Abstract
Carbon fiber running-specific prostheses (RSPs) have allowed individuals with lower extremity amputation (ILEA) to participate in running. It has been established that as running speed increases, leg stiffness (Kleg) remains constant while vertical stiffness (Kvert) increases in able-bodied runners. The Kvert further depends on a combination of the torsional stiffnesses of the joints (joint stiffness; Kjoint) and the touchdown joint angles. Thus, an increased understanding of spring-like leg function and stiffness regulation in ILEA runners using RSPs is expected to aid in prosthetic design and rehabilitation strategies. The aim of this study was to investigate stiffness regulation to various overground running speeds in ILEA wearing RSPs. Eight ILEA performed overground running at a range of running speeds. Kleg, Kvert and Kjoint were calculated from kinetic and kinematic data in both intact and prosthetic limbs. Kleg and Kvert in both limbs remained constant when running speed increased, while intact limbs in ILEA running with RSPs have a higher Kleg and Kvert than residual limbs. There were no significant differences in Kankle, Kknee and touchdown knee angle between the legs at all running speeds. Hip joints in both legs did not demonstrate spring-like function; however, distinct impact peaks were observed only in the intact leg hip extension moment at early stance phase, indicating that differences in Kvert between limbs in ILEA are due to attenuating shock with the hip joint. Therefore, these results suggest that ILEA using RSPs have a different stiffness regulation between the intact and prosthetic limbs during running.
Keywords: Lower extremity, spring-mass model, joint stiffness, amputees
Introduction
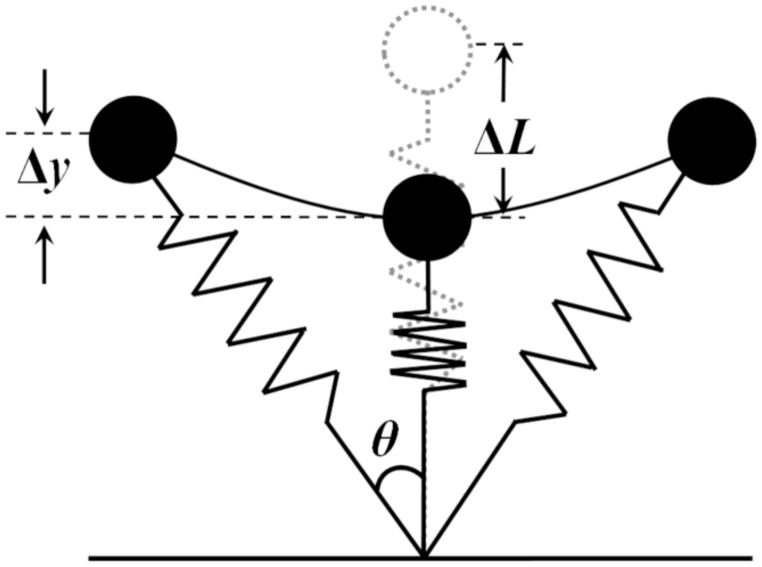
Recent carbon fiber running-specific prostheses (RSPs) have helped individuals with lower extremity amputation (ILEA) run by providing spring-like properties in their amputated leg. To describe the spring-like leg function during running, the whole body is often modeled with a “spring-mass model” which consists of a body mass supported by a spring (Figure 1; Blickhan, 1989; McMahon and Cheng, 1990). In this model, stiffness of the leg spring (leg stiffness; Kleg) is defined as the ratio of maximal vertical ground reaction force (Fpeak) to maximum leg compression (ΔL) during the stance phase. Previous studies have demonstrated that able-bodied humans adjust the spring-like behavior for different running speeds by increasing the angle swept (θ) by the stance limb while keeping Kleg nearly constant (He et al., 1991; Farley et al., 1993; McMahon and Cheng, 1990; Morin et al., 2005, 2006), indicating that the constant-stiffness leg spring may be a basic and invariant characteristic of running. However, it is still unknown whether ILEA using RSPs show similar responses between the amputated limb and the intact limb. A better understanding of spring-like leg behavior and stiffness regulation using RSPs will lead to identifying potential risk factors of amputee running and optimizing prosthesis designs to mitigate these risk factors and improve prosthesis and individual performance through rehabilitation strategies.
Figure 1.
Spring-mass model for running. The leg spring is compressed during the first half of the stance phase and rebounds during the second half. Maximal vertical displacement of the center of mass and leg spring compression during ground contact is represented by Δy and ΔL, respectively. Half of the angle swept by the leg spring during the ground contact is denoted by θ.
It has also been shown that vertical stiffness (Kvert; ratio of Fpeak to maximum vertical displacement of the center of mass at the middle of the stance phase) is a crucial parameter to determine the spring-like function in human running at a wide range of running speeds. Several studies have shown that vertical stiffness increases with an increase in running speed (Cavagna et al., 2005, 2012; He et al., 1991; Kuitunen et al., 2002; McGowan et al., 2012; Morin et al., 2005, 2006). In a multijointed system, Kvert further depends on a combination of the torsional stiffnesses of the joints (joint stiffness; Kjoint, Kuitunen et al., 2002; Butler et al., 2003; Farley et al., 1998; Farley and Morgenroth, 1999; Hobara et al., 2010). Past findings demonstrated that the changes in Kvert during running primarily depend on Kknee (Kuitunen et al., 2002; Arampatzis et al., 1999; Günther and Blickhan, 2002; Stefanyshyn and Nigg, 1998). Furthermore, Kvert is also influenced by the changes in touchdown joint angle because this angle changes the distance of the moment arm of the ground reaction force (GRF) at each joint (Farley et al., 1998; Hobara et al., 2010; Moritz and Farley, 2004).
Despite several studies examining Kvert during ILEA running (McGowan et al., 2012; Wilson et al., 2009), little is known about stiffness regulation at joint levels. Understanding spring-like leg behavior and stiffness regulation in ILEA using RSPs is important for evaluating their running ability and developing RSP designs and effective rehabilitation strategies. The aim of this study was to investigate stiffness regulation to various overground running speeds in ILEA wearing RSPs.
According to previous studies, RSPs generated lower vertical GRFs than intact limbs (Brüggemann et al., 2009; Grabowski et al., 2010; Weyand et al., 2009). Hence, in the present study we hypothesized that (1) leg stiffness would remain constant with an increase in running speed, but the leg stiffness in the intact limb would be greater than the residual limb, (2) vertical and joint stiffness in both limbs would increase with an increase in running speed, (3) vertical stiffness in the intact limb would be greater than the residual limb and (4) the difference in vertical stiffness would be associated with differences in knee stiffness.
Methods
Participants
Eight male subjects with unilateral transtibial amputation volunteered to participate in the experiment (Table 1). Each ILEA used his own RSP. The study was approved by the local ethics committee of the University of Maryland, College Park Institutional Review Board and prior to testing, written informed consent was obtained.
Table 1.
Subject characteristics.
| Subject | Age (years) | Height (m) | Total Mass (kg) | Running Experience (months) | RSP model | Amputated Limb | Cause of Amputation |
|---|---|---|---|---|---|---|---|
| 1 | 48 | 1.75 | 73.4 | 46 | Flex-Run | Right | Congenital |
| 2 | 31 | 1.71 | 67.9 | 48 | Flex-Run | Left | Trauma |
| 3 | 34 | 1.72 | 110.2 | 60 | Flex-Run | Left | Trauma |
| 4 | 27 | 1.8 | 73.8 | 9 | Cheetah | Left | Trauma |
| 5 | 23 | 1.88 | 85.3 | 9 | Cheetah | Right | Trauma |
| 6 | 27 | 1.84 | 85.3 | 3 | Flex-Run | Left | Trauma |
| 7 | 46 | 1.81 | 84.3 | 256 | Cheetah | Left | Trauma |
| 8 | 20 | 1.89 | 78 | 12 | Cheetah | Right | Trauma |
| Mean | 32.0 | 1.80 | 82.3 | 55.4 | |||
| SD | 10.2 | 0.07 | 82.3 | 84.0 |
Task and procedure
Participants were instructed to run overground on a 100 m long track at 2.5, 3.0 and 3.5 m/s (Figure 2). Each subject continuously ran around the track and data were recorded whenever the subjects passed through the capture volume. Five successful trials for each leg at each of the three running speeds were taken and averaged for further analysis. A successful trial was defined as the subject running within ±0.2 m/s of the prescribed speed within the track section containing the force platforms and stepping within the boundaries of the force platforms during the trial. The order for prescribed running speeds was randomized. To facilitate control of the desired running speed, predetermined speeds were governed using concurrent biofeedback. Using four sets of laser sensors around the track, the average speed over the track section was instantaneously calculated when the subjects ran past the sensors, and verbal cues were provided if subjects were outside of the target speed range. Subjects rested for as long as needed between speed conditions to reduce the effects of fatigue.
Figure 2.
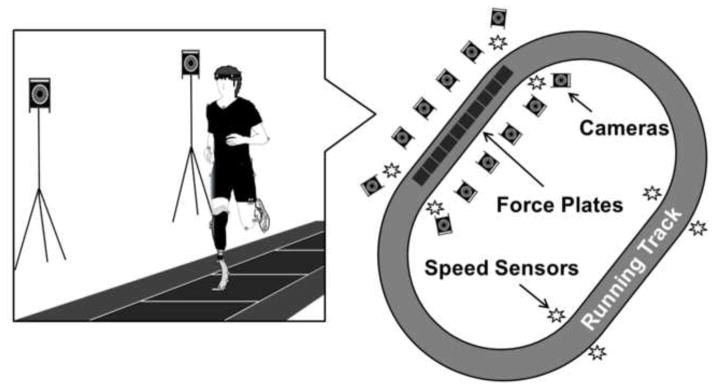
Schematic of experiment setup. Each subject ran around a 100m track containing 10 force plates that recorded ground reaction force data. Ten motion capture cameras collected 3D kinematic data and four sets of sensors around the track monitored running speed in real-time.
Data collection and analysis
Prior to beginning the experiment, retroreflective markers were placed bilaterally over the anterior and posterior iliac spines, heel, 3rd metatarsal head, 5th metatarsal head, and tip of the toe on the shoe. Marker clusters were placed bilaterally on the lateral thigh and shank segments. A static trial was collected prior to dynamic trials that included markers placed on the lateral and medial femoral condyles and the lateral and medial malleoli. On the amputated leg, the shank cluster was placed laterally on the socket and a marker was placed at the distal tip of the socket to define the long axis of the residual shank segment. According to a previous study (Buckley, 2000), the RSP “ankle” joint center was defined as the most acute point on the RSP curvature. Joint angular displacements were determined from the marker data using Visual3D (C-Motion, Germantown, MD) software.
Ten six-degree-of-freedom piezoelectric force platforms (60 cm × 50 cm each and the total measurement area of 600 cm × 50 cm; 9260AA6, Kistler, Amherst, NY) embedded in the track in series collected GRFs at 1000 Hz. The GRFs were filtered using a fourth order, zero lag low pass Butterworth filter with a cut-off frequency of 30 Hz. Further, we captured 3D positional data of the markers using a 10-camera motion capture system (Vicon, Centennial, CO) at 200 Hz. Raw marker data were filtered using a 4th order, zero lag low pass Butterworth filter with a cut-off frequency of 6 Hz.
From vertical GRF (vGRF) data, we determined step frequency (f), stance time (tc) and maximal vGRF (Fpeak) in both the intact and prosthetic limb (INT and PST, respectively). In the present study, we calculated Kleg utilizing the spring-mass model (Blickhan, 1989; Figure 1). During running, the peaks of vGRF and leg compression coincide in the middle of the ground contact phase. At this point, Kleg (N/m) can be calculated as the ratio of Fpeak to peak leg compression in the spring (ΔL) when the leg spring is maximally compressed:
| (1) |
ΔL was calculated from the maximum vertical displacement of the center of mass (COM; Δy), the initial length of the leg spring (L0), and half of the angle swept by the leg spring while it was in contact with the ground (θ):
| (2) |
with
| (3) |
where u is the speed of the body (m/s) and tc is the ground contact time at each step (He et al., 1991; Farley and Gonzalez, 1996; McMahon and Cheng, 1990). Based on the static standing trial, we calculated leg length in INT as the sum of lengths of the hip-knee-ankle joint centers to the ground. For the PST, we defined leg length as the sum of lengths of the hip-knee joint centers to the distal socket to the ground.
Following previous studies (Blickhan, 1989; Kuitunen et al., 2002; Morin et al., 2005; Farley and Gonzalez, 1996), Kvert was also calculated utilizing the spring-mass model. Assuming Fpeak and Δy coincide in the middle of the ground contact phase, the Kvert (N/m) can be calculated as
| (4) |
where Δy is the maximum vertical displacement of the COM during ground contact, which is obtained by integrating the vertical acceleration twice with respect to time (Cavagna, 1975). If Fpeak and maximal COM displacement did not coincide in the middle of the ground contact phase, we calculated the Kvert as the ratio of Fpeak and COM displacement between ground contact and the instant of Fpeak (Hobara et al., 2008, 2009, 2010, 2012).
Kjoint was calculated with the torsional spring model (Kuitunen et al., 2002; Farley et al., 1998; Farley and Morgenroth, 1999). The Kjoint (Nm/deg) was calculated as a change in the joint moment (ΔMjoint) divided by the change in joint angular displacement (Δθjoint) in the middle of the ground contact phase
| (5) |
In the present study, the joint moments were determined by utilizing rigid linked segment model, anthropomorphic data (Dempster, 1955), and inverse dynamics analysis (Winter, 1990). Since body mass influences the stiffness (Farley et al., 1993), Kleg, Kvert and Kjoint were divided by the subject’s body weight (BW).
Statistical methods
A two-way repeated measures ANOVA with two factors, running speed (3 levels) and limbs (2 levels), was performed to compare INT and PST at three running speeds. Bonferroni post-hoc multiple comparison was performed if a significant main effect was observed. Statistical significance was set at p < 0.05. Statistical analysis was executed using SPSS (IBM SPSS Statistics Version 19, SPSS Inc., Chicago, IL).
Results
Leg and vertical stiffness
Figure 3 shows vGRF-COM displacement curves during the ground contact in running at 2.5, 3.0 and 3.5 m/s, respectively. Both INT and PST compressed after touchdown, and vGRF increased with decreased COM displacement. The vGRF peaked at the moment of maximum leg compression (middle of the stance phase), and subsequently, the vGRF decreased with leg extension until take-off.
Figure 3.
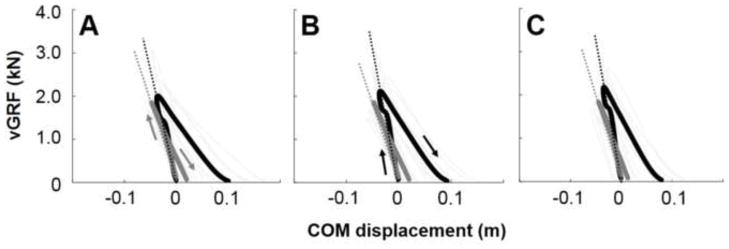
Time-normalized vGRF-COM displacement curves during the ground contact in running at 2.5 (A), 3.0 (B) and 3.5 m/s (C), respectively. Black thick (INT) and gray thick (PST) curves are means of 8 subjects. Gray thin curves represent mean curves for each individual in each leg. The slopes (dotted lines) of these curves represent vertical stiffness. Kvert is the slope of the vGRF-COM displacement curve in the leg compression phase
There was no significant main effect of running speed on Kleg, while there was a significant main effect of limbs (p < 0.05). Kleg was significantly greater in INT than PST at 2.5 and 3.5 m/s (Table 2; p < 0.05). No significant interaction existed between running speeds and limbs on Kleg. Fpeak showed significant main effects of running speed (p < 0.05) and limbs (p < 0.05). Fpeak was greater in INT compared to PST at each speed (Table 2). However, there was no significant speed by limb interaction effect on Fpeak. Statistical analyses revealed main effects of speed on ΔL, θ, f and tc (p < 0.01). However, there were no significant differences between limbs or interaction effects in these parameters (Table 2).
Table 2.
Comparison of kinetic and kinematic characteristics between the legs at all running speeds. Bold numbers and underlined bold numbers indicate significant differences between the limbs at p <0.05 and p <0.01, respectively.
| 2.5 m/s | 3.0 m/s | 3.5 m/s | ||||
|---|---|---|---|---|---|---|
| INT | PST | INT | PST | INT | PST | |
| Kleg, kN/BW/m | 0.03 (0.02) | 0.02 (0.01) | 0.03 (0.01) | 0.02 (0.01) | 0.03 (0.03) | 0.02 (0.01) |
| Kvert, kN/BW/m | 0.07 (0.03) | 0.05 (0.01) | 0.09 (0.05) | 0.05 (0.01) | 0.09 (0.03) | 0.05 (0.01) |
| Fpeak, N/BW | 2.54 (0.31) | 2.12 (0.22) | 2.68 (0.25)† | 2.29 (0.26) †* | 2.76 (0.35) † | 2.27 (0.22) ** |
| ΔL, m | 0.10 (0.02) | 0.11 (0.02) | 0.11 (0.02) | 0.12 (0.02) * | 0.12 (0.01) †# | 0.12 (0.02) ** |
| ΔCOM, m | 0.04 (0.01) | 0.05 (0.01) | 0.04 (0.01) | 0.05 (0.01) | 0.03 (0.01) | 0.04 (0.01) |
| tc, s | 0.27 (0.02) | 0.27 (0.03) | 0.25 (0.01) †† | 0.24 (0.02) ** | 0.23(0.02) ††## | 0.22 (0.02) **$$ |
| θ, deg. | 20.24 (2.03) | 20.52 (2.33) | 21.57 (1.62) † | 21.58 (2.02) | 23.27 (1.64) ## | 23.07 (2.23) **$$ |
| f, Hz | 2.73 (0.19) | 2.73 (0.13) | 2.76 (0.15) | 2.79 (0.14) * | 2.90 (0.15) ††## | 2.94 (0.15) **$$ |
| Kknee, Nm/BW/deg. | 0.09 (0.04) | 0.18 (0.19) | 0.10 (0.04) | 0.12 (0.06) | 0.11 (0.05) | 0.15 (0.12) |
| Kankle, Nm/BW/deg. | 0.17 (0.08) | 0.23 (0.04) | 0.17 (0.08) | 0.23 (0.04) | 0.17 (0.07) | 0.15 (0.12) |
| ΔMknee, Nm/BW | 2.37 (0.64) | 1.60 (0.59) | 2.53 (0.77) | 1.68 (0.65) | 2.45 (0.76) | 1.82 (0.64) |
| ΔMankle, Nm/BW | 2.44 (0.39) | 3.10 (0.69) | 2.58 (0.38) † | 3.33 (0.68) | 2.73 (0.50) † | 3.25 (0.67) |
| Δ θknee, deg. | 27.28 (7.27) | 15.53 (8.33) | 26.62 (7.15) | 16.85 (6.84) | 23.97 (7.43) †# | 16.27 (6.35) |
| Δ θankle, deg. | 17.60 (8.31) | 13.81 (3.04) | 17.68 (8.03) | 14.58 (3.03) | 18.06 (7.89) | 14.34 (3.14) |
| θhip, deg. | 142.38 (6.65) | 142.74 (6.89) | 140.61 (6.45) | 141.13 (6.12) | 138.17 (6.48) | 139.45 (5.29) |
| θknee, deg. | 167.65 (7.18) | 167.29 (7.91) | 166.90 (6.68) | 166.68 (7.69) | 164.85 (6.42) # | 165.32 (8.62) * |
| θankle, deg. | 99.08 (10.35) | 67.40 (10.99) | 98.99 (9.68) | 67.33 (10.83) | 99.38 (9.49) | 66.80 (10.90) *$ |
| Peak Mhip | 1.84 (0.31) | 0.97 (0.23) | 2.02 (0.28) | 1.17 (0.26) * | 2.36 (0.27) ††# | 1.29 (0.31) * |
significant differences from 2.5 m/s at p < 0.05 in INT.
significant differences from 2.5 m/s at p < 0.01 in INT.
significant differences between 3.0 m/s and 3.5 m/s at p < 0.05 in INT.
significant differences between 3.0 m/s and 3.5 m/s at p < 0.01 in INT
significant differences from 2.5 m/s at p < 0.05 in PST.
significant differences from 2.5 m/s at p < 0.01 in PST.
significant differences between 3.0 m/s and 3.5 m/s at p < 0.05 in PST.
significant differences between 3.0 m/s and 3.5 m/s at p < 0.01 in PST
There was no significant main effect of running speed on Kvert while there was a significant main effect of limbs. Kvert was significantly greater in INT than PST at 3.5 m/s (Table 2; p < 0.05). There were no significant main effects of running speed and limb on ΔCOM, indicating that differences in stiffness were due to differences in Fpeak rather than ΔCOM.
Joint stiffness
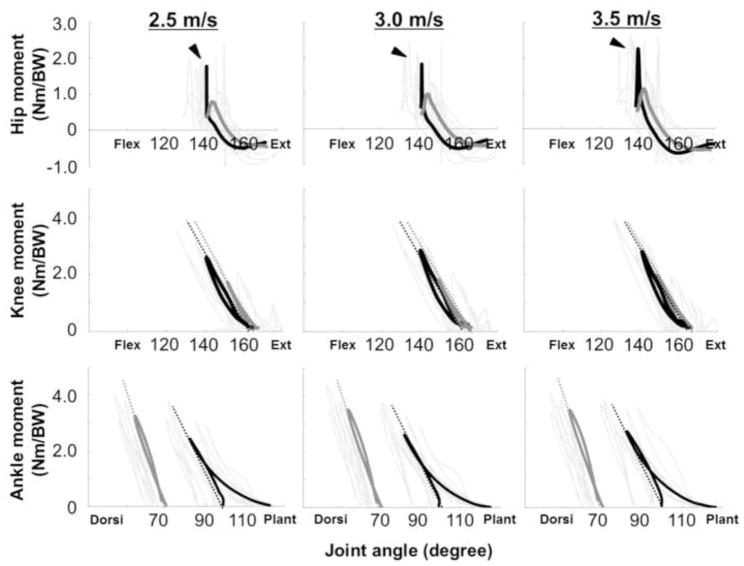
Figure 4 depicts moment-angle curves of the ankle, knee and hip joint during the ground contact in running at 2.5, 3.0 and 3.5 m/s, respectively. From the instant of touch down, the joints were flexed (dorsiflexed in ankle), and joint extension moments increased (plantarflexion moments in ankle). The joint moments of the knee and ankle peaked at maximum joint flexion (dorsiflexion), after which the joints began to extend (plantarflex) with decreases in joint moment until take-off. However, the hip joint in both legs did not show linear trends in the moment-angle relationships at any running speed; thus, we excluded the hip joint from joint stiffness calculations.
Figure 4.
Time-normalized moment-angle curves of the ankle, knee and hip joints during the ground contact in running at 2.5, 3.0 and 3.5 m/s, respectively. Arrows express the direction of the curve. In the figure, joint extension moments in each joint are represented as positive values. Black thick (INT) and gray thick (PST) curves are means of 8 subjects. Gray thin curves represent an individual value for each leg. The slopes (dotted lines) of these curves represent joint stiffnesses. Arrowheads indicate a peak hip extension moment at early stance phase in INT.
Kjoint is the slope of the moment-angular displacement curve in the leg compression phase. Table 2 shows comparisons of Kknee and Kankle between the legs across the three running speeds. Statistical analyses revealed that there were no significant main effects for running speeds or limb on Kknee and Kankle.
Joint kinetics and kinematics
ΔMknee was generally greater in INT than PST (p < 0.05), while there were no significant differences in ΔMankle between the legs. The ΔMknee remained constant in both legs; however, ΔMankle in INT increased 10.9% when the speed increased (p < 0.05; Table 2). Further, Δθknee was generally greater in INT than PST (2.5 m/s: p < 0.01, 3.0 m/s: p < 0.01, and 3.5 m/s: p < 0.05), while there were no significant differences in Δθankle between the limbs. Although Δθknee in INT decreased 13.8% when the speed increased (p < 0.05; Table 2), Δθankle remained constant in both limbs.
As shown in Table 2, ankle angle at touchdown (θankle) in PST was significantly extended compared to INT in all speeds (p < 0.01; Table 2). On the other hand, there was no significant bilateral difference in knee (θknee) and hip (θhip) angle at touchdown between the limbs.
Table 2 also shows a comparison of peak hip moments in early stance phase across three running speeds. The peak hip moment in early stance phase increased with increasing running speeds. Furthermore, the peak hip moment in early stance phase was generally greater in INT than PST at all running speeds (p < 0.01 at all speeds).
Discussion
The aim of this study was to investigate stiffness regulation to different overground running speeds in ILEA wearing RSPs. We found that Kleg remained constant in both legs for three speeds, but the Kleg in the intact limb was greater than the residual limb (Table 2). Current results support our first hypothesis, and agree with previous studies which stated that able-bodied runners keep Kleg constant for a range of running speeds (He et al., 1991; Farley et al., 1993; McMahon and Cheng, 1990; Morin et al., 2005, 2006). This suggests that a constant Kleg is a basic principle of running not only in able-bodied runners, but also in ILEA using RSPs. Maintaining a constant stiffness can be beneficial for the motor control of running when the running speed changes because other necessary kinematics and kinetics can be modulated with running speed without changing the stiffness.
Although changes in Kleg during running are associated with f and tc (Farley and Gonzalez, 1996; Morin et al., 2007), there were no significant differences in f and tc between the legs in any speed conditions in our study, indicating that differences in Kleg between limbs are not related to both f and tc. As reviewed by previous studies (Brughelli and Cronin, 2008a, b), the results would indicate that as speed increases, the angle swept by the leg increases, which increases the change in ΔL. Thus, as the Fpeak increases, the change in ΔL also increases, and therefore Kleg would not be altered significantly. Although our results contrast with a previous study (McGowan et al., 2012) which stated that there were no distinct differences in Kleg between the legs in ILEA running on a treadmill, the discrepancy between this study and our study may be explained by methodological differences. Further, current participants used their own RSP (2 in Cheetah, 4 in Flex-Run, 2 in Catapult), which is different from the previous study (4 in Cheetah, 1 in Sprinter and 1 in C-Sprint).
Our data showed that there was no significant main effect of running speed on Kvert (Table 2). Similarly, there was no significant main effect of running speed on Kknee and Kankle in both limbs (Table 2). These results contrast with our second hypotheses which stated that vertical and joint stiffness in both limbs would increase with an increase in running speed. The lack of main effect of running speed on Kvert, Kknee and Kankle might be due to the lower running speeds and smaller range of speeds in the present study compared with previous studies (Cavagna et al., 2005, 2012; He et al., 1991; Kuitunen et al., 2002; McGowan et al., 2012; Morin et al., 2005, 2006). Otherwise, small sample size might lead the invariant lower extremity stiffness according to the running speeds in the present study.
Kvert in INT was significantly greater than PST at 3.5 m/s (Table 2). However, the Kvert was not significantly greater at 2.5 and 3.0 m/s between the limbs. These results partly support our third hypothesis which stated that vertical stiffness in INT would be greater than PST. In other words, our third hypothesis was not fully supported at all speeds. A small sample size might have led to the non-significant differences in Kvert between the limbs.
Several studies demonstrated that changes in Kvert during running primarily depend on Kknee and not Kankle (Kuitunen et al., 2002; Arampatzis et al., 1999; Günther and Blickhan, 2002; Stefanyshyn and Nigg, 1998). However, as shown in Table 2, we observed no significant differences in Kjoint between the limbs at all running speeds. These results disagree with our final hypothesis, suggesting that differences in Kvert between the limbs probably depend upon something other than Kjoint in ILEA runners. Although Kvert is also influenced by the changes in touchdown joint angle (Farley et al., 1998; Hobara et al., 2010; McMahon et al., 1987; Moritz and Farley, 2004), the subjects in the present study were not likely to control Kvert by altering touchdown joint angles. In fact, there were no significant differences in θknee between the legs (Table 2). θankle in INT landed with more plantarflexed posture than PST; however, this comparison is dependent on how the prosthesis ankle joint and adjacent segments are defined. Moreover, the presence of a prosthetic limb may disrupt the normal joint/vertical stiffness relationships in running. Since identifying the major determinant of Kvert would be helpful in the development of more effective training methods both for able-bodied subjects (Hobara et al., 2011) and amputee athletes (Hobara et al., 2012), future studies should focus on investigating how to improve running performance in ILEAs.
A possible explanation for greater Kvert in the intact leg may be compensatory strategies involving the hip joint. In the present study, hip stiffness was not included in the stiffness calculations due to the lack of linearity between the moment-angle relationship of the hip joint in both legs. However, it is worthwhile to note that peak hip extension moment in INT demonstrated an obvious impact spike in early stance phase (arrowheads in Figure 4), while PST did not show such a distinct impact peak. This indicates the impact peak was not purely due to data processing artifacts (e.g. Bisseling & Hof, 2006; Edwards et al., 2011), else we would expect a distinct and consistent impact peak in both hips. These results suggest that the hip joints in ILEA with RSPs have a shock-absorbing function rather than a spring-like function. In fact, some previous studies demonstrated this unique compensatory strategy involving the hip joint during walking in transtibial amputees (Grumillier et al., 2008; Ventura et al., 2011). Further, Galli et al. (2008) demonstrated that a similar distinct impact of hip extension moment at early stance phase during walking was observed in subjects with Down syndrome, but not in a control group. The authors concluded that the extension moment pattern in the hip joint related to postural stability during dynamic movements. Therefore, less shock-absorption in INT might explain the differences in Kvert between the legs in ILEA running with RSPs. Furthermore, these compensatory movements may induce greater inefficiency and a higher injury risk during running as long term consequences.
There are some limitations in this study. First, although we observed that Kleg, Kvert and Kjoint remained constant in both legs of ILEA for all three speeds (2.5, 3.0 and 3.5 m/s), these results might be due to the fact that the range of speeds was very limited in the present study. Therefore, future studies should focus on investigating the effect of greater running speeds on spring-like leg behavior of ILEA running. Second, although we used the most acute curve point on the RSP (Buckley, 2000) to define the “ankle” joint center, other studies placed a marker as an “ankle” joint center on the prosthetic foot at the same height as the lateral malleolus of the intact limb during normal standing (Brüggemann et al., 2009; Buckley, 1999). Thus, caution needs to be taken regarding the interpretation and generalization of these findings. Finally, due to the limited number of transtibial amputees who can perform overground running at the prescribed speeds, only eight ILEAs with a great variation in the running experience of the participants (3–256 months) were available for the present study. Running with prostheses can take some time to adjust to, and this may affect the current results by introducing greater variability in the ILEA group regarding their running mechanics. To verify the compensatory strategies observed in this study and establish practical gait rehabilitation and optimization of prosthesis designs, there should be more subjects in future research.
In summary, the results of the present study suggest that Kleg, Kvert and Kjoint in both limbs remain constant when running speed increases. Intact limbs in ILEA running with RSPs have a higher Kleg and Kvert than residual limbs. Differences in the Kvert during running in ILEA might be due to attenuating shock with the hip joint, and not to differences in joint stiffness or touchdown joint angles.
Acknowledgments
This project was supported in part by NIH 1R03AR062321, Grant-in-Aid of Japan Society for the Promotion of Science, and Kyung Hee University International Scholars Program.
Footnotes
Conflict of interest
None of the authors have any conflicts of interest associated with this study.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Arampatzis A, Brüggemann GP, Metzler V. The effect of speed on leg stiffness and joint kinetics in human running. Journal of Biomechanics. 1999;32:1349–1353. doi: 10.1016/s0021-9290(99)00133-5. [DOI] [PubMed] [Google Scholar]
- Bisseling RW, Hof AL. Handling of impact forces in inverse dynamics. Journal of Biomechanics. 2006;39:2438–2444. doi: 10.1016/j.jbiomech.2005.07.021. [DOI] [PubMed] [Google Scholar]
- Blickhan R. The spring-mass model for running and hopping. Journal of Biomechanics. 1989;22:1217–1227. doi: 10.1016/0021-9290(89)90224-8. [DOI] [PubMed] [Google Scholar]
- Brüggemann GP, Arampatzis A, Emrich F, Potthast W. Biomechanics of double transtibial amputee sprinting using dedicated sprinting prostheses. Sports Technology. 2009;1:220–227. [Google Scholar]
- Brughelli M, Cronin J. Influence of running velocity on vertical, leg and joint stiffness: modelling and recommendations for future research. Sports Medicine. 2008a;38:647–657. doi: 10.2165/00007256-200838080-00003. [DOI] [PubMed] [Google Scholar]
- Brughelli M, Cronin J. A review of research on the mechanical stiffness in running and jumping: methodology and implications. Scandinavian Journal of Medicine and Science in Sports. 2008b;18:417–426. doi: 10.1111/j.1600-0838.2008.00769.x. [DOI] [PubMed] [Google Scholar]
- Buckley JG. Sprint kinematics of athletes with lower-limb amputations. Archives Physical Medicine and Rehabilitation. 1999;80:501–508. doi: 10.1016/s0003-9993(99)90189-2. [DOI] [PubMed] [Google Scholar]
- Buckley JG. Biomechanical adaptations of transtibial amputee sprinting in athletes using dedicated prostheses. Clinical Biomechanics. 2000;15:352–358. doi: 10.1016/s0268-0033(99)00094-7. [DOI] [PubMed] [Google Scholar]
- Butler RJ, Crowell HP, Davis IM. Lower extremity stiffness: Implication for performance and injury. Clinical Biomechanics. 2003;18:511–517. doi: 10.1016/s0268-0033(03)00071-8. [DOI] [PubMed] [Google Scholar]
- Cavagna GA. Force platforms as ergometers. Journal of Applied Physiology. 1975;39:174–179. doi: 10.1152/jappl.1975.39.1.174. [DOI] [PubMed] [Google Scholar]
- Cavagna GA, Heglund NC, Willems PA. Effect of an increase in gravity on the power output and the rebound of the body in human running. Journal of Experimental Biology. 2005;208:2333–2346. doi: 10.1242/jeb.01661. [DOI] [PubMed] [Google Scholar]
- Cavagna GA, Legramandi MA, La Torre A. An analysis of the rebound of the body in backward human running. Journal of Experimental Biology. 2012;215:75–84. doi: 10.1242/jeb.057562. [DOI] [PubMed] [Google Scholar]
- Dempster WT. Space requirements of the seated operator. Wright Patterson Air Force Base; 1955. WADC-TR-55-159. [Google Scholar]
- Edwards WB, Troy KL, Derrick TR. On the filtering of intersegmental loads during running. Gait & Posture. 2011;34:435–438. doi: 10.1016/j.gaitpost.2011.06.006. [DOI] [PubMed] [Google Scholar]
- Farley CT, Glasheen J, McMahon TA. Running springs: speed and animal size. Journal of Experimental Biology. 1993;185:71–86. doi: 10.1242/jeb.185.1.71. [DOI] [PubMed] [Google Scholar]
- Farley CT, Gonzalez O. Leg stiffness and stride frequency in human running. Journal of Biomechanics. 1996;29:181–186. doi: 10.1016/0021-9290(95)00029-1. [DOI] [PubMed] [Google Scholar]
- Farley CT, Houdijk HH, Van Strien C, Louie M. Mechanism of leg stiffness adjustment for hopping on surfaces of different stiffnesses. Journal of Applied Physiology. 1998;85:1044–1055. doi: 10.1152/jappl.1998.85.3.1044. [DOI] [PubMed] [Google Scholar]
- Farley CT, Morgenroth DC. Leg stiffness primarily depends on ankle stiffness during human hopping. Journal of Biomechanics. 1999;32:267–273. doi: 10.1016/s0021-9290(98)00170-5. [DOI] [PubMed] [Google Scholar]
- Galli M, Rigoldi C, Brunner R, Virji-Babul N, Giorgio A. Joint stiffness and gait pattern evaluation in children with Down syndrome. Gait and Posture. 2008;28:502–506. doi: 10.1016/j.gaitpost.2008.03.001. [DOI] [PubMed] [Google Scholar]
- Grabowski AM, McGowan CP, McDermott WJ, Beale MT, Kram R, Herr HM. Running-specific prostheses limit ground-force during sprinting. Biology Letters. 2010;6:201–204. doi: 10.1098/rsbl.2009.0729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grumillier C, Martinet N, Paysant J, André JM, Beyaert C. Compensatory mechanism involving the hip joint of the intact limb during gait in unilateral trans-tibial amputees. Journal of Biomechanics. 2008;41:2926–2931. doi: 10.1016/j.jbiomech.2008.07.018. [DOI] [PubMed] [Google Scholar]
- Günther M, Blickhan R. Joint stiffness of the ankle and the knee in running. Journal of Biomechanics. 2002;35:1459–1474. doi: 10.1016/s0021-9290(02)00183-5. [DOI] [PubMed] [Google Scholar]
- He JP, Kram R, McMahon TA. Mechanics of running under simulated low gravity. Journal of Applied Physiology. 1991;71:863–870. doi: 10.1152/jappl.1991.71.3.863. [DOI] [PubMed] [Google Scholar]
- Hobara H, Inoue K, Muraoka T, Omuro K, Sakamoto K, Kanosue K. Leg stiffness adjustment for a range of hopping frequencies in humans. Journal of Biomechanics. 2010;43:506–511. doi: 10.1016/j.jbiomech.2009.09.040. [DOI] [PubMed] [Google Scholar]
- Hobara H, Inoue K, Omuro K, Muraoka T, Kanosue K. Determinant of leg stiffness during hopping is frequency-dependent. European Journal of Applied Physiology. 2011;111:2195–2201. doi: 10.1007/s00421-011-1853-z. [DOI] [PubMed] [Google Scholar]
- Hobara H, Kato E, Kobayashi Y, Ogata T. Sex differences in relationships between passive ankle stiffness and leg stiffness during hopping. Journal of Biomechanics. 2012;45:2750–2754. doi: 10.1016/j.jbiomech.2012.09.008. [DOI] [PubMed] [Google Scholar]
- Hobara H, Kimura K, Omuro K, Gomi K, Muraoka T, Iso S, Kanosue K. Determinants of difference in leg stiffness between endurance- and power-trained athletes. Journal of Biomechanics. 2008;41:506–514. doi: 10.1016/j.jbiomech.2007.10.014. [DOI] [PubMed] [Google Scholar]
- Hobara H, Muraoka T, Omuro K, Gomi K, Sakamoto M, Inoue K, Kanosue K. Knee stiffness is a major determinant of leg stiffness during maximal hopping. Journal of Biomechanics. 2009;42:1768–1771. doi: 10.1016/j.jbiomech.2009.04.047. [DOI] [PubMed] [Google Scholar]
- Kuitunen S, Komi PV, Kyröläinen H. Knee and ankle joint stiffness in sprint running. Medicine and Science in Sports and Exercise. 2002;34:166–173. doi: 10.1097/00005768-200201000-00025. [DOI] [PubMed] [Google Scholar]
- McGowan CP, Grabowski AM, McDermott WJ, Herr HM, Kram R. Leg stiffness of sprinters using running-specific prostheses. Journal of Royal Society Interface. 2012;9:1975–1982. doi: 10.1098/rsif.2011.0877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMahon TA, Cheng GC. The mechanics of running: how does stiffness couple with speed? Journal of Biomechanics. 1990;23(S1):65–78. doi: 10.1016/0021-9290(90)90042-2. [DOI] [PubMed] [Google Scholar]
- McMahon TA, Valiant G, Frederick EC. Groucho running. Journal of Applied Physiology. 1987;62:2326–2337. doi: 10.1152/jappl.1987.62.6.2326. [DOI] [PubMed] [Google Scholar]
- Morin JB, Dalleau G, Kyrolainen H, Jeannin T, Belli A. A simple method for measuring stiffness during running. Journal of Applied Biomechanics. 2005;21:167–180. doi: 10.1123/jab.21.2.167. [DOI] [PubMed] [Google Scholar]
- Morin JB, Jeannin T, Chevallier B, Belli A. Spring-mass model characteristics during sprint running: correlation with performance and fatigue-induced changes. International Journal of Sports Medicine. 2006;27:158–165. doi: 10.1055/s-2005-837569. [DOI] [PubMed] [Google Scholar]
- Morin JB, Samozino P, Zameziati K, Belli A. Effects of altered stride frequency and contact time on leg-spring behavior in human running. Journal of Biomechanics. 2007;40:3341–3348. doi: 10.1016/j.jbiomech.2007.05.001. [DOI] [PubMed] [Google Scholar]
- Moritz CT, Farley CT. Passive dynamics change leg mechanics for an unexpected surface during hopping. Journal of Applied Physiology. 2004;97:1313–1322. doi: 10.1152/japplphysiol.00393.2004. [DOI] [PubMed] [Google Scholar]
- Stefanyshyn DJ, Nigg BM. Dynamic angular stiffness of the ankle joint during running and sprinting. Journal of Applied Biomechanics. 1998;14:292–299. doi: 10.1123/jab.14.3.292. [DOI] [PubMed] [Google Scholar]
- Ventura JD, Segal AD, Klute GK, Neptune RR. Compensatory mechanisms of transtibial amputees during circular turning. Gait and Posture. 2011;34:307–312. doi: 10.1016/j.gaitpost.2011.05.014. [DOI] [PubMed] [Google Scholar]
- Weyand PG, Bundle MW, McGowan CP, Grabowski A, Brown MB, Kram R, Herr H. The fastest runner on artificial legs: different limbs, similar function? Journal of Applied Physiology. 2009;107:903–911. doi: 10.1152/japplphysiol.00174.2009. [DOI] [PubMed] [Google Scholar]
- Wilson JR, Asfour S, Abdelrahman KZ, Gailey R. A new methodology to measure the running biomechanics of amputees. Prosthetics and Orthotics International. 2009;33:218–229. doi: 10.1080/03093640903107998. [DOI] [PubMed] [Google Scholar]
- Winter DA. In Biomechanics and Motor Control of Human Movement. New York: Wiley; 1990. [Google Scholar]