Abstract
Autophagy, a ubiquitous catabolic pathway involved in both cell survival and cell death, has been implicated in many age-associated diseases. Recent findings have shown autophagy to be crucial for proper insulin secretion and β-cell viability. Transgenic mice lacking autophagy in their β-cells showed decreased β-cell mass and suppressed glucose-stimulated insulin secretion. Several studies showed that stress can stimulate autophagy in β-cells: the number of autophagosomes is increased in different in vivo models for diabetes, such as db/db mice, mice fed high-fat diet, pdx-1 knockout mice, as well as in in vitro models of glucotoxicity and lipotoxicity. Pharmacological and molecular inhibition of autophagy increases the susceptibility to cell stress, suggesting that autophagy protects against diabetes-relevant stresses. Recent findings, however, question these conclusions. Pancreases of diabetics and β-cells exposed to fatty acids show accumulation of abnormal autophagosome morphology and suppression of lysosomal gene expression suggesting impairment in autophagic turnover. In this review we attempt to give an overview of the data generated by others and by us in view of the possible role of autophagy in diabetes, a role which depending on the conditions, could be beneficial or detrimental in coping with stress.
Keywords: autophagosomes, autophagy, β-cells, diabetes, fatty acids, insulin, lipotoxicity, lysosomes
Introduction: Autophagy in Diabetes
The rise in obesity in the developed and developing world has been associated with an increase in the prevalence of numerous diseases including heart disease, hypertension and type 2 diabetes [1]. A central strategy for studying the etiology of these pathologies has been to look for potentially pathogenic humoral factors or metabolites that are elevated during obesity. Among these, free fatty acids (FFAs) have attracted much attention, particularly in the context of pancreatic β-cells where they both impair insulin secretion [2–8] and induce cell death by apoptosis [9–12]. Lipotoxicity (or glucolipotoxicity when it is combined with high concentrations of glucose) is associated with induction of endoplasmic reticulum (ER) stress [13], mitochondrial network fragmentation [14], and oxidative damage to proteins [15]. Recent studies have shown that macroautophagy (hereafter named autophagy), a lysosome-mediated catabolic process implicated in the etiology of various age-associated diseases, is also implicated in lipotoxicity [16,17] and is associated with diabetes [18]. This finding elicited particular excitement because of the central roles autophagy is playing in both cell survival and death, and because of its regulation by major lipotoxic mediators, including ceramide [19], radical oxygen species (ROS) [20], ER stress [21] and mitochondrial fission [22,23]. The aim of this review is to display the evidence gathered on the effect of FFAs on autophagy and its possible relevance to type 2 diabetes. A question that deserves particular consideration is whether autophagy plays a protective role in β-cells to counteract stresses associated with diabetes, or whether it is a side effect of those stresses. In other words, does autophagy take part in the solution or in the problem of diabetes?
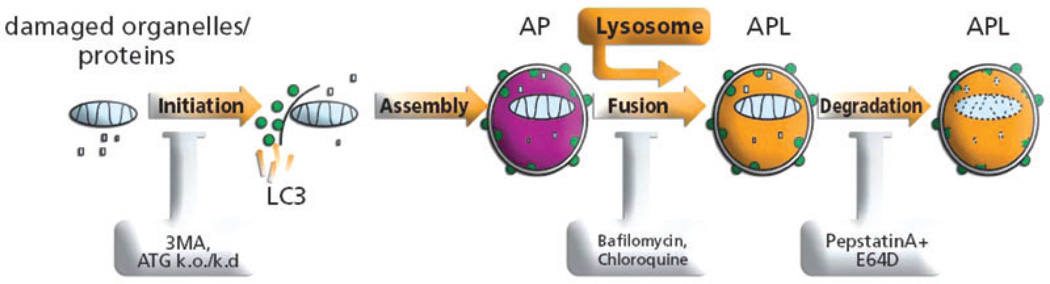
Autophagy is composed of several steps, starting with the formation of the phagophore, a small membrane which can originate either from the mitochondrial outer membrane or the ER, or the cytoplasmic membrane [24, 25, 26]. The phagophore evolves into the autophagosome, a double membrane engulfing a portion of the cytoplasm with its proteins and organelles. Following its formation, the autophagosome fuses with lysosomes to form the autophagolysosome (or autolysosome), an acidic compartment containing lysosomal proteases and lipases which sequester its content [27] (figure 1).
Figure 1.
Autophagy can be stimulated by starvation, radical oxygen species (ROS), endoplasmic reticulum (ER) stress, infection and rapamycin. The initiation of autophagy is marked by the generation of the isolation membrane to which LC3 is recruited. The isolation membrane is engulfing the organelle/s to be digested. The process proceeds with the fusion of the lysosome to the autophagosome (AP) to form the autophagolysosome (APL). Acidification of the autophagolysosome allows for the digestion of its content. The process can be inhibited at the stage of formation of the phagophore using ATG5DN or 3MA. Acidification as well as fusion of AP with lysosomes can be inhibited by chloroquine, bafilomycin, and digestion of the APL content can be inhibited by protease inhibitors such as pepstatin and E64D.
Basal autophagy occurs at low rate in all eukaryotes as a quality control mechanism to clear and recycle large damaged or redundant components of the cell including organelles, protein aggregates, ribosomes and lipids. Upon induction of stress such as amino acid starvation, ROS exposure or ER stress, autophagy is stimulated to protect the cell by increasing the availability of amino acids and by clearing accumulated damaged components. Autophagy is not exclusively linked to cell survival. Under chronic stress, it is sometimes associated with cell death (‘autophagic cell death’), although the meaning of autophagy in this case is still controversial [28]. Indeed in many cases, autophagy-associated cell death is the result of a blockage of autophagic flux rather than an induction of autophagosome formation.
The molecular mechanism regulating autophagy is only partially understood. The more thoroughly studied autophagy regulator is the mammalian target of rapamycin (mTOR), a central kinase in the insulin receptor signalling pathway, which inhibits autophagosome formation by inhibiting ULK1, one of the autophagy machinery kinases. Inhibition of mTOR by rapamycin is the most used way to stimulate autophagy without, at the same time, inducing plain stress. Interestingly, while mTOR inhibition affects a multitude of cellular functions, more specific ways of stimulating autophagy are not known. Indeed, the activity of the autophagic machinery seems to be mainly regulated post-translationally, by broad signalling pathways (notable alongside mTOR is PI3-kinase III), whereas the abundance of the autophagy components plays a minor role under physiological conditions [29,30].
The autophagic machinery has been extensively studied in yeast where it involves two dozens of autophagy-related genes (ATGs). A significant member of this family is the microtubule associated-protein 1 light chain 3 (LC3; the homologue of yeast ATG8), the only protein known to be associated with the autophagosome from its formation up to its maturation into autolysosome (figure 1). LC3 serves as a bona fide marker for autophagy; however, being unspecific it is usually not regarded as a reliable target for suppressing autophagy [30]. The more commonly used targets for the inhibition of autophagy are ATG5 and ATG7, which until recently were believed to be absolute requisites for autophagosome formation. An elegant study published last year, however, showed that even in the absence of ATG5 and ATG7, autophagy can occur to some extent [31], although not enough to prevent lethality shortly after birth of total knockouts of either ATG5 or ATG7 [32,33].
Tissue-specific knockouts of ATG5 or ATG7 have shown autophagy to be highly important for brain [34,35], heart [36,37] and liver [38] function (interestingly, in muscles, although absence of autophagy strongly hampered mitochondrial function, the whole animal did not show any apparent phenotype [39]). The physiologic relevance of autophagy in diseases such as Parkinson, Alzheimer, Huntington, heart diseases and cancer is also attracting attention, although the significance of altered autophagy in those diseases was not always clear [36,40–42]. Thus, for example, while it was originally reported that autophagy is stimulated during Parkinson and Huntington diseases, it has since become clear that the increase in autophagosomes observed during those diseases is caused by a decrease in autophagic flux rather than an increase in autophagosome formation [36]. For this reason, measurement of autophagic turnover has become a basic requirement to complement steady-state autophagy measurement.
Autophagy in Homeostasis
Steady-state (‘housekeeping’) autophagy has been recently shown to be important for the physiology as well as for the viability of pancreatic β-cells. Three separate studies reported impaired glucose tolerance in mice harbouring specific β-cell ATG7 deletion, due both to a decrease in β-cell mass and to impaired β-cell function [16,39,43]. In absence of autophagy, β-cells underwent apoptosis and displays suppression of glucose stimulated insulin secretion. Why is autophagy of such importance in β-cell homeostasis? While it is definitely possible that autophagy in general is crucial for β-cell function, some evidence points to mitophagy (autophagy of mitochondria) as being of particular significance.
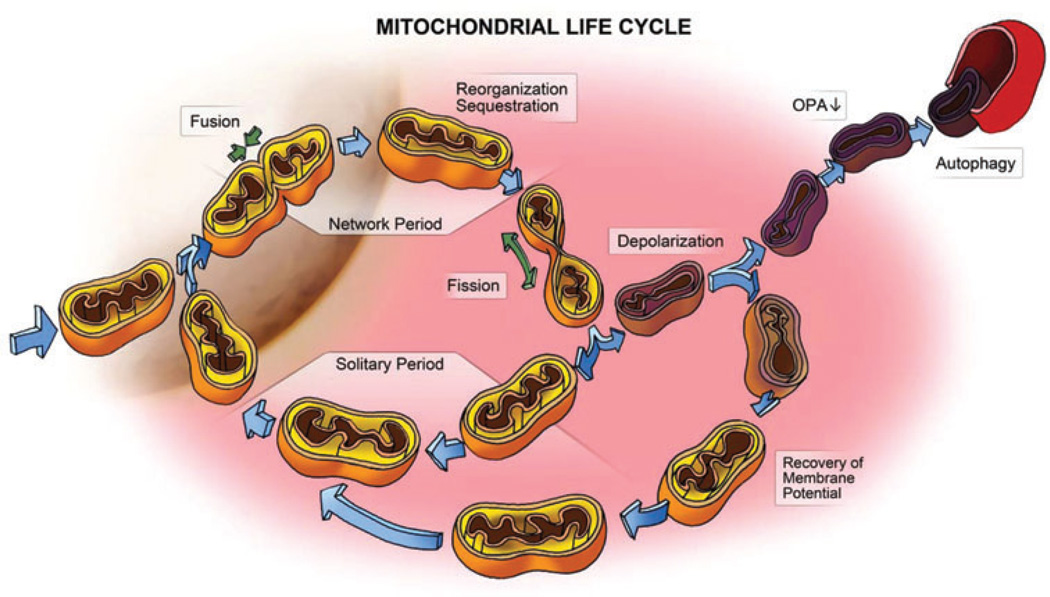
In β-cells mitochondria are arranged in a dense, web-like morphology, where they function as fuel sensors, tightly regulating insulin secretion in response to varying glucose concentrations. Maintenance of the quality of mitochondria in β-cells is therefore of utmost importance requiring the continuous activity of an intricate mechanism of selection that we have recently reported [44]. Quality control of mitochondria involves a cycle of constant fusion and fission of mitochondria with one another (“mitochondrial dynamics”), followed by selective mitophagy of those mitochondria that are depolarized and unable to re-fuse [22] (figure 2). Accordingly, any disruption of the quality control mechanism is expected to result in accumulation of damaged depolarized mitochondria. Corroborating this model is the observation that in the absence of mitochondrial fission autophagy is impaired, oxygen consumption is decreased and ROS damage is accumulated [44]. Remarkably, others have shown that disruption of autophagy leads to a similar phenotype. Knockout of ATG7 in β-cells elicited mitochondrial morphology deformation [39,43], and decrease in oxygen consumption along with increase in ROS [39]. The special role of ROS increase in insulin secretion impairment was shown by treating autophagy-deficient mice with N-Acotyl-Cysteine (NAC), a ROS scavenger, thereby protecting the animals from glucose intolerance [39].
Figure 2.
A schematic model of the life cycle of mitochondria and the roles of mitochondrial dynamics and autophagy in the segregation of dysfunctional mitochondria. Each mitochondrion cyclically shifts between a post-fusion state (Network Period) and a post-fission state (Solitary Period). A fusion event commonly lasts less than 2 min and is terminated by a fission event. Following a fission event, each daughter mitochondrion may either maintain intact membrane potential (yellow) or depolarize (purple). If it depolarizes, it is six times less likely to re-engage in further fusion events for the entire depolarization interval. A fraction of the daughter mitochondria that depolarize do recover and their fusion capacity is then restored (re-entering the cycle). However, if membrane potential depolarization is sustained, reduction in OPA1, a mitochondrial fusion protein, follows and elimination by autophagy occurs (for details see [22]).
Role of Autophagy during Stress
Stimulation of autophagy by fatty acids was not prevented by treatment with NAC and glutathione, two antioxidants, indicating that it is not mediated by ROS [17]. On the other hand, treatment of INS1 cells with the synthetic chaperone 4-PBA partially abolished the induction of autophagy by fatty acids, implying a role of ER stress in mediating the increase in autophagy by FFAs [17].
The implication of autophagy during stress was also reported, with various cellular and animal models of β-cell pathology showing increased autophagy. In islets isolated from Rab3−/− mice, autophagy was found to be involved in the degradation of insulin granules [45], while in islets isolated from PDX+/− mice autophagic cell death was reported [46]. More commonly used animal models, such as db/db mice and mice fed high-fat diet, also displayed an increase in autophagosome number in their β-cells [16]. In vitro, exposure of INS1 cells to high concentrations of glucose induced autophagy to allow the clearance of ubiquitinated aggregated proteins [47]. Similarly, long-term exposure to either palmitate or oleate strongly increased the number of autophagosomes in INS1 cells [16,17]. ATG5 knockdown enhanced the susceptibility to cell death by lipotoxicity, while further stimulation of autophagy using AKTI or rapamycin, two stimulators of autophagy, partially alleviated lipotoxicity [17]. The conclusion thereof was that increased autophagy in response to lipids serves as a protective mechanism from lipotoxicity. Consistent with this conclusion is the observation that β-cell-specific ATG7 knockout mice fed on high-fat diet worsened their diabetic symptoms [16].
Autophagic Turnover and Diabetes
While the importance of autophagy for properβ-cell function is undeniable, its role in the etiology of diabetic β-cell dysfunction recently received a new twist in a study conducted on pancreases retrieved from human cadavers. Electron microscope images of pancreatic sections of diabetics showed a massive increase in overloaded autophagosomes [18]. The cause of this abnormal autophagosome morphology was suggested to be alteration of autophagic maturation because of a decrease in lysosome formation. Diabetic β-cells harboured significantly reduced levels of various lysosomal genes, including cathepsins B and D, and of LAMP2, which are respectively involved in protein degradation and in fusion with the autophagosomes. Impaired autophagosome morphology and decrease in LAMP2 gene expression were also observed in islets treated with high concentrations of fatty acids for 24 h [18]. While these results are still too preliminary to be regarded as conclusive, they fit well with the observation that autophagic turnover is partially blocked by fatty acids in hepatocytes [48,49]. The effect of lipotoxicity on autophagic flux was assessed previously in β-cells; however, this was done using only qualitative techniques, showing that autophagic turnover was not totally blocked. We have recently measured the effect of fatty acids on the total cellular intensity of GFP in INS1 cells expressing GFP-LC3. This approach allows the estimation of autophagic turnover, GFP-LC3 being quenched and degraded in the acidic compartments of autophagolysosomes [50]. Our unpublished results show that palmitate suppressed autophagic flux in INS1 cells. The decrease in autophagic flux was associated with a decrease in lysosomal activity. Taking these observations into account, we suggest that the decrease in lysosomal function becomes a rate-limiting step halting autophagosome maturation. The reduction in autophagic degradation that ensues could account for more accumulation of damaged mitochondria and ER stress-damaged protein.
Conclusions
To conclude, autophagy is important for proper β-cell function and viability. It is also implicated in the pathology of the pancreas of type 2 diabetes in humans as well as in many animal and cellular models for diabetes. The nature of this implication is still controversial however. Originally, autophagy was reported to be activated in β-cells upon stress induction as a protective mechanism. Recently gathered evidence, however, suggests that the increase in autophagosomes observed in diabetics and in β-cells treated with FFAs could reflect a decrease in autophagic flux because of decreased lysosome formation. Such decrease contributes to the pathology rather than protects from it. More thorough studies, investigating autophagic flux and lysosomal state, are now required to determine the site of action of fatty acids and the possible implication to treatment of diabetes.
Acknowledgements
We would like to thank Dr. Danni Dagan for reviewing this, and providing us with his most valuable insights. We would also like to thank Erga Rivis for her assistance with the illustration in figure 1, and Erga Rivis Studio for providing us with scientific illustration services.
Footnotes
Conflict of Interests
The authors do not declare any conflict of interest relevant to this manuscript.
References
- 1.Ford ES, Giles WH, Dietz WH. Prevalence of the metabolic syndrome among US adults: findings from the third National Health and Nutrition Examination Survey. JAMA. 2002;287:356–359. doi: 10.1001/jama.287.3.356. [DOI] [PubMed] [Google Scholar]
- 2.Sako Y, Grill VE. A 48-hour lipid infusion in the rat time-dependently inhibits glucose-induced insulin secretion and B cell oxidation through a process likely coupled to fatty acid oxidation. Endocrinology. 1990;127:1580–1589. doi: 10.1210/endo-127-4-1580. [DOI] [PubMed] [Google Scholar]
- 3.Zhou YP, Grill VE. Long-term exposure of rat pancreatic islets to fatty acids inhibits glucose-induced insulin secretion and biosynthesis through a glucose fatty acid cycle. J Clin Invest. 1994;93:870–876. doi: 10.1172/JCI117042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhou YP, Grill V. Long term exposure to fatty acids and ketones inhibits B-cell functions in human pancreatic islets of Langerhans. J Clin Endocrinol Metab. 1995;80:1584–1590. doi: 10.1210/jcem.80.5.7745004. [DOI] [PubMed] [Google Scholar]
- 5.Elks ML. Chronic perifusion of rat islets with palmitate suppresses glucose-stimulated insulin release. Endocrinology. 1993;133:208–214. doi: 10.1210/endo.133.1.8319569. [DOI] [PubMed] [Google Scholar]
- 6.Las G, Mayorek N, Dickstein K, Bar-Tana J. Modulation of insulin secretion by fatty acyl analogs. Diabetes. 2006;55:3478–3485. doi: 10.2337/db06-0687. [DOI] [PubMed] [Google Scholar]
- 7.Mason TM, Goh T, Tchipashvili V, et al. Prolonged elevation of plasma free fatty acids desensitizes the insulin secretory response to glucose in vivo in rats. Diabetes. 1999;48:524–530. doi: 10.2337/diabetes.48.3.524. [DOI] [PubMed] [Google Scholar]
- 8.Paolisso G, Gambardella A, Amato L, et al. Opposite effects of short- and long-term fatty acid infusion on insulin secretion in healthy subjects. Diabetologia. 1995;38:1295–1299. doi: 10.1007/BF00401761. [DOI] [PubMed] [Google Scholar]
- 9.Schaffer JE. Lipotoxicity: when tissues overeat. Curr Opin Lipidol. 2003;14:281–287. doi: 10.1097/00041433-200306000-00008. [DOI] [PubMed] [Google Scholar]
- 10.Prentki M, Nolan CJ. Islet beta cell failure in type 2 diabetes. J Clin Invest. 2006;116:1802–1812. doi: 10.1172/JCI29103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.El-Assaad W, Buteau J, Peyot ML, et al. Saturated fatty acids synergize with elevated glucose to cause pancreatic beta-cell death. Endocrinology. 2003;144:4154–4163. doi: 10.1210/en.2003-0410. [DOI] [PubMed] [Google Scholar]
- 12.Shimabukuro M, Zhou YT, Levi M, Unger RH. Fatty acid-induced beta cell apoptosis: a link between obesity and diabetes. Proc Natl Acad Sci U S A. 1998;95:2498–2502. doi: 10.1073/pnas.95.5.2498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cnop M, Igoillo-Esteve M, Cunha DA, Ladriere L, Eizirik DL. An update on lipotoxic endoplasmic reticulum stress in pancreatic beta-cells. Biochem Soc Trans. 2008;36:909–915. doi: 10.1042/BST0360909. [DOI] [PubMed] [Google Scholar]
- 14.Molina AJ, Wikstrom JD, Stiles L, et al. Mitochondrial networking protects beta cells from nutrient induced apoptosis. Diabetes. 2009;58:2303–2315. doi: 10.2337/db07-1781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schrauwen P, Hesselink MK. Oxidative capacity, lipotoxicity, and mitochondrial damage in type 2 diabetes. Diabetes. 2004;53:1412–1417. doi: 10.2337/diabetes.53.6.1412. [DOI] [PubMed] [Google Scholar]
- 16.Ebato C, Uchida T, Arakawa M, et al. Autophagy is important in islet homeostasis and compensatory increase of beta cell mass in response to high-fat diet. Cell Metab. 2008;8:325–332. doi: 10.1016/j.cmet.2008.08.009. [DOI] [PubMed] [Google Scholar]
- 17.Choi SE, Lee SM, Lee YJ, et al. Protective role of autophagy in palmitate-induced INS-1 beta-cell death. Endocrinology. 2009;150:126–134. doi: 10.1210/en.2008-0483. [DOI] [PubMed] [Google Scholar]
- 18.Masini M, Bugliani M, Lupi R, et al. Autophagy in human type 2 diabetes pancreatic beta cells. Diabetologia. 2009;52:1083–1086. doi: 10.1007/s00125-009-1347-2. [DOI] [PubMed] [Google Scholar]
- 19.Scarlatti F, Bauvy C, Ventruti A, et al. Ceramide-mediated macroautophagy involves inhibition of protein kinase B and up-regulation of beclin 1. J Biol Chem. 2004;279:18384–18391. doi: 10.1074/jbc.M313561200. [DOI] [PubMed] [Google Scholar]
- 20.Scherz-Shouval R, Shvets E, Fass E, Shorer H, Gil L, Elazar Z. Reactive oxygen species are essential for autophagy and specifically regulate the activity of Atg4. EMBO J. 2007;26:1749–1760. doi: 10.1038/sj.emboj.7601623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yorimitsu T, Nair U, Yang Z, Klionsky DJ. Endoplasmic reticulum stress triggers autophagy. J Biol Chem. 2006;281:30299–30304. doi: 10.1074/jbc.M607007200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Twig G, Hyde B, Shirihai OS. Mitochondrial fusion, fission and autophagy as a quality control axis: the bioenergetic view. Biochim Biophys Acta. 2008;1777:1092–1097. doi: 10.1016/j.bbabio.2008.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Maiuri MC, Zalckvar E, Kimchi A, Kroemer G. Self-eating and self-killing: crosstalk between autophagy and apoptosis. Nat Rev Mol Cell Biol. 2007;8:741–752. doi: 10.1038/nrm2239. [DOI] [PubMed] [Google Scholar]
- 24.Hailey DW, Rambold AS, Satpute-Krishnan P, Mitra K, Sougrat R, Kim PK, et al. Mitochondria supply membranes for autophagosome biogenesis during starvation. Cell. 2010;141:656–667. doi: 10.1016/j.cell.2010.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ravikumar B, Moreau K, Jahreiss L, Puri C, Rubinsztein DC. Plasma membrane contributes to the formation of pre-autophagosomal structures. Nat Cell Biol. 2010;12:747–757. doi: 10.1038/ncb2078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hayashi-Nishino M, Fujita N, Noda T, Yamaguchi A, Yoshimori T, Yamamoto A. A subdomain of the endoplasmic reticulum forms a cradle for autophagosome formation. Nat Cell Biol. 2009;11:1433–1437. doi: 10.1038/ncb1991. [DOI] [PubMed] [Google Scholar]
- 27.He C, Klionsky DJ. Regulation mechanisms and signaling pathways of autophagy. Annu Rev Genet. 2009;43:67–93. doi: 10.1146/annurev-genet-102808-114910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kroemer G, Levine B. Autophagic cell death: the story of a misnomer. Nat Rev Mol Cell Biol. 2008;9:1004–1010. doi: 10.1038/nrm2527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mizushima N, Yoshimori T, Levine B. Methods in mammalian autophagy research. Cell. 2010;140:313–326. doi: 10.1016/j.cell.2010.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Klionsky DJ, Abeliovich H, Agostinis P, et al. Guidelines for the use and interpretation of assays for monitoring autophagy in higher eukaryotes. Autophagy. 2008;4:151–175. doi: 10.4161/auto.5338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nishida Y, Arakawa S, Fujitani K, et al. Discovery of Atg5/Atg7-independent alternative macroautophagy. Nature. 2009;461:654–658. doi: 10.1038/nature08455. [DOI] [PubMed] [Google Scholar]
- 32.Kuma A, Hatano M, Matsui M, et al. The role of autophagy during the early neonatal starvation period. Nature. 2004;432:1032–1036. doi: 10.1038/nature03029. [DOI] [PubMed] [Google Scholar]
- 33.Komatsu M, Waguri S, Ueno T, et al. Impairment of starvation-induced and constitutive autophagy in Atg7-deficient mice. J Cell Biol. 2005;169:425–434. doi: 10.1083/jcb.200412022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hara T, Nakamura K, Matsui M, et al. Suppression of basal autophagy in neural cells causes neurodegenerative disease in mice. Nature. 2006;441:885–889. doi: 10.1038/nature04724. [DOI] [PubMed] [Google Scholar]
- 35.Komatsu M, Waguri S, Chiba T, et al. Loss of autophagy in the central nervous system causes neurodegeneration in mice. Nature. 2006;441:880–884. doi: 10.1038/nature04723. [DOI] [PubMed] [Google Scholar]
- 36.Levine B, Kroemer G. Autophagy in the pathogenesis of disease. Cell. 2008;132:27–42. doi: 10.1016/j.cell.2007.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tanaka Y, Guhde G, Suter A, et al. Accumulation of autophagic vacuoles and cardiomyopathy in LAMP-2-deficient mice. Nature. 2000;406:902–906. doi: 10.1038/35022595. [DOI] [PubMed] [Google Scholar]
- 38.Komatsu M, Waguri S, Koike M, et al. Homeostatic levels of p62 control cytoplasmic inclusion body formation in autophagy-deficient mice. Cell. 2007;131:1149–1163. doi: 10.1016/j.cell.2007.10.035. [DOI] [PubMed] [Google Scholar]
- 39.Wu JJ, Quijano C, Chen E, et al. Mitochondrial dysfunction and oxidative stress mediate the physiological impairment induced by the disruption of autophagy. Aging. 2009;1:425–437. doi: 10.18632/aging.100038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Eskelinen EL, Saftig P. Autophagy: a lysosomal degradation pathway with a central role in health and disease. Biochim Biophys Acta. 2009;1793:664–673. doi: 10.1016/j.bbamcr.2008.07.014. [DOI] [PubMed] [Google Scholar]
- 41.Kundu M, Thompson CB. Autophagy: basic principles and relevance to disease. Annu Rev Pathol. 2008;3:427–455. doi: 10.1146/annurev.pathmechdis.2.010506.091842. [DOI] [PubMed] [Google Scholar]
- 42.Shintani T, Klionsky DJ. Autophagy in health and disease: a double-edged sword. Science. 2004;306:990–995. doi: 10.1126/science.1099993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jung HS, Chung KW, Won Kim J, et al. Loss of autophagy diminishes pancreatic beta cell mass and function with resultant hyperglycemia. Cell Metab. 2008;8:318–324. doi: 10.1016/j.cmet.2008.08.013. [DOI] [PubMed] [Google Scholar]
- 44.Twig G, Elorza A, Molina AJ, et al. Fission and selective fusion govern mitochondrial segregation and elimination by autophagy. EMBO J. 2008;27:433–446. doi: 10.1038/sj.emboj.7601963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Marsh BJ, Soden C, Alarcon C, et al. Regulated autophagy controls hormone content in secretory-deficient pancreatic endocrine beta-cells. Mol Endocrinol. 2007;21:2255–2269. doi: 10.1210/me.2007-0077. [DOI] [PubMed] [Google Scholar]
- 46.Fujimoto K, Hanson PT, Tran H, et al. Autophagy regulates pancreatic beta cell death in response to Pdx1 deficiency and nutrient deprivation. J Biol Chem. 2009;284:27664–27673. doi: 10.1074/jbc.M109.041616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kaniuk NA, Kiraly M, Bates H, Vranic M, Volchuk A, Brumell JH. Ubiquitinated-protein aggregates form in pancreatic beta-cells during diabetes-induced oxidative stress and are regulated by autophagy. Diabetes. 2007;56:930–939. doi: 10.2337/db06-1160. [DOI] [PubMed] [Google Scholar]
- 48.Singh R, Kaushik S, Wang Y, et al. Autophagy regulates lipid metabolism. Nature. 2009;458:1131–1135. doi: 10.1038/nature07976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Koga H, Kaushik S, Cuervo AM. Altered lipid content inhibits auotphagic vesicular fusion. FASEB J. 2010;24:3052–3065. doi: 10.1096/fj.09-144519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shvets E, Fass E, Elazar Z. Utilizing flow cytometry to monitor autophagy in living mammalian cells. Autophagy. 2008;4:621–628. doi: 10.4161/auto.5939. [DOI] [PubMed] [Google Scholar]