Abstract
Background
Starvation induces small bowel atrophy with increased intestinal epithelial apoptosis and decreased proliferation. Here, we examined these parameters after starvation in aged animals.
Methods
Sixty-four 6 week-old and 26 month-old C57BL/6 mice were randomly assigned to either an ad libitum fed or fasted group. The small bowel was harvested at 12, 48, and 72 hours following starvation. Proximal gut mucosal height was measured and epithelial cells counted. Apoptosis was identified by terminal deoxynucleotidyl transferase-mediated dUTP nick end labeling (TUNEL) staining. Proliferation was determined by immunohistochemical staining for proliferating cell nuclear antigen (PCNA). Comparison of fed vs. fasted and adult vs. old groups was done by one-way ANOVA with Tukey’s test and unpaired t-test. Significance was accepted at p<0.05.
Results
Aged mice had higher proximal gut weights, mucosal heights and cell numbers at baseline compared with the adult group (p<0.05). The rate of apoptosis was lower in the aged (p<0.05) while proliferation was not different between groups before starvation. After starvation, proximal gut wet weight decreased only in adult mice (p<0.05); Gut mucosal height and mucosal cell number decreased greater in adult than in aged mice (p<0.05). This was related to decreased proliferation only in the adult group (p<0.05). The fold of epithelial apoptosis increased was higher in the aged group than in the adult after starvation (p<0.05).
Conclusions
Gut mucosal kinetics change with age had lower rates of apoptosis and greater mucosal mass; the character of starvation-induced atrophy is diminished with aging.
Keywords: fasting, senescent, mucosal homeostasis, apoptosis, proliferation, TUNEL staining, PCNA staining
INTRODUCTION
Interest in geriatric nutrition has developed in the past 20 years in order to gain insight into the causes of nutritional deficiencies in the elderly. Several studies showed improved clinical outcome with nutrition supplementation.1, 2 Nutrition is a major concern in critically ill elderly patients which is typically given enterally.3 This practice, however, assumes a normally functioning gut; intestinal function such as nutrient absorption, maintenance of barrier permeability and immune secretion decrease with age under normal conditions, and are also likely related to the clinical outcome.
Aging is associated with changes in the integrity of the gastrointestinal tract that may result in diminished function. Age-induced changes include the gradual accumulation of random molecular damage, which evoke a range of general cellular responses such as growth arrest and eventually cell death by necrosis or apoptosis.4, 5
Ortega, et al6 presented that starvation impaired DNA, protein synthesis and enzyme activity in small intestine mucosa, and this change diminished with age; but, the effect of aging on gut epithelium homeostasis between cell death and proliferation is still unclear. We, therefore, chose to study a reproducible model of gut atrophy with clinical relevance for investigation, namely the effects of starvation. Small intestinal epithelial cell turnover depends on a balance between cell proliferation and cell death. Gut epithelial cells undergo mitosis in the intestinal crypts, and then migrate up the crypt-villus axis after differentiation. Gut epithelial cells undergo cell death via necrosis or apoptosis, presumably when senescence is reached. Apoptosis is distinguishable from necrosis in that it occurs without inflammation, and is the major mechanism for maintaining homeostasis in normal tissue in the face of ongoing cell growth and proliferation.7 However, the signals governing apoptosis and proximal gut epithelial homeostasis under normal conditions are not well delineated. In addition, many factors associated with aging such as inflammatory bowel disease, cancer, and trauma lead to dysregulation of cellular turnover between cell proliferation and apoptosis, further complicating the picture.
Starvation causes increased intestinal epithelial cell apoptosis in adult rats.8 In another study, we showed that proximal small intestine epithelium atrophy was induced by starvation in mice, which was associated with increased mucosal epithelial cell apoptosis and decreased cell proliferation.9 The aim of the current study was to identify whether proximal intestinal mucosal epithelium homeostasis changes with aging in response to starvation.
METHODS
Animals
Male C57BL6 mice (twenty-six 26 month-old, average weight 32.8 grams, and thirty-eight 6 week-old, average weight 26.2 g) were obtained from Harlan (Houston, Texas). Mice were individually acclimated for one week within a temperature-controlled cubicle with a 12-hour light-dark cycle. The protocol was approved by the Institutional Animal Care and Use Committee at the University of Texas Medical Branch, Galveston, Texas.
Experimental protocol
The mice were divided into the following groups: non-aged adult fed (n=8), aged fed (n=5), non-aged adult fasted (n=30) and aged fasted (n=21). Feeding groups had access to chow and water ad libitum. Fasted groups had chow withdrawn with access to water ad libitum. Mice were housed individually in cages with a wire-grid floor to prevent scatophagy in the experimental period, and were sacrificed by decapitation 12, 48 and 72 hours after removal of food.
The entire small bowel was excised, measured, weighed and divided into proximal and distal halves after flushing with ice cold phosphate-buffered saline (PBS). The first 1 cm of the proximal part was placed in 10% buffered formalin for histologic and immunohistochemical analysis. The subsequent 1 cm segment was excised, weighed and baked at 50°C for 3 days, then weighed again to obtain the dry weight. The ratio of the dry weight to wet weight was calculated, and the total proximal dry weight was obtained by product of total proximal wet weight and dry weight ratio.
Mucosal Histologic Morphology
Formalin fixed tissues were processed and embedded in paraffin. Four micrometer thick sections were stained with hematoxylin and eosin. With a calibrated eyepiece at 10× magnification, mucosa height including villus length and crypt depth was measured from 10 consecutive villi. Cell number was then counted in the same villi and crypt. The average value was used for each animal.
Immunohistochemistry
Sections of 3 µm were deparaffinized, rehydrated in graded alcohol (100%, 95%, and 70%) and washed with deionized water. Terminal deoxynucleotidyl transferase-mediated dUTP nick end labeling (TUNEL) staining10 (ApoTag, Oncor, San Francisco, CA) was applied to identify apoptotic cells. Deparaffinized sections were treated with proteinase K (20 µL/mL in phosphate-buffered saline [PBS]), and endogenous peroxidase activity was quenched with 2% H2O2 in PBS. Seventy-five microlites of equilibration buffer was placed on each section. TdT enzyme solution was applied and the section was incubated at 37°C for 1 hour. After incubation, sections were placed in stop/wash buffer and incubated with 55 µL anti-digoxigenin peroxidase at room temperature for 30 minutes. Sections were again washed with PBS, and diaminobenzidine-hydrogen peroxide was applied for color development. Sections were counterstained with hematoxylin and mounted for examination. Six sections of each block of tissue were stained at 40 µm intervals. In each section, 10 full-length villi were selected for counting TUNEL positive cells. Apoptotic cells were identified as those with brown staining of the nucleus, or apoptotic bodies, which are fragments of apoptotic cells engulfed by neighboring epithelial cells.
Proliferation was quantified in a similar way with immunohistochemical staining for proliferative cell nuclear antigen (PCNA). Deparaffinized and rehydrated sections were incubated with a horseradish peroxidase conjugated PCNA-antibody (SC-56, Santa Cruz, Santa Cruz, CA) at a 1:50 dilution overnight at 4°C, followed by washing in PBS and adding DAB-hydrogen peroxide for color development. After counterstaining and mounting, PCNA-positive cells were counted on 3 sections for each animal as described earlier. All examinations were carried out by blinded observers.
Statistics
Statistical analysis was performed with one-way ANOVA with Tukey’s test and unpaired t-test. Significance was accepted at p<0.05. Data are expressed as mean ± SEM.
RESULTS
All non-aged adult mice survived the experiment. Following food restriction, one aged animal died after 48 hours and three additional animals died after 72 hours. No significant differences in mortality were found between groups.
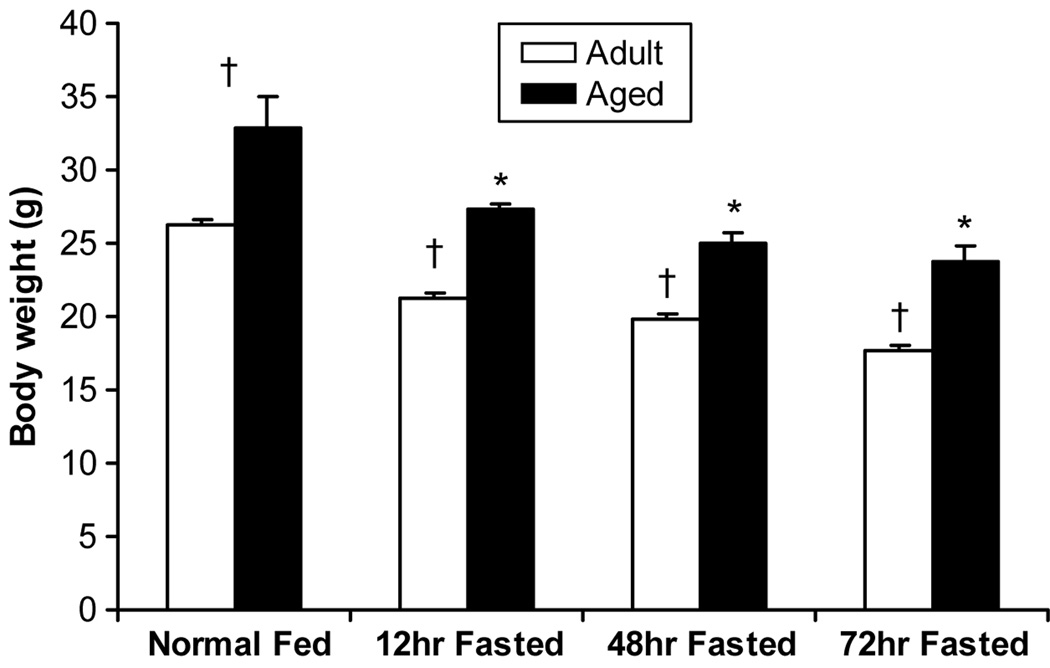
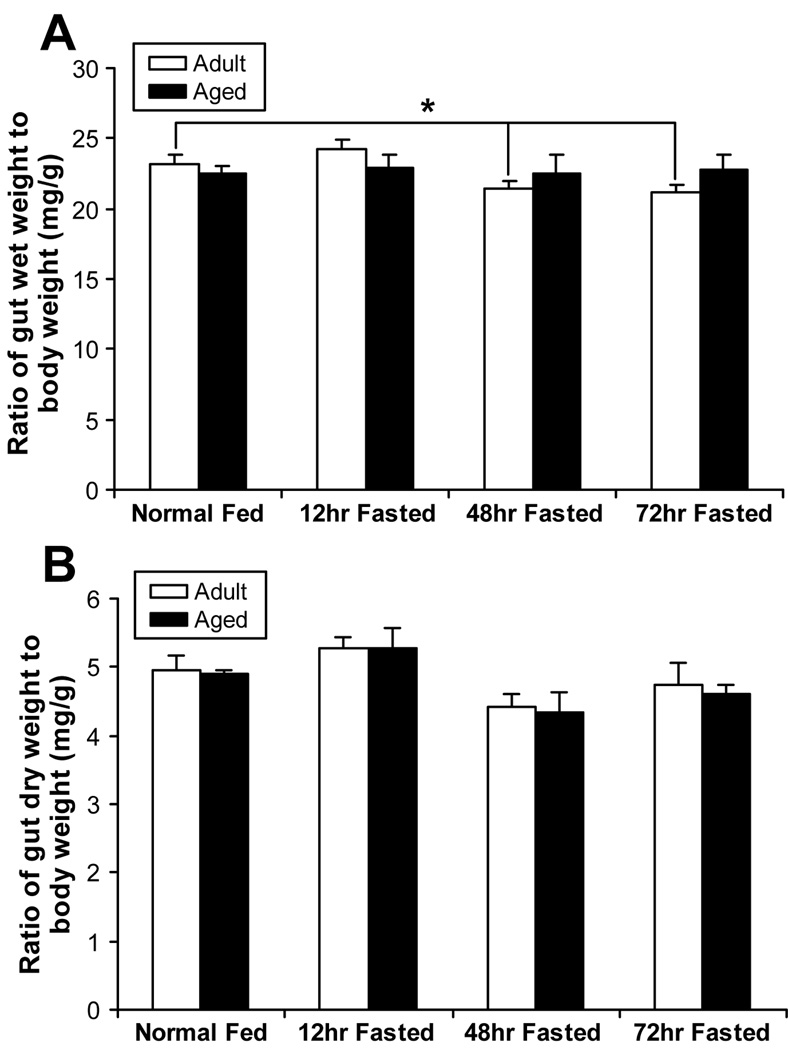
Body weight was 25% higher in the aged mice without starvation. After starvation, body weight significantly decreased in both groups at 12 hours, after which no further significant losses in weight occurred over the next 60 hours (Figure 1). The ratio of proximal small bowel wet weight to body weight was not different between the non-aged adult and aged adult mice before starvation. In non-aged adult mice, proportional bowel wet weight fell significantly with fasting while no changes were seen in the aged, indicating a diminished atrophic response in the aged. No changes were seen in the ratio of proximal small bowel dry weight to body weight in either group, intimating that the loss of wet weight in the non-aged group was fluid volume rather than non-fluid mass (Figure 2).
Figure 1.
Body weight was higher in the aged group before starvation (p<0.05). Both groups decreased after starvation at 12 hours as evidenced by within group comparisons (p<0.05). No significant changes were induced thereafter. * = p<0.05, significant difference compared to fed mice. † = p<0.05, significant difference between the non-aged adult group and aged group.
Figure 2.
A) The ratio of proximal gut wet and dry weight to body weight after starvation between adult and aged groups. * p<0.05, non-aged adult 48, 72 hours post fasted vs. normal groups. The ratio of proximal gut wet to body weight has not different between non-aged adult and aged groups over time after starvation.
B) The ratio of dry weight to body weight after starvation between adult and aged groups. The ratio of proximal gut dry weight to body weight has no different between non-aged adult and aged groups over the time course of starvation.
Intestinal morphologic change
Mucosal height and corresponding cell number were higher in aged mice without starvation (p<0.05). After starvation, mucosal height decreased 23.4±3.9% in aged mice, and 31.3±1.4% in adult mice 48 hours following removal of the food (p<0.05 for both within and between group comparisons). Epithelial cell number decreased in both groups (p<0.05) as well (Table 1). Though relative change of cell number between the two groups observed was insignificant, the degree of decreases in epithelial cell number in non-aged adult mice exceeded the aged mice 48 hours after starvation, again suggesting a greater atrophic response in the non-aged adult compared to aged mice.
Table 1.
Histological measurement of proximal small bowel
| Mucosal Height (µm) | Epithelial Cell Number | ||||||
|---|---|---|---|---|---|---|---|
| Mean±SEM | Relative % | Mean±SEM | Relative % | ||||
| Fed | Adult | 485±5 | Fed | Adult | 174±3 | ||
| Aged | 622±26* | Aged | 221±3* | ||||
| 12 hrs | Adult | 410±12† | −15.5±2.5 | 12 hrs | Adult | 162±2† | −7.4±1.1 |
| Aged | 470±22*† | −24.4±3.4 | Aged | 196±4*† | −11.4±1.9 | ||
| 48 hrs | Adult | 333±7† | −31.3±1.4 | 48 hrs | Adult | 148±3† | −17.9±1.3 |
| Aged | 477±24*† | −23.4±3.9* | Aged | 191±6*† | −11.8±2.3* | ||
| 72 hrs | Adult | 388±9† | −20.0±1.9 | 72 hrs | Adult | 150±4† | −16.3±1.8 |
| Aged | 484±9*† | −22.2±1.4 | Aged | 179±4*† | −14.7±1.0 | ||
: p<0.05, significant difference between non-aged adult and aged.
: p<0.05, significant difference compared to fed groups.
Mucosal height and cell number are higher in aged group at base line (p<0.05), which significantly decreases after starvation in both groups (p<0.05). The relative change of mucosal height and cell number were significantly different between the aged and adult groups 48 hours after starvation.
Cell death and proliferation
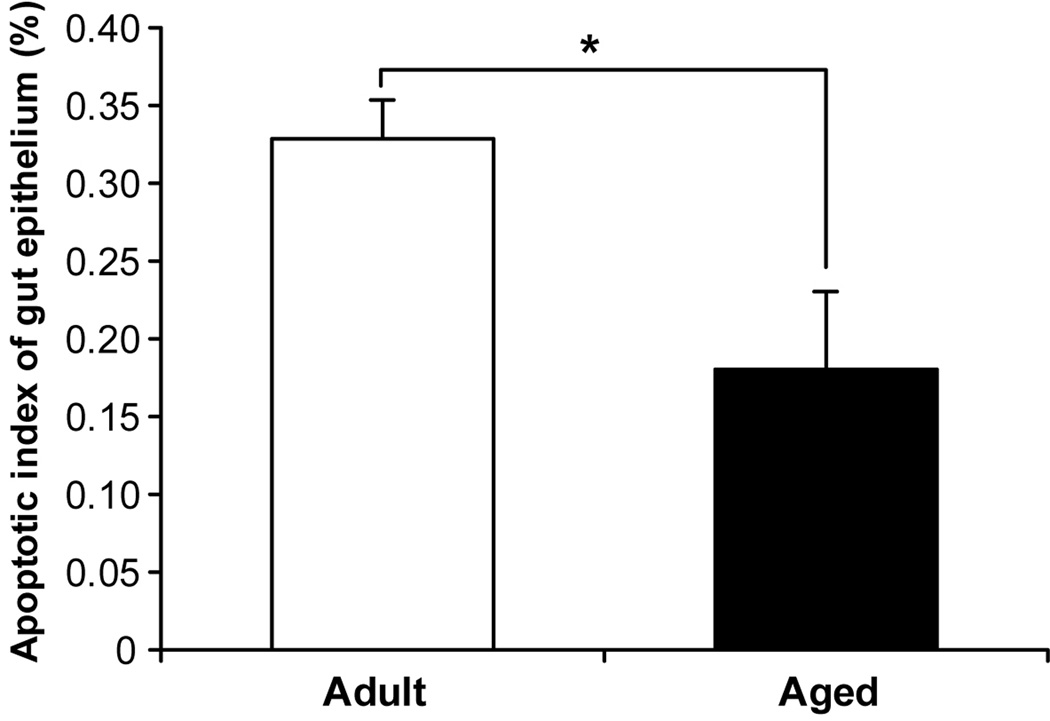
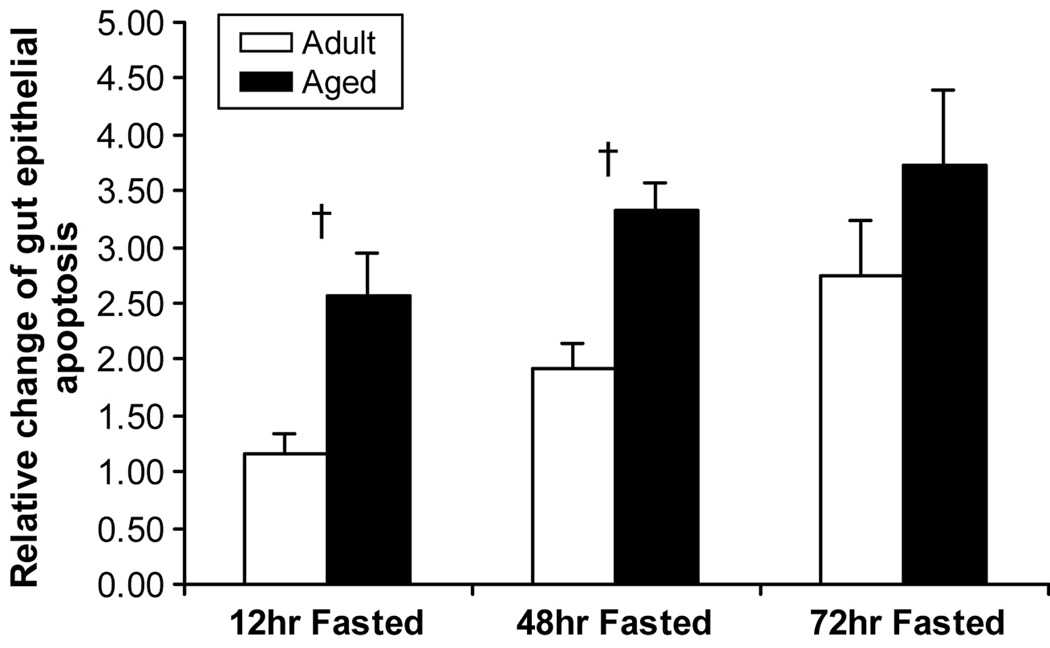
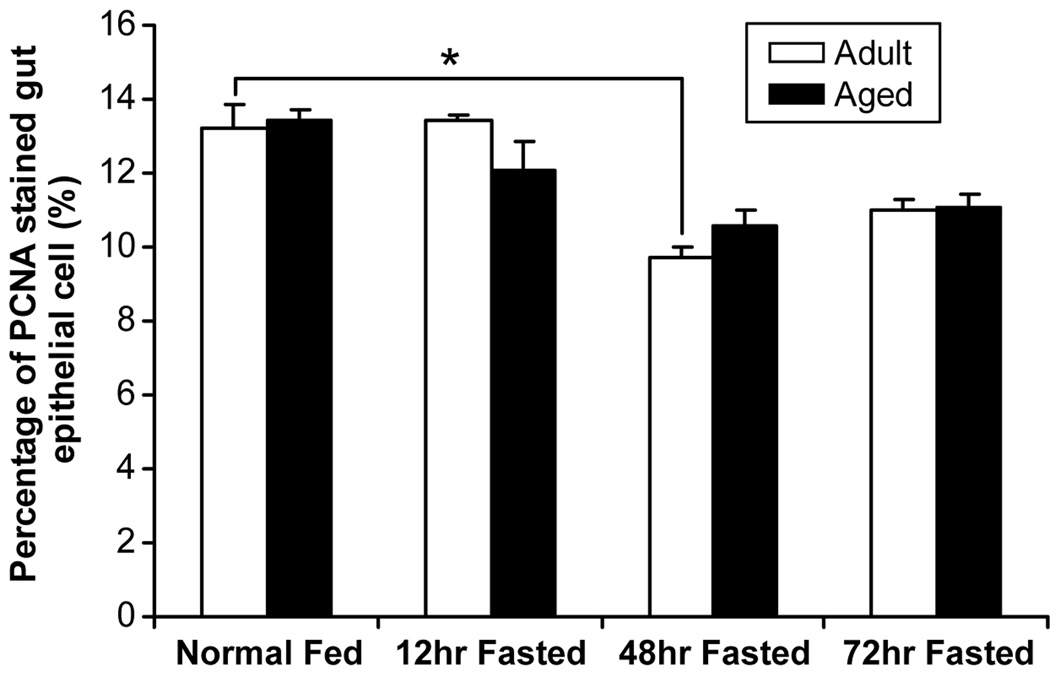
Apoptotic index was lower before starvation in the aged mice (p<0.05) (Figure 3), while proliferation was not different between groups. Starvation caused increased apoptosis in both adult and aged groups. Since there is a significant difference of apoptosis which occurred in intestinal epithelia with age, data must be “calibrated” before the effect of starvation is estimated. Compared to the apoptotic index at baseline in the adult and aged groups, the relative fold-rate changes in apoptosis after fasting increased more in aged (2.6±0.4-fold at 12 hours; 3.3±0.2-fold at 48 hours) than in non-aged adults (1.2±0.2-fold at 12 hours; 1.9±0.2-fold at 48 hours) (p<0.05) (Figure 4). No significant difference in cell proliferation was found in either the non-aged adult or aged groups across time, except proliferation decreased in non-aged adults at 48 hours after starvation (Figure 5).
Figure 3.
Aged mice have a lower rate of apoptosis in the small bowel epithelium without starvation (p<0.05). * = p<0.05, non-aged adult vs. aged groups.
Figure 4.
Fold-rate changes in rate of apoptosis in non-aged adult and aged mice after starvation. Aged mice have a greater relative increase in the rate of apoptosis at 12 and 48 hours after starvation compared to non-aged adult mice (p<0.05). + = p<0.05, adult vs. aged groups.
Figure 5.
Cell proliferation has not significantly different between aged and adult groups at any time point. The proliferation index was significantly decreased in the non-aged adult group 48 hours after starvation, but not at any other time point (* = p< 0.05, adult group vs. baseline). No significant changes in proliferation were seen in the aged group at any time point.
DISCUSSION
We found that before starvation, aged mice have a greater overall weight and proximal bowel weight compared to non-aged adult mice with no proportional differences. In response to starvation, overall body weight and gut wet weight fell in both groups at 12 hours, but did not fall significantly thereafter. However, after starvation relative gut wet weight and epithelial cell number fell more in the non-aged adult mice compared to the aged, suggesting a diminished atrophic response to starvation in the aged. To define the associated changes in gut epithelial cell number kinetics, we examined the rates of gut epithelial cell apoptosis and proliferation. Before starvation, we found that small bowel epithelial cell apoptosis was diminished in the aged compared to the non-aged adult mice. After starvation, relative rates of apoptosis were actually higher in the aged, while decreases in proliferation were higher in the non-aged adults. We showed previously that starvation induces proximal small intestinal epithelial atrophy, which is related to increased epithelial cell apoptosis and decreased proliferation in non-aged adult rats.11 In the current study, we found that aged mice have a lower apoptotic index under normal conditions, but have an increased apoptotic index in response to starvation. Secondly, we found that atrophy was greater in non-aged adult mice, indicating that overall responses to changes in cell turnover such as those seen with starvation are slowed with aging, perhaps because of diminished responsiveness in proliferation pathways and not apoptosis.
In this study, we found that proximal guts wet weight decreased after starvation but not dry weight in non-aged adult mice. That is perhaps as much about cell volume or interstitial volume alternation, followed by decreased micro environment fluid volume. Garcia-Arumi E et al12 showed that starvation caused blood flow decreased in small intestine within 9 days. We also showed that relative change of gut mucosa height and epithelia number decreased after starvation, which corresponded with proximal gut wet weight change. However, gut mucosa only represents the part of the proximal gut dry weight. We did not measure the protein content of gut mucosa in this experiment based on the same reason.
Aging is characterized by progressive deterioration in the structure and function of many organs within the body. Cells are exposed to many intrinsic and extrinsic stresses that may lead to the accumulation of errors and other damage.13 Martin et al14 found an age-related decrease in the apoptotic response of stem cells to low-dose irradiation. Our data showed that aged mice had differing characteristics compared to the adult group at baseline. Proximal small bowel epithelial cells respond to starvation distinguished in adult from aged mice. Aged mice showed relative increases in apoptosis although proliferation was not similarly reduced. We speculate that gut epithelial cells are less responsive to cell death signals under normal conditions while susceptibility to these signals is retained and actually increases with starvation. Similarly, gut epithelial cells in aged mice are less responsive to signals that typically decrease proliferation.
The mechanisms regulating intestinal epithelial homeostasis are complicated and influenced by internal and external factors. Gastrointestinal hormones (gastrin, cholecystokinin, secretin) and peptides (somatostatin, enteroglucagon) stimulate epithelial cell growth in the small intestine.15 Complete starvation causes mucosal atrophy and loss of mucosal height.16 In an in vitro study, glutamine starvation induced apoptosis through specific caspase activation in rat intestinal epithelial cells.17 The effects of aging on starvation initiated mucosal turnover have not been defined. Other studies showed that starvation resulted in a smaller decrease in DNA labeling of crypt cells in aging rats18 as proliferation decreased. In this present study, we showed that atrophy is not as active in the aged mouse compared to the adult mouse, but this relatively diminished atrophic response is due more to lack of changes in proliferation rather than to increased apoptosis.
Heller et al19 reported that proximal intestinal hyperplasia occurred in 33-month-old diet-restricted rats. Using our model, we found gut epithelial cellularity was relatively increased under normal conditions in aged mice versus adult mice, but the degree of decreases reduced after starvation. Holt et al18 showed that a 60% food restriction causes the gut epithelial apoptotic index to increase in aged rats. In this present study, epithelial apoptotic index increased in both aged and adult mice after starvation. Xiao et al20 presented that enterocyte turnover with increased proliferation and decreased apoptosis in the colonic mucosa were associated with aging. Those contradictions might be explained by different experimental designs, method, animal spices, age and tissue.
The clinical relevance of starvation and aging in inducing cell apoptosis has been investigated in several studies.15, 21 Increased apoptosis in small intestinal epithelial cells is associated with increased bidirectional permeability of the intestinal barrier,22, 23 which leads to decreased intraluminal nutrient uptake,24 and to increased permeability resulting in bacterial translocation.25 Translocation of enteric bacteria, toxins, and gut-derived factors carried through the intestinal barrier under these conditions may increase morbidity and mortality.26 All of these takes together emphasizing implicates the importance of enteral feeding in aged patients, which may diminish small intestine epithelial cell apoptosis.
In conclusion, we showed that starvation induced epithelial cell changes diminished in the aged mice. However, signaling associated with apoptosis and proliferation is altered with aging in response to starvation especially aged mice are less responsive to signals that typically decrease proliferation. These findings suggest that aging is associated with differing baseline characteristics and responsiveness of the gut mucosa to stimuli that might be related to changes in clinical outcomes in the elderly.
Acknowledgments
This study was supported by grants from the National Institutes of Health (P50 GM-60338, R01 GM-56687 and T32 GM008256) and Shriners Hospitals for Children (8660).
REFERENCES
- 1.Bourdel-Marchasson I, Barateau M, Rondeau V, et al. A multi-center trial of the effects of oral nutritional supplementation in critically ill older inpatients. GAGE Group. Groupe Aquitain Geriatrique d'Evaluation. Nutrition. 2000;16:1–5. doi: 10.1016/s0899-9007(99)00227-0. [DOI] [PubMed] [Google Scholar]
- 2.Nourhashemi F, Andrieu S, Rauzy O, et al. Nutritional support and aging in preoperative nutrition. Curr Opin Clin Nutr Metab Care. 1999;2:87–92. doi: 10.1097/00075197-199901000-00015. [DOI] [PubMed] [Google Scholar]
- 3.Howard L, Malone M. Clinical outcome of geriatric patients in the United States receiving home parenteral and enteral nutrition. Am J Clin Nutr. 1997;66:1364–1370. doi: 10.1093/ajcn/66.6.1364. [DOI] [PubMed] [Google Scholar]
- 4.Potten CS, Loeffler M. Stem cells: attributes, cycles, spirals, pitfalls and uncertainties. Lessons for and from the crypt. Development. 1990;110:1001–1020. doi: 10.1242/dev.110.4.1001. [DOI] [PubMed] [Google Scholar]
- 5.Smith JR, Pereira-Smith OM. Replicative senescence: implications for in vivo aging and tumor suppression. Science. 1996;273:63–67. doi: 10.1126/science.273.5271.63. [DOI] [PubMed] [Google Scholar]
- 6.Ortega MA, Nunez MC, Suarez MD, Gil A, Sanchez-Pozo A. Age-related response of the small intestine to severe starvation and refeeding in rats. Ann Nutr Metab. 1996;40:351–358. doi: 10.1159/000177944. [DOI] [PubMed] [Google Scholar]
- 7.Kerr JF, Wyllie AH, Currie AR. Apoptosis: a basic biological phenomenon with wide-ranging implications in tissue kinetics. Br J Cancer. 1972;26:239–257. doi: 10.1038/bjc.1972.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Iwakiri R, Gotoh Y, Noda T, et al. Programmed cell death in rat intestine: effect of feeding and fasting. Scand J Gastroenterol. 2001;36:39–47. doi: 10.1080/00365520150218048. [DOI] [PubMed] [Google Scholar]
- 9.Jeschke MG, Debroy MA, Wolf SE, Rajaraman S, Thompson JC. Burn and starvation increase programmed cell death in small bowel epithelial cells. Dig Dis Sci. 2000;45:415–420. doi: 10.1023/a:1005445501016. [DOI] [PubMed] [Google Scholar]
- 10.Wolf SE, Ikeda H, Matin S, et al. Cutaneous burn increases apoptosis in the gut epithelium of mice. J Am Coll Surg. 1999;188:10–16. doi: 10.1016/s1072-7515(98)00260-9. [DOI] [PubMed] [Google Scholar]
- 11.Wu XW, Spies M, Herndon DN, Thompson JC, Wolf SE. Starvation induced mucosal atrophy and gut epithelial cell apoptosis is diminished in IL-1 receptor knockout mice. Surg Forum. 2001;(LII):17–20. [Google Scholar]
- 12.Garcia-Arumi E, Quiles M, Lopez-Hellin J, Andreu AL, Arbos MA, Schwartz S. Effect of starvation on organ blood flow in the senescent rat. Arch Physiol Biochem. 1998;105:337–341. [PubMed] [Google Scholar]
- 13.Lajtha LG. Stem cell concepts. Differentiation. 1979;14:23–34. doi: 10.1111/j.1432-0436.1979.tb01007.x. [DOI] [PubMed] [Google Scholar]
- 14.Martin K, Kirkwood TB, Potten CS. Age changes in stem cells of murine small intestinal crypts. Exp Cell Res. 1998;241:316–323. doi: 10.1006/excr.1998.4001. [DOI] [PubMed] [Google Scholar]
- 15.Mezzarobba V, Bielicki G, Jeffrey FM, et al. Lack of effect of ageing on acetate oxidation in rat skeletal muscle during starvation: a (13)C NMR study. J Exp Biol. 2000;203(Pt 6):995–1001. doi: 10.1242/jeb.203.6.995. [DOI] [PubMed] [Google Scholar]
- 16.Inoue Y, Grant JP, Snyder PJ. Effect of glutamine-supplemented total parenteral nutrition on recovery of the small intestine after starvation atrophy. JPEN J Parenter Enteral Nutr. 1993;17:165–170. doi: 10.1177/0148607193017002165. [DOI] [PubMed] [Google Scholar]
- 17.Papaconstantinou HT, Chung DH, Zhang W, et al. Prevention of mucosal atrophy: role of glutamine and caspases in apoptosis in intestinal epithelial cells. J Gastrointest Surg. 2000;4:416–423. doi: 10.1016/s1091-255x(00)80022-0. [DOI] [PubMed] [Google Scholar]
- 18.Holt PR, Yeh KY, Kotler DP. Altered controls of proliferation in proximal small intestine of the senescent rat. Proc Natl Acad Sci U S A. 1988;85:2771–2775. doi: 10.1073/pnas.85.8.2771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Heller TD, Holt PR, Richardson A. Food restriction retards age-related histological changes in rat small intestine. Gastroenterology. 1990;98:387–391. doi: 10.1016/0016-5085(90)90829-p. [DOI] [PubMed] [Google Scholar]
- 20.Xiao ZQ, Moragoda L, Jaszewski R, Hatfield JA, Fligiel SE, Majumdar AP. Aging is associated with increased proliferation and decreased apoptosis in the colonic mucosa. Mech Ageing Dev. 2001;122:1849–1864. doi: 10.1016/s0047-6374(01)00323-2. [DOI] [PubMed] [Google Scholar]
- 21.Tepper CG, Seldin MF, Mudryj M. Fas-mediated apoptosis of proliferating, transiently growth-arrested, and senescent normal human fibroblasts. Exp Cell Res. 2000;260:9–19. doi: 10.1006/excr.2000.4990. [DOI] [PubMed] [Google Scholar]
- 22.Sun Z, Wang X, Wallen R, et al. The influence of apoptosis on intestinal barrier integrity in rats. Scand J Gastroenterol. 1998;33:415–422. doi: 10.1080/00365529850171053. [DOI] [PubMed] [Google Scholar]
- 23.Sun Z, Wang X, Deng X, et al. The influence of intestinal ischemia and reperfusion on bidirectional intestinal barrier permeability, cellular membrane integrity, proteinase inhibitors, and cell death in rats. Shock. 1998;10:203–212. doi: 10.1097/00024382-199809000-00009. [DOI] [PubMed] [Google Scholar]
- 24.Carter EA, Hatz RA, Yarmush ML, Tompkins RG. Injury-induced inhibition of small intestinal protein and nucleic acid synthesis. Gastroenterology. 1990;98:1445–1451. doi: 10.1016/0016-5085(90)91074-g. [DOI] [PubMed] [Google Scholar]
- 25.Zapata-Sirvent RL, Hansbrough JF, Greenleaf GE, Grayson LS, Wolf P. Reduction of bacterial translocation and intestinal structural alterations by heparin in a murine burn injury model. J Trauma. 1994;36:1–6. doi: 10.1097/00005373-199401000-00001. [DOI] [PubMed] [Google Scholar]
- 26.Magnotti LJ, Upperman JS, Xu DZ, Lu Q, Deitch EA. Gut-derived mesenteric lymph but not portal blood increases endothelial cell permeability and promotes lung injury after hemorrhagic shock. Ann Surg. 1998;228:518–527. doi: 10.1097/00000658-199810000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]