Abstract
BACKGROUND
Recent studies suggest that HPA-1a–specific, low-avidity maternal antibodies not detectable by conventional methods can cause neonatal alloimmune thrombocytopenia (NAIT). We performed studies to further define the incidence and clinical significance of this type of antibody.
STUDY DESIGN AND METHODS
Surface plasmon resonance analysis was used to detect low-avidity antibodies in HPA-1a–negative, “antibody-negative” mothers of suspected NAIT cases. The ability of antibodies detected to promote immune destruction of human platelets (PLTs) was examined in a newly developed NOD/SCID mouse model.
RESULTS
Among 3478 suspected cases of NAIT, 677 HPA-1a–negative mothers were identified. HPA-1a–specific antibodies were detected by conventional antibody testing in 616 cases (91%). Low-avidity HPA-1a–specific antibodies were identified in 18 of the remaining 61 cases (9%). Clinical follow-up on 13 cases showed that eight were referred because of suspected NAIT and five because the mother’s sister had previously had an infant with NAIT. Only six infants born to the 13 sensitized mothers had clinically significant thrombocytopenia at birth. Three of four low-avidity antibodies tested in the mouse caused accelerated clearance of HPA-1a/a but not HPA-1b/b PLTs. Only 3 of 12 mothers with low-avidity HPA-1a antibodies were positive for HLA-DRB3*0101.
CONCLUSIONS
The findings confirm previous reports that low-avidity HPA-1a antibodies can cause NAIT but show that the presence of such an antibody does not predict that an infant will be affected. The low incidence of HLA-DRB3*0101 in this cohort (p < 0.0001) suggests that women negative for DRB3*0101 may be predisposed to produce low-avidity HPA-1a antibodies.
Neonatal alloimmune thrombocytopenia (NAIT), caused by maternal immunization against platelet (PLT)-specific antigens inherited by a fetus from its father, occurs once in approximately 1000 births.1–3 Although many cases are mild, approximately half of the affected infants have bleeding symptoms and up to 10% experience intracranial hemorrhage.4,5 The first human PLT antigen (HPA) shown to be capable of triggering maternal antibodies and causing NAIT was HPA-1a (originally called PlA1) described by Shulman and colleagues in 1962.6 Since that time, at least 27 different HPA antigens have been implicated in NAIT.7–14 However, fetal–maternal incompatibility for HPA-1a continues to be the most common cause of this disorder, even though only approximately 2% of women of Northern European and African American descent are HPA-1a negative and are at risk of producing HPA-1a antibodies. Using flow cytometry and solid-phase assays, it is now possible to detect HPA-1a antibodies with high sensitivity and specificity.8,15,16 However, infants thought to have NAIT are sometimes born to HPA-1a–negative mothers who lack detectable HPA-1a antibodies.17 Socher and coworkers, in 2009,18 described two such cases and, using surface plasmon resonance (SPR) analysis, obtained evidence that low-avidity maternal HPA-1a antibodies not detectable in conventional serologic assays can be the cause of fetal PLT destruction in such instances. More recently, Bakchoul and colleagues19 described seven similar cases in which low-avidity HPA-1a antibodies appear to have caused NAIT. It is important that the prevalence of low-avidity HPA-1a antibodies in pregnancy and their role in NAIT be defined as fully as possible to optimize management of thrombocytopenic infants born to HPA-1a–negative, antibody-negative mothers. Here, we describe findings made in a cohort of “seronegative” HPA-1a–negative mothers referred for study because they gave birth to an infant with a clinical picture typical of NAIT or were carrying an infant thought to be at risk for thrombocytopenia.
MATERIALS AND METHODS
Patients
Blood samples from parents of infants suspected of having NAIT and, in some cases, buccal swab DNA samples, were referred to the Platelet and Neutrophil Immunology Laboratory of the BloodCenter of Wisconsin for diagnostic testing. Clinical histories were obtained by verbal and written communication with attending physicians.
Serologic studies
Maternal serum samples from suspected NAIT cases submitted to the Platelet and Neutrophil Immunology Laboratory were tested for PLT-reactive and glycoprotein-specific antibodies as previously described8 using flow cytometric analysis and/or modified capture enzyme-linked immunosorbent assay (ELISA).15,16,20
PLT alloantigen typing
PLT genotyping for antigens of the HPA-1-6, HPA-9, and HPA-15 systems was carried out by the Molecular Diagnostic Laboratory of BloodCenter of Wisconsin with in-house allelic discrimination assays using fluorescently labeled detection probes.21
Detection of HPA-1a–specific antibodies using SPR analysis
SPR was performed using an SPR instrument (Biacore 3000, GE Healthcare, Piscataway, NJ).18 HPA-1a/a and HPA-1b/b GPIIb/IIIa was isolated from PLTs of group O donors using concanavalin A–Sepharose (Sigma-Aldrich, St Louis, MO)22 and immunoaffinity chromatography with the complex-specific monoclonal antibody (MoAb) AP223 followed by elution at neutral pH with elution buffer (Gentle AG/AB, Thermo Scientific Pierce Protein Research Products, Rockford, IL) and was provided by Gen-Probe GTI Diagnostics, Inc. (Waukesha, WI). Approximately 7000 resonance units of purified HPA-1a/a or HPA-1b/b GPIIb/IIIa in 10 mmol/L sodium acetate, pH 4.0, was immobilized via carboxymethylated dextran on Biacore CM5 chips using a primary amine coupling kit (GE Healthcare, Pittsburgh, PA). Human immunoglobulin (Ig)G fractions were isolated from serum using two sequential purifications with an IgG purification kit (Melon Gel, Thermo Scientific, Rockford, IL). Purified IgG was dialyzed against buffer containing 10 mmol/L phosphate buffer, 2.7 mmol/L potassium chloride, and 140 mmol/L sodium chloride, pH 7.4. The purified IgG was diluted 1:8 in running buffer and was injected for 150 seconds at a flow rate of 5 μL/min followed by 150 seconds of running buffer (phosphate-buffered saline [PBS]) to allow for antibody dissociation. Antigen was regenerated with two 30-second pulses of 200 mmol/L sodium carbonate. Each surface was stable for more than 100 injection-regeneration cycles. Binding assays were performed at least four times with each sample. The net SPR signal was obtained by computer-assisted subtraction of the signal obtained with HPA-1b/b GPIIb/IIIa from that obtained with HPA-1a/a GPIIb/IIIa.
Measurement of immune clearance of human PLTs in NOD/SCID mice
Studies were carried out as described previously24 with several modifications. Briefly, a 200-μL suspension containing 4 × 108 human PLTs from a healthy HPA-1a/a or HPA-1b/b group O donor was infused into the retroorbital cavity of an anesthetized 6- to 8-week-old NOD/SCID mouse (The Jackson Laboratory, Bar Harbor, ME). After 30 minutes, a tail blood sample was collected to determine the baseline level of circulating human PLTs (Time 0). Human IgG isolated from 200 μL of serum as described above for the SPR studies was then injected into the other retroorbital plexus. Circulating PLTs were processed from 10 to 20 μL of tail vein blood at 5 and 24 hours after injection of human IgG and the content of human PLTs relative to total PLTs was determined by flow cytometry using the GPIIb/IIIa-specific MoAb AP2 labeled with the fluorochrome AlexaFluor 488.25
Approvals
Studies involving human subjects and animals were approved by the institutional review board of the Blood-Center of Wisconsin and the Institutional Animal Care and Use Committee of the Medical College of Wisconsin, respectively.
RESULTS
Case selection
In a cohort of 3478 cases referred for NAIT evaluation, 677 families were identified in which the mother was HPA-1a negative (HPA-1b/1b) and the father possessed at least one HPA-1a allele (19% of referred NAIT cases) (Fig. 1). HPA-1a–specific antibodies were detected in sera from 616 (91%) of these mothers using conventional serologic methods, including flow cytometry and modified capture ELISA. In 61 cases (9%), maternal HPA-1a antibodies were not detected. Further studies were performed on maternal serum from these cases.
Fig. 1.
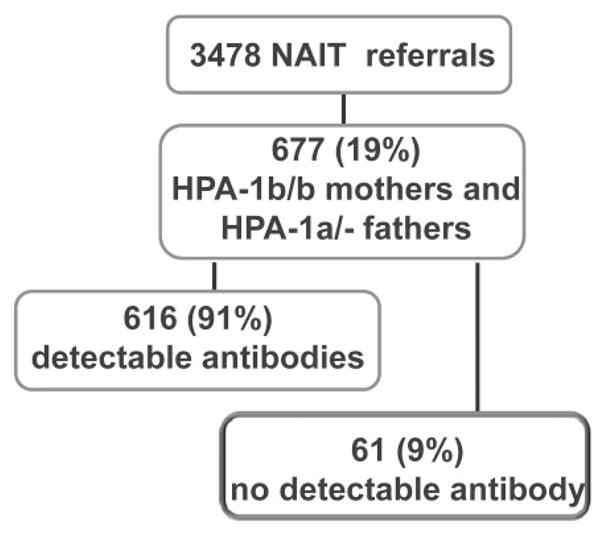
Cohorts of maternal–paternal pairs referred because of suspicion of NAIT. Sixty-one HPA-1a–negative mothers had no HPA-1a antibodies detected in conventional assays. Serum from these individuals was subjected to SPR analysis.
Standardization of the SPR assay
IgG isolated from sera that contained HPA-1a–specific antibodies readily detectable by standard serologic methods was perfused over Biacore chips to which HPA-1a–positive and HPA-1a–negative GPIIb/IIIa had been immobilized and sensorgrams were generated. As shown in Fig. 2, these antibodies preferentially recognized the HPA-1a–positive target and produced a signal that increased rapidly (a consequence of antibody binding) and then tailed off slowly when the antibody-containing perfusate was replaced by buffer. To compare the sensitivity of SPR and standard serology for antibody detection, serial dilutions of an HPA-1a antibody from an NAIT case (Fig. 2A) were tested against HPA-1a/1a and HPA-1b/1b targets using flow cytometry and SPR analysis. The maximum dilutions of antibody that could be detected using SPR and flow cytometry were 1:1000 and 1:200, respectively, indicating that SPR was approximately five times more sensitive than flow cytometry for detection of this particular antibody (data not shown).
Fig. 2.
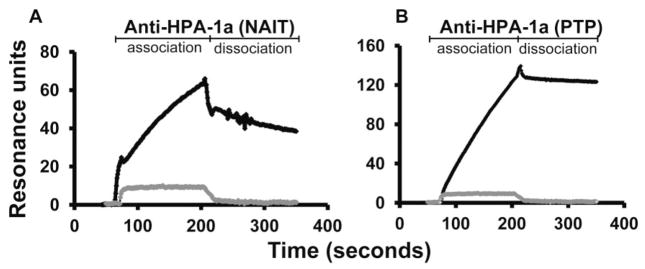
Typical SPR sensorgrams obtained with HPA-1a antibodies detectable by standard serology. Purified IgG diluted 1:8 in PBS was injected at a flow rate of 5 μL/min over HPA-1a/a and HPA-1b/b GPIIb/IIIa. The SPR signal obtained with HPA-1–negative GPIIb/IIIa was subtracted from that obtained with HPA-1a–positive GPIIb/IIIa to obtain net SPR values. (A) Pattern obtained with serum from the mother of an infant with NAIT. (B) Pattern obtained with serum from a patient with post-transfusion purpura. Gray tracing is signal obtained with serum from a normal individual.
SPR analysis of serum from antibody-negative, HPA-1a–negative mothers
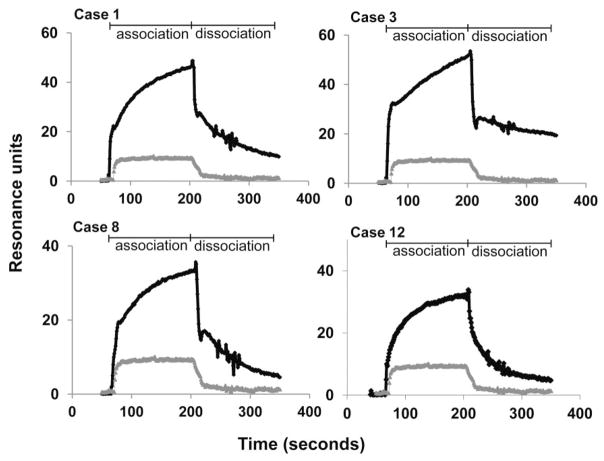
Sensorgrams were generated using IgG from the 61 HPA-1a–negative mothers who were antibody negative by conventional serology and IgG from 30 normal subjects. As shown in Fig. 3, serum from 18 mothers produced a maximum signal against HPA-1a/1a GPIIb/IIIa that exceeded the mean value obtained with IgG from 30 normal donors by more than 2 SD. Representative sensor-grams are shown in Fig. 4, where it can be seen that the on rates for binding of the maternal antibodies (a function of the concentrations of antibody and target antigen) were comparable to those obtained with serologically detectable HPA-1a antibodies shown in Fig. 2. However, the off rates for the maternal antibodies (a function of antibody avidity) were much more rapid, dissociation being almost complete in most cases within 200 seconds of beginning the perfusion with buffer.
Fig. 3.
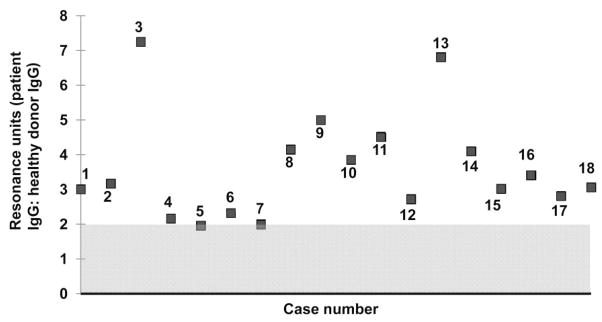
Serum from 18 HPA-1a–negative, “antibody-negative” mothers reacted preferentially against HPA-1a–positive GPIIb/IIIa when tested by SPR. Values shown on the abscissa indicate the ratio of SPR signals obtained with patient IgG at 150 seconds postinjection to the mean signal obtained with serum from 30 healthy individuals. Area shaded gray indicates range of values obtained with normal sera ±2 SD.
Fig. 4.
Representative sensorgrams obtained with low-avidity HPA-1a–specific maternal antibodies. Tracings shown are net of the signal obtained against HPA-1a/a GPIIb/IIIa. Gray tracing indicates sensorgram obtained with normal serum.
Clinical characteristics of NAIT cases associated with low-avidity maternal HPA-1a antibodies
Clinical information characterizing 13 of the 18 NAIT cases in which low-avidity maternal HPA-1a antibodies were detected was obtained from available files and by telephone and written communications with referring physicians. Because of the time delay between referral of samples for serologic testing and testing by SPR analysis, it was not possible to obtain clinical follow-up on five cases. The clinical review showed that 8 of the 13 cases (Group 1, Cases 1–8) had been referred because an infant was born with thrombocytopenia (six cases) or was felt to be at risk for thrombocytopenia because a sibling born previously had experienced NAIT (two cases). The five remaining cases (Group 2, Cases 9–13) had been referred for testing because the mother was pregnant and had a sister who had previously given birth to an infant diagnosed with NAIT. Clinical findings in Group 1 (Cases 1–8) are summarized in Table 1. One infant (Case 6) had a normal PLT count at birth. In this case, a maternal blood sample was tested at 6 months’ gestation because a previous infant by a different father had severe thrombocytopenia and intracranial hemorrhage at birth. Postnatal typing showed that the infant’s PLT type was HPA-1b/b and it was therefore not at risk for NAIT. A second infant (Case 1) was born with a PLT count of 141 × 109/L. Testing in this case was done at 5 months’ gestation because a previous infant had experienced severe thrombocytopenia and bleeding at birth. The mother was given intravenous (IV) immunoglobulin throughout the second pregnancy. The remaining six infants had postnatal PLT count nadirs ranging from 8 × 109 to 61 × 109/L (mean, 32 × 109/L; median, 33 × 109/L). Five infants had petechial hemorrhages in the skin and/or mucous membranes but none had bleeding from other sites. Two infants (Cases 2 and 8) were given maternal PLT transfusions, which produced satisfactory elevations in PLT levels. One infant (Case 3) was given random-donor PLTs, which failed to produce satisfactory PLT increments. This infant was then treated with IVIG and subsequently recovered. Case 5 had both thrombocytopenia and hemolysis caused by D sensitization and was treated with exchange transfusion. In Cases 2, 4, 5, and 7, time to achieve a stable PLT count of at least 100 × 109/L ranged from 4 to 10 days. Case 8 was given weekly transfusions of maternal PLTs, which produced satisfactory PLT increments. However, thrombocytopenia recurred repeatedly until PLTs finally stabilized in the normal range at approximately 10 weeks of age.
TABLE 1.
Cases referred because of NAIT
| Case | Para/gravida | Sample: month of gestation | HPA-1 type of father | HPA-1 type of infant | PLT nadir (×109/L) | Bleeding symptoms | Prenatal Rx | Postnatal therapy | Days to recovery (>100 × 109/L) | DRB3 * 0101 | HLA abs | Comments |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2/1 | 5 | NT | NA | 141 | None | IVIG | None | NA | Positive | Positive | First infant had severe NAIT. No serologic studies done. |
| 2 | 1/1 | 9 | HPA-1a/a | HPA-1a/b | 26 | Petechiae 3+ | Maternal PLT Tx ×1 | 5 | Negative | Negative | ||
| 3 | 2/2 | 9 | HPA-1a/b | NA | 38 | None | PLTs, IVIG | NA | Negative | Positive | First child had “mild” TP. No studies done. Second child had “poor” response to PLT Tx, then responded to IVIG. | |
| 4 | 1/1 | 9 | HPA-1a/b | NA | 38 | Petechiae 2+ | None | 4 | Negative | Positive | ||
| 5 | 2/2 | 9 | HPA-1a/a | HPA-1a/a | 18 | Petechiae 1+ | Exchange Tx PLT Tx | 10 | Negative | Negative | D immunization detected during second pregnancy. Anemic (DAT positive) and thrombocytopenic at birth. | |
| 6 | 2/1 | 6 | HPA-1a/b | HPA-1b/b | 200 | None | None | NA | Negative | Negative | Previous child by a different father had severe NAIT and ICH leading to disability. Second child had normal PLTs at birth. Typing showed it was HPA-1a negative and first child was HPA-1a positive. | |
| 7 | 1/1 | 9 | HPA 1a/a | HPA 1a/b | 61 | Petechiae 2+ | None | 10 | Negative | Positive | Second child born 2 years later had petechiae and PLTs 61 × 109/L at birth. Recovered in 3 days without Rx. No serologic studies done. | |
| 8 | 1/1 | 9 | HPA-1a/a | HPA-1a/b | 8 | Petechiae 2+ | Maternal PLT Tx ×8 | 70 | NA | Negative | Maternal PLT Txs given ×5 over 10 weeks produced good PLT increments but counts dropped to <30 × 109/L over 3–6 days. Not clear whether maternal PLTs were adequately washed. |
abs = antibodies; NA = not available; NT = not tested; Rx = prescription; Tx = transfusion.
As noted, the Group 2 cases were studied about midpregnancy because an HPA-1a–negative sister had previously given birth to an infant with NAIT. Findings made in this group are summarized in Table 2. Four infants had a normal PLT count at birth. The fifth infant (Case 12) had a PLT level of 125 × 109/L and achieved a normal PLT count in 3 days without specific treatment. The mother had been given IVIG throughout the pregnancy because the fetus had been typed as HPA-1a/b.
TABLE 2.
Cases referred because of NAIT in an infant born to a sister
| Case | Para/gravida | Sample: month of gestation | HPA type of father | HPA type of infant | Prenatal prescription | PLT count at birth (×109/L) | DRB3*0101 | HLA abs |
|---|---|---|---|---|---|---|---|---|
| 9 | 2/2 | 5 | HPA-1a/a | HPA-1a/b | IVIG | 260 | Negative | Negative |
| 10 | 1/1 | 7 | HPA-1a/a | HPA-1a/b | 208 | Positive | Negative | |
| 11 | 1/1 | 4 | HPA-1a/a | HPA-1a/b | 235 | Positive | Positive | |
| 12 | 1/1 | 4 | HPA-1a/b | HPA-1a/b | IVIG | 125 | Negative | Negative |
| 13 | 2/2 | 6 | HPA-1a/a | HPA-1a/b | 250 | Negative | Negative |
abs = antibodies.
Optimization of the NOD/SCID mouse model for study of low-avidity HPA-1a antibodies
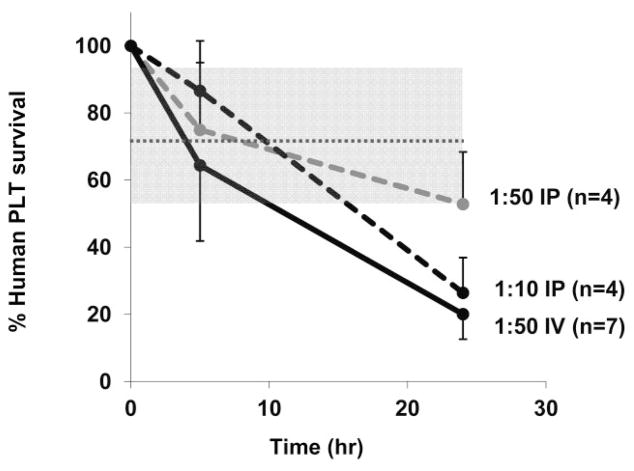
To determine whether low-avidity antibodies detectable only by SPR are capable of destroying human PLTs in vivo, we used a NOD/SCID mouse model.24,25 Because this mouse strain lacks xenoantibodies that ordinarily cause rapid destruction of transfused blood cells from other species, transfused human PLTs survive normally in its circulation for at least 24 hours,24,25 during which time destruction of HPA-1a PLTs by anti-HPA-1a25 and by drug-dependent human antibodies24 can be demonstrated. High-titer human antibodies promote rapid PLT clearance in this model when injected by the intraperitoneal (IP) route.25 However, we were concerned that IP injection might be suboptimal for the study of PLT destruction mediated by low-avidity antibodies because the requirement for antibody to diffuse from the peritoneum into the blood would significantly reduce contact between the injected antibody and circulating PLTs. To examine this issue, we performed a preliminary study in which we compared the ability of a “conventional” HPA-1a antibody from an NAIT case to promote clearance of HPA-1a/a–positive PLTs when injected by the IP and IV routes. As shown in Fig. 5, when antibody (diluted 1/50) was injected IP, clearance of HPA-1a PLTs at 24 hours was not different from that of HPA-1a–negative PLTs. When the same amount of antibody was injected IV, however, PLT clearance was readily demonstrated at 24 hours. Approximately the same rate of clearance was seen when five times this amount of antibody was injected IP, suggesting that IV infusion is approximately five times as effective as IP injection in promoting PLT destruction.
Fig. 5.
A conventional HPA-1a antibody clears PLTs more efficiently when injected IV than when given IP. Human PLTs were transfused to NOD/SCID mice followed by IV or IP injection of a conventional HPA-1a antibody. At 24 hours, PLT survival was shortened when antibody diluted 1/50 was given IV but not when the same quantity of antibody was given IP (p < 0.01). Five times as much antibody (1/10) given IP caused PLT clearance comparable to a smaller amount (1/50) given IV. Values shown are average of multiple determinations ±1.0 SD. Shaded gray area depicts range of PLT survival at 24 hours after injection of normal serum (mean ± 2.0 SD).
Low-avidity HPA-1a antibodies from three of four mothers tested caused accelerated clearance of HPA-1a–positive but not HPA-1a–negative PLTs in the NOD/SCID mouse
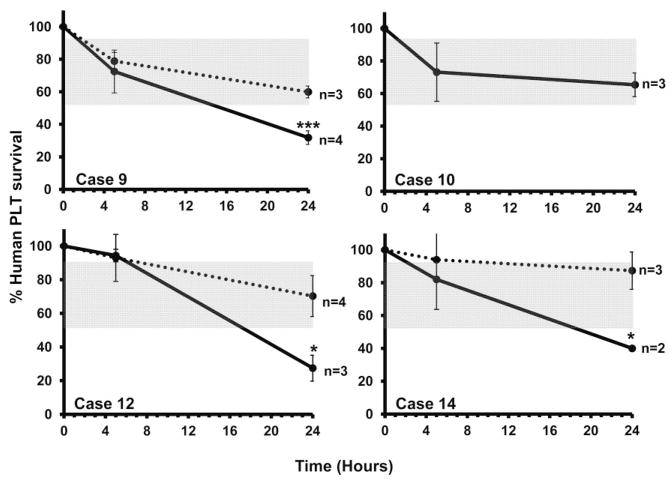
On the basis of these findings, IV infusion was used to investigate the ability of low-avidity HPA-1a antibodies to promote destruction of HPA-1a–positive PLTs. The relatively large volume of serum required for the combined SPR and mouse studies limited the number of patients that could be studied in this way. A further limitation was that five maternal samples (Cases 1, 3, 4, 7, and 11) could not be used in mouse studies because they contained Class I HLA antibodies that could have affected survival of both HPA-1a–positive and -negative PLTs. Because of these limitations, we were able to study only four maternal samples in the mouse (Cases 9, 10, and 12, Table 2; and Case 14, Fig. 3) diagnosed as NAIT but not included in Table 1 because detailed clinical information could not be obtained. As shown in Fig. 6, maternal IgG from Cases 9, 12, and 14 caused accelerated clearance of HPA-1a–positive, but not HPA-1a–negative PLTs, a difference that was significant at 24 hours, but not at 5 hours. In contrast, maternal IgG from Case 10 had no effect on clearance of HPA-1a–positive or HPA-1a–negative PLTs.
Fig. 6.
Low-avidity HPA-1a antibodies from three mothers promoted clearance of HPA-1a/a (——) but not HPA-1b/b (······) PLTs. At 24 hours, survival of HPA-1a/a PLTs but not HPA-1b/b PLTs was significantly shortened after infusion of IgG from mothers of Cases 9, 12, and 14 but was unaffected by IgG from the mother of Case 10. Values shown are mean of multiple determinations ±1.0 SD. Shaded gray area depicts range of PLT survival at 24 hours after injection of normal serum. ***p < 0.001, *p < 0.05.
IV infusion was superior to intraperitoneal injection for demonstrating the ability of low-avidity HPA-1a antibodies to promote HPA-1a–positive PLT clearance in the mouse
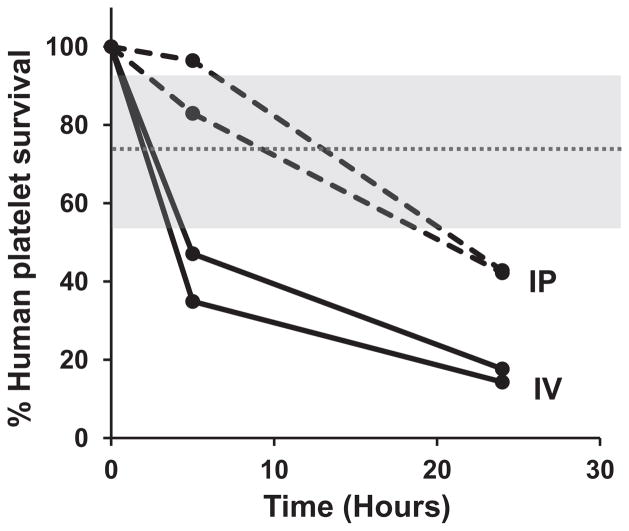
Findings shown in Fig. 5 show that a conventional HPA-1a antibody cleared PLTs more effectively when given IV than when given IP. To determine whether this is also true of low-avidity antibodies, we performed a separate study using IgG from the mother of Case 9, which had been obtained postnatally in relatively large quantity and, like the prenatal sample, lacked HPA-1a antibody detectable by standard serology. As shown in Fig. 7, at both 5 and 24 hours, PLT clearance was significantly more rapid when antibody was given IV than when an equal quantity was injected IP.
Fig. 7.
Low-avidity IgG from Case 9 promoted clearance of HPA-1a/a PLTs more effectively when given IV (——) than when injected IP (----). Shaded gray area depicts range of PLT survival at 24 hours after injection of normal serum (mean ± 2.0 SD).
DISCUSSION
Our studies identified six infants (Cases 2–5, 7, and 8 in Table 1) who had severe or moderately severe neonatal thrombocytopenia when born to HPA-1a–negative women whose serum contained low-avidity antibodies reacting preferentially with HPA-1a–positive GPIIb/IIIa using SPR for antibody detection. Another infant (Case 1), whose mother had been given IVIG throughout pregnancy, had a slightly low PLT count (141 × 109/L) at birth. None of the infants had any other condition that might have affected PLT levels except for Case 5 in which hemolysis due to D incompatibility was present, necessitating an exchange transfusion. Five of the six thrombocytopenic infants had petechial hemorrhages in the skin and mucosal surfaces but none experienced bleeding from other sites. All but one of the more severely affected infants achieved a stable PLT count within 4 to 10 days of birth. The sixth (Case 8) is of particular interest because this infant had the lowest PLT count at birth (8 × 109/L) and remained thrombocytopenic for about 10 weeks except when PLT levels were elevated after transfusion of maternal PLTs. While not unprecedented, this is an exceptionally long recovery time, even for an infant born to a mother with a conventional HPA-1a antibody. We are aware of instances in which prolonged thrombocytopenia proved to be a consequence of failure to wash maternal PLTs sufficiently well to remove all maternal antibody but it was not possible to determine whether this might have explained the persistence of thrombocytopenia in Case 8. Together, these findings are consistent with the possibility that severe to moderately severe neonatal thrombocytopenia in six infants (Cases 2–5, 7, and 8) was caused by low-avidity antibodies specific for HPA-1a and that thrombocytopenia may have been ameliorated in a seventh case (Case 1) by prenatal IVIG treatment.
We were surprised to learn that five of the 13 cases in which follow-up information was obtained were referred for testing in midpregnancy because an HPA-1a–negative sister had previously given birth to an infant with NAIT. Each of the infants was HPA-1a–positive and was therefore at risk to experience NAIT. As shown in Table 2, four of the infants were born with a normal PLT count and the fifth (Case 12), whose mother had been given IVIG during the last 3 months of pregnancy, had only mild thrombocytopenia (125 × 109/L). It is apparent from Fig. 3 that maternal antibodies in these cases (Cases 9–13) were at least as potent (as judged from the SPR signals) as those from mothers whose infants were born with thrombocytopenia.
It was possible to test the ability of low-avidity HPA-1a–specific antibodies to promote destruction of HPA-1a–positive PLTs in the NOD/SCID mouse with only four maternal samples (Cases 9, 10, 12, and 14) because insufficient sample was available or because maternal Class I HLA antibodies were present. As shown in Fig. 6, antibodies from Cases 9, 12, and 14 caused accelerated clearance of HPA-1a–positive, but not HPA-1a–negative, PLTs at 24 hours but the antibody from Case 10 did not. Maternal samples from Cases 9, 10, and 12 were obtained in midpregnancy for testing because a sister had given birth to an infant with NAIT. In Case 10, no prenatal treatment was given and the infant was born with a normal PLT count. In Cases 9 and 12, IVIG was administered during the last 4 months of pregnancy. The Case 9 infant had a normal count at birth and the Case 12 infant was slightly thrombocytopenic (PLT count, 125 × 109/L). Case 14 was one of those in which it was not possible to obtain clinical information. It can be speculated that the infants from Cases 9 and 12 might have had more severe thrombocytopenia had IVIG not been given, in which case the findings are consistent with the possibility there would be a potential correlation between detection of an antibody in SPR and the likelihood of NAIT. Further studies to test this possibility will be of interest but comparisons of this type have the obvious limitation that the quantity and quality of antibody present in a maternal IgG sample will not necessarily reflect the antibody status of the fetus.
Maternal typing for the Class II HLA antigen DRB3*0101 (formerly designated DR52a) was performed in 12 of the 13 cases in which clinical information was obtained. Only three mothers (25%) were DRB3*0101 positive, about the incidence expected in the general population (33%). Absence of an association between DRB3*0101 and sensitization to HPA-1a is surprising because previous studies of women who produced serologically detectable HPA-1a antibodies have shown that more than 90% are positive for DRB3*0101.1,2,26,27 Women positive for DRB3*0101 are more than 100 times more likely than women who lack it to produce a serologically detectable HPA-1a antibody in response to immunization during pregnancy,28 a difference that appears to be accounted for by the high affinity of the DR3*0101 antigen for a peptide generated from integrin-β3 of HPA-1a–positive individuals by intracellular processing.28,29 As shown in Table 3, the lower incidence of DRB3*0101 in mothers who formed low-avidity HPA-1a antibodies was significant compared to the reported incidence of this antigen in women who produce antibodies that can be detected by standard antibody assays. The findings suggest that women lacking DRB3*0101 who are incompatible with their fetus for HPA-1a may be predisposed to produce low-avidity antibodies against this antigen.
TABLE 3.
Percentage of DRB3*0101-positive women who generate conventionally detectable maternal HPA-1a antibodies is significantly different from those who generate low-avidity antibodies
| Reference | Country | DRB3*0101 positive | Total | % positive |
|---|---|---|---|---|
| L’Abbe27 | Canada | 32 | 35 | 91.4* |
| Braud26 | France | 52 | 52 | 100* |
| Williamson1 | England | 43 | 44 | 97.7* |
| Kjeldsen-Kragh2 | Norway | 180 | 198 | 90.1* |
| Current study | United States | 3 | 12 | 25 |
p value < 0.0001 calculated with exact Fisher’s test.
Our findings support the recent reports by Socher and colleagues18 and Bakchoul and colleagues19 that low-avidity HPA-1a antibodies can cause fetal PLT destruction leading to NAIT and that the pathogenicity of such antibodies can be demonstrated in the NOD/SCID mouse model.19 Findings made in Cases 9 to 13 of our study and in the NOD/SCID mouse suggest, however, that not all low-avidity HPA-1a antibodies are capable of causing neonatal thrombocytopenia and raise the possibility that production of low-avidity HPA-1a antibodies by at-risk women carrying an HPA-1a–positive fetus may be a relatively common occurrence.
Although recognition of low-avidity antibodies specific for PLT glycoproteins is a new development, low-avidity antibodies specific for red blood cells (RBCs), referred to in some contexts as “high-titer low-avidity” antibodies30,31 have been recognized by RBC serologists for many years as IgG immunoglobulins that react weakly with RBCs of most persons, sometimes at very high dilutions. Specificity of these antibodies is often difficult to determine, but some appear to recognize well-defined, alloantigens such as York, Chido, McCoy, JMH, Holley, and others.30,31 In general, it is thought that high-titer low-avidity antibodies reactive with RBCs rarely cause destruction of incompatible RBCs31,32 and that their main significance is that they can complicate procurement of compatible blood for patients requiring transfusions. It seems possible that low-avidity antibodies reactive with RBCs usually fail to cause hemolysis because the huge mass of RBCs in the circulation prevents low-avidity immunoglobulins from accumulating on the cell surface at a density above the threshold needed for RBC clearance. With PLTs, which have a total mass about 1/300 that of RBCs, this could be a less significant limitation, allowing some low-avidity antibodies that are efficiently transferred across the placenta to cause fetal PLT destruction.
Acknowledgments
This work was supported by Grant HL-13629 (RHA) and 11GRNT7690032 (JAP).
We appreciate the assistance of Maria Gitter who identified unresolved NAIT referrals for this study. JAP and RHA each contributed to research design, oversight of laboratory studies, interpretation of results, and manuscript preparation; AK contributed to research design and interpretation of findings made and performed laboratory studies; DN performed laboratory studies; DB contributed to oversight of laboratory studies and interpretation of results; JM and BRC identified study candidates, provided laboratory and clinical information on patients selected for study, and critiqued the manuscript.
ABBREVIATIONS
- NAIT
neonatal alloimmune thrombocytopenia
- SPR
surface plasmon resonance
Footnotes
CONFLICTS OF INTEREST
The authors have no disclaimers to make or conflicts to disclose.
References
- 1.Williamson LM, Hackett G, Rennie J, Palmer CR, Maciver C, Hadfield R, Hughes D, Jobson S, Ouwehand WH. The natural history of fetomaternal alloimmunization to the platelet-specific antigen HPA-1a (PlA1, Zwa) as determined by antenatal screening. Blood. 1998;92:2280–7. [PubMed] [Google Scholar]
- 2.Kjeldsen-Kragh J, Killie MK, Tomter G, Golebiowska E, Randen I, Hauge R, Aune B, Øian P, Dahl LB, Pirhonen J, Lindeman R, Husby H, Haugen G, Grønn M, Skogen B, Husebekk A. A screening and intervention program aimed to reduce mortality and serious morbidity associated with severe neonatal alloimmune thrombocytopenia. Blood. 2007;110:833–9. doi: 10.1182/blood-2006-08-040121. [DOI] [PubMed] [Google Scholar]
- 3.Dreyfus M, Kaplan C, Verdy E, Schlegel N, Durand-Zaleski I, Tchernia G. Frequency of immune thrombocytopenia in newborns: a prospective study. Immune Thrombocytopenia Working Group. Blood. 1997;89:4402–6. [PubMed] [Google Scholar]
- 4.Mao C, Guo J, Chituwo BM. Intraventricular haemorrhage and its prognosis, prevention and treatment in term infants. J Trop Pediatr. 1999;45:237–40. doi: 10.1093/tropej/45.4.237. [DOI] [PubMed] [Google Scholar]
- 5.Bussel J. Diagnosis and management of the fetus and neonate with alloimmune thrombocytopenia. J Thromb Haemost. 2009;7 (Suppl 1):253–7. doi: 10.1111/j.1538-7836.2009.03380.x. [DOI] [PubMed] [Google Scholar]
- 6.Shulman NR, Aster RH, Pearson HA, Hiller MC. Immunoreactions involving platelet. VI. Reactions of maternal isoantibodies responsible for neonatal purpura. Differentiation of a second platelet antigen system. J Clin Invest. 1962;41:1059–69. doi: 10.1172/JCI104556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kaplan C. The role of the low-frequency antigens in neonatal alloimmune thrombocytopenia. ISBT Sci Ser. 2007;2:106–10. [Google Scholar]
- 8.Peterson JA, Gitter ML, Kanack A, Curtis B, McFarland J, Bougie D, Aster R. New low-frequency platelet glycoprotein polymorphisms associated with neonatal alloimmune thrombocytopenia. Transfusion. 2010;50:324–33. doi: 10.1111/j.1537-2995.2009.02438.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Peterson JA, Pechauer SM, Gitter ML, Kanack A, Curtis BR, Reese J, Kamath VM, McFarland JG, Aster RH. New platelet glycoprotein polymorphisms causing maternal immunization and neonatal alloimmune thrombocytopenia. Transfusion. 2012;52:1117–24. doi: 10.1111/j.1537-2995.2011.03428.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kekomaki R, Raivio P, Kero P. A new low-frequency platelet alloantigen, Vaa, on glycoprotein IIbIIIa associated with neonatal alloimmune thrombocytopenia. Transfus Med. 1992;2:27–33. doi: 10.1111/j.1365-3148.1992.tb00131.x. [DOI] [PubMed] [Google Scholar]
- 11.Bertrand G, Jallu V, Saillant D, Kervran D, Martageix C, Kaplan C. The new platelet alloantigen Cab a: a single point mutation Gln 716 His on the alpha 2 integrin. Transfusion. 2009;49:2076–83. doi: 10.1111/j.1537-2995.2009.02240.x. [DOI] [PubMed] [Google Scholar]
- 12.Jallu V, Dusseaux M, Kaplan C. A new Ser472Asn (Cab2[a+]) polymorphism localized within the alphaIIb “thigh” domain is involved in neonatal thrombocytopenia. Transfusion. 2011;51:393–400. doi: 10.1111/j.1537-2995.2010.02815.x. [DOI] [PubMed] [Google Scholar]
- 13.Kroll H, Feldmann K, Zwingel C, Hoch J, Bald R, Bein G, Bayat B, Santoso S. A new platelet alloantigen, Swi(a), located on glycoprotein Ia identified in a family with fetal and neonatal alloimmune thrombocytopenia. Transfusion. 2011;51:1745–54. doi: 10.1111/j.1537-2995.2010.03038.x. [DOI] [PubMed] [Google Scholar]
- 14.Sachs UJ, Bakchoul T, Eva O, Giptner A, Bein G, Aster RH, Gitter M, Peterson J, Santoso S. A point mutation in the EGF-4 domain of beta(3) integrin is responsible for the formation of the Sec(a) platelet alloantigen and affects receptor function. Thromb Haemost. 2012;107:80–7. doi: 10.1160/TH11-08-0542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Davoren A, Curtis BR, Aster RH, McFarland JG. Human platelet antigen-specific alloantibodies implicated in 1162 cases of neonatal alloimmune thrombocytopenia. Transfusion. 2004;44:1220–5. doi: 10.1111/j.1537-2995.2004.04026.x. [DOI] [PubMed] [Google Scholar]
- 16.Curtis BR, McFarland JG. Detection and identification of platelet antibodies and antigens in the clinical laboratory. Immunohematology. 2009;25:125–35. [PubMed] [Google Scholar]
- 17.Mueller-Eckhardt C, Kayser W, Forster C, Mueller-Eckhardt G, Ringenberg C. Improved assay for detection of platelet-specific PlA1 antibodies in neonatal alloimmune thrombocytopenia. Vox Sang. 1982;43:76–81. [PubMed] [Google Scholar]
- 18.Socher I, Andrei-Selmer C, Bein G, Kroll H, Santoso S. Low-avidity HPA-1a alloantibodies in severe neonatal alloimmune thrombocytopenia are detectable with surface plasmon resonance technology. Transfusion. 2009;49:943–52. doi: 10.1111/j.1537-2995.2008.02065.x. [DOI] [PubMed] [Google Scholar]
- 19.Bakchoul T, Kubiak S, Krautwurst A, Roderfeld M, Siebert HC, Bein G, Sachs UJ, Santoso S. Low-avidity anti-HPA-1a alloantibodies are capable of antigen-positive platelet destruction in the NOD/SCID mouse model of alloimmune thrombocytopenia. Transfusion. 2011;51:2455–61. doi: 10.1111/j.1537-2995.2011.03171.x. [DOI] [PubMed] [Google Scholar]
- 20.Curtis BR, Edwards JT, Hessner MJ, Klein JP, Aster RH. Blood group A and B antigens are strongly expressed on platelets of some individuals. Blood. 2000;96:1574–81. [PubMed] [Google Scholar]
- 21.Ruan L, Pei B, Li Q. Multicolor real-time polymerase chain reaction genotyping of six human platelet antigens using displacing probes. Transfusion. 2007;47:1637–42. doi: 10.1111/j.1537-2995.2007.01335.x. [DOI] [PubMed] [Google Scholar]
- 22.Fitzgerald LA, Leung B, Phillips DR. A method for purifying the platelet membrane glycoprotein IIb-IIIa complex. Anal Biochem. 1985;151:169–77. doi: 10.1016/0003-2697(85)90067-3. [DOI] [PubMed] [Google Scholar]
- 23.Pidard D, Montgomery RR, Bennett JS, Kunicki TJ. Interaction of AP-2, a monoclonal antibody specific for the human platelet glycoprotein IIb/IIIa complex, with intact platelets. J Biol Chem. 1983;258:12582–6. [PubMed] [Google Scholar]
- 24.Bougie DW, Nayak D, Boylan B, Newman PJ, Aster RH. Drug-dependent clearance of human platelets in the NOD/scid mouse by antibodies from patients with drug-induced immune thrombocytopenia. Blood. 2010;116:3033–8. doi: 10.1182/blood-2010-03-277764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Newman PJ, Aster R, Boylan B. Human platelets circulating in mice: applications for interrogating platelet function and survival, the efficacy of antiplatelet therapeutics, and the molecular basis of platelet immunological disorders. J Thromb Haemost. 2007;5 (Suppl 1):305–9. doi: 10.1111/j.1538-7836.2007.02466.x. [DOI] [PubMed] [Google Scholar]
- 26.Braud V, Chevrier D, Cesbron A, Bignon JD, Kaplan C, Valentin N, Muller JY. Susceptibility to alloimmunization to platelet HPA-1a antigen involves TAP1 polymorphism. Hum Immunol. 1994;41:141–5. doi: 10.1016/0198-8859(94)90007-8. [DOI] [PubMed] [Google Scholar]
- 27.L’Abbe D, Tremblay L, Filion M, Busque L, Goldman M, Décary F, Chartrand P. Alloimmunization to platelet antigen HPA-1a (PIA1) is strongly associated with both HLA-DRB3*0101 and HLA-DQB1*0201. Hum Immunol. 1992;34:107–14. doi: 10.1016/0198-8859(92)90036-m. [DOI] [PubMed] [Google Scholar]
- 28.Maslanka K, Yassai M, Gorski J. Molecular identification of T cells that respond in a primary bulk culture to a peptide derived from a platelet glycoprotein implicated in neonatal alloimmune thrombocytopenia. J Clin Invest. 1996;98:1802–8. doi: 10.1172/JCI118980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rayment R, Kooij TW, Zhang W, Siebold C, Murphy MF, Allen D, Willcox N, Roberts DJ. Evidence for the specificity for platelet HPA-1a alloepitope and the presenting HLA-DR52a of diverse antigen-specific helper T cell clones from alloimmunized mothers. J Immunol. 2009;183:677–86. doi: 10.4049/jimmunol.0801473. [DOI] [PubMed] [Google Scholar]
- 30.Moulds MK. Serological investigation and clinical significance of high-titer, low-avidity (HTLA) antibodies. Am J Med Technol. 1981;47:789–95. [PubMed] [Google Scholar]
- 31.Rolih S. A review: antibodies with high-titer, low-avidity characteristics. Immunohematology. 1990;6:59–67. [PubMed] [Google Scholar]
- 32.Lee WM, Grindle K, Pappas T, Marshall DJ, Moser MJ, Beaty EL, Shult PA, Prudent JR, Gern JE. High-throughput, sensitive, and accurate multiplex PCR-microsphere flow cytometry system for large-scale comprehensive detection of respiratory viruses. J Clin Microbiol. 2007;45:2626–34. doi: 10.1128/JCM.02501-06. [DOI] [PMC free article] [PubMed] [Google Scholar]