Abstract
Purpose
Following the report that continuous exposure of naltrexone (NTX) to drug-dependent pregnant women is safe and effective, the work was designed to develop NTX-loaded controlled delivery systems capable of making NTX available from 1 month to 4 months or greater by a single parenteral administration. Such drug-delivery systems will be useful in alleviating problems such as fetal alcohol syndrome in pregnant women and other problems associated with alcoholism.
Methods
These studies were designed to investigate in vitro drug availability and microsphere degradation (investigated by gel permeation chromatography(GPC) peak areas of water-soluble fragments released into incubation medium, changes in molecular weight with degradation time, and changes in the glass transition temperature with degradation time) of NTX-loaded poly(D,L-lactide-co-glycolide) (PDLLAGA) microspheres.
Results
Data showed that in vitro drug availability and degradation were affected by the initial molecular weight of the copolymers, the type of copolymers (lactide-co-glycolide ratio), the source of the polymer (the manufacturer), and the nature of the drug (anhydrous versus regular NTX).
Conclusion
Drug-development scientists interested in NTX-loaded microspheres for the design of controlled release devices using these polyesters should take adequate cognizance of the variables that affect drug availability from NTX-loaded microspheres. The copolymers are suitable for the fabrication of NTX-loaded microspheres capable of sustained drug release from 30 to 150 days.
Keywords: Poly(d, l-lactide-co-glycolide), controlled drug release, glass transition temperature, polyester
Introduction
Report has shown that ~7.5% of the US population (about 14 million Americans) abuse and/or are dependent on alcohol. Alcohol-related deaths account for about 5% of all deaths in the United States. Aside from human suffering, which is difficult to quantify, it is estimated that alcohol dependence costs the society about 116 billion dollars per year1. Efforts for the treatment of alcoholism include detoxification, non-pharmacological (psychosocial) treatment methods, and pharmacotherapy. The effectiveness of psychosocial treatment methods has not been established, they have met with little or no success in treating alcoholism. The focus of medication development for addictive disorders, such as alcoholism, has moved from withdrawal to relapse prevention2. Only three medications are officially available for the treatment of alcoholism: disulfiram (Antabuse), naltrexone (NTX) (REVIA®), and calcium acetylhomotaurinate (Acamprosate). Disulfiram is a deterrent medication that alters body’s response to alcohol, making its ingestion unpleasant. Consequently, its effectiveness in treating alcoholism is limited because of the reluctance by the patients to take it. Medications that reduce alcohol consumption or anticraving medications are by-products of the advances in neuroscience research and point to the possibility for the development of medications to improve the effectiveness of concurrent psychosocial or behavioral treatment of alcoholism3,4. Glutamate antagonist, acamprosate, has been investigated and found to be very promising. The effectiveness of acamprosate in the treatment of alcoholics has been reported5,6. Acamprosate has been approved in Europe and United States for the treatment of alcoholism.
It is known that opiate addiction is a very serious problem all over the world. Narcotic antagonists, compounds having chemical structures similar to those of opiates and which can preferentially occupy the body’s opiate receptors and then block the euphoric effects of opiates thereby making the use of opiates pleasureless with the concomitant removal of addiction, have been found useful for the treatment of addiction7,8. NTX is a safer and more potent narcotic antagonist compared with naloxone and cyclazocine; hence, it is the most promising narcotic antagonist used for the treatment of narcotic addiction9,10.
However, oral NTX hydrochloride, as REVIA™ tablet, has been associated with a high early dropout rate. It has been reported that treatment outcomes with NTX were not significantly better than placebo unless patients were segregated according to adherence to treatments: those who adhered to treatment protocol by taking the medication regularly performed significantly better than those who took placebo only11,12. Thus compliance is critical to the efficacy of NTX. This problem is important in that alcoholics show particularly low rates of medication compliance. Consequently, there is need for a new drug-delivery system for NTX in the treatment of alcohol dependence in the light of variable compliance with oral medication in this patient population: a drug-delivery system that can deliver a lower constant dose for a long time. Aside from the issue of compliance, orally administered NTX is highly extracted. Thus hepatic metabolism (>98% metabolized) will result in having a very low concentration of the parent drug in the brain13. In fact, the bioavailability of an oral NTX has been reported to be very low, which is due to the extensive first-pass metabolism14. An injectable long-acting delivery system will make NTX bypass the liver. Another advantage of controlled delivery systems in the administration of NTX is the high probability of achieving and maintaining a constant and predictable drug plasma concentration, which can ensure therapeutic success. It has been reported that a wide fluctuation in drug plasma levels occurs with orally administered NTX given daily to alcoholics15.
The problems associated with the use of NTX in the treatment of alcoholism, as highlighted above, are true of the use of the drug for treating narcotic addiction. Thus compliance is a serious problem with NTX because no euphoric effects are associated with the drug in the management of addiction and there are no observable pharmacological consequences for not taking it. Thus it has been used most successfully with only a select subpopulation of highly motivated individuals. For NTX to live up to its initial promise for treating either opiate addiction or alcoholism, efforts should be geared toward removing the decision-making process from the patients vis-à-vis whether to continue medication or to discontinue treatment.
Over the years, frantic efforts have been made to develop controlled drug-delivery systems for NTX with the hope of circumventing the limitations highlighted earlier, but some of them are not successful16–18.
Reports on NTX controlled drug-delivery systems based on polyesters that have met with some measures of success in clinical trials have been given. Four healthy non-smokers, who reported no history of drug and alcohol dependence, were recruited to a placebo-controlled, double-blind, outpatient trial involving NTX (52 mg) microcapsules19. No adverse effects were reported. Further studies on the same microcapsules containing either 103 mg or 206 mg of NTX were carried out in comparison with placebo in cocaine-dependent subjects20. The controlled release formulations of NTX were well-tolerated and resulted in plasma levels of 1 ng/mL or greater for >3 weeks. Moreover, opiate receptor blockade was observed. The clinical utility of the same NTX-loaded microcapsule formulation (produced by BIOTEK, Inc., Woburn, MA) has been reported21. It was shown that microcapsules containing 206 mg of NTX, in combination with psychotherapy (coping skills sessions), significantly reduced the percentage of heavy drinking days in alcoholics with minimal side effects in the participants. The same NTX-loaded microcapsule formulation, dubbed Depotrex®, containing either 192 or 384 mg of NTX was tested in heroin-dependent patients22. Plasma levels of NTX remained above 1 ng/mL for approximately 3 and 4 weeks, respectively, after administration of 192 or 384 mg of NTX. Further, the controlled release formulation of NTX (Depotrex®) was found safe and effective, with a long-lasting (about a month) antagonism of intravenous heroin. Naltrel® (a long-acting formulation of NTX) has been approved for the treatment of alcoholism. Vivitrex®/Vivitrol®, an extended-release microsphere formulation of NTX for the treatment of alcoholism, produced by Alkermes, Inc., Cambridge, MA, was found to maintain stable and pharmacologically relevant plasma concentrations of NTX for at least 28 days23. Further, changes in μ-opioid receptor density did not impact its efficacy in suppressing morphine-induced analgesia in the rat.
Other polymers have been investigated for the design of controlled delivery systems for NTX with some measures of success9,24.
All the controlled delivery systems discussed earlier provided either in vitro or in vivo sustained availability of NTX for up to 1 month. Recently, eight cases of NTX implant treatment showed significant benefits in managing pregnant heroin-dependent patients who found it difficult to shift away from heroin-dependent use patterns25. The implants comprised NTX encapsulated in poly(D,L-lactide) microspheres compressed into pellets. Each implant consisted of 10 pellets. Positive obstetric and neonatal outcomes were associated with the management of pregnant women who received the NTX implant. Most of the women received two 1.8 g NTX implants subcutaneously and remained heroin-free throughout their pregnancies. It was concluded that neonatal and maternal outcomes of the treatment of pregnant women with sustained delivery systems for NTX provided a viable alternative to methadone maintenance treatment in the pregnant heroin users. Hulse et al.26 provided data on NTX implant (two 1.8 g NTX pellets) and blood NTX and 6-β-naltrexol levels during pregnancy. Blood NTX and 6-β-naltrexol were measured at intervals for 247 days following implant administration. Blood NTX levels remained above 2 ng/mL for 247 days post-implant, which covered 214 days of gestation, because the pregnant patient received her implant 33 days prior to conception. Obstetric and neonatal outcomes associated with the implant treatments were positive, showing that a single implant procedure at or around conception would provide adequate protection against relapse back to dependent heroin use over most of the pregnancy. A collaborative effort between a research group in Spain27 and another research group in the United Kingdom28 obtained similar results.
The accounts presented earlier indicate that NTX is effective and safe even when administered to pregnant women continuously for up to 6 months for the treatment of heroin addiction. Further, reports have shown that polymeric NTX controlled delivery systems, based on poly(lactide-co-glycolide), are biocompatible29–31. We believe that it is possible to develop effective method(s) to salvage NTX, a drug that is active and useful for the treatment of alcoholism and opiate dependence. This approach may compliment other new drugs being developed for the treatment of alcoholism and save a lot of drug discovery and development cost. We have embarked on the investigation of colloidal polymeric controlled delivery systems (nanospheres and microspheres) for NTX. We have reported on the design of NTX-loaded hydrolyzable cross-linked nanospheres suitable for targeted drug delivery32,33 for the treatment of alcoholism and opiate addiction. We have extended the work to microspheres: drug-delivery devices capable of sustaining the availability of NTX from 1 month to 4 months or greater by a single parenteral administration. We were encouraged by the safety and effectiveness of continuous availability of NTX to drug-dependent pregnant women26. Babies delivered by alcoholic expectant women are exposed to fetal alcohol syndrome, aside from the suffering the mothers experience because of alcoholism. Moreover, it is of interest to study the differences in the effectiveness of controlled delivery system for NTX when the availability is for 1 month or 4 months or greater.
Materials and methods
Materials
The term PLGA is often used for general copolymers composed of lactide and glycolide units regardless of their composition. It is customary to designate poly(D,L-lactide-co-glycolide) as PDLLGA and poly(L-lactide-co-glycolide) as PLLGA. We have adopted this nomenclature in this article. PDLLGA copolymers (different molar ratios of D,L-lactide to glycolide) were used: 50:50 up to 90:10. They were obtained from either Birmingham Polymers, Inc., Birmingham, Alabama or Polysciences, Inc., Warrington, Pennsylvania as shown in Table 1. Poly(vinyl alcohol) (PVA; average molecular weight, 30,000–70,000; Sigma, Atlanta, Georgia), methylene chloride (Aldrich), and acetone (99.9%; Aldrich, Atlanta, Georgia) were used as received. NTX base (anhydrous: Code 3940; water content, 0.3%) and NTX base (regular: Code 4605; water content, 5%) were obtained from Mallinckrodt, Inc., St. Louis, MO, and used as received.
Table 1.
Poly (D,L-lactide-co-glycolide) copolymers used for the fabrication of microspheres.
| Microspheres | Copolymer composition | Initial molecular weight (g/mol)a | Amount of drug incorporated (mg) | Inherent viscosity dL/g (manufacturer) |
|---|---|---|---|---|
| PDLLGA-90:10-0 | 90:10 | 120,778 | 0 | 0.85 (Birmingham Polymers, Inc.) |
| PDLLGA-90:10-200 | 90:10 | 120,778 | 200 | 0.85 (Birmingham Polymers, Inc.) |
| PDLLGA-90:10-200 | 90:10 | 85,415 | 200 | 0.64 (Birmingham Polymers, Inc.) |
| PDLLGA-90:10-200 | 90:10 | 66,572 | 200 | 0.54 (Birmingham Polymers, Inc.) |
| PDLLGA-90:10-0 | 90:10 | 80,145 | 0 | 0.20 (Polysciences, Inc.) |
| PDLLGA-90:10-200 | 90:10 | 80,145 | 200 | 0.20 (Polysciences, Inc.) |
| PDLLGA-65:35-0 | 65:35 | 30,600 | 0 | 0.42 (Birmingham Polymers, Inc.) |
| PDLLGA-65:35-200 | 65:35 | 30,600 | 200 | 0.42 (Birmingham Polymers, Inc.) |
| PDLLGA-50:50-0 | 50:50 | 28,960 | 0 | 0.61 (Birmingham Polymers, Inc.) |
| PDLLAGA-50:50-200 | 50:50 | 28,960 | 200 | 0.61 (Birmingham Polymers, Inc.) |
Determined using GPC when the copolymers were received.
Methods
Preparation of microspheres
Empty microspheres or NTX-loaded microspheres were prepared by an oil-in-water emulsion solvent evaporation method34,35. PDLLGA of different molar ratios of D,L-lactide to glycolide were used. Two grams of PDLLGA were dissolved in 20 mL of methylene chloride, followed by the dispersion of 200 mg of NTX in the polymer solution. The polymer solution was added slowly to 50 mL of 0.5% of PVA solution being stirred at 800 revolutions per minute (rpm) to obtain an oil-in-water emulsion. After stirring for 10 min, the stirring rate was reduced to 400 rpm and continued overnight to allow the methylene chloride to evaporate slowly. The microspheres formed were filtered and vacuum-dried.
Particle size analysis
The average particle size and size distribution were determined by light-scattering method34,36 using an Accusizer Model 770 Particle Sizer and a Branson Sonifier 250. Approximately 20–50 mg of microsphere sample was added to a 20-mL scintillation vial. A solution (10 mL) containing 0.1% (w/w) Triton X-100 in distilled water was added to the scintillation vial. The sample was continuously sonicated for 30 ± 10 sec at 10 power using the Branson Sonifier 250 under pulse mode until the microspheres were thoroughly dispersed. The sample was added dropwise to the particle sizer until particle count of 4000 mL−1 was achieved. The system was then set on autodilution during data collection. The value reported is an average of two determinations.
Scanning electron microscopy
The shape and morphology of the microspheres were investigated using scanning electron microscope34,35,37. The microsphere samples were mounted on double side carbon disks and then coated with 20 nm of gold by a gold sputtering coater. The scanning electron micrograph (SEM) was prepared using a JEOL JSM-6400 scanning electron microscope under 5 keV and 15 mm working distance.
Loading efficiency
Loading efficiency was determined by precipitating the solution of the microspheres in ethanol. The supernatant was centrifuged for 1 h and quantitation was done spectrophotometrically at 280 nm according to the standard curve prepared from a solution of NTX.
where Mactual is the actual amount of NTX in each batch of the microspheres as determined experimentally, and Mtheoretical is the theoretical amount of NTX in each batch of the microspheres calculated from the quantity added during the fabrication process.
In vitro availability of NTX
Studies on in vitro release of NTX from the microspheres were carried out in 50-mL cylindrical tubes containing 40 mL of buffer (PBS, pH 7.4) containing a known weight of NTX-loaded microspheres. The dispersion was incubated at 37°C in a Labquake tube shaker capable of 360° rotation34. At predetermined time intervals, 2 mL sample was taken out and replaced with 2 mL fresh buffer solution maintained at the same temperature. Quantitation was at 280 nm according to the standard curve prepared from a solution of NTX in PBS buffer, pH 7.4. Each release experiment was carried out in triplicate.
Microsphere degradation studies
A known weight of the microspheres was suspended in 2 mL of buffer (PBS, pH 7.4) in an Eppendorf tube. All tubes were incubated at 37°C. Enough samples were prepared such that one tube could be taken out at time intervals; the time intervals were made to correspond to the time intervals used for the in vitro NTX availability studies. After centrifugation, the aqueous phase was collected for gel permeation chromatographic (GPC) analysis, while the degraded microsphere was dried and kept for further analysis. Mass loss was determined gravimetrically by comparing the dry weight remaining at a specific time interval with the initial weight.
Gel permeation chromatography
Molecular weights of the PDLLGA copolymers and the degraded fragments were characterized by GPC (Waters 2690 separation modules). Waters 2410 differential refractive index detector was used. In the analysis of aqueous phase containing degraded PDLLGA copolymer fragments, 0.1 M NaNO3 aqueous solution was used as eluent, at a flow rate of 0.7 mL/min. Two Waters analytical columns, UltraHydrogel 120 and UltraHydrogel Linear, were used. The operating temperature for both the columns and the detector was set to 35°C. The molecular weight was calibrated by a series of PEG standards (Waters). Samples from the supernatant (aqueous) of the degradation studies were used as obtained. For the analysis of the residual copolymers as microspheres degradation progressed, the same separation modules and detector were used. Tetrahydrofuran (THF) was used as the eluent at a flow rate of 0.7 mL/min. Two PolyLab analytical columns were used. The molecular weight was calibrated by a series of polystyrene standard (PolyLab®). The samples were prepared by dissolving the dried microspheres in THF at a concentration of 0.05–0.2% w/v and filtered through a 0.45-μm cellulose membrane filter38.
Differential scanning calorimetry
Materials retrieved by drying the remaining part of the degraded microspheres were characterized by differential scanning calorimetry (DSC) using TA instruments DSC-2920 under a nitrogen atmosphere at a temperature scanning rate of 20°C/min. The midpoint values are reported as Tg.
Results and discussion
In vitro availability
Biodegradable polymers have received a lot of attention in the design of devices for spatial placement and temporal delivery of many classes of bioactive agents because they can be reabsorbed by or eliminated easily from the body and there is no need for a surgical intervention29–31,39. Aside from the issues of biodegradability, biocompatibility, and easy elimination from the body, poly(lactic acid), poly(glycolic acid), and their copolymers are a very fascinating class of biodegradable polymers because various combinations of formulation and process variables can be employed in the design of drug-delivery systems (microspheres, nanospheres, slabs, etc.) capable of controlled drug release ranging from days to months or years40,41. A review of NTX long-acting formulations (drug availability is for about 1 month) based on polyesters in the treatment of alcohol dependence has been given42. This research work is an attempt to develop microsphere formulations for NTX: some capable of sustaining the release of NTX for a short time (1 month) and others for up to 4 months or longer by a single administration. Successful developments of the formulations will provide a unique opportunity to compare them in the clinical setting. PDLLGA copolymers used in this work were not made in-house but purchased. Consequently, changes in the physicochemical properties investigated were in accordance with what the availability of the commercial products would allow.
Figure 1 shows the influence of copolymer molecular weight and source of copolymer on the in vitro availability of NTX base (regular). The copolymer composition was kept constant at 90:10 (D,L-lacitde:glycolide). Table 1 shows the copolymer compositions, the sources, and the molecular weights of the copolymers determined when received from the manufacturers. For microspheres fabricated from copolymers made by the same supplier (Birmingham Polymers, Inc.), molecular weight has no effect on drug release. The multiphasic drug-release profile is characteristic of microsphere (made from polyester copolymer) drug-delivery systems34,43,44. Figure 1 shows that microspheres made from copolymers of the same composition but different supplier (Polysciences, Inc. or Birmingham Polymers, Inc.; Table 1) exhibited different drug-release profiles. The NTX-loaded microspheres prepared from polyester made by Polysciences, Inc. showed a burst effect, which lasted for about 10 days. It was followed by a period of diffusion-controlled release. The release rate slowed down at about 28 days; then a constant release profile occurred again till about 99 days when what appeared to be a degradation-controlled phase occurred. The release profile is complex and multiphasic. Another interesting phenomenon is that the initial molecular weight of this copolymer (90:10, intrinsic viscosity (IV) 0.20) from Polysciences, Inc. is greater than that of 90:10 (IV 0.54) from Birmingham Polymers, Inc.; yet, the release rate up to 123 days was faster. This observation underscores the statement that any change in the source of materials of formulation necessitated extensive testing to prove equivalence. All the formulations sustained the in vitro availability of NTX for up to 4.5 months.
Figure 1.
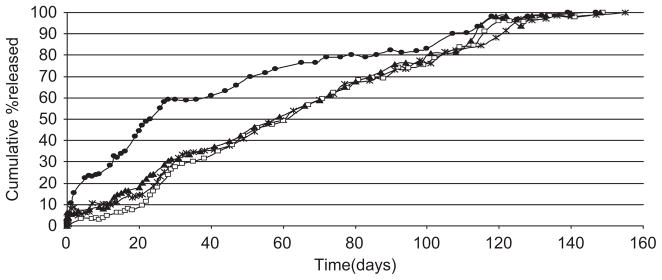
Influence of copolymer molecular weight and intrinsic viscosity [using a single copolymer composition (PDLLGA-90:10)] on the release of regular naltrexone from microspheres [● (0.20 dL/g); □ (0.64 dL/g); ▶ (0.85 dL/g); * (0.54 dL/g); (ε: 0.06% to 7.19%)].
Figure 2 shows that there is no difference in the in vitro availability of anhydrous NTX from copolymers of the same composition (PDLLGA-90:10) but different molecular weights and sources (Polysciences, Inc. and Birmingham Polymers, Inc.). As shown in Figure 3, the effect of copolymer composition (i.e. the ratio of lactic acid to glycolic acid) is not much for PDLLGA 50:50 and PDLLGA 65:35. The release of regular NTX was rapid, and ended within 25 days. It is monophasic. However, the effect of copolymer composition becomes very discernible and pronounced at high ratio of lactic acid to glycolic acid (PDLLGA 90:10), which may be due to alteration in the crystallinity, hydrophobicity, and biodegradability of the polyesters34,45. In vitro and in vivo availabilities of leuprorelin acetate from microspheres prepared form PLGA 75:25 were faster than those fabricated from PLGA 90:1046.
Figure 2.
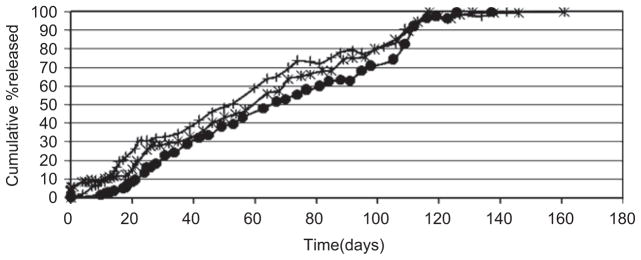
Influence of copolymer molecular weight and intrinsic viscosity [using a single copolymer composition (PDLLGA-90:10)] on the release of anhydrous naltrexone from microspheres [● (0.85 dL/g); + (0.20 dL/g); * (0.64 dL/g); (ε: 0.1% to 3.5%)].
Figure 3.
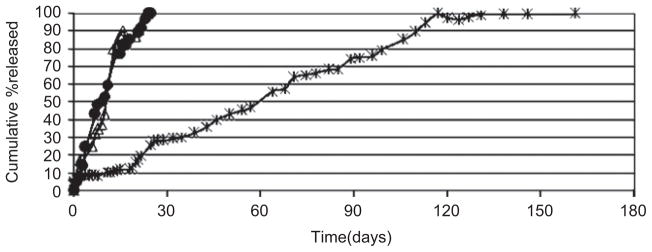
Influence of copolymer composition on the release of regular naltrexone from PDLLGA microspheres [● 50:50 (0.61 dL/g); △ 65:35 (0.42 dL/g); * 90:10 (0.85 dL/g); (ε: 0.16% to 3.12%)].
Particle size analysis, scanning electron microscopy, and encapsulation efficiency studies
Figure 4 shows the typical particle size distribution and SEM of NTX-loaded microspheres investigated in this work. The microspheres have smooth surfaces and are spherical. Table 2 shows the average particle size and the encapsulation efficiency. Particle size data and encapsulation efficiency are reported as average of two determinations. It appears that there is a trend of decrease in particle size with increase in the hydrophilicity of the microspheres fabricated from Birmingham copolymers loaded with regular NTX (PDLLGA (90:10) Bpi (R) < PDLLGA (65:35) Bpi (R) < PDLLGA (50:50) Bpi (R)). The trend is reversed in the loading efficiency ((PDLLGA (90:10) Bpi (R) > PDLLGA (65:35) Bpi (R) > PDLLGA (50:50) Bpi (R)). Comparing microspheres fabricated from Polysciences copolymer (90:10) with Birmingham copolymer (90:10) loaded with regular NTX (200 mg), it appears that the particle size of PDLLGA (90:10) Poly sci. (R) is less than PDLLGA (90:10) Bpi (R). The trend is similar for encapsulation efficiency. Comparing microspheres fabricated from Polysciences copolymer (90:10) with Birmingham copolymer (90:10) loaded with anhydrous NTX (200 mg), it appears that the particle size of PDLLGA (90:10) Poly sci. (AN) is higher than PDLLGA (90:10) Bpi (AN). The trend is reversed for encapsulation efficiency. The data on particle size analysis and encapsulation efficiency could not suggest the type of trend seen in in vitro availability data that could be attributed to the type of drug or source of the materials. A more comprehensive study with factorial statistical experimental design will be probably more revealing.
Figure 4.
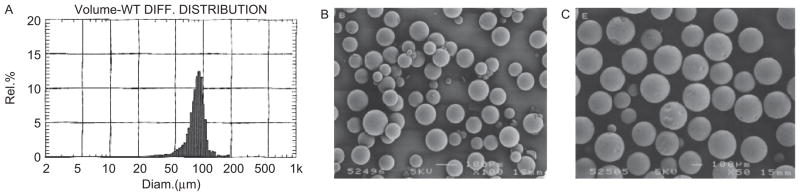
A typical particle size distribution of regular naltrexone (NTX)-loaded microspheres (A: PDLLGA-90:10) and typical scanning electron micrographs of regular NTX-loaded microspheres (B: PDLLGA-65:35) and (C: PDLLGA-90:10).
Table 2.
Influence copolymer composition and copolymer source on average particle size and the encapsulation efficiency of PDLLGA naltrexone (NTX)-loaded microspheres (average of two determinations).
| Copolymers | Particle size (μm) | Encapsulation efficiency (%) |
|---|---|---|
| PDLLGA (90:10)Poly sci. (AN) | 86.13 | 63.87 |
| PDLLGA (90:10)Poly sci. (R) | 58.41 | 58.82 |
| PDLLGA (90:10)Bpi (AN) | 64.37 | 68.55 |
| PDLLGA (90:10)Bpi (R) | 62.51 | 75.87 |
| PDLLGA (65:35)Bpi (R) | 67.57 | 65.07 |
| PDLLGA (50:50)Bpi (R) | 76.15 | 58.22 |
R = Regular NTX; AN = anhydrous NTX.
Degradation studies
Degradation products of PDLLGA in aqueous phase
The water-soluble low-molecular-weight materials that appeared in the water phase were monitored by aqueous phase GPC. The most typical aqueous GPC results of the samples have two peaks; some samples contain a third peak in between which appears after a period of degradation (data not shown). The molecular weights of the two peaks that appear in most of the samples are around 1100 and 630 g/mol. The molecular weights of these peaks vary little between different copolymer compositions, various intrinsic viscosities (i.e. average molecular weights of the copolymers), or manufacturer, and with or without drug loading. They did not change with time in the incubation medium. It seems these materials are from the microsphere copolymers and are not associated with the release of the drug or degradation of the copolymers. The samples from early period of the degradation study usually do not have the third peak and the third peak only appears after certain periods of degradation of the microspheres. The molecular weight of this peak ranged from 650 to 870. The area of this peak was also characterized by GPC; it is a measure of microsphere degradation47.
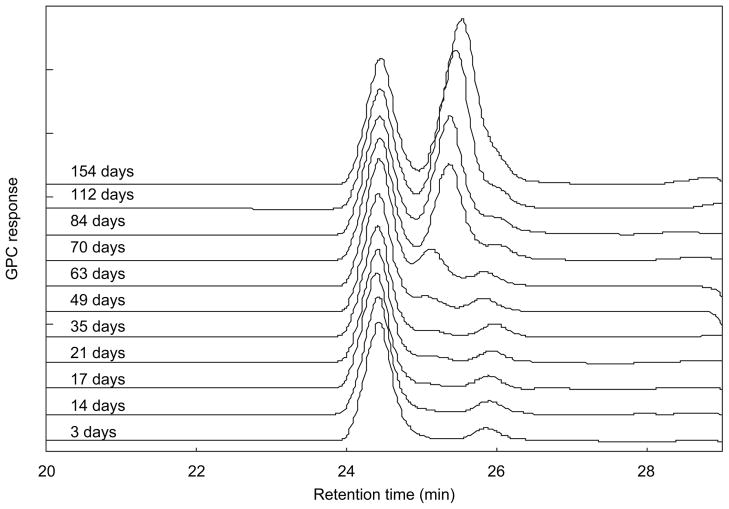
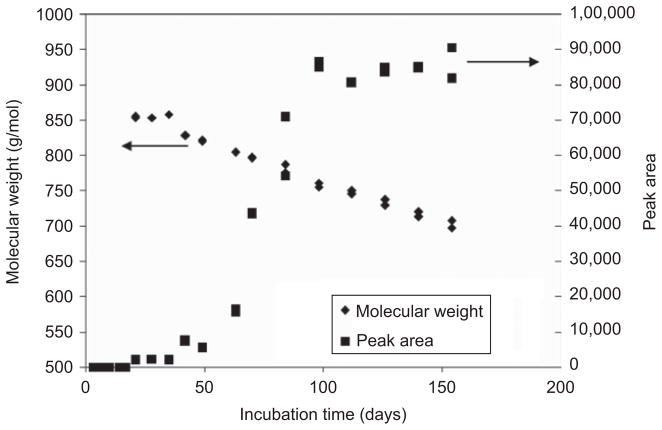
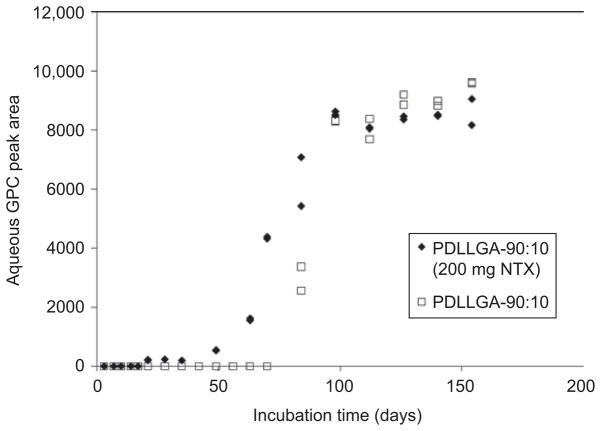
Figure 5 is an overlay of a series of aqueous GPC elution profiles at different degradation times for PDLLGA-90:10 (prepared with 200 mg regular NTX base) microspheres (IV 0.85 dL/g, from Birmingham Polymers, Inc.). It can be seen that the molecular weight of the third peak is decreasing steadily but slightly with time after 40 days of incubation, and the value that started at 850 g/mol dropped to 700 g/mol at the end of the study (154 days). The area of this peak (which changes with time) indicates the relative amount of the water-soluble materials in the incubation medium. Figure 6 is a plot of the change in molecular weight and the change in peak area of the aqueous degradation products with incubation time. Similar results were obtained in the study of PDLLGA-90:10 without NTX and PDLLGA-90:10 with anhydrous NTX (data not shown).
Figure 5.
Aqueous phase gel permeation chromatographic (GPC) overlay of elution profiles of PDLLGA-90:10 (prepared with 200 mg regular naltrexone) microsphere at different incubation time.
Figure 6.
Changes in molecular weights and peak areas of the aqueous phase degradation products from PDLLGA-90:10 (prepared with 200 mg regular naltrexone) microsphere at different incubation time.
As shown in Figures 5 and 6, the material corresponding to the third peak becomes detectable by GPC after 20 days of incubation (which corresponds to the change in the in vitro release isotherms in Figures 1 and 2). The amount of the material did not change much before 50 days; thereafter, the peak area increased dramatically. This increase slowed down and stopped after 100 days of incubation, and then became constant. Obviously, the materials corresponding to this third GPC peak are the low-molecular-weight water-soluble materials released from the microspheres. The molecular weight of the low-molecular-weight water-soluble materials also decreased with time almost linearly (Figure 6). The behavior of these low-molecular-weight PDLLGA water-soluble fractions demonstrated a dramatic change between 50 and 100 days of incubation. To investigate this observation further, the microspheres from the degradation studies were retrieved and dried. The molecular weights of the retrieved microsphere materials were measured by organic phase GPC using THF as eluent, as reported in the next section; glass transition temperature was investigated as well by DSC. Our interest in investigating the molecular weight changes of retrieved microsphere materials with degradation time is also due to the report that in vitro and in vivo degradation profiles of PLGA are similar in terms of molecular weight decrease48–51.
Molecular weight of microspheres
Results show that the molecular weight of the microspheres does not change significantly before 50 days or after 100 days of the degradation study for the PDLLGA-90:10 (prepared with 200 mg regular NTX) microspheres (data not shown). However, between 50 and 100 days, the molecular weight value dropped dramatically. This change was observed at the same time range as the corresponding change of the peak area of water-soluble degradation fragments found in the aqueous phase. This observation confirms the belief that the degradation of the PLGA microspheres is usually slow at the beginning, and then when the incubation reaches a certain time (here 50 days for PDLLGA-90:10 prepared with 200 mg of regular NTX), the process is significantly accelerated.
Glass transition temperature (Tg)
Tg is a very important property of polymeric materials. Many properties of polymeric materials change significantly when the temperature changes across the glass transition temperature. For the biodegradable controlled drug-release materials such as polyesters, the glass transition temperature of the material decreases in accordance with the Fox–Flory equation47,52: Tg decreases with decrease in molecular weight.
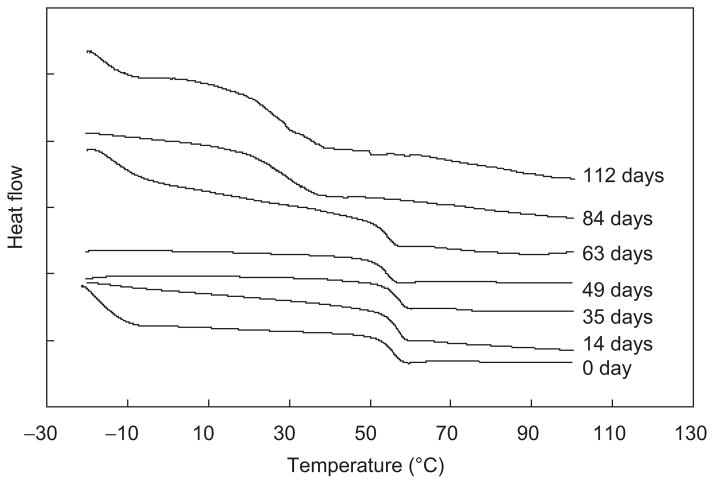
Figure 7 is an overlay of DSC thermograms of materials remaining from PDLLGA-90:10 (prepared with 200 mg of regular NTX) microspheres after degradation for different days. A similar change in profile was observed for the glass transition temperatures of the retrieved microsphere materials (Figure 7) and the amount of the materials in the aqueous phase solution at various incubation time periods (GPC peak area) (data not shown). It is an indication of the progressive nature of microsphere degradation with time. The initial glass transition temperature of the PDLLGA-90:10 microsphere (prepared with 200 mg of regular NTX) is around 57°C (Figure 7). This glass transition temperature did not change appreciably for 60 days of incubation. Then, the Tg dropped dramatically from 57°C to a level below 30°C in the time frame of 60 to 80 days of the degradation study. This change is associated with the degradation process. Thus the degradation process decreases the molecular weight of the polymer and then lowers the glass transition temperature. As the glass transition temperature decreases, it is easier for the water molecules to diffuse into microspheres and for the degraded fragments to move out of the microspheres, thereby accelerating the degradation process.
Figure 7.
An overlay of DSC thermograms for PDLLGA-90:10 (prepared with 200 mg of regular naltrexone) microspheres remaining after degradation for different days.
Data on in vitro availability show the cumulated percentage of NTX released from PDLLGA-90:10 (prepared with 200 mg of NTX) microspheres plotted versus time (Figures 1–3). In the beginning of the release study, there is a brief burst release. After the burst release, a diffusion-controlled period is observed (characterized by a slow rate of release). After about 20 days of drug release, acceleration of drug release began, which could be attributed to degraded copolymer, lowering of glass transition temperature, and faster diffusion out of the drug as more water gained access to the structure of the microspheres. The reason for the long period of nearly constant NTX release in PDLLGA-90:10 microspheres (Figures 1–3) might be due to decrease in the molecular weight of the microsphere materials immediately the microspheres got in contact with the release medium, but material loss (complete degradation) did not occur until a critical molecular weight was reached. Thus NTX was being released via diffusion though the increasing water-filled pores of the microspheres. Accelerated release was also seen at the end of the release studies when complete breakdown of the microspheres occurred (around 100 days).
Effect of copolymer composition
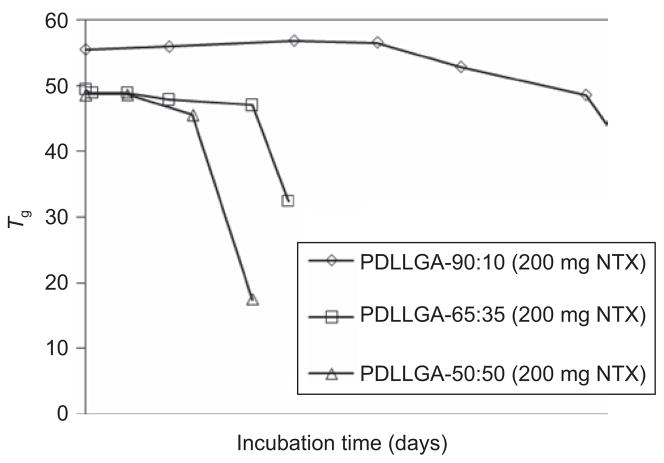
Three different copolymer compositions were studied in this work: PDLLAGA-90:10, PDLLAGA-65:35, and PDLLAGA -50:50. The rate of degradation is expected to reflect the hydrophilicity of the copolymers. Data were obtained on the aqueous phase GPC peak areas for the fragments released from PDLLGA microspheres of different copolymer compositions. With increasing amount of glycolide unit in the copolymers, the degradation process became significantly accelerated (data not shown). The degradation pattern was also confirmed by molecular weight and glass transition temperature changes of the microspheres with incubation time. Changes in the molecular weight of the microspheres at different incubation time were observed (data not shown), a reflection of the different glycolide content. A dramatic drop in molecular weight occurred after 50 days of incubation in the case of PDLLGA-90:10 (200 mg NTX). For PDLLGA-65:35 (200 mg NTX), within 20 days the molecular weight started to fall rapidly; the corresponding time was 14 days for PDLLGA-50:50 (200 mg NTX). The decrease in glass transition temperatures of the microspheres with incubation time (Figure 8) parallels the decrease in molecular weight with incubation time (data not shown). The release of drug is also accelerated with increasing hydrophilic fraction in the copolymers (Figure 3).
Figure 8.
Changes in the glass transition temperature of PDLLGA microspheres of different copolymer compositions (lactide:glycolide ratio = 90:10, 65:35, and 50:50) as a function of time.
Effect of drug loading on microsphere degradation
Loading of drug in the microsphere polymer matrix may have significant effects on the mechanism of drug release, as well as degradation behavior of the polymer matrix. The PDLLGA-90:10 copolymer was selected as microsphere matrix material to study the effect of drug loading. The amount of drug used in the fabrication of the microspheres was 200 mg. Figure 9 shows the differences in the degradation of the PDLLGA-90:10 microspheres with (200 mg NTX) and without the drug. The degraded water-soluble fragments of PDLLGA-90:10 appeared in the aqueous phase on Day 20 of incubation and significant increase in the amount of the fragments started on Day 50 (Figure 9). Comparing with the microspheres prepared with the same copolymer but without drug, no decomposed water-soluble fragment of PDLLGA-90:10 was discernible until 70 days. Then it increased significantly. Very similar phenomena were observed for the copolymer molecular weight (Figure 10) and the glass transition temperature (data not shown). Drug loading accelerated the degradation of the microspheres, possibly due to the plasticizing effect of the drug followed by decrease in Tg53.
Figure 9.
Effect of drug loading on the degradation of naltrexone (NTX)-loaded PDLLGA-90:10 microspheres.
Figure 10.
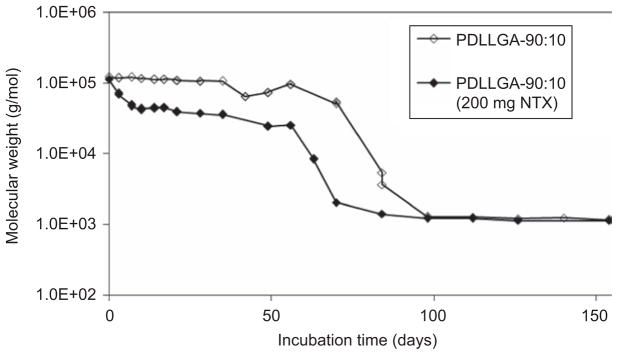
Changes in molecular weight of PDLLGA-90:10 microspheres with and without drug loading.
Effect of the polymer IV
Aside from the composition of the copolymers and drug loading, other properties of the copolymer are also important in the degradation of microspheres and the release behavior. With fixed composition and without drug loading, two PDLLGA-90:10 copolymer microspheres from different sources and different intrinsic viscosities were compared in the degradation studies. PDLLGA-90:10 (Bm) bought from Birmingham Polymers, Inc. has an IV of 0.85 dL/g and PDLLGA-90:10 (Poly) bought from Polysciences, Inc. has a lower IV of 0.20 dL/g. GPC results show that PDLLGA-90:10 (Bm) has a higher initial molecular weight of 120,778 g/mol compared with 80,145 g/mol of PDLLAGA-90:10 (Poly) (Table 1). The PDLLGA-90:10 (Bm) microspheres gave a higher glass transition temperature profile than PDLLAGA-90:10 (Poly) microspheres. The molecular weight of PDLLGA-90:10 (Bm) microspheres (which has a higher initial molecular weight and prepared with 200 mg regular NTX) slightly decreased with time in the first 50 days of immersion in PBS buffer (data not shown); in contrast, PDLLGA-90:10 (Poly) microspheres prepared with 200 mg regular NTX, with a lower initial molecular weight, exhibited a continuous decrease in molecular weight right from the beginning of the degradation studies (data not shown). The differences in the molecular weight profile with incubation time are reflected in the changes in the glass transition temperature profiles (data not shown). These data explain the influence of the source of copolymers and initial molecular weight of the copolymers on in vitro availability of regular NTX (Figure 1).
Conclusion
The data obtained from studies on NTX-loaded PDLLGA microspheres reveal that the in vitro availability and degradation (investigated by the GPC peak areas of water-soluble fragments released into incubation medium, changes in molecular weight with degradation time, and changes in the glass transition temperature with degradation time) were affected by the initial molecular weight of the copolymers, the type of copolymers (lactide-co-glycolide ratio), the source of the copolymer, and the nature of the drug (anhydrous NTX versus regular NTX). A drug-development scientist interested in the development of NTX-loaded microspheres for the design of controlled release devices using these polyesters should take adequate cognizance of the variables highlighted earlier. The copolymers are suitable for the fabrication of NTX-loaded microspheres capable of sustained drug release from 30 to 150 days.
Footnotes
Declaration of interest
Supported by NIH/NIAAA Grant # 5 R21AA13407-03. This work was carried out in a laboratory supported by NIH/NCRR Grant # 1C06RR020608-01
References
- 1.Weinrieb RM, O’Brien CP. Naltrexone in the treatment of alcoholism. Annu Rev Med. 1997;48:477–487. doi: 10.1146/annurev.med.48.1.477. [DOI] [PubMed] [Google Scholar]
- 2.Fuller RK, Hiller-Sturmhöfel S. Alcoholism treatment in the United States. An overview. Alcohol Res Health. 1999;23:69–77. [PMC free article] [PubMed] [Google Scholar]
- 3.Johnson BA, Ait-Daoud N. Medications to treat alcoholism. Alcohol Res Health. 1999;23:99–106. [PMC free article] [PubMed] [Google Scholar]
- 4.Litten RZ, Allen J, Fertig J. Pharmacotherapies for alcohol problems: a review of research with focus on developments since 1991. Alcohol Clin Exp Res. 1996;20:859–876. doi: 10.1111/j.1530-0277.1996.tb05264.x. [DOI] [PubMed] [Google Scholar]
- 5.Swift RM. Drug therapy for alcohol dependence. N Engl J Med. 1999;340:1482–1490. doi: 10.1056/NEJM199905133401907. [DOI] [PubMed] [Google Scholar]
- 6.Paille FM, Guelfi JD, Perkins AC, Royer RJ, Steru L, Parot P. Double-blind randomized multicentre trial of acamprosate in maintaining abstinence from alcohol. Alcohol Alcohol. 1995;30:239–247. [PubMed] [Google Scholar]
- 7.Milne GM, Jr, Johnson MR. Narcotic antagonists and analgesia. Annu Rep Med Chem. 1976;11:23–32. [Google Scholar]
- 8.O’Brien CP, Greenstein R, Ternes J, Woody GE. Clinical pharmacology of narcotic antagonists. Ann N Y Acad Sci. 1978;311:232–240. doi: 10.1111/j.1749-6632.1978.tb16779.x. [DOI] [PubMed] [Google Scholar]
- 9.Maa YF, Heller J. Controlled release of naltrexone pamoate from linear poly(ortho ester) J Control Release. 1990;14:21–23. [Google Scholar]
- 10.Chien YW. Long-acting parenteral drug formulations. J Parenter Sci Technol. 1981;35:106–139. [PubMed] [Google Scholar]
- 11.Volpicelli JR, Rhines KC, Rhines JS, Volpicelli LA, Alterman AI, O’Brien CP. Naltrexone and alcohol dependence. Role of subject compliance. Arch Gen Psychiatry. 1997;54:737–742. doi: 10.1001/archpsyc.1997.01830200071010. [DOI] [PubMed] [Google Scholar]
- 12.Chick J, Anton R, Checinski K, Croop R, Drummond DC, Farmer R, Labriola D, Marshall J, Moncrieff J, Morgan MY, Peters T, Ritson B. A multicentre, randomized, double-blind, placebo-controlled trial of naltrexone in the treatment of alcohol dependence or abuse. Alcohol Alcohol. 2000;35:587–593. doi: 10.1093/alcalc/35.6.587. [DOI] [PubMed] [Google Scholar]
- 13.Johnson DAW. Observations on the use of long-acting depot neuroleptic injections in the maintenance therapy of schizophrenia. J Clin Psychiatry. 1984;5:13–21. [PubMed] [Google Scholar]
- 14.Wall ME, Brine DR, Perez-Reyes M. Metabolism and disposition of naltrexone in man after oral and intravenous administration. Drug Metab Dispos. 1981;9:369–375. [PubMed] [Google Scholar]
- 15.Verbey K. The clinical pharmacology of naltrexone: pharmacology and pharmacodynamics. NIDA Res Monogr. 1980;28:147–158. [PubMed] [Google Scholar]
- 16.Wise DL. Development of drug delivery systems for use in treatment of narcotic addiction. A culmination. In: Wise DL, editor. Biopolymeric Controlled Release Systems. Vol. 1. CRC; Boca Raton, Florida: 1984. pp. 115–181. [Google Scholar]
- 17.Martin WR, Sandquist VL. A sustained release depot for narcotic antagonists. Arch Gen Psychiatry. 1974;30:31–33. doi: 10.1001/archpsyc.1974.01760070019003. [DOI] [PubMed] [Google Scholar]
- 18.Chiang CN, Kishimoto A, Barnett G, Hollister LE. Implantable narcotic antagonists: a possible new treatment for narcotic addiction. Psychopharmacol Bull. 1985;21:672–675. [PubMed] [Google Scholar]
- 19.Heishman SJ, Francis-Wood A, Keenan RM, Chiang CN, Terrill JB, Tai B, Henningfield JE. Safety and pharmacokinetics of a new formulation of depot naltrexone. In: Harris LS, editor. Problems of Drug Dependence. Vol. 2. 1994. p. 82. NIDA Research Monograph, No. 141. [Google Scholar]
- 20.Alim TN, Tai B, Chiang CN, Green T, Rosse RB, Lindquist T, Deutsch SI. Tolerability study of a depot form of naltrexone substance abusers. In: Harris LS, editor. Problems of Drug Dependence. Vol. 2. 1995. p. 253. NIDA Research Monograph, No. 153. [Google Scholar]
- 21.Kranzler HR, Modesto-Lowe V, Nuwayser ES. Sustained-release naltrexone for alcoholism treatment: a preliminary study. Alcohol Clin Exp Res. 1998;22:1074–1079. [PubMed] [Google Scholar]
- 22.Comer SD, Collins ED, Kleber HD, Nuwayser ES, Kerrigan JH, Fischman MW. Depot naltrexone: long-lasting antagonism of the effects of heroin in humans. Psychopharmacology (Berl) 2002;159:351–360. doi: 10.1007/s002130100909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bartus RT, Emerich DF, Hotz J, Blaustein M, Dean RL, Perdomo B, Basile AS. Vivitrex, an injectable, extended-release formulation of naltrexone, provides pharmacokinetic and pharmacodynamic evidence of efficacy for 1 month in rats. Neuropsychopharmacology. 2003;28:1973–1982. doi: 10.1038/sj.npp.1300274. [DOI] [PubMed] [Google Scholar]
- 24.Bennett DB, Li X, Adams NW, Kim SW, Hoes CJT, Feijen J. Biodegradable polymeric drugs for naltrexone. J Control Release. 1991;16:43–52. [Google Scholar]
- 25.Hulse G, O’Neil G. Using naltrexone implants in the management of the pregnant heroin user. Aust N Z J Obstet Gynaecol. 2002;42:569–573. doi: 10.1111/j.0004-8666.2002.548_14.x. [DOI] [PubMed] [Google Scholar]
- 26.Hulse GK, Arnold-Reed DE, O’Neil G, Hansson RC. Naltrexone implant and blood naltrexone levels over pregnancy. Aust N Z J Obstet Gynaecol. 2003;43:386–388. doi: 10.1046/j.0004-8666.2003.00121.x. [DOI] [PubMed] [Google Scholar]
- 27.Carreño JE, Alvarez CE, Narciso GI, Bascarán MT, Díaz M, Bobes J. Maintenance treatment with depot opioid antagonists in subcutaneous implants: an alternative in the treatment of opioid dependence. Addict Biol. 2003;8:429–438. doi: 10.1080/13556210310001646402. [DOI] [PubMed] [Google Scholar]
- 28.Foster J, Brewer C, Steele T. Naltrexone implants can completely prevent early (1-month) relapse after opiate detoxification: a pilot study of two cohorts totalling 101 patients with a note on naltrexone blood levels. Addict Biol. 2003;8:211–217. doi: 10.1080/1355621031000117446. [DOI] [PubMed] [Google Scholar]
- 29.Schwope AD, Wise DL, Howes JF. Lactic/glycolic acid polymers as narcotic antagonist delivery systems. Life Sci. 1975;17:1877–1885. doi: 10.1016/0024-3205(75)90473-7. [DOI] [PubMed] [Google Scholar]
- 30.Yamaguchi K, Anderson JM. Biocompatibility studies of naltrexone sustained release formulations. J Control Release. 1992;19:299–314. [Google Scholar]
- 31.Schmidt C, Wenz R, Nies B, Moll F. Antibiotic in vivo/in vitro release, histocompatibility and biodegradation of gentamicin implants based on lactic acid polymers and copolymers. J Control Release. 1995;37:83–94. [Google Scholar]
- 32.Yin W, Akala EO, Taylor RE. Design of naltrexone-loaded hydrolyzable crosslinked nanoparticles. Int J Pharm. 2002;244:9–19. doi: 10.1016/s0378-5173(02)00297-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Akala EO, Wang H, Adedoyin A. Disposition of naltrexone after intravenous bolus administration in Wistar rats, low-alcohol-drinking rats and high-alcohol-drinking rats. Neuropsychobiology. 2008;58:81–90. doi: 10.1159/000159776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Akala EO, Scott KR. Studies on α-tocopherol-loaded biodegradable microspheres. Pharm Pharmacol Lett. 2001;2:72–75. [Google Scholar]
- 35.Yoncheva K, Lambov N, Miloshev S. Modification of biodegradable poly(malate) and poly(lactic-co-glycolic acid) microparticles with low molecular polyethylene glycol. Drug Dev Ind Pharm. 2009;35:449–454. doi: 10.1080/03639040802438357. [DOI] [PubMed] [Google Scholar]
- 36.Gungor OnS, Okyar A, Erturk-Toker S, Baktir G, Ozsoy Y. Ondansetron-loaded chitosan microspheres for nasal antiemetic drug delivery: an alternative approach to oral and parenteral routes. Drug Dev Ind Pharm. 2010;36:806–813. doi: 10.3109/03639040903517906. [DOI] [PubMed] [Google Scholar]
- 37.Fan Y, Shan-Guang W, Yu-Fang P, Feng-Lan S, Tao L. Preparation and characteristics of erythromycin microspheres for lung targeting. Drug Dev Ind Pharm. 2009;35:639–645. doi: 10.1080/03639040802512243. [DOI] [PubMed] [Google Scholar]
- 38.Diab R, Hamoudeh M, Boyron O, Elaissari A, Fessi H. Microencapsulation of cytarabine using poly(ethylene glycol)–poly(epsilon-caprolactone) diblock copolymers as surfactant agents. Drug Dev Ind Pharm. 2010;36:456–469. doi: 10.3109/03639040903261989. [DOI] [PubMed] [Google Scholar]
- 39.Reed AM, Gilding DK. Biodegradable polymers for use in surgery—poly(glycolic)/poly(lactic acid) homo and copolymers: 2. In vitro degradation. Polymer. 1981;22:494–498. [Google Scholar]
- 40.Tice TR, Mason DW, Gilly RM. Clinical use and future of parenteral microsphere delivery systems. In: Prescott LF, Nimmo WS, editors. Novel Drug Delivery and its Therapeutic Application. John Wiley & Sons; England: 1989. pp. 223–233. [Google Scholar]
- 41.Merkli A, Tabatabay C, Gunny R, Heller J. Biodegradable polymers for the controlled release of ocular drugs. Prog Polym Sci. 1998;23:563–580. [Google Scholar]
- 42.Johnson BA. Naltrexone long-acting formulation in the treatment of alcohol dependence. Ther Clin Risk Manag. 2007;3:741–749. [PMC free article] [PubMed] [Google Scholar]
- 43.Dunne M, Corrigan I, Ramtoola Z. Influence of particle size and dissolution conditions on the degradation properties of polylactide-co-glycolide particles. Biomaterials. 2000;21:1659–1668. doi: 10.1016/s0142-9612(00)00040-5. [DOI] [PubMed] [Google Scholar]
- 44.Sheshala R, Peh KK, Darwis Y. Preparation, characterization, and in vivo evaluation of insulin-loaded PLA–PEG microspheres for controlled parenteral drug delivery. Drug Dev Ind Pharm. 2009;35:1364–1374. doi: 10.3109/03639040902939213. [DOI] [PubMed] [Google Scholar]
- 45.Kitchell JP, Wise DL. Poly(lactic/glycolic acid) biodegradable drug–polymer matrix systems. Meth Enzymol. 1985;112:436–448. doi: 10.1016/s0076-6879(85)12034-3. [DOI] [PubMed] [Google Scholar]
- 46.Okada H, Doken Y, Ogawa Y, Toguchi H. Preparation of three-month depot injectable microspheres of leuprorelin acetate using biodegradable polymers. Pharm Res. 1994;11:1143–1147. doi: 10.1023/a:1018936815654. [DOI] [PubMed] [Google Scholar]
- 47.Park TG. Degradation of poly(lactic-co-glycolic acid) microspheres: effect of copolymer composition. Biomaterials. 1995;16:1123–1130. doi: 10.1016/0142-9612(95)93575-x. [DOI] [PubMed] [Google Scholar]
- 48.Kenley R, Lee M, Mahoney TH, Sanders L. Poly(lactide-co-glycolide) decomposition kinetics in vivo and in vitro. Macromolecules. 1987;20:2403–2406. [Google Scholar]
- 49.Therin M, Christel P, Li S, Garreau H, Vert M. In vivo degradation of massive poly(alpha-hydroxy acids): validation of in vitro findings. Biomaterials. 1992;13:594–600. doi: 10.1016/0142-9612(92)90027-l. [DOI] [PubMed] [Google Scholar]
- 50.Tsuji H, Ikada Y. Properties and morphology of poly(3-lactide) 4. Effect of structural parameters on long-term hydrolysis of poly(3-lactide) in phosphate buffered solution. Polym Degradation Stability. 2000;67:179–189. [Google Scholar]
- 51.Cam D, Hyon SH, Ikada Y. Degradation of high molecular weight poly(3-lactide) in alkaline medium. Biomaterials. 1995;16:833–843. doi: 10.1016/0142-9612(95)94144-a. [DOI] [PubMed] [Google Scholar]
- 52.Jamshidi K, Hyon SH, Ikada Y. Thermal characterization of polylactides. Polymer. 1988;29:2229–2234. [Google Scholar]
- 53.Kranz H, Ubrich N, Maincent P, Bodmeier R. Physicomechanical properties of biodegradable poly(D,L-lactide) and poly(D,L-lactide-co-glycolide) films in the dry and wet states. J Pharm Sci. 2000;89:1558–1566. doi: 10.1002/1520-6017(200012)89:12<1558::aid-jps6>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]