Abstract
Background
Vascular dysfunction is a surrogate marker of early-stage atherosclerosis. Serum leukocyte count is a non-traditional risk factor of cardiovascular (CV) disease and has predictive value for CV outcome. The aim of this study was to investigate the relationship between leukocyte count and peripheral vascular dysfunction.
Methods and Results
In this cross-sectional study, 357 individuals without known CV disease and with low Framingham risk (10-year hard coronary artery disease risk <10%) were identified. Vascular function was measured by assessing reactive hyperemia-induced vasodilation (reactive hyperemia index, RHI). In 105 individuals with vascular dysfunction (29.4%), the median leukocyte count was significantly higher than in those with normal RHI (6.4×109/L vs. 6.0×109/L; P=0.04). The neutrophil count was the strongest predictor of impaired vascular function among leukocyte subtypes (odds ratio [OR], 2.70; 95% confidence interval [CI]: 1.58–4.60, P<0.001). In a multivariate logistic regression model, the highest quintile of neutrophil count (OR, 2.36; 95% CI: 1.35–4.12; P=0.003), body mass index (OR, 1.05; 95% CI: 1.01–1.09; P=0.009) and systolic blood pressure (OR, 0.97; 95% CI: 0.97–0.99; P<0.001) were independently predictive for vascular dysfunction.
Conclusions
The highest quintile of leukocyte count is independently associated with vascular dysfunction in individuals with low CV risk. This suggests that subclinical inflammation affects vascular function. Leukocyte count may be useful for personalized risk stratification.
Keywords: Framingham risk, Leukocyte count, Vascular function
Atherosclerosis is the primary cause of mortality and morbidity in cardiovascular (CV) disease.1 A large body of evidence has highlighted the key role of systemic low-grade inflammation in all phases of atherosclerosis, and raised levels of inflammatory markers have been associated with poor outcome.2–4 Endothelial dysfunction, characterized by decreased nitric oxide bioavailability, is the earliest detectable functional disturbance in the natural history of atherosclerosis.5 In the last decade, evidence has been accumulating to support the hypothesis that the presence of endothelial dysfunction represents a major promoter for atherosclerosis and thrombosis, and is an independent predictor for future CV events in several types of patients.6 Importantly, elevated levels of inflammatory markers are associated with reduced basal and stimulated nitric oxide release from arterial endothelial cells through various mechanisms.7 Therefore, chronic inflammation may serve as an underlying mechanism for endothelial dysfunction.8 Leukocyte count is a common blood test in clinical practice. High leukocyte count may reflect a chronic inflammatory state and may contribute directly to atherosclerosis through specific mechanisms.9 In prior decades, a number of studies investigated the value of leukocyte count and subtypes for the prediction of CV risk, independent of traditional risk factors, in participants with high CV risk or in the secondary prevention setting.10–12 There have been no studies, however, that have directly explored the effect of leukocyte count on vascular function in low-risk subjects. The purpose of this study was to investigate the relationship between leukocyte count and vascular dysfunction, a surrogate of early atherosclerosis, in individuals with low CV risk.
Methods
Subjects
In this cross-sectional study, we identified patients who underwent assessment of vascular function at the Mayo Clinic in Rochester. Only patients with low Framingham risk (Framingham risk score, FRS; 10-year hard coronary artery disease risk <10%) were included. Exclusion criteria were diabetes, documented CV disease (coronary artery disease, cerebral vascular disease and peripheral arterial disease), uncontrolled hypertension, heart failure, pregnancy, and inflammatory disorders. This study was approved by the Mayo Clinic Institutional Review Board.
Demographic, Clinical and Laboratory Characteristics
Demographic characteristics (age, sex, ethnicity), CV risk factors and history of CV disease were recorded in all patients. Measurements related to CV risk included resting arterial blood pressure (BP), heart rate, body mass index (BMI), lipid profile including total, high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C) and triglyceride, fasting blood glucose and serum creatinine. Total and differential leukocyte counts were assessed using the standard Coulter counter technique (Coulter LH 700; Beckman Coulter, Miami, FL, USA). Blood samples were obtained from individuals in the fasting state after measurements of vascular function.
Risk Factors and Risk Stratification
The risk factors were defined as: (1) hypertension, resting BP≥140/90 mmHg or treatment with anti-hypertensive medications; (2) diabetes mellitus, fasting blood glucose level ≥126 mg/dl and/or treatment with insulin or oral hypoglycemic agents; (3) family history, presence of coronary artery disease in first-degree relatives <55 years of age (male) or <65 years of age (female); (4) hyperlipidemia, serum LDL-C ≥160 mg/dl or treatment with lipid-lowering drugs; and (5) cigarette smoking, current smoking or smoking cessation <1 year.
The CV risk was assessed using FRS13 and only individuals with low risk (10-year hard coronary artery disease risk <10%) were enrolled. Metabolic syndrome was defined according to the recent international statement.14
Measurement of Peripheral Vascular Function
Patients were instructed to start fasting at least 12 h before measurement and to refrain from smoking and strenuous exercise during that time period. All vasoactive medications were discontinued at least 24 h prior to testing. Peripheral arterial tonometry (PAT) signals were obtained using the EndoPAT 2000 device (Itamar Medical, Caesarea, Israel).
A PAT finger probe was placed on each index finger. Pulsatile volume changes of the distal digit induced pressure alterations in the finger cuff, which were sensed by pressure transducers and transmitted to and recorded by the EndoPAT 2000 device. Vascular function was measured via a reactive hyperemia (RH) PAT index (RHI) as previously described.15 The ratio of the PAT signal after cuff release compared with baseline was calculated through a computer algorithm automatically normalizing for baseline signal and indexed to the contralateral arm. The calculated ratio (RHI) is a validated measure of vascular function, with higher RHI correlating with better vascular function. RHI ≤1.67 is generally used as a cutoff point for endothelial dysfunction.
The RH-PAT data were collected and analyzed on computer in an operator-independent manner. Determination of reproducibility of RH-PAT measurements has been described previously.16
Statistical Analysis
The normality of all distributions was assessed using Kolmogorov-Smirnov test. Data are presented as mean ± SD or median for continuous variables and frequencies, group percentages for discrete variables. Because leukocyte count and differential count were non-normally distributed, these variables are presented as geometric mean with 20th and 80th percentile ranks and are further categorized into quintiles. The highest quintile is defined as above the 80th percentile of the variable (quintile 5; Q5). Two-sample t-tests or rank-sum tests were used to test group differences in continuous variables; the Pearson chi-squared test was used for discrete variables. All tests were 2-tailed and P<0.05 was considered significant. Spearman correlation tests were used to analyze bivariate associations between changes in RHI and changes in other variables. Four multivariate logistic regression models were used to identify independent determinants of endothelial dysfunction. In model A, age, gender, BMI, current smoking, systolic BP (SBP), total cholesterol, HDL-C, and the leukocyte count (as a continuous variable) were included. In model B, age, gender, BMI, current smoking, SBP, total cholesterol, HDLC, and the neutrophil count (as a continuous variable) were included. In model C, age, gender, BMI, current smoking, SBP, total cholesterol, HDL-C and the highest quintile of the leukocyte count were included. In model D, age, gender, BMI, current smoking, SBP, total cholesterol, HDL-C and the highest quintile of the neutrophil count were included. We report the odds ratio (OR) and their confidence intervals (CI). Variables with a high level of association were not included in the same model. JMP 8.0 (SAS Institute, Cary, NC, USA) was used for statistical analysis.
Results
Demographic, Clinical and Laboratory Characteristics
A total of 357 patients were eligible for analysis. Among them, 259 (72.5%) were outpatients at a preventive cardiology clinic, 78 (21.9%) were healthy volunteers, and 20 (5.6%) were outpatients at an internal medicine clinic. Of them, 105 (29.4%) had endothelial dysfunction (RHI ≤ 1.67). The demographic, clinical and laboratory characteristics are listed in Table 1. Compared to patients with normal vascular function, fewer patients with vascular dysfunction had hypertension (21.9% vs. 34.5%, P=0.01); they were more overweight (BMI 28.6± 6.0 kg/m2 vs. 27.0±5.6 kg/m2, P=0.02); were younger (age 45.3±14.3 years vs. 48.5±13.3 years (P=0.04); had lower SBP (115.9±14.5 mmHg vs. 121.4±15.3 mmHg, P=0.01); and had lower HDL-C (54.0±18.2 mg/dl vs. 58.7±16.6 mg/dl, P=0.02). Ten-year Framingham risk was not significantly different between the 2 groups (2.2±1.9 and 2.5±2.1, P=0.18).
Table 1.
Subject Characteristics
| Endothelial dysfunction (n=105) | Normal vascular function (n=252) | P-value | |
|---|---|---|---|
| Age (years) | 45.3±14.3 | 48.5±13.3 | 0.04 |
| Sex (male) | 31 (29.5) | 74 (29.4) | 0.98 |
| Ethnicity (Caucasian) | 98 (93.3) | 232 (92.1) | 0.67 |
| Hypertension | 23 (21.9) | 87 (34.5) | 0.01 |
| Smoking | 8 (7.6) | 10 (4.0) | 0.15 |
| Menopausal women | 136 (31.4) | 103 (40.9) | 0.09 |
| Metabolic syndrome | 32 (30.5) | 56 (22.2) | 0.09 |
| SBP (mmHg) | 115.9±14.5 | 121.4±15.3 | 0.01 |
| DBP (mmHg) | 72.2±9.6 | 74.7±10.4 | 0.03 |
| BMI (kg/m2) | 28.6±6.0 | 27.0±5.6 | 0.02 |
| Medications | |||
| Aspirin | 36 (34.3) | 101 (40.1) | 0.31 |
| RAAS inhibitor | 14 (13.3) | 34 (13.5) | 0.97 |
| β-blocker | 21 (20.0) | 50 (19.8) | 0.97 |
| CCB | 16 (15.2) | 32 (12.7) | 0.52 |
| Statins | 26 (24.8) | 62 (24.6) | 0.96 |
| Glucose (mg/dl) | 96.6±11.3 | 95.7±10.7 | 0.56 |
| TCH (mg/dl) | 192.1±44.7 | 198.9±40.7 | 0.16 |
| TG (mg/dl) | 122.2±87.2 | 120.6±74.8 | 0.86 |
| HDL-C (mg/dl) | 54.0±18.2 | 58.7±16.6 | 0.02 |
| LDL-C (mg/dl) | 115.0±39.2 | 116.8±36.6 | 0.71 |
| Creatinine (mg/dl) | 0.9±0.2 | 0.9±0.2 | 0.69 |
| Framingham risk (%) | 2.2±1.9 | 2.5±2.1 | 0.18 |
Data given as mean ± SD or n (%).
BMI, body mass index; CCB, calcium channel blocker; DBP, diastolic blood pressure; HDL-C, serum high-density lipoprotein cholesterol; LDL-C, serum low-density lipoprotein cholesterol; RAAS, renin-angiotensin-aldosterone system; SBP, systolic blood pressure; TCH, serum total cholesterol; TG, serum triglycerides.
Leukocyte Count and Vascular Dysfunction
In the overall patients, the median (20th–80th percentile) of leukocyte count, neutrophil, monocyte and lymphocyte count were as follows: 6.1 (4.9–7.4) 109/L, 3.5 (2.6–4.6) 109/L, 0.46 (0.37–0.59) 109/L and 1.85 (1.34–2.28) 109/L, respectively. In individuals with impaired endothelium-dependent vascular function, the median leukocyte count and neutrophil were significantly higher compared to those with normal function (Table 2). In a univariate model, the highest quintile of leukocyte count (OR, 2.12; 95% CI: 1.26–3.67, P=0.004) and neutrophil (OR, 2.68; 95% CI: 1.58–4.60, P<0.001) were significantly associated with vascular dysfunction (Figure 1). In a bivariate correlation analysis, RHI was weakly and negatively correlated with leukocyte and neutrophil counts (Figure 2). From the first quintile to the fifth quintile of leukocyte count, RHI was 2.10, 2.08, 2.10, 2.09, and 1.91, respectively. From the first quintile to the fifth quintile of neutrophil count, RHI was 2.17, 2.09, 2.01, 2.09, and 1.92, respectively.
Table 2.
Blood Cell Counts
| Blood cell count median (20th–80th percentiles) | Endothelial dysfunction (n=105) | Normal vascular function (n=252) | P-value |
|---|---|---|---|
| Leukocyte (109/L) | 6.4 (4.9–8.1) | 6.0 (4.9–7.1) | 0.04 |
| Neutrophil (109/L) | 3.6 (2.7–5.2) | 3.4 (2.6–4.4) | 0.02 |
| Monocyte (109/L) | 0.48 (0.38–0.61) | 0.46 (0.37–0.58) | 0.29 |
| Lymphocyte (109/L) | 1.7 (1.3–2.3) | 1.9 (1.4–2.3) | 0.24 |
| Erythrocyte (1012/L) | 4. 5 (4.2–5.0) | 4.5 (4.2–4.9) | 0.65 |
| Hemoglobin (g/L) | 13.7 (12.7–14.7) | 13.7 (12.8–14.8) | 0.85 |
| Platelet (109/L) | 243.0 (195.6–287) | 248.0 (204.0–293.8) | 0.70 |
Figure 1.
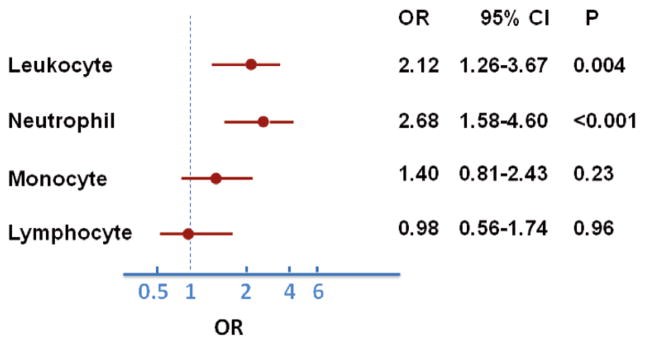
Univariate model for the influence of the highest quintile of leukocyte count and subtypes on vascular dysfunction. (●) Odds ratio (OR); (—) 95% confidence interval (CI). The highest quintile of leukocyte count and neutrophil count were positively associated with vascular dysfunction. The neutrophil count had the strongest predictive value.
Figure 2.
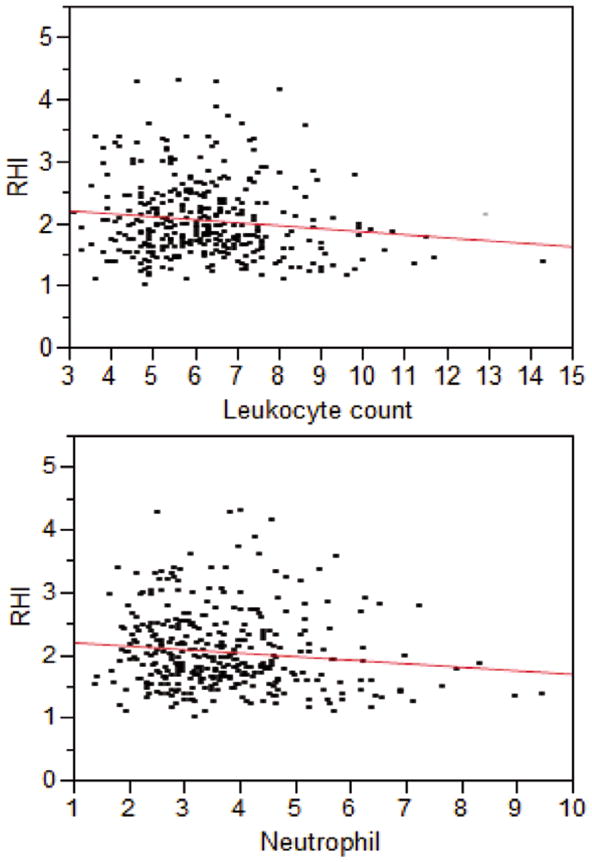
Reactive hyperemia index (RHI) was inversely correlated with leukocyte count (109/L) (r=−0.10, P=0.05) and with neutrophil count (109/L) (r=−0.14, P=0.01).
Multivariate Logistic Regression Analysis
On multivariate analysis, the following significant associations were found to be independent determinants of vascular dysfunction (Table 3): in model A, SBP (OR, 0.97; 95% CI: 0.96–0.99, P=0.001) and BMI (OR, 1.06; 95% CI: 1.02–1.10, P=0.005); in model B, SBP (OR, 0.97; 95% CI: 0.96–0.99, P=0.001), BMI (OR, 1.05; 95% CI: 1.01–1.09, P=0.02) and neutrophil count (OR, 1.20; 95% CI: 1.01–1.43, P=0.04); in model C, SBP (OR, 0.97; 95% CI: 0.96–0.99, P=0.001), BMI (OR, 1.05; 95% CI: 1.01–1.09, P=0.02) and the highest quintile of leukocyte count (OR, 1.91; 95% CI: 1.09–3.37, P=0.02); and in model D, SBP (OR, 0.97; 95% CI: 0.96–0.99, P<0.001), BMI (OR, 1.05; 95% CI: 1.01–1.09, P=0.009) and the highest quintile of neutrophil count (OR, 2.36; 95% CI: 1.35–4.12, P=0.003).
Table 3.
Logistic Multivariate Regression for Independent Predictors of Vascular Dysfunction
| Model A | Model B | Model C | Model D | |||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| OR | 95% CI | OR | 95% CI | OR | 95% CI | OR | 95% CI | |
| Age (years) | 0.99 | 0.97–1.01 | 0.99 | 0.97–1.01 | 0.99 | 0.97–1.01 | 0.99 | 0.97–1.01 |
| Sex (male) | 1.22 | 0.69–2.16 | 1.17 | 0.66–2.08 | 1.21 | 0.68–2.14 | 1.28 | 0.73–2.23 |
| SBP (mmHg) | 0.97† | 0.96–0.99 | 0.97† | 0.96–0.99 | 0.97† | 0.96–0.99 | 0.97† | 0.96–0.99 |
| BMI (kg/m2) | 1.06† | 1.02–1.10 | 1.05* | 1.01–1.09 | 1.05* | 1.01–1.09 | 1.05† | 1.01–1.09 |
| Smoking | 1.64 | 0.60–4.51 | 1.62 | 0.59–4.44 | 1.66 | 0.61–4.51 | 1.56 | 0.56–4.32 |
| TCH (mg/dl) | 0.99 | 0.99–1.01 | 0.99 | 0.99–1.01 | 0.99 | 0.99–1.01 | 0.99 | 0.99–1.01 |
| HDL-C (mg/dl) | 0.99 | 0.97–1.01 | 0.99 | 0.97–1.01 | 0.99 | 0.97–1.01 | 0.99 | 0.97–1.01 |
| Leukocyte (109/L) | 1.08 | 0.93–1.26 | – | – | – | – | – | – |
| Neutrophil (109/L) | – | – | 1.20* | 1.01–1.43 | – | – | – | – |
| Leukocyte (Q5) | – | – | – | – | 1.91* | 1.09–3.37 | – | – |
| Neutrophil (Q5) | – | – | – | – | – | – | 2.36† | 1.35–4.12 |
Discussion
In this study we found a highly significant association between leukocyte count and vascular dysfunction in patients with low CV risk. Moreover, in a multivariate model, the highest quintile of neutrophil count was the strongest predictor for early atherosclerosis (vascular dysfunction) in these patients, stronger than commonly recognized CV risk factors such as smoking or dyslipidemia. The current study supports a role for inflammation in early atherosclerosis in humans.
The healthy endothelium is an active organ with important homeostatic functions.17 Normal vascular function reflects the ability of the endothelium to protect against vascular injury and the initiation of atherosclerosis.18,19 CV risk factors may trigger a chronic inflammatory process that is accompanied by endothelial dysfunction.20,21 Leukocytes and leukocyte subtypes are not only inflammatory markers, but also play important roles in inflammation due to their effects or functions that collectively represent a major mechanism of innate immunity. Mechanisms for leukocyte-induced vascular injury have been described in several experimental models.22–24 In previous clinical studies, the relation between leukocyte count and endothelial function has been investigated. Walker et al reported that impaired acetylcholine-induced endothelium-dependent dilation in the forearm was related to higher white blood cell count among healthy middle-aged and older adults.25 We have previously demonstrated that smoking is associated with epicardial coronary endothelial dysfunction and elevated white blood cell count in patients with chest pain and early coronary artery disease.26 Elkind et al found that relative elevations in leukocyte count were associated with a reduction in brachial artery flow-mediated dilation among stroke-free patients, and speculated that the associations between flow-mediated dilation and leukocyte count are more prominent in individuals at relatively lower risk of atherosclerotic disease.27 In the current study, we extended previous observations to a low-risk patient group and assessed vascular function at the digital artery. We found a strong and independent association between leukocyte count and digital vascular function, thus providing evidence that elevated leukocyte count may be involved in early atherosclerosis in these patients.
Given the known prognostic significance of vascular function, 28 it may be inappropriate to consider individuals with the highest quintile of leukocyte count as being at “low CV risk”. A stricter primary prevention intervention strategy may be reasonable for this group, and deserves further study. Furthermore, because measurement of leukocyte count is a common, low-cost and simple test, it may be helpful to use this for CV risk assessment in individuals classified as low risk using traditional risk stratification tools.
In a previous study from our group, the early phase of obesity in an experimental model was characterized by coronary endothelial dysfunction.29 In the current study, BMI was independently associated with vascular dysfunction. Visceral fat tissue leads to production of pro-atherogenic adipokines that are important contributors to oxidative stress and chronic inflammation, 30 both factors with an important harmful impact on endothelial function.
It is worth noting that we found a positive association between RHI and SBP, an association not seen with other techniques to measure endothelial function. Hamburg et al, however, reported a similar relationship of RHI with BP in a large cohort of patients.31 This may reflect the distinct circulatory and vascular responses assessed with the EndoPAT technique. Although this is speculative we think that high BP may increase pulse amplitude in the process of hyperemic response to ischemia due to changes in the structure of digital arteries. Moreover, the use of anti-hypertensive medications could have different influences on the digital circulation.
Study Limitations
This single-center, observational study has limitations inherent to non-randomized trials. The majority of individuals were recruited from the preventive cardiology clinic, but the detailed information of clinical manifestations was not available. Moreover, multiple regression analysis may mitigate bias after adjustment of confounding factors, but unmeasured factors (eg, psychosocial stressors of daily life, menstrual cycle), which may have an impact on vascular function,32 leave room for residual bias.
The C-reactive protein (CRP) level, a widely used marker of inflammation, was not available to subjects in this study. In a recent study using similar technology to assess vascular function, CRP was not associated with RHI.33 Our previous work also found that cigarette smoking increased leukocyte count and impaired endothelial function without any elevation of CRP.26 Bo et al reported that high-sensitivity CRP was not independently associated with subclinical atherosclerosis.34 In contrast, Clapp et al reported the direct effects of CRP was to enhance vascular function via increased nitric oxide production in vitro.35 The current study was not designed to address the mechanism of the interaction between high leukocyte count and impaired vascular function (inflammation, oxidative stress or others). Therefore, the lack of CRP information does not affect the main results of the current study.
Furthermore, the majority of patients were Caucasian. Thus the conclusion of the current study should not be extrapolated to subjects of different ethnicity.
Conclusion
A relatively high leukocyte count is independently associated with vascular dysfunction in persons at low CV risk. This suggests that leukocyte count may be a useful tool for CV screening in persons at low CV risk. The present results also provide potential insights into the mechanisms by which inflammation contributes to atherogenesis.
Acknowledgments
Research of the investigators is supported by the National Institute of Health (NIH Grant HL-92954 and AG-31750 to A.L., and NIH Grants DK-73608, HL-77131, and HL-085307 to L.O.L.). J.L. received a scholarship from the China Scholarship Council (No. 2010811095) and was supported by the “Beijing Nova program” of Beijing Municipal Science and Technology Commission (A2007079), Beijing China. A.J.F. was supported by the Walter and Gertrud Siegenthaler Foundation, the young academics Support Committee of the University of Zurich, and the Swiss foundation for medical-biological scholarships (SSMBS; SNSF No PASMP3_132551).
Footnotes
Disclosures
A.L. is a member of the Itamar medical advisory board. The other authors report no potential conflicts of interest and have nothing to disclose.
References
- 1.Lim S, Despres JP, Koh KK. Prevention of atherosclerosis in overweight/obese patients: In need of novel multi-targeted approaches. Circ J. 2011;75:1019–1027. doi: 10.1253/circj.cj-10-1240. [DOI] [PubMed] [Google Scholar]
- 2.Yeh ET, Anderson HV, Pasceri V, Willerson JT. C-reactive protein: Linking inflammation to cardiovascular complications. Circulation. 2001;104:974–975. doi: 10.1161/01.cir.104.9.974. [DOI] [PubMed] [Google Scholar]
- 3.Whiteley W, Chong WL, Sengupta A, Sandercock P. Blood markers for the prognosis of ischemic stroke: A systematic review. Stroke. 2009;40:e380–e389. doi: 10.1161/STROKEAHA.108.528752. [DOI] [PubMed] [Google Scholar]
- 4.Manabe I. Chronic inflammation links cardiovascular, metabolic and renal diseases. Circ J. 2011;75:2739–2748. doi: 10.1253/circj.cj-11-1184. [DOI] [PubMed] [Google Scholar]
- 5.Lerman A, Zeiher AM. Endothelial function: Cardiac events. Circulation. 2005;111:363–368. doi: 10.1161/01.CIR.0000153339.27064.14. [DOI] [PubMed] [Google Scholar]
- 6.Rubinshtein R, Kuvin JT, Soffler M, Lennon RJ, Lavi S, Nelson RE, et al. Assessment of endothelial function by non-invasive peripheral arterial tonometry predicts late cardiovascular adverse events. Eur Heart J. 2010;31:1142–1148. doi: 10.1093/eurheartj/ehq010. [DOI] [PubMed] [Google Scholar]
- 7.Eiserich JP, Baldus S, Brennan ML, Ma W, Zhang C, Tousson A, et al. Myeloperoxidase, a leukocyte-derived vascular NO oxidase. Science. 2002;296:2391–2394. doi: 10.1126/science.1106830. [DOI] [PubMed] [Google Scholar]
- 8.Prasad A, Zhu J, Halcox JP, Waclawiw MA, Epstein SE, Quyyumi AA. Predisposition to atherosclerosis by infections: Role of endothelial dysfunction. Circulation. 2002;106:184–190. doi: 10.1161/01.cir.0000021125.83697.21. [DOI] [PubMed] [Google Scholar]
- 9.Libby P, Okamoto Y, Rocha VZ, Folco E. Inflammation in atherosclerosis: Transition from theory to practice. Circ J. 2010;74:213–220. doi: 10.1253/circj.cj-09-0706. [DOI] [PubMed] [Google Scholar]
- 10.Wheeler JG, Mussolino ME, Gillum RF, Danesh J. Associations between differential leucocyte count and incident coronary heart disease: 1764 incident cases from seven prospective studies of 30,374 individuals. Eur Heart J. 2004;25:1287–1292. doi: 10.1016/j.ehj.2004.05.002. [DOI] [PubMed] [Google Scholar]
- 11.Liu Y, Imanishi T, Ikejima H, Tsujioka H, Ozaki Y, Kuroi A, et al. Association between circulating monocyte subsets and in-stent restenosis after coronary stent implantation in patients with ST-elevation myocardial infarction. Circ J. 2010;74:2585–2591. doi: 10.1253/circj.cj-10-0544. [DOI] [PubMed] [Google Scholar]
- 12.Nozawa N, Hibi K, Endo M, Sugano T, Ebina T, Kosuge M, et al. Association between circulating monocytes and coronary plaque progression in patients with acute myocardial infarction. Circ J. 2010;74:1384–1391. doi: 10.1253/circj.cj-09-0779. [DOI] [PubMed] [Google Scholar]
- 13.Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation. 2002;106:3143–3421. [PubMed] [Google Scholar]
- 14.Alberti KG, Eckel RH, Grundy SM, Zimmet PZ, Cleeman JI, Donato KA, et al. Harmonizing the metabolic syndrome: A joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation. 2009;120:1640–1645. doi: 10.1161/CIRCULATIONAHA.109.192644. [DOI] [PubMed] [Google Scholar]
- 15.Bonetti PO, Pumper GM, Higano ST, Holmes DR, Jr, Kuvin JT, Lerman A. Noninvasive identification of patients with early coronary atherosclerosis by assessment of digital reactive hyperemia. J Am Coll Cardiol. 2004;44:2137–2141. doi: 10.1016/j.jacc.2004.08.062. [DOI] [PubMed] [Google Scholar]
- 16.Bonetti PO, Barsness GW, Keelan PC, Schnell TI, Pumper GM, Kuvin JT, et al. Enhanced external counterpulsation improves endothelial function in patients with symptomatic coronary artery disease. J Am Coll Cardiol. 2003;41:1761–1768. doi: 10.1016/s0735-1097(03)00329-2. [DOI] [PubMed] [Google Scholar]
- 17.Flammer AJ, Anderson T, Celermajer DS, Creager MA, Deanfield J, Ganz P, et al. The assessment of endothelial function: From research into clinical practice. Circulation. 2012;126:753–767. doi: 10.1161/CIRCULATIONAHA.112.093245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Reriani MK, Lerman LO, Lerman A. Endothelial function as a functional expression of cardiovascular risk factors. Biomark Med. 2010;4:351–360. doi: 10.2217/bmm.10.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li J, Flammer AJ, Nelson RE, Gulati R, Friedman PA, Thomas RJ, et al. Normal vascular function as a prerequisite for the absence of coronary calcification in patients free of cardiovascular disease and diabetes. Circ J. 2012;76:2705–2710. doi: 10.1253/circj.cj-12-0683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Konduracka E, Galicka-Latala D, Cieslik G, Rostoff P, Fedak D, Sieradzki J, et al. Effect of atorvastatin on endothelial function and inflammation in long-duration type 1 diabetic patients without coronary heart disease and arterial hypertension. Diabetes Obes Metab. 2008;10:719–725. doi: 10.1111/j.1463-1326.2007.00798.x. [DOI] [PubMed] [Google Scholar]
- 21.Li J, Flammer AJ, Lennon RJ, Nelson RE, Gulati R, Friedman PA, et al. Comparison of the effect of the metabolic syndrome and multiple traditional cardiovascular risk factors on vascular function. Mayo Clin Proc. 2012;87:968–975. doi: 10.1016/j.mayocp.2012.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sandoo A, Carroll D, Metsios GS, Kitas GD, Veldhuijzen van Zanten JJ. The association between microvascular and macrovascular endothelial function in patients with rheumatoid arthritis: A cross-sectional study. Arthritis Res Ther. 2011;13:R99. doi: 10.1186/ar3374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Masuda M, Takahashi H. Adhesion of leukocytes to endothelial cells in atherosclerosis. Rinsho Byori. 1998;46:1149–1155. [PubMed] [Google Scholar]
- 24.DiStasi MR, Ley K. Opening the flood-gates: How neutrophil-endothelial interactions regulate permeability. Trends Immunol. 2009;30:547–556. doi: 10.1016/j.it.2009.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Walker AE, Seibert SM, Donato AJ, Pierce GL, Seals DR. Vascular endothelial function is related to white blood cell count and myeloperoxidase among healthy middle-aged and older adults. Hypertension. 2010;55:363–369. doi: 10.1161/HYPERTENSIONAHA.109.145870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lavi S, Prasad A, Yang EH, Mathew V, Simari RD, Rihal CS, et al. Smoking is associated with epicardial coronary endothelial dysfunction and elevated white blood cell count in patients with chest pain and early coronary artery disease. Circulation. 2007;115:2621–2627. doi: 10.1161/CIRCULATIONAHA.106.641654. [DOI] [PubMed] [Google Scholar]
- 27.Elkind MS, Sciacca RR, Boden-Albala B, Tondella ML, Feikin DR, Fields BS, et al. Leukocyte count is associated with reduced endothelial reactivity. Atherosclerosis. 2005;181:329–338. doi: 10.1016/j.atherosclerosis.2005.01.013. [DOI] [PubMed] [Google Scholar]
- 28.Lind L, Berglund L, Larsson A, Sundstrom J. Endothelial function in resistance and conduit arteries and 5-year risk of cardiovascular disease. Circulation. 2011;123:1545–1551. doi: 10.1161/CIRCULATIONAHA.110.984047. [DOI] [PubMed] [Google Scholar]
- 29.Galili O, Versari D, Sattler KJ, Olson ML, Mannheim D, McConnell JP, et al. Early experimental obesity is associated with coronary endothelial dysfunction and oxidative stress. Am J Physiol Heart Circ Physiol. 2007;292:H904–H911. doi: 10.1152/ajpheart.00628.2006. [DOI] [PubMed] [Google Scholar]
- 30.Li ZL, Woollard JR, Ebrahimi B, Crane JA, Jordan KL, Lerman A, et al. Transition from obesity to metabolic syndrome is associated with altered myocardial autophagy and apoptosis. Arterioscler Thromb Vasc Biol. 2012;32:1132–1141. doi: 10.1161/ATVBAHA.111.244061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hamburg NM, Palmisano J, Larson MG, Sullivan LM, Lehman BT, Vasan RS, et al. Relation of brachial and digital measures of vascular function in the community: The Framingham Heart Study. Hypertension. 2011;57:390–396. doi: 10.1161/HYPERTENSIONAHA.110.160812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hashimoto M, Akishita M, Eto M, Ishikawa M, Kozaki K, Toba K, et al. Modulation of endothelium-dependent flow-mediated dilatation of the brachial artery by sex and menstrual cycle. Circulation. 1995;92:3431–3435. doi: 10.1161/01.cir.92.12.3431. [DOI] [PubMed] [Google Scholar]
- 33.Hamburg NM, Keyes MJ, Larson MG, Vasan RS, Schnabel R, Pryde MM, et al. Cross-sectional relations of digital vascular function to cardiovascular risk factors in the Framingham Heart Study. Circulation. 2008;117:2467–2474. doi: 10.1161/CIRCULATIONAHA.107.748574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bo M, Corsinovi L, Brescianini A, Sona A, Astengo M, Dumitrache R, et al. High-sensitivity C-reactive protein is not independently associated with peripheral subclinical atherosclerosis. Angiology. 2009;60:12–20. doi: 10.1177/0003319708322387. [DOI] [PubMed] [Google Scholar]
- 35.Clapp BR, Hirschfield GM, Storry C, Gallimore JR, Stidwill RP, Singer M, et al. Inflammation and endothelial function: Direct vascular effects of human C-reactive protein on nitric oxide bioavailability. Circulation. 2005;111:1530–1536. doi: 10.1161/01.CIR.0000159336.31613.31. [DOI] [PubMed] [Google Scholar]


