 72 h, and 10 mg/kg mefloquine at 96 h.
For a first assessment of whether haemoglobin type affected the pharmacokinetics of artesunate and dihydroartemisinin, we collected a venous blood sample 1 h after the first dose of artesunate from patients enrolled in 2009 into prechilled fluoride-oxalate tubes (Becton Dickinson, Franklin Lakes, NJ, USA). We separated plasma immediately and stored it in liquid nitrogen for measurements of artesunate and dihydroartemisinin concentration by high-throughput liquid chromatography with tandem mass spectrometry after solid-phase extraction.17
For all patients, thick blood films were made every 6 h until the asexual parasite density was undetectable (ie, no parasites were recorded per 500 leucocytes). Parasite clearance curves were derived from these parasite density counts.18 The parasite clearance half-life—ie, the time for parasite density to decrease by 50%—was calculated with the parasite clearance estimator developed by the Worldwide Antimalarial Resistance Network. This instrument calculates the parasite clearance rate constant for each patient, on the basis of the linear part of the loge parasite density-time profile.19 In collaboration with Dondorp and colleagues,1 we obtained parasite density counts and calculated half-life values for patients treated with the same drug regimen in Pailin, western Cambodia, and Wang Pha, western Thailand, in 2007 and 2008. Additionally, we compared our findings with those from elsewhere in western Thailand,2 but did not make formal comparisons because patients received different doses of artemisinins.
We did high-performance liquid chromatography (D-10 haemoglobin analyser; Bio-Rad, Hercules, CA, USA) with 5 μL haemolysate to establish haemoglobin type (two copies of haemoglobin A [HbAA], one copy of haemoglobin A and one of HbE [HbAE], or two copies of HbE [HbEE]). We used a QiaAmp kit (Qiagen, Valencia, CA, USA) to extract DNA from 200 μL haemolysate. A 596 bp fragment of G6PD that can contain the X chromosome-linked Viangchan mutation (G6PD*VC; 871G→A; encoding about 10% G6PD enzyme activity) was amplified by PCR to identify patients with the mutation.20,21 The primer pair was 5′-GACGTCCGTGATGAGAAGG-3′ and 5′-CCTACCATCCCACCTCT-3′. For PCR, we denatured initial DNA at 94°C for 2 min, and subsequently did 38 cycles of denaturation at 94°C for 20 s, annealing at 62°C for 10 s, with extension at 72°C for 1.5 min and final extension at 72°C for 5 min. Amplified products were sequenced on an ABI 3730XL automatic sequencer (Applied Biosystems, Foster City, CA, USA). The presence of only the wild-type G6PD allele defined wild-type individuals; the presence of both wild-type and mutant alleles defined heterozygotes (when the patient was female); and the presence of only the mutant allele defined hemizygotes (when the patient was male) or homozygotes (when female). The 3.7 kb deletional determinant of α-thalassaemia (α-thalassaemia−3.7kb) was detected with a nested PCR protocol to identify heterozygotes (−α/αα) and homozygotes (−α/−α).22
By use of 18 microsatellite loci on 14 chromosomes, we genotyped all P falciparum isolates to identify clones and establish their relatedness, as done previously.5 This number of variable markers provides enough power to distinguish parasite genotypes.23 Fluorescently labelled PCR products were detected with an ABI 3100 Genetic Analyzer (Life Technologies, Carlsbad, CA, USA). Alleles were assigned and analysed with GeneMapper software (version 3.2).24 Minor alleles were scored only when their signal was greater than 33.3% of the height of the primary allele.25 We defined multiple-clone infections as the presence of at least two loci that had two or more alleles.
The clustering of 110 P falciparum clones was estimated from the microsatellite data with the Structure package (version 2.2), which uses bayesian approaches to infer the most likely number of populations (K) represented in the total sample and then measures the probability that individual parasites come from each of these K populations.26 We used an admixture model, which assumes that a fraction of each individual's genome is drawn from K population clusters. We calculated and plotted an individual's estimated membership fraction in each of the K inferred clusters. We did ten replicate runs at each K from one to eight with 50000 burn-in steps, with 100000 subsequent iterations. We used log-likelihood values to assess the most likely number of population clusters.24 Assessment of half-life in different patients infected with parasites of the same (or very similar) genotypes provides a straightforward means to estimate the proportion of half-life variation that can be attributed to parasite genetic factors. We identified groups of highly related parasites (differing at two or fewer of the loci assayed) infecting two or more patients.
In 2010 (when techniques were available in our laboratory), we did an ex-vivo assay. We prepared stock solutions of dihydroartemisinin, chloroquine diphosphate, mefloquine, and quinine (Sigma, Steinheim, Germany) in 70% ethanol. We prepared two-fold serial dilutions of these stock solutions in culture water (Lonza, Walkersville, MD, USA). The final concentrations were 0.09–100 nM for dihydroartemisinin, 2.4–2500 nM for chloroquine diphosphate, 1.2–1250 nM for mefloquine, and 4.8–5000 nM for quinine. We added 50 μL of each drug solution in duplicate to 96-well, flat-bottomed plates (Costar 3595, Corning, Lowell, MA, USA), air-dried them in a laminar-flow hood, and stored them for up to 1 month at 4°C. Every plate included two drug-free wells as negative controls for each drug. We validated the quality of every batch of plates by measuring the antimalarial drug responses of the 3D7 P falciparum clone.
For the ex-vivo SYBR Green Fluorescence I-based method of screening,27 we put 200 μL erythrocyte suspensions (final parasitaemia 0.5–1%; final haematocrit 1%) into the 96-well plates. The plates were incubated for 72 h at 37°C in 5% CO2, and then stored at −20°C. Later, they were thawed and the contents carefully resuspended. After removing a 100 μL cell suspension from each well, we added 100 μL of SYBR Green I (Invitrogen) in lysis buffer (0.02% vol/vol). The 2X lysis buffer contained Tris (20 mM; pH 7.5), EDTA (edetic acid; 5 mM), saponin (0.008% wt/vol), and Triton X-100 (0.08% vol/vol). We incubated plates at ambient temperature for 1 h and then quantified SYBR Green I signals with a FLUOstar OPTIMA instrument (BMG Labtech, Cary, NC, USA). We calculated the concentration at which the drugs inhibited 50% of parasite growth (IC50) with the inhibitory sigmoid Emax model, with estimation of the IC50 through non-linear regression with an online standard function of the R software (ICEstimator version 1.2).28 Ex-vivo susceptibility was deemed to be reduced when IC50 was more than 100 nM with chloroquine, more than 500 nM with quinine, more than 30 nM with mefloquine, and more than 10 nM with dihydroartemisinin.
Statistical analysis
To compare half-life values between study sites, we used the Mann-Whitney U test. We analysed correlations with Spearman correlation tests. We analysed prevalence data by Fisher's exact test. These analyses were done with GraphPad Prism (version 5.04).
In a linear regression model, we defined certain continuous variables for erythrocyte polymorphisms: haemoglobin type (HbAA=0, HbAE=1, HbEE=2), α-thalassaemia−3.7kb genotype (wild-type=0, heterozygote=1, homozygote=2), and G6PD*VC genotype (wild-type=0, heterozygote=1, hemizygote=2). To account for the possible effects of acquired immunity on half-life in our study population, we used three surrogates for increased likelihood of exposure to P falciparum: male sex, older age, and residence in Kravanh or Veal Veng districts, which are close to forested regions. We thus defined binary variables: male versus female sex, age 21 years or older versus less than 21 years, and residence versus non-residence in Kravanh and Veal Veng districts. We also defined the binary variable year 2010 versus year 2009. This analysis was done with R software (version 2.14.0).
To account for the effects of covariates (sex, age, residence, erythrocyte polymorphisms) on half-life in different parasite groups, we regressed these variables against half-life and then did an ANOVA with the residuals. Although the regression analysis accounts for the effects of parasite groups on half-life, it only crudely measures the contribution of parasite genetics (ie, heritability). For improved measurement of this contribution, we did a twin analysis5 to estimate the heritability of artemisinin resistance in Pailin. We estimated the heritability of half-life, the proportion of variation in this trait that can be attributed to parasite genetics, with the mean-squared terms of the ANOVA,5 and estimated SDs for heritability of half-life in accordance with Lynch and Walsh.29 We deemed significant a p value of less than 0.05.
This study is registered with ClinicalTrials.gov, number NCT00341003.
Role of the funding source
The sponsor had no role in study design, data collection, data analysis, data interpretation, or writing of the report. The corresponding author had full access to all data in the study and the final responsibility for the decision to submit for publication.
Results
We assessed 3504 individuals from all six districts of Pursat province seeking treatment for malaria symptoms. 712 people (20.3%) were diagnosed with falciparum malaria, but 544 (76.4%) of these patients were not enrolled because of low parasite densities or other exclusion criteria (appendix). The remaining 168 patients were predominantly men with a median age of 24 years (table), which is consistent with our previous findings (unpublished). 103 patients (61.3%) had at least one erythrocyte polymorphism (appendix). Compliance was good and no patients vomited after taking the drugs.
Table. Characteristics of patients and parasitological data.
| Patients (n=168) | |
|---|---|
| Men | 145 (86%) |
|
| |
| Age (years) | 24 (18–39) |
|
| |
| Residence in Kravanh or Veal Veng districts | 90 (54%) |
|
| |
| Haemoglobin (g/L) | 139 (122–152) |
|
| |
| Haemoglobin phenotype | |
| AA | 96 (57%) |
| AE | 65 (39%) |
| EE | 7 (4%) |
|
| |
| α-thalassaemia genotype | |
| αα/αα | 123 (73%) |
| −α/αα | 43 (26%) |
| −α/−α | 2 (1%) |
|
| |
| G6PD*VC | |
| Wild-type | 141 (84%) |
| Heterozygous | 7 (4%) |
| Hemizygous | 20 (12%) |
| Homozygous | 0 |
|
| |
| Parasitaemia | |
| Initial density of Plasmodium falciparum (per μL) | 50 498 (27 940–123 276) |
| P falciparum gametocytaemia | 26 (16%) |
| Plasmodium vivax co-infection* | 13 (8%) |
|
| |
| Parasite clearance | |
| Parasite clearance time (h) | 78 (60–90)† |
| Half-life (h) | 5.85 (5.54–6.18)‡ |
Data are n (%) or median (IQR), unless otherwise stated.
Median density of P vivax was 400 per μL (IQR 48–2060).
One patient had undetectable parasitaemia at 48 h, but the parasitaemia at 42 h could not be counted because of slide damage and so the parasite clearance time could not be established.
Geometric mean (95% CI).
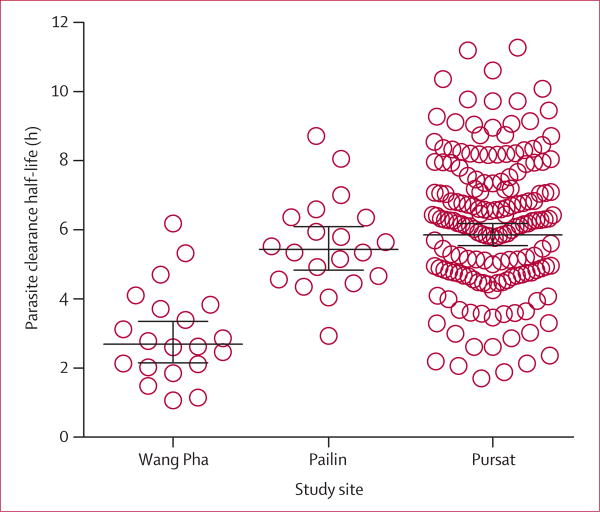
Half-life ranged from 1.71 to 11.28 h (figure 1) and did not correlate with initial parasite densities (r=0.073; p=0.346). Half-life in Pursat (geometric mean 5.85 h, 95% CI 5.54–6.18; n=168) was similar to that in Pailin (5.43 h, 4.84–6.10; n=20; p=0.109) but longer than that in Wang Pha (2.16 h, 2.69–3.36; n=20; p<0.0001; figure 1) and elsewhere in western Thailand (geometric mean 3.7 h, 95% CI 3.6–3.8; n=292) in 2010.2 In Pursat, 108 (64%) patients had a half-life longer than the geometric mean of patients in Pailin. All patients in our study achieved undetectable parasitaemia by 126 h without the need for rescue treatment.
Figure 1. Parasite clearance half-life.
Bars denote geometric mean and 95% CIs. Values for Pailin and Wang Pha were calculated from raw data obtained from Dondorp and colleagues.1
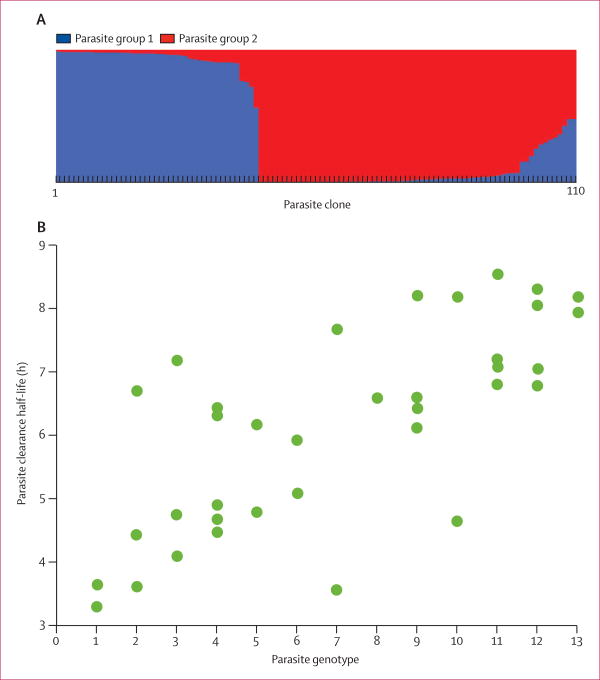
Of the 168 P falciparum isolates obtained directly from patients, 58 were genotyped as multiclonal infections. The microsatellite markers differentiated the remaining 110 clonal isolates into two clusters, parasite group 1 (PG1) and parasite group 2 (PG2), on the basis of their genetic similarity (figure 2). PG1 had significantly less genetic variation as measured by expected heterozygosity (0.50, SD 0.28) than did PG2 (0.75, 0.14; Z=−0.291; p=0.004). Additionally, PG1 had fewer alleles per locus (5.0, SD 2.8) than did PG2 (8.0, 3.0; Z=−0.277; p=0.006). Patients infected with PG1 parasites (n=15) had higher median concentrations than did those infected with PG2 parasites (n=32) of both artesunate (95.80 ng/mL vs 63.10 ng/mL; p=0.043) and dihydroartemisinin (929.0 ng/mL vs 688.5 ng/mL; p=0.641) in their plasma 1 h after the first artesunate dose. PG1 was associated with an increase in half-life of 0.79 h (95% CI 0.07–1.52; p=0.033), male sex with a rise of 0.96 h (0.05–1.97; p=0.064), HbE with a lengthening of 0.55 h (0.06–1.16; p=0.078), and the year 2010 with an increase of 0.68 h (−0.05 to 1.41; p=0.068; appendix). This regression analysis estimated that PG1 accounted for 4% of half-life variation.
Figure 2. Genetic analyses of Plasmodium falciparum clones.
(A) Clustering of 110 clones on the basis of analysis of 18 microsatellite markers. (B) Parasite clearance half-life in 39 of the clones that we were able to classify into 13 groups of very similar parasite genotypes. Genotype groups were ranked between one and 13 on the basis of increasing mean half-life.
We classified 39 of the 110 P falciparum clones into 13 groups of twin parasite genotypes, each containing highly related clones differing at two loci or fewer of the microsatellite markers and identified in two to five patients. Half-life varied between genotype groups (figure 2). We recorded significantly less variation in half-life in patients infected with parasites with highly related or identical genotypes (F=2.95; p=0.010) and derived a mean heritability of 0.40 (SD 0.17).
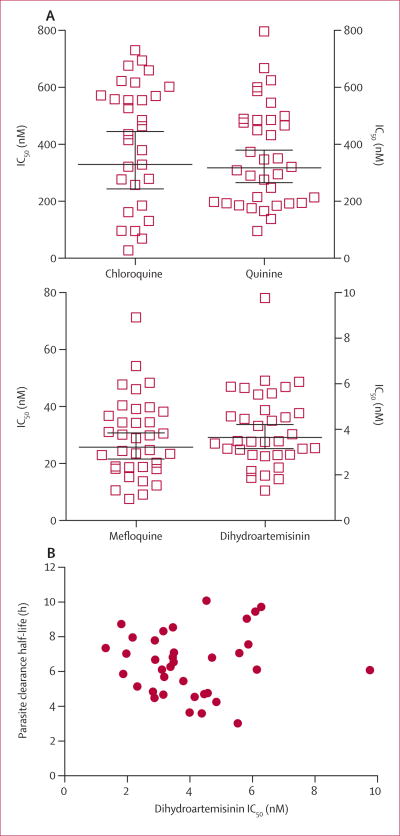
In ex-vivo drug assays done in 2010, 26 (86.7%) of 30 parasite isolates had reduced ex-vivo susceptibility to chloroquine, six (17.1%) of 35 to quinine, and 19 (54.3%) of 35 to mefloquine (figure 3). These profiles match what is expected for parasites in this region.30 We noted significant positive correlations between IC50 values for dihydroartemisinin and those for quinine (r=0.596; n=35; p=0.0002), but not with those for mefloquine (r=0.309; n=35; p=0.071) or chloroquine (r=0.188; n=30; p=0.320). IC50 values for dihydroartemisinin showed no correlation with half-life (r=0.013; n=36; p=0.941; Figure 3) and did not differ between PG1 and PG2 parasites (appendix). Geometric mean IC50 values for mefloquine were significantly higher in parasites from PG2 (36.59 nM; n=12) than in those from PG1 (16.46 nM; n=13; p=0.0007; appendix). Ten of 12 PG2 parasites showed reduced ex-vivo susceptibility to mefloquine.
Figure 3. Ex-vivo susceptibility of Plasmodium falciparum isolates to antimalarial drugs.
(A) P falciparum isolates were tested in a conventional ex-vivo assay for their responses to chloroquine, quinine, mefloquine, and dihydroartemisinin. Bars denote geometric mean and 95% CIs. (B) Dihydroartemisinin IC50 values for parasite isolates against corresponding half-life values. IC50=concentration at which drugs inhibited 50% growth.
Discussion
The time taken to clear P falciparum parasites in patients from Pursat is lengthy, with half-life as long as 11.28 h, suggesting that the artemisinin-resistance phenotype is well established outside Pailin (panel; appendix). We have identified a genetically defined cluster of parasites (PG1) that is significantly associated with increased half-life; a heritability analysis suggested that roughly 40% of half-life variation can be attributed to parasite genetics. Additionally, some host factors increase half-life, although not significantly.
Our findings have several important implications. First, although PG1 accounts for only 4% of half-life variation, the identification of this parasite cluster further corroborates the genetic basis for the artemisinin-resistance phenotype and suggests that our understanding of the epidemiology of artemisinin resistance will benefit from detailed investigations of parasite population structure through analyses of single-nucleotide polymorphisms and whole-genome sequencing. The evidence for a strong effect of parasite genetics on half-life is consistent with a recent study,34 in which a strongly selected region on chromosome 13 was identified that explained much variation in parasite clearance rates in western Thailand.
Second, the fairly low genetic diversity of PG1 parasites suggests that their high prevalence in Pursat is probably due to a population bottleneck or founder effect, rather than selection due to artemisinin pressure in the larger parasite population. Third, some host factors that we measured could confound phenotype–genotype correlation studies aimed at identification of parasite molecular markers of artemisinin resistance in western Cambodia. Additional investigations of erythrocyte polymorphisms and acquired immunity could therefore be warranted. The additional finding that PG2 parasites have significantly higher IC50 values with mefloquine than do PG1 parasites suggests that use of this drug might have increased the prevalence of PG2 parasites in western Cambodia.
One limitation of our study is that we did not obtain detailed data to investigate whether pharmacokinetics accounts for some half-life variation. However, our finding that concentrations of artesunate and dihydroartemisinin were higher 1 h after the first dose of artesunate in patients infected with PG1 than in those infected with PG2 parasites suggests that the increased half-life values in patients infected with PG1 parasites are not due to reduced drug exposure. Additionally, we have not adequately accounted for the potential effects of immunity or parasite sequestration on half-life. Adaptive immune responses could be simultaneously removing parasites from circulation as artemisinin is taken and begins to kill parasites. Dependent on the stage of parasite development at the time of the first artesunate dose, parasites can rapidly sequester together and thus disappear from the peripheral blood.35 Immunity and sequestration could shorten half-life and account for the rapid clearance of some parasite populations irrespective of whether they cluster with PG1 or PG2. Age might not be an adequate surrogate for acquired immunity in western Cambodia because malaria is infrequently acquired in this region (appendix). We are now working to identify an in-vitro correlate of parasite-clearing immunity, which is a high priority for parasite clearance studies in southeast Asia.
Detailed assessments of parasite population structure that account for the effects of pharmacokinetics, erythrocyte polymorphisms, acquired immunity, and parasite sequestration on half-life could help to accurately identify artemisinin-resistant and artemisinin-susceptible parasite clones, which are needed to elucidate the biochemical and genetic determinants of slow parasite clearance. Results from these investigations could drive the development of new antimalarial drugs that kill artemisinin-resistant parasites and support public health efforts to prevent their spread. The presence of detectable gametocytaemia in 15% of patients suggests that parasites are transmitted before malaria treatment, and that single-dose primaquine treatment should be considered for routine use because of its gametocytocidal activity.36 Future work should investigate the haemolytic potential of single-dose primaquine treatment in patients with the G6PD*VC mutation and other deficiency traits, which are common in Cambodia and neighbouring countries.
Mapping the spread or emergence of artemisinin-resistant parasites necessitates an enormous effort to produce sufficiently large datasets for estimation of half-life and monitoring of changes with time. We screened roughly 3500 individuals seeking treatment from a large western Cambodian province in 2 years to detect the association of PG1 parasites with long half-life. Such clinical studies are logistically difficult, time-consuming, and costly to do in different places over time, especially in regions such as western Cambodia where malaria endemicity is fairly low. Nevertheless, only clinical studies can provide the relevant in-vivo half-life data and sufficient amounts of parasite DNA needed to investigate the genetic basis of artemisinin resistance. Along with high-quality phenotype data, high-resolution genotyping data are urgently needed to identify a molecular marker of artemisinin resistance. This marker will enable large-scale surveillance efforts to map the present distribution of artemisinin-resistant parasites and contain their spread.
Artemisinins are the most potent compounds in the antimalarial drug arsenal and no suitable replacements are expected any time soon. Therefore, the slowing of parasite clearance rates is alarming because it suggests that parasites are developing artemisinin resistance. This worsening phenotype will result in increased numbers of parasites surviving artemisinin treatment, exposing an ever-increasing parasite biomass to the few effective partner drugs of artemisinin-based combination therapy that remain (eg, piperaquine and lumefantrine). This scenario increases the probability that parasites will develop resistance to these drugs and that available artemisinin-based combination therapy might soon be unable to eliminate all parasites from patients with falciparum malaria. Studies are now needed to establish whether slow parasite clearance rates result in parasitological treatment failures, which unfortunately could herald an era of increased malaria morbidity in southeast Asia.
Supplementary Material
Panel: Research in context.
Systematic review
We searched PubMed with the terms “artemisinin” and “resistance” and limited our search to clinical trials. We used no date or language restrictions. We identified 94 reports from around the world. In Cambodia, the only reports of artemisinin resistance as defined by delayed parasite clearance after treatment with an artemisinin (monotherapy or combination therapy) are from Battambang and Pailin provinces.1,31,32 A standard method for comparison of parasite clearance rates between different sites was clearly needed after these studies. From the parasite clearance curve, the parasite clearance rate and parasite clearance half-life have now been defined as suitable parameters.18,19 The only study that has reported these values in southeast Asia was done in western Thailand and in Pailin, Cambodia.2 This study showed that parasite clearance rates are slowing in western Thailand and predicts that they will become as slow as those reported in Pailin in 2–6 years. A 2012 study has reported these values in Mali,33 where slow parasite clearance rates were not recorded.
Interpretation
Our large study provides evidence of heritable artemisinin resistance in a second province in western Cambodia. It also establishes a baseline half-life value for Pursat province, where longitudinal studies will continue to monitor whether artemisinin resistance is becoming more prevalent or is worsening. Additionally, our findings suggest that some host factors, such as erythrocyte polymorphisms, affect half-life. Both the effect of host factors on half-life and the parasite population clusters recorded in western Cambodia could be important variables in investigations of phenotype–genotype associations of artemisinin resistance.
Acknowledgments
This study was supported by the Division of Intramural Research, National Institute of Allergy and Infectious Diseases, National Institutes of Health. We thank Wenjuan Gu, Robert W Gwadz, Ky Kien Hong, Jennifer L Kirk, Pech Eng Ly, Prum Phoeun, Koeut Savuth, Siv Sovannaroth, Thomas E Wellems, and Jordan A Zuspann for their efforts in support of this work.
Footnotes
For parasite clearance estimator see https://www.wwarn.org/research/parasite-clearance-estimator
See Online for appendix
Contributors: CA, SSr, SSu, PL, CZ, SM, JMA, NL, HJ, JS, X-zS, NJW, AMD, SD, and RMF contributed to study design. SSr, SSu, CZ, and SM gathered clinical data. CA and JM genotyped patients. CA, ESP, TJCA, and JM genotyped parasites. PL undertook ex-vivo drug assays. CA, ESP, KS, PL, NL, TJCA, MPF, JM, and RMF analysed data. CA, KS, PL, TJCA, MPF, JM, and RMF wrote the report.
Conflicts of interest: We declare that we have no conflicts of interest.
References
- 1.Dondorp AM, Nosten F, Yi P, et al. Artemisinin resistance in Plasmodium falciparum malaria. N Engl J Med. 2009;361:455–67. doi: 10.1056/NEJMoa0808859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Phyo AP, Nkhoma S, Stepniewska K, et al. Emergence of artemisinin-resistant malaria on the western border of Thailand: a longitudinal study. Lancet. 2012;379:1960–66. doi: 10.1016/S0140-6736(12)60484-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.WHO. Global plan for artemisinin resistance containment (GPARC) Geneva: World Health Organization; 2011. [Google Scholar]
- 4.National Institute of Statistics. General population census of Cambodia 2008 provisional population totals. [accessed July 28, 2012];2008 Aug; http://www.stat.go.jp/english/info/meetings/cambodia/pdf/pre_rep1.pdf.
- 5.Anderson TJ, Nair S, Nkhoma S, et al. High heritability of malaria parasite clearance rate indicates a genetic basis for artemisinin resistance in western Cambodia. J Infect Dis. 2010;201:1326–30. doi: 10.1086/651562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sanguansermsri T, Flatz G, Flatz SD. Distribution of hemoglobin E and beta-thalassemia in Kampuchea (Cambodia) Hemoglobin. 1987;11:481–86. doi: 10.3109/03630268708998008. [DOI] [PubMed] [Google Scholar]
- 7.Sanguansermsri T, Flatz SD, Flatz G. The hemoglobin E belt at the Thailand-Kampuchea border: ethnic and environmental determinants of hemoglobin E and beta-thalassemia gene frequencies. Gene Geogr. 1987;1:155–61. [PubMed] [Google Scholar]
- 8.Lachant NA, Tanaka KR. Impaired antioxidant defense in hemoglobin E-containing erythrocytes: a mechanism protective against malaria? Am J Hematol. 1987;26:211–19. doi: 10.1002/ajh.2830260302. [DOI] [PubMed] [Google Scholar]
- 9.Cheng ML, Ho HY, Tseng HC, Lee CH, Shih LY, Chiu DT. Antioxidant deficit and enhanced susceptibility to oxidative damage in individuals with different forms of alpha-thalassaemia. Br J Haematol. 2005;128:119–27. doi: 10.1111/j.1365-2141.2004.05257.x. [DOI] [PubMed] [Google Scholar]
- 10.Laosombat V, Sattayasevana B, Chotsampancharoen T, Wongchanchailert M. Glucose-6-phosphate dehydrogenase variants associated with favism in Thai children. Int J Hematol. 2006;83:139–43. doi: 10.1532/IJH97.A20513. [DOI] [PubMed] [Google Scholar]
- 11.O'Neill PM, Barton VE, Ward SA. The molecular mechanism of action of artemisinin—the debate continues. Molecules. 2010;15:1705–21. doi: 10.3390/molecules15031705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chotivanich K, Udomsangpetch R, Dondorp A, et al. The mechanisms of parasite clearance after antimalarial treatment of Plasmodium falciparum malaria. J Infect Dis. 2000;182:629–33. doi: 10.1086/315718. [DOI] [PubMed] [Google Scholar]
- 13.Chotivanich K, Udomsangpetch R, McGready R, et al. Central role of the spleen in malaria parasite clearance. J Infect Dis. 2002;185:1538–41. doi: 10.1086/340213. [DOI] [PubMed] [Google Scholar]
- 14.Buffet PA, Milon G, Brousse V, et al. Ex vivo perfusion of human spleens maintains clearing and processing functions. Blood. 2006;107:3745–52. doi: 10.1182/blood-2005-10-4094. [DOI] [PubMed] [Google Scholar]
- 15.Cohen S, McGregor IA, Carrington S. Gamma-globulin and acquired immunity to human malaria. Nature. 1961;192:733–37. doi: 10.1038/192733a0. [DOI] [PubMed] [Google Scholar]
- 16.WHO Communicable Diseases Cluster. Severe falciparum malaria. Trans R Soc Trop Med Hyg. 2000;94(suppl 1):S1–90. [PubMed] [Google Scholar]
- 17.Lindegardh N, Dondorp AM, Singhasivanon P, White NJ, Day NP. Validation and application of a liquid chromatographic-mass spectrometric method for determination of artesunate in pharmaceutical samples. J Pharm Biomed Anal. 2007;45:149–53. doi: 10.1016/j.jpba.2007.04.030. [DOI] [PubMed] [Google Scholar]
- 18.White N. The parasite clearance curve. Malar J. 2011;10:278. doi: 10.1186/1475-2875-10-278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Flegg JA, Guerin PJ, White NJ, Stepniewska K. Standardizing the measurement of parasite clearance in falciparum malaria: the parasite clearance estimator. Malar J. 2011;10:339. doi: 10.1186/1475-2875-10-339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Poon MC, Hall K, Scott CW, Prchal JT. G6PD Viangchan: a new glucose 6-phosphate dehydrogenase variant from Laos. Hum Genet. 1988;78:98–99. doi: 10.1007/BF00291246. [DOI] [PubMed] [Google Scholar]
- 21.Matsuoka H, Nguon C, Kanbe T, et al. Glucose-6-phosphate dehydrogenase (G6PD) mutations in Cambodia: G6PD Viangchan (871G>A) is the most common variant in the Cambodian population. J Hum Genet. 2005;50:468–72. doi: 10.1007/s10038-005-0279-z. [DOI] [PubMed] [Google Scholar]
- 22.Crompton PD, Traore B, Kayentao K, et al. Sickle cell trait is associated with a delayed onset of malaria: implications for time-to-event analysis in clinical studies of malaria. J Infect Dis. 2008;198:1265–75. doi: 10.1086/592224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Anderson TJ, Williams JT, Nair S, et al. Inferred relatedness and heritability in malaria parasites. Proc Biol Sci. 2010;277:2531–40. doi: 10.1098/rspb.2010.0196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mu J, Awadalla P, Duan J, et al. Recombination hotspots and population structure in Plasmodium falciparum. PLoS Biol. 2005;3:e335. doi: 10.1371/journal.pbio.0030335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Anderson TJ, Haubold B, Williams JT, et al. Microsatellite markers reveal a spectrum of population structures in the malaria parasite Plasmodium falciparum. Mol Biol Evol. 2000;17:1467–82. doi: 10.1093/oxfordjournals.molbev.a026247. [DOI] [PubMed] [Google Scholar]
- 26.Pritchard JK, Stephens M, Donnelly P. Inference of population structure using multilocus genotype data. Genetics. 2000;155:945–59. doi: 10.1093/genetics/155.2.945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Smilkstein M, Sriwilaijaroen N, Kelly JX, Wilairat P, Riscoe M. Simple and inexpensive fluorescence-based technique for high-throughput antimalarial drug screening. Antimicrob Agents Chemother. 2004;48:1803–06. doi: 10.1128/AAC.48.5.1803-1806.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Le Nagard H, Vincent C, Mentre F, Le Bras J. Online analysis of in vitro resistance to antimalarial drugs through nonlinear regression. Comput Methods Programs Biomed. 2011;104:10–18. doi: 10.1016/j.cmpb.2010.08.003. [DOI] [PubMed] [Google Scholar]
- 29.Lynch M, Walsh B. Genetics and analysis of quantitative traits. 1. Sunderland, MA: Sinauer Associates; 1998. pp. 581–92. [Google Scholar]
- 30.Lim P, Wongsrichanalai C, Chim P, et al. Decreased in vitro susceptibility of Plasmodium falciparum isolates to artesunate, mefloquine, chloroquine, and quinine in Cambodia from 2001 to 2007. Antimicrob Agents Chemother. 2010;54:2135–42. doi: 10.1128/AAC.01304-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Noedl H, Se Y, Schaecher K, Smith BL, Socheat D, Fukuda MM. Evidence of artemisinin-resistant malaria in western Cambodia. N Engl J Med. 2008;359:2619–20. doi: 10.1056/NEJMc0805011. [DOI] [PubMed] [Google Scholar]
- 32.Noedl H, Se Y, Sriwichai S, et al. Artemisinin resistance in Cambodia: a clinical trial designed to address an emerging problem in southeast Asia. Clin Infect Dis. 2010;51:e82–89. doi: 10.1086/657120. [DOI] [PubMed] [Google Scholar]
- 33.Maiga AW, Fofana B, Sagara I, et al. No evidence of delayed parasite clearance after oral artesunate treatment of uncomplicated falciparum malaria in Mali. Am J Trop Med Hyg. 2012;87:23–28. doi: 10.4269/ajtmh.2012.12-0058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cheeseman IH, Miller BA, Nair S, et al. A major genome region underlying artemisinin resistance in malaria. Science. 2012;336:79–82. doi: 10.1126/science.1215966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Beaudry JT, Fairhurst RM. Microvascular sequestration of Plasmodium falciparum. Blood. 2011;117:6410. doi: 10.1182/blood-2010-09-305102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Maude RJ, Socheat D, Nguon C, et al. Optimising strategies for Plasmodium falciparum malaria elimination in Cambodia: primaquine, mass drug administration and artemisinin resistance. PLoS One. 2012;7:e37166. doi: 10.1371/journal.pone.0037166. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.