Abstract
Object
Chiari malformation Type I (CM-I) is characterized by hindbrain deformity. We investigated the effects of craniocervical decompression surgery on the anatomical features of hindbrain deformity with a prospective MRI study of patients with CM-I.
Methods
A prospective longitudinal study was conducted in 48 patients with CM-I (39 with syringomyelia) treated with craniocervical decompression. Clinical examinations and cervical MRI were performed before surgery and 1 week, 3–6 months, and annually after surgery. Hindbrain deformity was defined by tonsillar ectopia, pointed cerebellar tonsils, and/or cervicomedullary protuberance. The length of the clivus, basiocciput (sphenooccipital synchondrosis to basion), supraocciput (internal occipital protuberance to opisthion), and anteroposterior (AP) width of CSF pathways at the foramen magnum were measured and compared with those from 18 healthy volunteers (control group).
Results
Before surgery, the patients’ posterior fossa bones were short and their CSF pathways were narrow. All patients had tonsillar ectopia (mean [± SD] 12.3 ± 5.1 mm; normal 0.3 ± 1.0). The majority of patients had pointed tonsils and more than two-thirds exhibited a cervicomedullary protuberance. Clivus and basiocciput lengths were significantly shorter than the values obtained in the control group. However, the supraocciput length did not differ significantly from control measurements. The mean bulbopontine sulcus distance superior to the basion was 9.5 ± 2.6 mm (vs 13.6 ± 2.8 mm in controls; p < 0.0001). The AP widths of the CSF pathways at the level of the foramen magnum were significantly narrowed. After surgery, CSF pathways significantly expanded both ventrally and dorsally. By 3–6 months after surgery, pointed tonsils became round, cervicomedullary protuberance disappeared, and tonsillar ectopia diminished by 51% (to 6.0 ± 3.3 mm; p < 0.0001).
Conclusions
The cerebellar tonsils and brainstem assumed a normal appearance within 6 months after craniocervical decompression. These findings support the concept that the CM-I is not a congenital malformation of the neural elements but rather an acquired malformation that arises from pulsatile impaction of the cerebellar tonsils into the foramen magnum. Clinical trial registration no.: NCT00001327.
Keywords: Chiari malformation Type I, syringomyelia, decompression, magnetic resonance imaging, surgical
Chiari malformation Type I was originally described as a cerebellar deformity characterized by elongated, conical tonsils that extended across the foramen magnum and into the spinal canal in patients with hydrocephalus.2 Magnetic resonance imaging later revealed that patients with clinical findings of CM-I often have a protrusion on the dorsal surface of the medulla and infrequently have hydrocephalus.3 A previous study indicates that the hindbrain position may move inferiorly or superiorly after craniocervical surgery,3 but did not address changes in the morphology of the tonsils or brainstem that occur in response to surgery. The present study was designed to characterize changes in hindbrain morphology that occur after craniocervical decompression surgery that preserves the arachnoid membrane and includes an expansile duraplasty.4
Methods
The study was part of clinical trial no. NCT00001327 and was approved by the institutional review board of the National Institute of Neurological Disorders and Stroke. The study was designed with a lower age limit of 18 years of age but a special exemption from the institutional review board permitted one 16-year-old to enter the study. Informed consent was obtained from all participants and from the parents of the minor patient. Forty-eight patients with CM-I (39 with syringomyelia) were evaluated before and after the surgical procedure of craniocervical decompression, preservation of the arachnoid membrane, and duraplasty. The surgical procedure included removal of enough bone at the foramen magnum, adjacent suboccipital bone, and bone from the upper cervical lamina to decompress the inferior part of the cerebellar hemispheres and the entire posterior surface of the cerebellar tonsils; laminectomy included C-1 and often the superior part of the lamina of C-2. Ultrasound imaging was performed before dural opening to ensure that bony decompression was adequate and to evaluate for obstructive membranes within the subarachnoid space. The arachnoid was left intact and an autologous dural graft was used. Intradural exploration was not performed, and no attempt was made to surgically shrink the cerebellar tonsils.
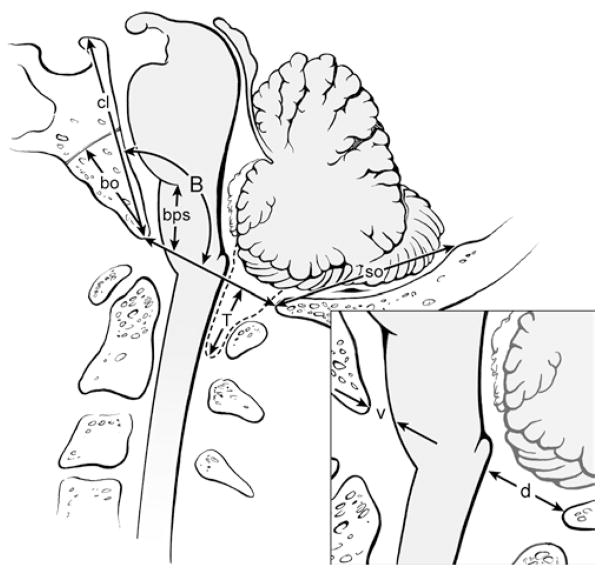
The patients’ mean age at surgery was 36.8 ± 11.2 years (range 16–62 years). Clinical and radiological assessments were performed before surgery and 1 week, 3–6 months (mean 4.0 ± 1.5 months), a nd a nnually a fter surgery. Annual clinical and imaging assessments were protocol-related and deemed necessary to evaluate for possible long-term changes in neurological function and hindbrain morphology. Three independent examiners evaluated T1-weighted, midsagittal MR images of the posterior fossa and cervical spine to characterize the cerebellar tonsils as being rounded or pointed, to detect the presence of dorsal cervicomedullary protuberance, and to measure tonsillar ectopia and medullary position relative to the basion in patients and 18 healthy volunteers (Fig. 1). The level of the foramen magnum was determined before surgery by drawing a line between the basion and opisthion (the McRae line). This method could not be used after surgery because the opisthion was removed in the surgical procedure. The postoperative McRae line was established by extending a line posterior to the basion that reproduced the angle (Boogaard angle) between the posterior surface of the clivus and the McRae line that was found before surgery (Figs. 1 and 2).1
Fig. 1.
Diagram of the measurements taken in the midsagittal plane. Linear measurements (mm) included: cerebellar tonsillar ectopia (T, maximum extension of the cerebellar tonsils caudal to the foramen magnum); bulbopontine sulcus height (bps, distance from basion to pontomedullary junction); supraocciput length (so, internal occipital protuberance to opisthion); clivus length (cl, apex of dorsum sellae to basion); basiocciput length (bo, sphenooccipital synchondrosis to basion); AP width of the ventral subarachnoid space (v, inset), and AP width of the dorsal subarachnoid space (d, inset). The Boogaard angle (B, inner angle formed by the clivus, basion, and opisthion) was also measured.
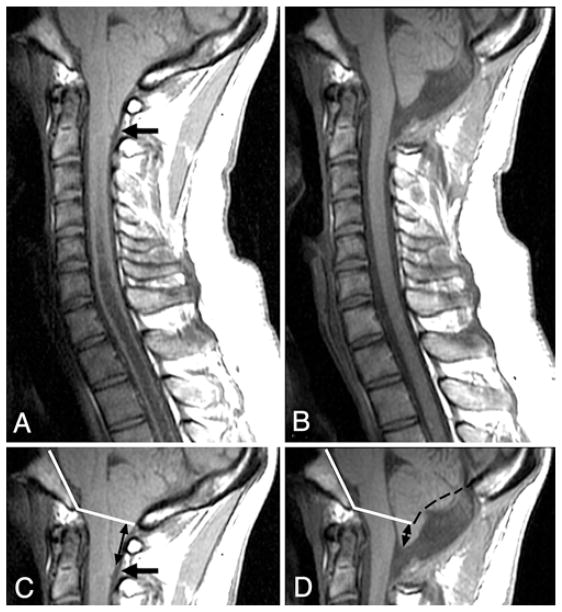
Fig. 2.
A and B Midsagittal T1-weighted MR images of the posterior fossa and cervical spine obtained before (A) and 6 months after (B) craniocervical decompression. Abnormally shaped (pointed) tonsils, dorsal cervicomedullary protuberance (arrow), and obliterated CSF spaces at the foramen magnum are evident preoperatively (A). Following surgery (B), the tonsils have assumed a normal shape, the cervicomedullary protuberance has disappeared, and CSF spaces including the foramen of Magendie have enlarged. C and D: The upper portions of the images in A and B, respectively, showing the Boogaard angle (inner angle between intersecting white lines in C and D) that was established before surgery (C) and was used to determine the level of the foramen magnum after surgery (D). In D, black dashes were added to indicate the position of the inner table of the supraocciput before surgery. Tonsillar ectopia was much reduced after surgery (D, black arrows).
Statistical Analysis
Data are expressed as the mean ± SD. Values from patients before and after surgery were compared with those from healthy volunteers by using the unpaired Student t-test. Measured values from patients before surgery were compared with those obtained after surgery by using the paired Student t-test. A p value ≤ 0.05 was considered significant.
Results
The mean duration of follow-up was 3.6 ± 3.1 years (range 0.2–12.2 years). Before surgery, the cerebellar tonsils extended 12.3 mm (mean, range 5.0–22.7 mm) below the foramen magnum (Table 1). The cerebellar tonsils were pointed in 81% of patients and dorsal cervicomedullary protuberance was present in 71% of patients (Fig. 2A). Pointed tonsils were associated with greater tonsillar ectopia (13.2 ± 4.4 mm) than rounded tonsils (8.4 ± 5.9 mm, p < 0.02, unpaired t-test). However, patients with (11.7 ± 5.0 mm) or without (13.9 ± 5.0 mm, p < 0.2) dorsal cervicomedullary protuberance had a similar degree of tonsillar ectopia. Pointed tonsils and cervicomedullary protuberance were absent in all controls. The bulbopontine sulcus was inferiorly placed in patients (9.5 ± 2.6 mm vs 13.6 ± 2.8 mm in controls; p < 0.0001) (Table 1). The clivus (38.6 ± 3.4 vs 43.2 ± 3.5 mm in controls; p < 0.0001) and basiocciput (19.7 ± 3.3 vs 26.3 ± 4.4 mm in controls; p < 0.0001) were abnormally short in patients, although the supraocciput was not significantly shortened (40.1 ± 4.0 vs 41.5 ± 4.4 mm in controls; nonsignificant). The ventral CSF space AP width at the foramen magnum was 1.7 ± 0.8 mm (vs 6.2 ± 1.8 in controls; p < 0.0001), and dorsal CSF space was absent in all but 4 patients (0.08 ± 0.3 mm vs 5.2 ± 2.6 mm in controls) (Figs. 1, 2, and 3; Table 1). Following surgery, removal of suboccipital bone superior to the opisthion measured 26 ± 4 mm (range 17–35 mm). Removal of the suboccipital bone extended superiorly roughly two-thirds of the distance from the opisthion to the torcula, shortening the suboccipital bone to 34% ± 11% of its original length. Cerebrospinal fluid pathways at the foramen magnum expanded both ventrally (2.7 ± 0.9 mm; p < 0.0001, paired t-test) and dorsally (12.0 ± 4.5 mm; p < 0.0001) in all patients. Cerebellar tonsils ascended a mean distance of 6.3 mm (p < 0.0001; paired t-test), with tonsillar ectopia diminishing by 51% (Fig. 2C and D). The bulbopontine sulcus ascended a mean distance of 1.2 mm relative to the basion (p < 0.0001). Pointed cerebellar tonsils became rounded and dorsal cervicomedullary protuberance resolved in all cases (Table 1). Normalization of the shape of the cerebellar tonsils and cervicomedullary junction occurred within 6 months of surgery (Figs. 2B and 3 right).
TABLE 1.
Hindbrain morphology in CM-I patients before and after decompression surgery*
| Feature | Patients
|
Control Group (N = 18) | ||
|---|---|---|---|---|
| Preop (N = 48) | 1 wk Postop† (N = 42) | 3–6 mos Postop (N = 48) | ||
| tonsillar ectopia (mm) | 12.3 ± 5.1 | 9.0 ± 4.1 (p < 0.0001)‡ | 6.0 ± 3.3 (p < 0.0001)‡ | 0.3 ± 1.0 |
| tonsillar shape | ||||
| round | 9 | 16 | 48 | 18 |
| pointed | 39 | 26 | 0 | 0 |
| presence of cervicomedullary protuberance | ||||
| yes | 34 | 22 | 0 | 0 |
| no | 14 | 20 | 48 | 18 |
| bulbopontine sulcus distance superior to basion (mm) | 9.5 ± 2.6 (p < 0.0001)§ | 9.9 ± 2.5 (p < 0.02)‡ (p < 0.0001)§ | 10.7 ± 2.3 (p < 0.0001)‡ (p < 0.0007)§ | 13.6 ± 2.8 |
| AP width of CSF pathway (mm) | ||||
| ventral | 1.7 ± 0.8 (p < 0.0001)§ | 1.9 ± 0.9 (p < 0.0001)§ | 2.7 ± 0.9 (p < 0.0001)‡ (p < 0.0001)§ | 6.2 ± 1.8 |
| dorsal | 0.08 ± 0.3 (p < 0.0001)§ | 6.1 ± 4.3 (p < 0.0001)‡ | 12.0 ± 4.5 (p < 0.0001)‡ (p < 0.0001)§ | 5.2 ± 2.6 |
| clivus length (mm) | 38.6 ± 3.4 (p < 0.0001)§ | NA | NA | 43.2 ± 3.5 |
| basiocciput length (mm) | 19.7 ± 3.3 (p < 0.0001)§ | NA | NA | 26.3 ± 4.4 |
| supraocciput length (mm) | 40.1 ± 4.0 | NA | NA | 41.5 ± 4.4 |
Measurements are expressed as the mean ± SD. Values for tonsillar shape and presence of cervicomedullary protuberance are numbers of patients. Abbreviation: NA = not applicable.
Six patients did not undergo imaging 1 week postoperatively.
Significant when compared with patients’ parameter before surgery (paired t-test).
Significant when patients’ parameter is compared with control group parameter (unpaired t-test).
Fig. 3.
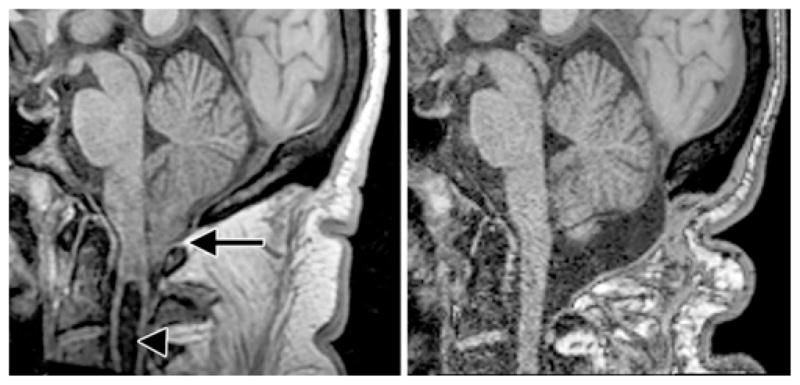
Midsagittal T1-weighted MR images of the posterior fossa and upper cervical spine obtained in a patient with CM-I before (left) and 6 months after (right) craniocervical decompression. The preoperative image demonstrates pointed, ectopic cerebellar tonsils (arrow), impression of the anterior medulla against the tip of the odontoid process, absence of CSF spaces at the foramen magnum, and a cervical syrinx (arrowhead). Postoperatively, the tonsils have ascended and become rounded, anterior compression has resolved, CSF spaces have expanded, and the syrinx has completely resolved. The CSF spaces in the superior half of the posterior fossa are unchanged.
Discussion
Abnormal development of the inferior part of the posterior fossa accounts for the decrease in volume of the posterior fossa associated with CM-I.5,6 In CM-I the cerebellar tonsils obstruct the subarachnoid space and alter CSF flow dynamics.4,7,8 Pulsatile CSF flow through the dorsal subarachnoid pathways is blocked at the level of the foramen magnum, and pulsatile CSF flow through the ventral subarachnoid space is constricted at this level.4 During cardiac systole and brain expansion, this narrowing of the CSF pathways limits the normally rapid compensatory passage of CSF from the noncompliant intracranial cavity, across the foramen magnum, and into the more compliant spinal canal. As a result, in patients with CM-I the normal increase in brain volume during cardiac systole creates pulsatile caudal displacement of the hindbrain through the foramen magnum and into the spinal canal.4,7,8 This caudal force applies a mechanical load to the posterior and inferior part of the cerebellum at the level of the foramen magnum (Figs. 2A and 3 left). In contrast, neural structures within the superior part of the posterior fossa, including the pons and superior surface of the cerebellar hemispheres, are not compressed or displaced.5 The foramen magnum provides the neural elements and CSF with access to the spinal canal, which has greater fluid compliance and, consequently, lower CSF pulse pressure than the intracranial cavity. The impaction of the cerebellar tonsils into the foramen magnum during each cardiac cycle occurs 100,000–120,000 times per day in adults. Its primary effects on tonsillar shape and secondary effects on the dorsal surface of the junction of the medulla and upper portion of the spinal cord manifest themselves as a chronic disorder, CM-I.
We demonstrate here that the shape of the cerebellar tonsils and medulla undergo a gradual recovery to normalcy after surgical expansion of the posterior fossa and subsequent creation of normal CSF pathway dimensions at the level of the foramen magnum (Figs. 2 and 3). The amount of bone removed in this study was associated with the hindbrain assuming a normal morphology. However, the minimum amount of bone that would permit the hindbrain to return to a normal morphology was not established in this study. It is unknown if removal of lesser amounts of bone using the surgical technique of extraarachnoid craniocervical decompression and duraplasty would have achieved the same results. We did not encounter any cases of cerebellar slump, which appears to require adhesion of the posterior-inferior surface of the cerebellum to the dura and was reported by Williams using the type of craniocervical decompression in which the dura and arachnoid are opened and not closed.3 Tonsillar ectopia and cervicomedullary protuberance resolved in all patients within 6 months of surgery.
Recovery of a normal shape after decompression surgery indicates that the dysmorphic features of the hindbrain in CM-I are caused not by an inherent neural malformation of the cerebellar tonsils but by a primary structural deformity of the posterior fossa and pulsatile impaction of the cerebellar tonsils into the foramen magnum.
Conclusions
Decompressive surgery restores CSF flow dynamics across the foramen magnum to normal, eliminates the impaction of the tonsils in the foramen magnum, and allows the cerebellum and the dorsal surface of the junction of the medulla and upper portion of the cervical segment of the spinal cord to recover to normal morphology. The relief of impaction of the tonsils into the foramen magnum does not restore a normal shape to the cerebellum and medulla immediately, but requires a period of up to 6 months. These observations indicate that the morphological abnormalities of the cerebellar tonsils and dorsal cervicomedullary junction are acquired, not congenital, and that CM-I should not be considered a primary CNS “malformation.”
Acknowledgments
This research was supported by the Intramural Research Program of the National Institute of Neurological Disorders and Stroke at the National Institutes of Health.
Abbreviations used in this paper
- AP
anteroposterior
- CM-I
Chiari malformation Type I
Footnotes
Disclosure
The authors report no conflict of interest concerning the materials or methods used in this study or the findings specified in this paper.
Author contributions to the study and manuscript preparation include the following. Conception and design: Heiss, Oldfield. Acquisition of data: Heiss, Suffredini. Analysis and interpretation of data: all authors. Drafting the article: Heiss, Suffredini. Critically revising the article: all authors. Reviewed submitted version of manuscript: all authors. Approved the final version of the manuscript on behalf of all authors: Heiss. Statistical analysis: Suffredini, Bakhtian. Administrative/technical/material support: Bakhtian, Oldfield. Study supervision: Heiss, Oldfield.
References
- 1.Bogdanov EI, Heiss JD, Mendelevich EG, Mikhaylov IM, Haass A. Clinical and neuroimaging features of “idiopathic” syringomyelia. Neurology. 2004;62:791–794. doi: 10.1212/01.wnl.0000113746.47997.ce. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chiari H. Concerning changes in the cerebellum due to hydrocephalus of the cerebrum. Dtsch Med Wochenschr. 1891;17:1172–1175. (Ger) [Google Scholar]
- 3.Duddy MJ, Williams B. Hindbrain migration after decompression for hindbrain hernia: a quantitative assessment using MRI. Br J Neurosurg. 1991;5:141–152. doi: 10.3109/02688699108998460. [DOI] [PubMed] [Google Scholar]
- 4.Heiss JD, Patronas N, DeVroom HL, Shawker T, Ennis R, Kammerer W, et al. Elucidating the pathophysiology of syringomyelia. J Neurosurg. 1999;91:553–562. doi: 10.3171/jns.1999.91.4.0553. [DOI] [PubMed] [Google Scholar]
- 5.Noudel R, Jovenin N, Eap C, Scherpereel B, Pierot L, Rousseaux P. Incidence of basioccipital hypoplasia in Chiari malformation type I: comparative morphometric study of the posterior cranial fossa. Clinical article. J Neurosurg. 2009;111:1046–1052. doi: 10.3171/2009.2.JNS08284. [DOI] [PubMed] [Google Scholar]
- 6.Nyland H, Krogness KG. Size of posterior fossa in Chiari type 1 malformation in adults. Acta Neurochir (Wien) 1978;40:233–242. doi: 10.1007/BF01774749. [DOI] [PubMed] [Google Scholar]
- 7.Oldfield EH. Editorial. Cerebellar tonsils and syringomyelia. J Neurosurg. 2002;97:1009–1010. doi: 10.3171/jns.2002.97.5.1009. [DOI] [PubMed] [Google Scholar]
- 8.Oldfield EH, Muraszko K, Shawker TH, Patronas NJ. Pathophysiology of syringomyelia associated with Chiari I malformation of the cerebellar tonsils. Implications for diagnosis and treatment. J Neurosurg. 1994;80:3–15. doi: 10.3171/jns.1994.80.1.0003. [DOI] [PubMed] [Google Scholar]