Abstract
Cancer patients often suffer from pain and most will be prescribed μ-opioids. μ-opioids are not satisfactory in treating cancer pain and are associated with multiple debilitating side effects. Recent studies show that μ and δ opioid receptors are separately expressed on IB4 (−) and IB4 (+) neurons which control thermal and mechanical pain, respectively. In this study we investigated IB4 (+) and IB4 (−) neurons in mechanical and thermal hypersensitivity in an orthotopic mouse oral cancer model. We used a δ opioid receptor agonist and a P2X3 antagonist to target IB4 (+) neurons and to demonstrate that this subset plays a key role in cancer-induced mechanical allodynia, but not in thermal hyperalgesia. Moreover, selective removal of IB4 (+) neurons using IB4-SAP impacts cancer-induced mechanical but not thermal hypersensitivity. Our results demonstrate that peripherally administered pharmacological agents targeting IB4 (+) neurons, such as a selective δ-opioid receptor agonist or P2X3 antagonist, might be useful in treating oral cancer pain.
Perspective
To clarify the mechanisms of oral cancer pain, we examined the differential role of IB4 (+) and IB4 (−) neurons. Characterization of these two subsets of putative nociceptors is important for further development of effective clinical cancer pain relief.
Keywords: δ-opioid receptor (DOR), μ-opioid receptor (MOR), NGF, isolectin B4, cancer pain
Introduction
Pain represents one of the worst symptoms for cancer patients and remains the most difficult to treat. Opioid analgesics (e.g., morphine) that target μ opioid receptors (MORs) are the most common therapy for cancer pain. Although μ opioids are initially effective for cancer pain management, they are associated with undesired side effects, including opioid tolerance, physical dependence, nausea, respiratory depression, constipation, and immunosuppression11, 14. There is no effective analgesic regimen for treating intractable cancer pain. As a result, patients often experience agonizing pain and resultant debilitation if tolerance to MORs agonists develops.
The contribution by δ opioid receptor (DORs) to cancer pain is not as well characterized as that of MORs. Some studies report that DOR agonists produce potent analgesia in several pain conditions including bone cancer pain in animal models9, 15, 30, 56. Moreover, DOR agonists produce minimal side effects and do not lead to tolerance13, 15, 30. These characteristics make them a promising alternative for the treatment of cancer pain.
Recently it has been shown that MORs and DORs are expressed in different subsets of putative nociceptors that serve distinct functions 46. DORs are located on IB4 (+) neurons and DOR agonists produce analgesia to mechanical pain with no effect on thermal pain. In contrast, MORs are expressed on IB4 (−) neurons and MOR agonists reduce heat pain without affecting mechanical pain. However, the complete segregation between MORs and DORs in mice has been challenged recently 59. Nevertheless, IB4 (+) and IB4 (−) neurons differ in their neurochemical expression, sensitivity to neurotrophins, electrophysiological properties, and anatomical locations, which might ultimately lead to distinct functions 18, 21, 35, 41, 52, 53. IB4 (−) neurons express TrkA receptors that bind nerve growth factor (NGF), depend on NGF for survival, express substance P (SP) and calcitonin gene-related peptide (CGRP) 4, 36. In contrast, IB4 (+) neurons express receptors for glial cell line-derived neurotrophic factor (GDNF), depend on GDNF for survival, express P2X3 receptors, and have poor expression of SP and CGRP 3, 5, 8, 22, 37, 58. Centrally, IB4 (+) neurons terminate predominantly in inner lamina II whereas IB4 (−) neurons terminate in lamina I and outer lamina (II) 36, 51. Despite distinct characteristics of the two subsets, the specific function of IB4 (+) and IB4 (−) neurons is not known in oral cancer pain.
NGF, which primarily acts on IB4(−)/TrkA(+) neurons, plays a role in pancreatic cancer pain 62 as well as bone cancer pain 23, 34, 47. Neutralizing NGF attenuates both spontaneous and movement-evoked pain in mice with both cancer 23, 34, 47. Oral squamous cell carcinoma (OSCC), which is characterized by mechanical allodynia and is clinically distinct from bone cancer, also expresses high levels of NGF16, 28, 61. The limited antinociceptive effect of anti-NGF in our OSCC mouse paw model 61 implies that IB4 (−)/TrkA (+) neurons are not solely responsible for carcinoma-induced mechanical pain. The involvement of IB4 (+) neurons in oral cancer pain has never been studied. It is also unknown whether OSCC cells secrete neurotrophins from the GDNF family that can activate and sensitize IB4 (+) neurons. GDNF and neurturin are particularly of interest as they both maintain a subset of IB4 (+)/ P2X3 (+) neurons that do not express CGRP 2, 32. In addition, GDNF has been shown to reduce mechanical thresholds of IB4 (+) neurons 7, 38.
In the present study, we examined the role of IB4 (+) and IB4 (−) neurons in processing mechanical and thermal nociception following OSCC supernatant injection in mice. In addition, we evaluated whether pharmacological agents such as P2X3 antagonist and DOR agonist are effective against OSCC-induced nociception.
Materials and Methods
Experimental animals
42 male C57BL/6 mice (6–8 weeks old, Charles River Laboratories, Hollister, CA) were used in this study. They were exposed to a light-dark cycle (L:D 12:12-h) and kept in a temperature-controlled room with food and water ad libitum. All procedures were approved by the New York University Institutional Animal Care and Use Committee.
Cell culture
The human tongue SCC cell line, HSC-3 (ATCC, Manassas, VA), and human normal oral keratinocytes (NOK) were cultured at 37 °C with 5% CO2. Both cell cultures were grown to confluence and then washed to remove all unattached cells. The media for both SCC and NOK cell cultures were replaced with Defined Keratinocyte–Serum Free Media (SFM) and then further incubated for 72 h prior to supernatant collection for behavioral testing and ELISA, as we previously described 29.
Intrathecal administration of IB4-saporin
2μl of IB4-SAP (1.2mg/ml, 53% saporin/mole IB4) or 3μl unconjugated saporin (SAP) (1mg/ml) (Advanced Targeting Systems, San Diego, CA) was diluted with PBS to a total volume of 8μl were anesthetized with 2.5% isoflurane. With the use of a Hamilton syringe, IB4-SAP (n=6 mice) or SAP (control, n=6 mice) was injected into the subarachnoid space on the midline between the L4 and L5 vertebrae. All behavioral testing of IB4-SAP and SAP treated mice was performed 14 days after injection.
Supernatant injection and behavioral testing
50μl supernatant from HSC-3 or NOK cells was injected in the mid-plantar right hind paw of each mouse (n=6 in each group) 29. Our previous study demonstrated that HSC-3 supernatant induced mechanical allodynia for a duration of 3 or more hours 29. In this current study, mechanical sensitivity was measured 1 hr post-injection using an electronic von Frey anesthesiometer (IITC Life Science, Woodland Hills, CA). The withdrawal-threshold was defined as the force (g) that was sufficient to elicit a withdrawal response. Six measurements were taken for each animal. Thermal sensitivity was measured using a paw thermal stimulator (Hargreaves’ Apparatus, Department of Anesthesiology, UC San Diego, La Jolla, CA). Mice were placed in plastic chambers on a heated glass surface (25°C). A radiant heat source was focused on the hindpaw and latency to withdraw was measured as the average of 6 trials per animal taken ≥ 5 minutes apart. The cutoff latency was set at 20 seconds to avoid tissue damage. In all behavioral experiments, the observer was blind to the treatment groups.
Drugs
TNP-ATP is a selective P2X antagonist which potently blocks P2X1, P2X3, and heteromeric P2X2/3 receptors. SNC80 is a highly selective agonist for DORs. Naltrindole (NTI) is a selective DOR antagonist. SNC80 (Tocris Bioscience, Ellisville, MI) was dissolved in sterile acidic (0.2% HCl) saline solution. NTI (Sigma, St. Louis, MO), TNP-ATP (Sigma, St. Louis, MO), and NGF neutralizing antibody (Mab 256, R&D Systems, San Jose, CA) were mixed directly into 50μl of cancer supernatant and injected into the right hind paw of the mice 1h prior to behavioral testing. 10nmol of SNC80, 0.2nmol NTI, 2μmol TNP-ATP, and 12.5μg anti-NGF were administered to each animal. Drug dosage was based on previous findings 1, 24, 46.
IB4 labeling
Animals were euthanized with 4% isoflurane and perfused with cold 0.1M PBS solution followed by 4% paraformaldehyde (PFA). The spinal cord was removed, post-fixed in 4% PFA, and cryo-protected in sucrose gradient (20%–50%) at 4°C. Serial frozen spinal cord sections (12μm) were cut on a cryostat and thaw-mounted on gelatin-coated slides for processing. Following tissue sectioning, spinal cord sections were briefly rinsed in PBS and then incubated in 5% goat serum in PBS with 0.1% Triton X-100 for 1h followed by incubation overnight in IB4-FITC (1:400 Sigma, St. Louis, MO). The specificity of IB4-FITC was reported in previous studies 25, 55, and was confirmed by distinct patterns of IB4 and SP labeling in the spinal cord (data not shown). Image analysis was performed using NIH Image J software. The area of staining was outlined and pixel density within the selected area was then measured and divided by the total area. Data were collected from 6 randomly selected sections from 3 animals per treatment group.
ELISA measurement of GDNF and Neurturin
Supernatant content of GDNF and neurturin from both HSC-3 cells and NOKs was measured using human GDNF (RayBiotech, Inc., Norcross, GA) and neurturin ELISA kit (Antigenix America, Inc, Huntingston Sta, NY), respectively. The optical density of the standards and samples was read at 450 nm wavelength using a Model 680 Microplate Reader (Bio-Rad Laboratories, Inc., Hercules, CA). All samples (n=3) were run in quadruplicate.
Statistical analysis
The statistics software SigmaPlot for Windows (version 11.0) was used to perform Students’ t test, or one-way ANOVA for Multiple Comparisons. Significance level was set at P < 0.05. Results are presented as mean ± SEM.
Results
OSCC supernatant induces both mechanical and thermal hypersensitivity
Consistent with our previous findings 29, intraplantar injection of OSCC supernatant induced significant mechanical allodynia as shown by decreased paw withdrawal thresholds to mechanical stimulation (Fig. 1A). In addition, we showed that OSCC supernatant produced thermal hyperalgesia. The paw withdrawal latency to noxious heat stimulus in OSCC supernatant-treated mice was shorter than vehicle treated ones (Fig. 1B).
Figure 1.
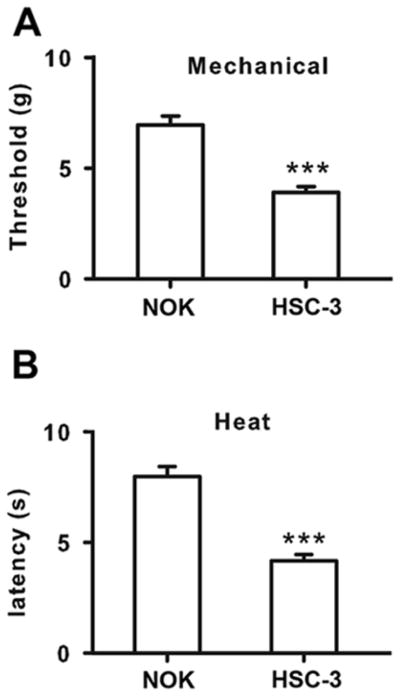
OSCC supernatant injected into the mice hind paws resulted in both mechanical and thermal hypersensitivity. A. OSCC supernatant decreased paw withdrawal latency to mechanical stimulus compared with normal oral keratinocytes (NOK) supernatant injection in mice. B. OSCC supernatant decreased paw withdrawal latency to thermal stimulus compared with NOK supernatant injection in mice. ***P<0.001
Both IB4 (+) and IB4 (−) neurons mediate OSCC supernatant-induced mechanical allodynia
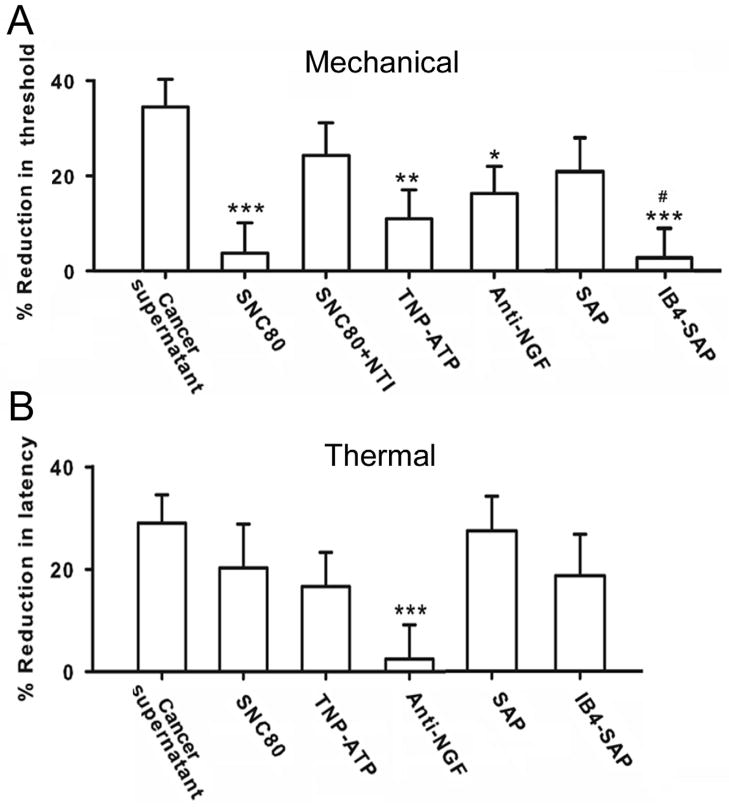
We first treated mice with DOR selective agonist SNC80 mixed in OSCC supernatant. SNC80 significantly inhibited OSCC supernatant-induced mechanical allodynia (Fig. 2A, n=6). The anti-nociceptive action of SNC80 on mechanical allodynia was blocked by co-administration of a low dose of the DOR selective antagonist naltrindole (Fig. 2A). Since a proportion of IB4 (+) neurons express the purinergic receptor P2X3, we used the P2X3 antagonist TNP-ATP to determine whether antagonism of P2X3 blocks OSCC induced mechanical hypersensitivity. TNP-ATP also reduced OSCC supernatant induced mechanical allodynia (Fig. 2A). Involvement of IB4 (+) nociceptors in OSCC-induced mechanical hypersensitivity is further confirmed by selective ablation of IB4 (+). Two weeks after the intrathecal administration of IB4-SAP, IB4 (+) neurons in the dorsal horn of the spinal cord degenerated. As shown in figure 3, IB4 (+) labeling was significantly decreased in the spinal cord of IB4-SAP treated mice compared to SAP-treated controls. Pretreatment with IB4-SAP (n=6) eliminated mechanical hypersensitivity induced by cancer supernatant (Fig. 2A) while mechanical hypersensitivity remained in SAP injected controls. The mechanical thresholds of SAP control mice were not significantly different from naïve control mice after OSCC supernatant injection.
Figure 2.
IB4 (+) and IB4 (−) neurons in OSCC-induced nociceptive behaviors. A. OSCC-induced mechanical allodynia was attenuated by DOR selective agonist SNC80 and P2X3 antagonist TNP-ATP. The effect of SNC80 was blocked by coadministration of DOR selective antagonist naltrindole (NTI). Note that anti-NGF was also effective in blocking OSCC-induced mechanical allodynia. Removal of IB4 (+) neurons by IB4-SAP significantly abolished OSCC-induced mechanical allodynia. B. SNC80 or TNP-ATP was not effective against thermal hyperalgesia. In contrast, anti-NGF significantly blocked OSCC-induced thermal hypersensitivity. Removal of IB4 (+) neurons by IB4-SAP showed no effect on OSCC-induced thermal hyperalgesia. IB4-SAP and SAP treated mice exhibited similar reduction after OSCC-supernatant injection. *P<0.05; **P<0.01; ***P<0.001, groups were compared with OSCC-supernatant injection alone. # P<0.05; IB4-SAP vs. SAP treatment group.
Figure 3.
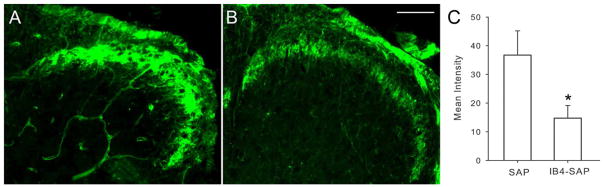
Two weeks after intrathecal IB4-SAP injection, IB4 staining in the superficial dorsal horn of L4 and L5 spinal segments was reduced. A. Spinal cord section of SAP treated control mice. B. Spinal cord section of IB4-SAP treated mice. C. Immuno-intensity was reduced after IB4-SAP compared with SAP treated mice. *P<0.05; Scale bar=100μm.
Neutralization of NGF in the OSCC supernatant also effectively decreased cancer supernatant-induced mechanical hypersensitivity (Fig. 2A), indicating that IB4 (−)/TrkA (+) nociceptors are involved as well.
IB4 (+) neurons do not participate in cancer supernatant induced thermal hyperalgesia
Neither SNC-80 (DOR selective agonist) nor TNP-ATP (P2X3 selective antagonist) exhibited efficacy against thermal hypersensitivity induced by cancer supernatant (Fig. 2B). In addition, IB4-SAP treated mice still showed thermal hypersensitivity after OSCC supernatant injection and their paw withdrawal latency did not differ from mice injected with nonconjugated saporin controls (Fig. 2B). In contrast, anti-NGF significantly reduced OSCC supernatant-induced thermal hyperalgesia (Fig. 2B).
Increased neurturin but not GDNF in oral SCC supernatant
It is known that GDNF reduces mechanical sensitivity thresholds of IB4 (+) nociceptors 7, 38, we further investigated whether OSCC cells secrete higher than normal levels of GDNF. OSCC supernatant had similar GDNF concentration compared to normal oral keratinocyte supernatant (Fig. 4A). In contrast, neurturin levels were higher in OSCC cells compared with supernatant from normal oral keratinocytes (Fig. 4B, P < 0.05).
Figure 4.
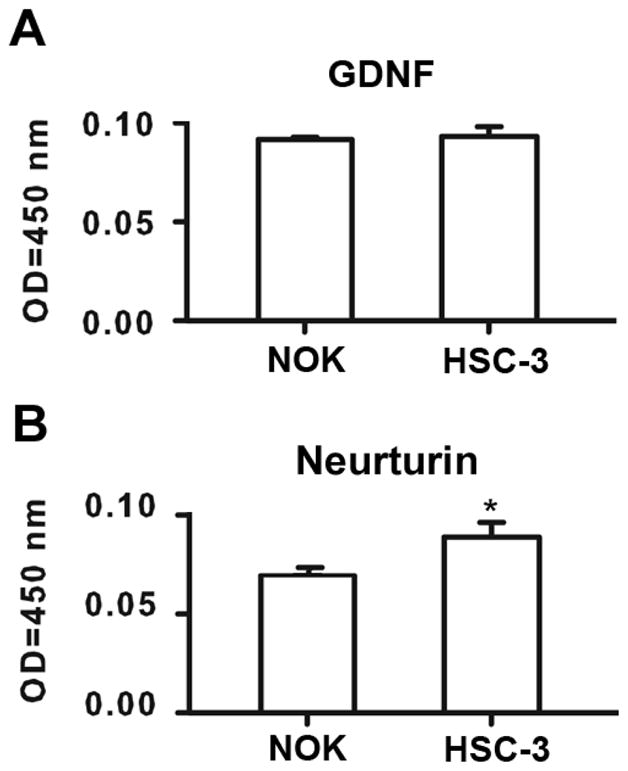
Neurotrophin concentration in OSCC cells. A. OSCC supernatant had similar GDNF concentration compared with that of NOKs. OD=optical density. B. OSCC supernatant had higher neurturin concentration compared with that of NOKs. Values are expressed in OD values measured at 450 nm wavelength.
Discussion
In this study we provide evidence that IB4 (+) and IB4 (−) nociceptors are differentially involved in OSCC-induced pain. While both neuronal subpopulations are responsible for mechanical allodynia induced by OSCC supernatant in our mouse model, IB4 (−)/TrkA (+) neurons exclusively mediated thermal hyperalgesia.
We have shown from our previous studies that OSCC causes mechanical allodynia in both a mouse paw and a tongue model 29, 61. However the cellular source of nociceptive mediators is difficult to isolate due to the heterogeneous nature of the cancer microenvironment. Other than cancer cells, the cancer microenvironment includes many cellular types such as immune cells, fibroblasts, and endothelial cells. All these cells can secrete factors that directly excite or sensitize nociceptors. Here by injecting OSCC supernatant directly into the mouse paw, we clearly demonstrate that mediators secreted by cancer cells alone are sufficient to cause mechanical and thermal nociception in mice.
We then employed several pharmacological approaches to identify the subpopulation of putative nociceptors that are involved in OSCC-induced mechanical and thermal hypersensitivity. While it is still a question as to whether DORs are expressed exclusively on IB4 (+) neurons in mice 46, a selective DOR agonist effectively decreased OSCC-induced mechanical allodynia. In mice DRG neurons, the majority of IB4 (+) nociceptors express P2X3 receptors 8, 58. We therefore used the P2X3 receptor antagonist to target IB4 (+) nociceptors as our second pharmacologic approach. P2X3 receptor antagonist also attenuated OSCC-induced mechanical allodynia. Lastly, to confirm the involvement of IB4 (+) neurons in OSCC-induced mechanical allodynia, IB4 (+) neurons were eliminated using IB4-Saporin conjugates. We conclude that mechanical allodynia is decreased by IB4-SAP treatment. In contrast to the results found with mechanical allodynia, the DOR agonist, P2X3 antagonist, and IB4-SAP treatment showed no significant effect on OSCC-induced thermal hypersensitivity. Consistent with previous findings on IB4 (+) neurons 12, 46, our study suggests that IB4 (+) neurons do not play a significant role in OSCC-induced thermal nociception. Our finding that anti-NGF successfully abolishes OSCC-induced thermal hyperalgesia suggests that in the setting of oral cancer, thermal hyperalgesia is mediated by IB4 (−)/TrkA (+) neurons.
The involvement of IB4 (−)/TrkA (+) neurons in mechanical pain is not surprising. Cancers of different histological types, including OSCC, produce high levels of NGF, and NGF neutralizing antibodies decrease cancer-induced mechanical pain 23, 34, 47, 61. Peripherally administered NGF produces robust mechanical hypersensitivity in mice, rats, and humans 6, 19, 27, 33, 54, suggesting the role of IB4 (−)/TrkA (+) neurons in mechanical nociception.
While Scherrer and colleagues found complete segregation of IB4 (+)/DOR (+) and IB4 (−) /MOR (+) neurons in mechanical and thermal processing 46, a more recent study found overlap between MOR and DOR expression in mice DRGs 59. However, this finding of overlap could not fully explain our results, and functional differences between IB4 (−) and IB4 (+) subsets have been reported extensively 18, 21, 35, 41, 52, 53. In agreement with that of Scherrer et al., we found that DOR agonism inhibited mechanical allodynia but not thermal hyperalgesia. Furthermore, functional convergence of IB4 (+) and IB4 (−) neurons was only found in mechanical allodynia but not thermal hyperalgesia. Lastly, unlike in the rats where IB4 (+) and TrkA (+) neurons overlap substantially21, 53, it has been reported that there is little overlap between these two subpopulations in mice 22, 37, 43. It appears that IB4 (+) neurons might be lacking receptors necessary for thermal processing in mice. Indeed, IB4 (+) neurons express few TRPV1 channels10, 32, 60, 63, but the role of IB4 (+) neurons in thermal sensitivity remains unclear 10, 18, 53, 60.
Discrepancy in Scherrer and colleagues’ and current experimental results might reflect differences in the routes of drug delivery. While Scherrer and colleagues used spinal administration of opioid agonists 46, we administered all drugs into the paw. Therefore, the difference between our study and Scherrer et al.’s might result from functional differences between the central and peripheral terminals of nociceptors. Although peripheral administered drugs could have a central effect, in our case, opioid agonists would have a direct effect on peripheral terminals first.
Differences in the pain behavioral models could also contribute to the disparate findings. We injected cancer supernatant containing a complex mixture of algogenic chemicals; therefore, cross activation or sensitization by chemical mediators on the two subsets of neurons is likely to occur. In addition to endothelin, NGF, proteases, IL-6, and TNF-α, as we have previously reported 29, 44, 45, 61, OSCC releases neurturin which might activate IB4 (+) neurons. Since GDNF acts on IB4 (+) neurons to produce mechanical allodynia 25, we investigated whether cancer supernatant contains a high level of GDNF. Both normal oral keratinocytes and HSC-3 cell supernatant produced and secreted a low concentration of GDNF demonstrating that the activity of IB4 (+) neurons is not likely driven by GDNF in our model. Future studies should scan the full spectrum of mediators secreted by cancer cells and their actions on IB4 (+) and IB4 (−) neurons which might differentially modulate thermal and mechanical nociception. It has been reported that IB4 (+) neurons express receptors for a number of mediators including neurturin 32, ATP 8, 58, bradykinin 57 and VEGF 31. Therefore, IB4 (+) neurons are likely to be activated by a multiple mediators in the cancer microenvironment.
It should be noted that there are differences in the nociceptive circuitry and neurochemical expression patterns in trigeminal ganglia and DRG. In rats, there are substantial differences in SP, CGRP, and IB4 expression patterns between the DRG and trigeminal ganglia 43. In mice, only 10% of the peptidergic (i.e. contains SP or CGRP) and IB4 (+) neurons overlap in trigeminal ganglia43. While this percentage is comparable to what has been reported in mouse DRGs 22, 37 the lack of information regarding other nociceptive markers in trigeminal ganglia and DRGs prevented us from drawing further conclusions. We have used a paw model in this oral cancer pain study because there are well established mechanical and thermal nociceptive assays for this model. A more complete understanding of the putative nociceptors mediating oral cancer pain will require an anatomically correct oral cancer model and validated thermal and nociceptive assays.
Our finding that peripherally administered DOR agonists and P2X3 antagonists are effective in reducing OSCC-induced mechanical hypersensitivity has significant clinical implications. Function or movement provoked (mechanical) pain represents a major clinical problem in oral cancer patients who often experience difficulty with eating, drinking, swallowing, and speaking, and experience a much lower quality of life 16, 20. Oral cancer patients report pain as the worst symptom, and in their final months of life, 85% of these patients suffered from pain 48. While μ-opioids are initially effective in alleviating pain, tolerance develops quickly, doses escalate, and side effects abound.
DOR-selective agonists have been shown to produce potent analgesia when administered intrathecally 42, 46, 50, 56 and subcutaneously 26, 39, 40, with a reduction or absence in the development of physical dependence 17, reduced constipation 49, and reduced respiratory depression 13. Additionally, systemic application of a δ-opioid receptor agonist exhibited much higher potency than morphine in treating bone cancer induced mechanical pain and had fewer side effects 9. In this study, we demonstrated for the first time that DORs and P2X3 receptors play a role in oral cancer pain. Peripheral action of the DOR agonist and P2X3 antagonist might also increase efficacy and minimize unwanted effects. Accordingly, analgesics targeting IB4 (+) neurons present a promising avenue for future drug development to treat oral cancer pain.
Conclusion
IB4 (+) and IB4 (−) neurons are differentially involved in OSCC-induced pain. Mechanical hypersensitivity is mediated by both IB4 (+) and IB4 (−) neurons, while thermal hypersensitivity is mediated predominantly by IB4 (−) neurons. Pharmacological agents targeting IB4 (+) neurons exhibit great therapeutic potential in oral cancer pain treatment.
Acknowledgments
This work was funded by NIH/NIDCR R21DE01856
Footnotes
Disclosures
The authors declare no conflict of interest for these studies.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Adriaenssens E, Vanhecke E, Saule P, Mougel A, Page A, Romon R, Nurcombe V, Le Bourhis X, Hondermarck H. Nerve growth factor is a potential therapeutic target in breast cancer. Cancer Res. 2008;68:346–351. doi: 10.1158/0008-5472.CAN-07-1183. [DOI] [PubMed] [Google Scholar]
- 2.Albers KM, Davis BM. The skin as a neurotrophic organ. Neuroscientist. 2007;13:371–382. doi: 10.1177/10738584070130040901. [DOI] [PubMed] [Google Scholar]
- 3.Albers KM, Woodbury CJ, Ritter AM, Davis BM, Koerber HR. Glial cell-line-derived neurotrophic factor expression in skin alters the mechanical sensitivity of cutaneous nociceptors. J Neurosci. 2006;26:2981–2990. doi: 10.1523/JNEUROSCI.4863-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bennett DL, Koltzenburg M, Priestley JV, Shelton DL, McMahon SB. Endogenous nerve growth factor regulates the sensitivity of nociceptors in the adult rat. Eur J Neurosci. 1998;10:1282–1291. doi: 10.1046/j.1460-9568.1998.00139.x. [DOI] [PubMed] [Google Scholar]
- 5.Bennett DL, Michael GJ, Ramachandran N, Munson JB, Averill S, Yan Q, McMahon SB, Priestley JV. A distinct subgroup of small DRG cells express GDNF receptor components and GDNF is protective for these neurons after nerve injury. J Neurosci. 1998;18:3059–3072. doi: 10.1523/JNEUROSCI.18-08-03059.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bergmann I, Reiter R, Toyka KV, Koltzenburg M. Nerve growth factor evokes hyperalgesia in mice lacking the low-affinity neurotrophin receptor p75. Neuroscience Letters. 1998;255:87–90. doi: 10.1016/s0304-3940(98)00713-7. [DOI] [PubMed] [Google Scholar]
- 7.Bogen O, Joseph EK, Chen X, Levine JD. GDNF hyperalgesia is mediated by PLCgamma, MAPK/ERK, PI3K, CDK5 and Src family kinase signaling and dependent on the IB4-binding protein versican. Eur J Neurosci. 2008;28:12–19. doi: 10.1111/j.1460-9568.2008.06308.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bradbury EJ, Burnstock G, McMahon SB. The expression of P2X3 purinoreceptors in sensory neurons: effects of axotomy and glial-derived neurotrophic factor. Mol Cell Neurosci. 1998;12:256–268. doi: 10.1006/mcne.1998.0719. [DOI] [PubMed] [Google Scholar]
- 9.Brainin-Mattos J, Smith ND, Malkmus S, Rew Y, Goodman M, Taulane J, Yaksh TL. Cancer-related bone pain is attenuated by a systemically available delta-opioid receptor agonist. Pain. 2006;122:174–181. doi: 10.1016/j.pain.2006.01.032. [DOI] [PubMed] [Google Scholar]
- 10.Breese NM, George AC, Pauers LE, Stucky CL. Peripheral inflammation selectively increases TRPV1 function in IB4-positive sensory neurons from adult mouse. Pain. 2005;115:37–49. doi: 10.1016/j.pain.2005.02.010. [DOI] [PubMed] [Google Scholar]
- 11.Breivik H. Opioids in cancer and chronic non-cancer pain therapy – indications and controversies. Acta Anaesthesiologica Scandinavica. 2001;45:1059–1066. doi: 10.1034/j.1399-6576.2001.450902.x. [DOI] [PubMed] [Google Scholar]
- 12.Cavanaugh DJ, Lee H, Lo L, Shields SD, Zylka MJ, Basbaum AI, Anderson DJ. Distinct subsets of unmyelinated primary sensory fibers mediate behavioral responses to noxious thermal and mechanical stimuli. Proceedings of the National Academy of Sciences. 2009;106:9075–9080. doi: 10.1073/pnas.0901507106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cheng PY, Wu D, Decena J, Soong Y, McCabe S, Szeto HH. Opioid-induced stimulation of fetal respiratory activity by [D-Ala2]deltorphin I. Eur J Pharmacol. 1993;230:85–88. doi: 10.1016/0014-2999(93)90413-c. [DOI] [PubMed] [Google Scholar]
- 14.Christo PJ, Mazloomdoost D. Cancer Pain and Analgesia. Annals of the New York Academy of Sciences. 2008;1138:278–298. doi: 10.1196/annals.1414.033. [DOI] [PubMed] [Google Scholar]
- 15.Codd EE, Carson JR, Colburn RW, Stone DJ, Van Besien CR, Zhang SP, Wade PR, Gallantine EL, Meert TF, Molino L, Pullan S, Razler CM, Dax SL, Flores CM. JNJ-20788560 [9-(8-azabicyclo[3.2.1]oct-3-ylidene)-9H-xanthene-3-carboxylic acid diethylamide], a selective delta opioid receptor agonist, is a potent and efficacious antihyperalgesic agent that does not produce respiratory depression, pharmacologic tolerance, or physical dependence. J Pharmacol Exp Ther. 2009;329:241–251. doi: 10.1124/jpet.108.146969. [DOI] [PubMed] [Google Scholar]
- 16.Connelly ST, Schmidt BL. Evaluation of pain in patients with oral squamous cell carcinoma. J Pain. 2004;5:505–510. doi: 10.1016/j.jpain.2004.09.002. [DOI] [PubMed] [Google Scholar]
- 17.Cowan A, Zhu XZ, Mosberg HI, Omnaas JR, Porreca F. Direct dependence studies in rats with agents selective for different types of opioid receptor. J Pharmacol Exp Ther. 1988;246:950–955. [PubMed] [Google Scholar]
- 18.Dirajlal S, Pauers LE, Stucky CL. Differential response properties of IB(4)-positive and -negative unmyelinated sensory neurons to protons and capsaicin. J Neurophysiol. 2003;89:513–524. doi: 10.1152/jn.00371.2002. [DOI] [PubMed] [Google Scholar]
- 19.Dyck PJ, Peroutka S, Rask C, Burton E, Baker MK, Lehman KA, Gillen DA, Hokanson JL, O’Brien PC. Intradermal recombinant human nerve growth factor induces pressure allodynia and lowered heat-pain threshold in humans. Neurology. 1997;48:501–505. doi: 10.1212/wnl.48.2.501. [DOI] [PubMed] [Google Scholar]
- 20.Epstein JB, Elad S, Eliav E, Jurevic R, Benoliel R. Orofacial pain in cancer: part II--clinical perspectives and management. J Dent Res. 2007;86:506–518. doi: 10.1177/154405910708600605. [DOI] [PubMed] [Google Scholar]
- 21.Fang X, Djouhri L, McMullan S, Berry C, Waxman SG, Okuse K, Lawson SN. Intense isolectin-B4 binding in rat dorsal root ganglion neurons distinguishes C-fiber nociceptors with broad action potentials and high Nav1.9 expression. J Neurosci. 2006;26:7281–7292. doi: 10.1523/JNEUROSCI.1072-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Golden JP, Hoshi M, Nassar MA, Enomoto H, Wood JN, Milbrandt J, Gereau RWt, Johnson EM, Jr, Jain S. RET signaling is required for survival and normal function of nonpeptidergic nociceptors. J Neurosci. 2010;30:3983–3994. doi: 10.1523/JNEUROSCI.5930-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Halvorson KG, Kubota K, Sevcik MA, Lindsay TH, Sotillo JE, Ghilardi JR, Rosol TJ, Boustany L, Shelton DL, Mantyh PW. A blocking antibody to nerve growth factor attenuates skeletal pain induced by prostate tumor cells growing in bone. Cancer Res. 2005;65:9426–9435. doi: 10.1158/0008-5472.CAN-05-0826. [DOI] [PubMed] [Google Scholar]
- 24.Honore P, Mikusa J, Bianchi B, McDonald H, Cartmell J, Faltynek C, Jarvis MF. TNP-ATP, a potent P2X3 receptor antagonist, blocks acetic acid-induced abdominal constriction in mice: comparison with reference analgesics. Pain. 2002;96:99–105. doi: 10.1016/s0304-3959(01)00434-1. [DOI] [PubMed] [Google Scholar]
- 25.Joseph EK, Chen X, Bogen O, Levine JD. Oxaliplatin Acts on IB4-Positive Nociceptors to Induce an Oxidative Stress-Dependent Acute Painful Peripheral Neuropathy. The Journal of Pain. 2008;9:463–472. doi: 10.1016/j.jpain.2008.01.335. [DOI] [PubMed] [Google Scholar]
- 26.Joseph EK, Levine JD. Mu and delta opioid receptors on nociceptors attenuate mechanical hyperalgesia in rat. Neuroscience. 2010;171:344–350. doi: 10.1016/j.neuroscience.2010.08.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kerr BJ, Souslova V, McMahon SB, Wood JN. A role for the TTX-resistant sodium channel Nav 1.8 in NGF-induced hyperalgesia, but not neuropathic pain. Neuroreport. 2001;12:3077–3080. doi: 10.1097/00001756-200110080-00019. [DOI] [PubMed] [Google Scholar]
- 28.Kolokythas A, Cox DP, Dekker N, Schmidt BL. Nerve Growth Factor (NGF) and Tyrosine Kinase A (Trk A) Receptor in Oral Squamous Cell Carcinoma: Is There an Association with Perineural Invasion? Journal of Oral and Maxillofacial Surgery. 2010;68:1290–1295. doi: 10.1016/j.joms.2010.01.006. [DOI] [PubMed] [Google Scholar]
- 29.Lam DK, Schmidt BL. Serine proteases and protease-activated receptor 2-dependent allodynia: A novel cancer pain pathway. Pain. 2010;149:263–272. doi: 10.1016/j.pain.2010.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Le Bourdonnec B, Windh RT, Leister LK, Zhou QJ, Ajello CW, Gu M, Chu GH, Tuthill PA, Barker WM, Koblish M, Wiant DD, Graczyk TM, Belanger S, Cassel JA, Feschenko MS, Brogdon BL, Smith SA, Derelanko MJ, Kutz S, Little PJ, DeHaven RN, DeHaven-Hudkins DL, Dolle RE. Spirocyclic delta opioid receptor agonists for the treatment of pain: discovery of N,N-diethyl-3-hydroxy-4-(spiro[chromene-2,4′-piperidine]-4-yl) benzamide (ADL5747) J Med Chem. 2009;52:5685–5702. doi: 10.1021/jm900773n. [DOI] [PubMed] [Google Scholar]
- 31.Lin J, Li G, Den X, Xu C, Liu S, Gao Y, Liu H, Zhang J, Li X, Liang S. VEGF and its receptor-2 involved in neuropathic pain transmission mediated by P2X2/3 receptor of primary sensory neurons. Brain Research Bulletin. 2010;83:284–291. doi: 10.1016/j.brainresbull.2010.08.002. [DOI] [PubMed] [Google Scholar]
- 32.Lindfors PH, Voikar V, Rossi J, Airaksinen MS. Deficient nonpeptidergic epidermis innervation and reduced inflammatory pain in glial cell line-derived neurotrophic factor family receptor alpha2 knock-out mice. J Neurosci. 2006;26:1953–1960. doi: 10.1523/JNEUROSCI.4065-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Malik-Hall M, Dina OA, Levine JD. Primary afferent nociceptor mechanisms mediating NGF-induced mechanical hyperalgesia. Eur J Neurosci. 2005;21:3387–3394. doi: 10.1111/j.1460-9568.2005.04173.x. [DOI] [PubMed] [Google Scholar]
- 34.Mantyh WG, Jimenez-Andrade JM, Stake JI, Bloom AP, Kaczmarska MJ, Taylor RN, Freeman KT, Ghilardi JR, Kuskowski MA, Mantyh PW. Blockade of nerve sprouting and neuroma formation markedly attenuates the development of late stage cancer pain. Neuroscience. 2010;171:588–598. doi: 10.1016/j.neuroscience.2010.08.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mogil JS, Breese NM, Witty MF, Ritchie J, Rainville ML, Ase A, Abbadi N, Stucky CL, Seguela P. Transgenic expression of a dominant-negative ASIC3 subunit leads to increased sensitivity to mechanical and inflammatory stimuli. J Neurosci. 2005;25:9893–9901. doi: 10.1523/JNEUROSCI.2019-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Molliver DC, Radeke MJ, Feinstein SC, Snider WD. Presence or absence of TrkA protein distinguishes subsets of small sensory neurons with unique cytochemical characteristics and dorsal horn projections. J Comp Neurol. 1995;361:404–416. doi: 10.1002/cne.903610305. [DOI] [PubMed] [Google Scholar]
- 37.Molliver DC, Wright DE, Leitner ML, Parsadanian AS, Doster K, Wen D, Yan Q, Snider WD. IB4-Binding DRG Neurons Switch from NGF to GDNF Dependence in Early Postnatal Life. Neuron. 1997;19:849–861. doi: 10.1016/s0896-6273(00)80966-6. [DOI] [PubMed] [Google Scholar]
- 38.Moore BA, Albers KM, Davis BM, Grandis JR, Togel S, Bauer AJ. Altered inflammatory gene expression underlies increased susceptibility to murine postoperative ileus with advancing age. Am J Physiol Gastrointest Liver Physiol. 2007;292:G1650–1659. doi: 10.1152/ajpgi.00570.2006. [DOI] [PubMed] [Google Scholar]
- 39.Pacheco DF, Duarte ID. Delta-opioid receptor agonist SNC80 induces peripheral antinociception via activation of ATP-sensitive K+ channels. Eur J Pharmacol. 2005;512:23–28. doi: 10.1016/j.ejphar.2005.02.018. [DOI] [PubMed] [Google Scholar]
- 40.Pacheco DF, Reis GM, Francischi JN, Castro MS, Perez AC, Duarte ID. delta-Opioid receptor agonist SNC80 elicits peripheral antinociception via delta(1) and delta(2) receptors and activation of the l-arginine/nitric oxide/cyclic GMP pathway. Life Sci. 2005;78:54–60. doi: 10.1016/j.lfs.2005.04.032. [DOI] [PubMed] [Google Scholar]
- 41.Park CK, Kim MS, Fang Z, Li HY, Jung SJ, Choi SY, Lee SJ, Park K, Kim JS, Oh SB. Functional expression of thermo-transient receptor potential channels in dental primary afferent neurons: implication for tooth pain. J Biol Chem. 2006;281:17304–17311. doi: 10.1074/jbc.M511072200. [DOI] [PubMed] [Google Scholar]
- 42.Porreca F, Filla A, Burks TF. Studies in vivo with dynorphin-(1–9): analgesia but not gastrointestinal effects following intrathecal administration to mice. Eur J Pharmacol. 1983;91:291–294. doi: 10.1016/0014-2999(83)90481-8. [DOI] [PubMed] [Google Scholar]
- 43.Price TJ, Flores CM. Critical evaluation of the colocalization between calcitonin gene-related peptide, substance P, transient receptor potential vanilloid subfamily type 1 immunoreactivities, and isolectin B4 binding in primary afferent neurons of the rat and mouse. J Pain. 2007;8:263–272. doi: 10.1016/j.jpain.2006.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Quang PN, Schmidt BL. Endothelin-A Receptor Antagonism Attenuates Carcinoma-Induced Pain Through Opioids in Mice. J Pain. 2010;11:663–671. doi: 10.1016/j.jpain.2009.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Quang PN, Schmidt BL. Peripheral endothelin B receptor agonist-induced antinociception involves endogenous opioids in mice. Pain. 2010;149:254–262. doi: 10.1016/j.pain.2010.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Scherrer G, Imamachi N, Cao Y-Q, Contet C, Mennicken F, O’Donnell D, Kieffer BL, Basbaum AI. Dissociation of the Opioid Receptor Mechanisms that Control Mechanical and Heat Pain. Cell. 2009;137:1148–1159. doi: 10.1016/j.cell.2009.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sevcik MA, Ghilardi JR, Peters CM, Lindsay TH, Halvorson KG, Jonas BM, Kubota K, Kuskowski MA, Boustany L, Shelton DL, Mantyh PW. Anti-NGF therapy profoundly reduces bone cancer pain and the accompanying increase in markers of peripheral and central sensitization. Pain. 2005;115:128–141. doi: 10.1016/j.pain.2005.02.022. [DOI] [PubMed] [Google Scholar]
- 48.Shedd DP, Carl A, Shedd C. Problems of terminal head and neck cancer patients. Head Neck Surg. 1980;2:476–482. doi: 10.1002/hed.2890020606. [DOI] [PubMed] [Google Scholar]
- 49.Sheldon RJ, Riviere PJ, Malarchik ME, Moseberg HI, Burks TF, Porreca F. Opioid regulation of mucosal ion transport in the mouse isolated jejunum. J Pharmacol Exp Ther. 1990;253:144–151. [PubMed] [Google Scholar]
- 50.Shook JE, Pelton JT, Lemcke PK, Porreca F, Hruby VJ, Burks TF. Mu opioid antagonist properties of a cyclic somatostatin octapeptide in vivo: identification of mu receptor-related functions. J Pharmacol Exp Ther. 1987;242:1–7. [PubMed] [Google Scholar]
- 51.Silverman JD, Kruger L. Selective neuronal glycoconjugate expression in sensory and autonomic ganglia: relation of lectin reactivity to peptide and enzyme markers. J Neurocytol. 1990;19:789–801. doi: 10.1007/BF01188046. [DOI] [PubMed] [Google Scholar]
- 52.Snider WD, McMahon SB. Tackling pain at the source: new ideas about nociceptors. Neuron. 1998;20:629–632. doi: 10.1016/s0896-6273(00)81003-x. [DOI] [PubMed] [Google Scholar]
- 53.Stucky CL, Lewin GR. Isolectin B(4)-positive and -negative nociceptors are functionally distinct. J Neurosci. 1999;19:6497–6505. doi: 10.1523/JNEUROSCI.19-15-06497.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Svensson P, Cairns BE, Wang K, Arendt-Nielsen L. Injection of nerve growth factor into human masseter muscle evokes long-lasting mechanical allodynia and hyperalgesia. Pain. 2003;104:241–247. doi: 10.1016/s0304-3959(03)00012-5. [DOI] [PubMed] [Google Scholar]
- 55.Tarpley JW, Kohler MG, Martin WJ. The behavioral and neuroanatomical effects of IB4-saporin treatment in rat models of nociceptive and neuropathic pain. Brain Research. 2004;1029:65–76. doi: 10.1016/j.brainres.2004.09.027. [DOI] [PubMed] [Google Scholar]
- 56.Tung AS, Yaksh TL. In vivo evidence for multiple opiate receptors mediating analgesia in the rat spinal cord. Brain Res. 1982;247:75–83. doi: 10.1016/0006-8993(82)91029-0. [DOI] [PubMed] [Google Scholar]
- 57.Vellani V, Zachrisson O, McNaughton PA. Functional bradykinin B1 receptors are expressed in nociceptive neurones and are upregulated by the neurotrophin GDNF. The Journal of Physiology. 2004;560:391–401. doi: 10.1113/jphysiol.2004.067462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Vulchanova L, Riedl MS, Shuster SJ, Stone LS, Hargreaves KM, Buell G, Surprenant A, North RA, Elde R. P2X3 is expressed by DRG neurons that terminate in inner lamina II. European Journal of Neuroscience. 1998;10:3470–3478. doi: 10.1046/j.1460-9568.1998.00355.x. [DOI] [PubMed] [Google Scholar]
- 59.Wang HB, Zhao B, Zhong YQ, Li KC, Li ZY, Wang Q, Lu YJ, Zhang ZN, He SQ, Zheng HC, Wu SX, Hokfelt TG, Bao L, Zhang X. Coexpression of delta- and mu-opioid receptors in nociceptive sensory neurons. Proc Natl Acad Sci U S A. 2010;107:13117–13122. doi: 10.1073/pnas.1008382107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Woodbury CJ, Zwick M, Wang S, Lawson JJ, Caterina MJ, Koltzenburg M, Albers KM, Koerber HR, Davis BM. Nociceptors lacking TRPV1 and TRPV2 have normal heat responses. J Neurosci. 2004;24:6410–6415. doi: 10.1523/JNEUROSCI.1421-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ye Y, Dang D, Zhang J, Viet CT, Lam DK, Dolan JC, Gibbs JL, Schmidt BL. Nerve growth factor links oral cancer progression, pain, and cachexia. Mol Cancer Ther. 2011;10:1667–1676. doi: 10.1158/1535-7163.MCT-11-0123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhu Z, Friess H, diMola FF, Zimmermann A, Graber HU, Korc M, Buchler MW. Nerve growth factor expression correlates with perineural invasion and pain in human pancreatic cancer. J Clin Oncol. 1999;17:2419–2428. doi: 10.1200/JCO.1999.17.8.2419. [DOI] [PubMed] [Google Scholar]
- 63.Zwick M, Davis BM, Woodbury CJ, Burkett JN, Koerber HR, Simpson JF, Albers KM. Glial cell line-derived neurotrophic factor is a survival factor for isolectin B4-positive, but not vanilloid receptor 1-positive, neurons in the mouse. J Neurosci. 2002;22:4057–4065. doi: 10.1523/JNEUROSCI.22-10-04057.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]