Abstract
Cerebrovascular risk factors and stroke are highly prevalent with advancing age and stroke may be more common than Alzheimer’s disease, particularly amongst older men. While stroke mortality continues to decline, the prevalence of individuals with various vascular risk factors continues to rise and many are undiagnosed or undertreated. Asymptomatic cerebrovascular brain injury that includes asymptomatic brain infarction, white matter hyperintensities and even accelerated brain atrophy is even more frequent than clinical stroke. Moreover, the impact of cerebrovascular risk factors on brain injury appears to begin in middle life and additively increases the likelihood of later life dementia. This review focuses on the use of neuroimaging and genetics to understand the impact of asymptomatic vascular risk factors on the trajectories of cognitive aging as well as incident cognitive impairment, stroke and mortality. Results of this review emphasize the need for early detection and treatment of vascular risk factors to improve the cognitive health of our rapidly aging population.
Keywords: Magnetic Resonanace Imaging, white matter hyperintensities, cerebrovascular disease, pathophysiology
INTRODUCTION
Growth in the number of individuals over 65 years of age in the world is unprecedented. For example, in Italy nearly 20% of the population is over age 65 [1]. In the United States, approximately 330 individuals per hour turn age 60 and as of 2010 as many as 45 million were 65 years of age or older [2], resulting in an expected near doubling of the population age 65 and older by 2030[1]. The percentage of individuals over age 65 is increasing even more rapidly in developing countries [1]. It is, therefore, timely to consider diseases of aging, such as cognitive impairment.
Complaints of cognitive impairment, particularly memory loss, are common to older individuals [3]. Cross-sectional epidemiological studies suggest linear age-related decline in memory performance, although remarkable differences in individual trajectories of decline exist [4]. These data have been used to suggest that the results of at least some of the studies of cognitive aging may be contaminated by the inclusion of individuals with incipient disease [4].
While Alzheimer’s disease (AD) is clearly the major cause for cognitive impairment amongst the elderly [5–7], current evidence suggests that an individual’s lifetime risk for cerebrovascular disease (CVD) may be similar or even higher than AD [8] and these two diseases commonly occur in the same individual [9]. Although stroke is considered the clinical hallmark of CVD, it is important to recognize that the full spectrum of CVD includes clinically asymptomatic cerebral infarction, white matter hyperintensities (WMH) and even accelerated brain atrophy [10], that are common to advancing age amongst cognitively normal community dwelling individuals [11].
The potential impact of clinically asymptomatic cerebrovascular injury was first identified by Hachinksi and colleagues [12–18]. In this series of seminal articles, Hachinski’s group described the presence of attenuated white matter signals seen on cerebral x-ray computed tomography which they called “leuko-araiosis” to indicate reduced x-ray absorption in cerebral white matter. First reports found significant associations between leuko-araiosis and vascular risk factors. In addition, the presence of leuko-araiosis was associated with cognitive impairment amongst older individuals with and without dementia. Finally, this group was the first to examine the pathologic consequences of this unique imaging finding [18]. Coincident to the discovery of leuko-araiosis, Awad and colleagues identified “incidental subcortical lesions” on MRI [19] (currently described as white matter hyperintensities; WMH) and noted that these lesions were significantly associated with advancing age and vascular disease. Pathologic study of these lesions also suggested a possible vascular etiology to which they ascribed to the state of “état criblé” [20].
These two series of seminal and seemingly independent, but coincident observations, heralded a new era in medical research relating to the risk and consequences of asymptomatic cerebrovascular brain injury as evaluated by various non-invasive neuroimaging methods. Subsequent studies focused on more fully describing the association between vascular risk factors and leukoaraiosis or WMH in mostly clinical samples [21–28]. Studies of essentially healthy and cognitively normal individuals, however, did not begin until sometime later.
My own work began in 1992 with the publication of a novel method for the measurement of brain volume and WMH from MRI [29]. After an initial report of age-related differences in brain structure amongst optimally healthy older individuals [30], we examined the impact of hypertension on brain structure [31]. Even within this select group of patients with well-controlled hypertension, we identified significant reductions in brain volume and expansion of cerebral ventricles. A number of individuals had extensive WMH, but the finding of brain atrophy associated with hypertension remained even when subjects with extensive WMH were removed from the analysis. The observation that WMH were common to older individuals with hypertension led to a follow-up study of 51 very healthy older individuals free of chronic medical illnesses and not taking any prescription medications [32]. We hypothesized that if vascular disease caused WMH, these individuals would have very little WMH. Using quantitative analysis of WMH volume, however, we identified a bimodal distribution, with 10% of subjects with extensive WMH burden. Further evaluation of this cohort found that systolic blood pressure (SBP), even within the range of normal, was associated with increased WMH when adjusting for age. In addition, WMH volume was significantly and negatively associated with cognitive performance after adjusting for age and education. Individuals with extensive WMH (greater than the 95% confidence interval for age) also had significantly higher SBP and significantly lower frontal glucose metabolism. The finding that systolic blood pressure, even when in the normal range for age, was a significant independent predictor of WMH volume suggests that the effect of systolic blood pressure may operate along a continuum. A later publication from the ARIC study using qualitative WMH measures confirmed this finding [33, 34]. Our findings also confirmed previous observations suggesting that WMH must be extensive to have an impact on brain structure and function [35]. These results suggested that vascular risk factors, even within the normal range, may impact brain structure and function among older individuals, possibly increasing risk for late life dementia.
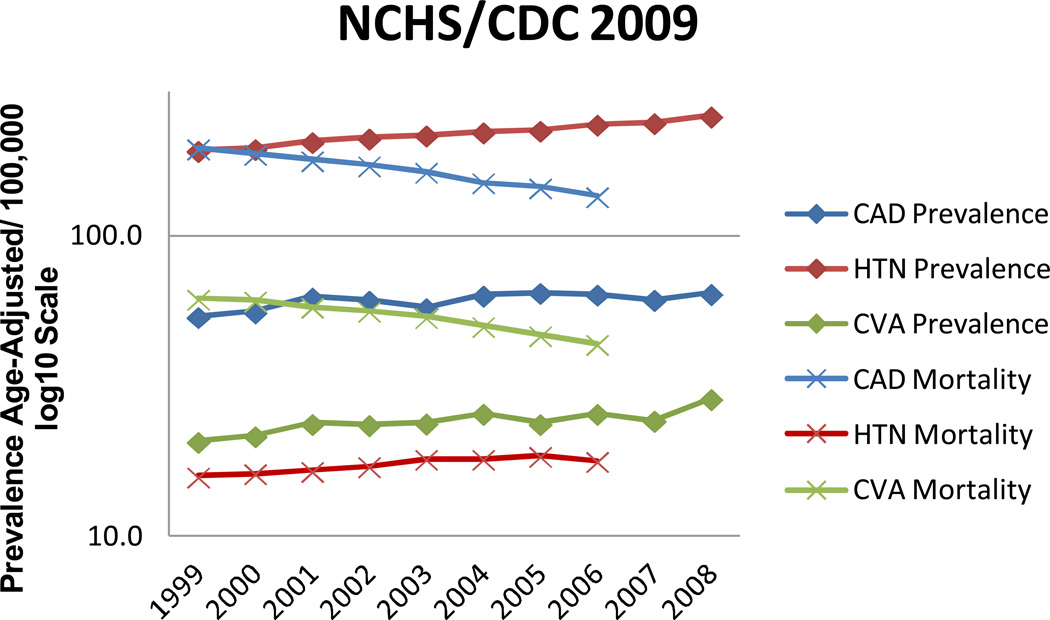
It was evident from these early studies that clinically silent CVD was ubiquitous to older individuals, but the impact on brain function was somewhat subtle, particularly for individuals who were otherwise healthy. In response to this observation, I refocused my research activities to the study of larger subject cohorts that included individuals with varying degrees of medical health. With this focus, I sought collaborations with established, longitudinal programs in order to increase the size of the study populations and broaden health inclusion, as well as to acquire middle-life health risk data that would allow me to assess life course effects of CVD on brain structure and cognition. The rationale for this approach was two-fold. First, the prevalence of vascular risk factors, particularly hypertension has a prevalence of nearly 10% amongst individuals 20–34 years of age [36], is generally unrecognized in this age group, similarly undertreated [37] and increases in prevalence steadily with advancing age. This results in an increased prevalence of individuals surviving to advanced age with multiple systemic vascular injuries. Second, the impact of vascular risk factors may be greatest when sustained over decades. This hypothesis may be extremely relevant given the reduction in vascular mortality over the last decade [37] resulting in an increasing prevalence of individuals living to more advanced age with comorbid vascular disease burdens (Figure 1).
Figure 1.
Center for Disease Control, National Health Statistics 2009 prevalence figures for trends in cardiovascular disease and mortality for the decade beginning in 1999. Note that mortality declined steadily while prevalence has remained or increased slightly over this decade of observation.
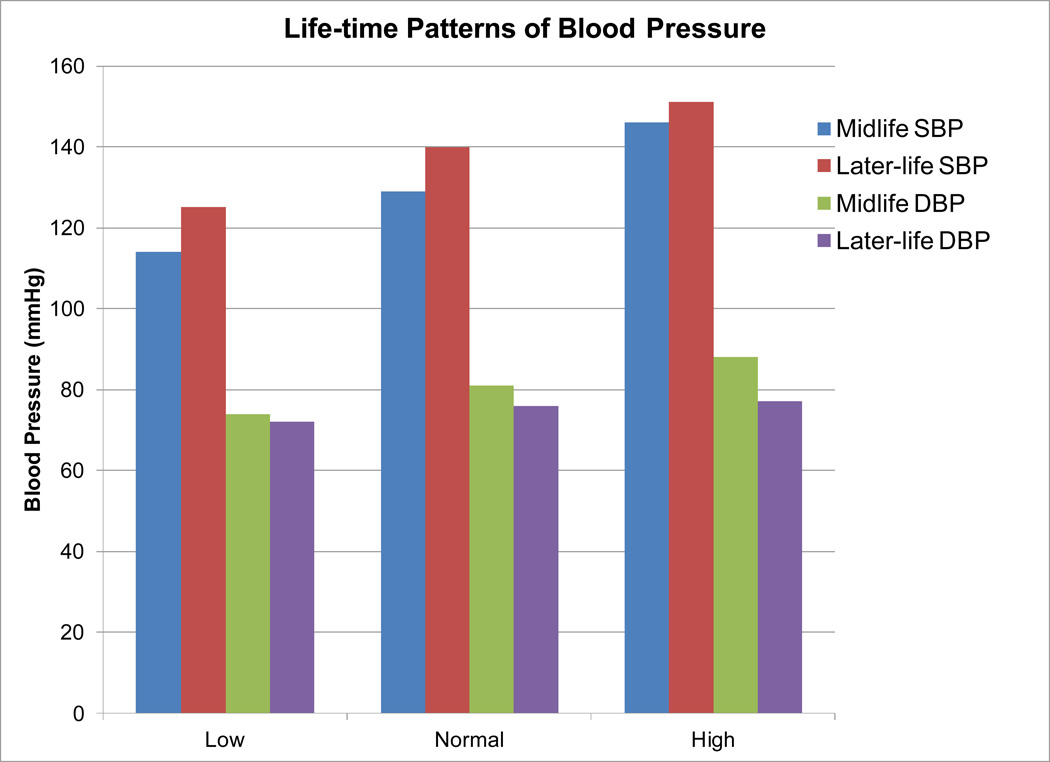
I was fortunate to begin this work with Dr. Dorit Carmelli, principal investigator of the NHLBI Twin Study. Together, Dr. Carmelli and I developed a research program that focused on MRI measures of CVD-associated brain injury [38–43]. Using a method developed by Lenore Launer [44], we identified patterns of blood pressure regulation identified in middle-life (Figure 2) and investigated the impact of these patterns on later-life brain structure and function. Two observations were apparent from these analyses. First, the impact of blood pressure pattern was most strong for systolic blood pressure [38]. Second, there was a nearly linear association between blood pressure pattern and MRI measures of brain injury as well as the prevalence of associated systemic vascular diseases such as coronary artery disease, peripheral vascular disease and stroke. Longitudinal analyses confirmed initial findings [43]. Finally, there were significant associations between blood pressure patterns and impairments in cognitive function including clinically significant memory impairment such as mild cognitive impairment (MCI) that were equal in magnitude to the effect of apolipoprotein E4 genotype [45]. These results confirmed my initial hypothesis that vascular risk factors result in untoward effects that impact brain structure and function. These findings, however, extended current observations to include clinically relevant cognitive impairment (confirmed by a later community based study [46, 47]) suggesting that more aggressive treatment of vascular risk factors may result in reduced risk for later-life cognitive impairment. Moreover, these results pointed to an important facet of hypertension care: the pattern of early life SBP appeared to be sustained in this cohort, despite 78% of those with high BP receiving treatment [38].
Figure 2.
Life-time pattern of systolic and diastolic blood pressures for three categories determined in middle life. Note that 78% of individuals in the high blood pressure category received treatment.
Study of twins also allowed us to examine potential genetic influences on brain structure. In this seminal work, we found that structural brain measures such as intracranial, brain and WMH volumes were highly heritable [48–52]. The notion that genetic influences might extend to later-life was an unexpected finding suggesting that a substantial proportion of brain aging may be under genetic control. We also hypothesized that these influences could be modified through environmental interactions or other complex genetic traits. Evidence for this hypothesis comes from two additional studies. First, Carmelli et al., found that differences in brain injury among monozygotic twins were significantly associated with differences in vascular risk factor prevalence [39]. Second, longitudinal studies of heritability estimates of brain volume strongly suggest a significant environmental influence [53]. These initial findings indicated that there exist strong genetic influences on brain aging which may place certain individuals at higher risk for poor cognitive aging. The fact that these genetic influences may be modified by environmental influences and other associated and possibly treated complex traits also suggests that the increased genetic vulnerability to brain aging may be ameliorated by preventive therapy [45]. The ability to extend the findings from the NHLBI Twins Study to the general population, however, was limited by the fact that the NHLBI subjects were Caucasian, male, veterans and twins.
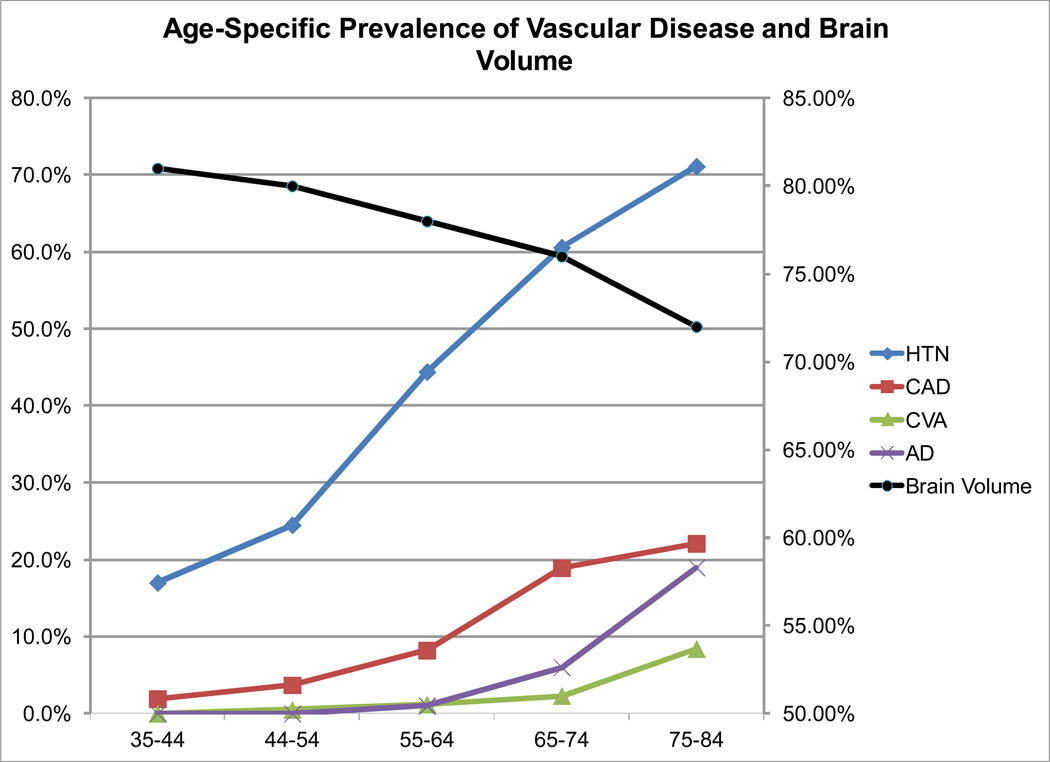
In order to expand the relevance of my research, I joined with Dr. Philip Wolf to study the participants of the Framingham Heart Study (FHS), a community based population study which would assure that further results of my work would have relevance to the general population. The FHS satisfied four important needs of my evolving research program: the population is considered generally representative; it included both men and women; it had considerable longitudinal data; and it included both parent and offspring members (and now the third generation) enabling further study of the genetics of brain structure. Through serial studies, we were able to confirm that CVD risk factors lead to accelerating brain aging and cognitive impairment in the general population [11, 54–64]. Moreover, we extended current understanding of vascular influences on brain structure and function, by identifying that noticeable untoward influences begin at 55 years of age [55, 56] (Figure 3). This subsequent finding underscores the potent influence of vascular risk factors on cognitive aging that likely begin at least a decade before similar influences due to Alzheimer’s disease [5, 65]. The clinical relevance of vascular risk factors on brain structure and function were further supported by the finding of longitudinal influences [64] and significant associations with clinically relevant outcomes such as MCI, dementia, stroke and even death [66].
Figure 3.
Age-specific prevalence of vascular disease and brain volumes from the offspring cohort of the Framingham Heart Study (modified after DeCarli et al. [11]).
Results from the FHS confirmed that the untoward effects of vascular risk factors on brain structure and function were true for both men and women, but also led to the novel observation that the impact of vascular risks on brain structure and function may begin earlier than expected. The FHS studies and those of the NHLBI Twins consistently show elevated blood pressure as the leading cause of brain atrophy, increased WMH volume, silent brain infarction and cognitive impairment. Recognizing that vascular risk factors are prevalent and often detectable at a relatively young age, these results may have substantial public health ramifications relating to the time and extent of risk factor treatment.
Both the NHLBI and FHS studies examined predominately Caucasian individuals. My ability to collaborate productively with the FHS encouraged other collaborations with epidemiologically based studies such as the Northern Manhattan Stroke Study (NOMAS), the Washington Heights Innwood Columbia Aging Projects (WHICAP) and the Chicago Healthy Aging Projects (CHAP) which included mixed racial and ethnic populations. Research with these groups confirmed the prominent role of cerebrovascular disease on cognition [67–84] seemingly independent of race or ethnicity [77, 85] suggesting that racial and ethnic differences in prevalent and potentially treatable vascular risk factors [86] may explain similar differences in prevalent dementia [87].
My experience with epidemiological research helped me to further refine questions regarding the impact of CVD on brain structure and function, allowing for more targeted research focusing on specific causal pathways that epidemiological studies cannot answer. For example, we developed new methods to examine the spatial extent of WMH in order to further analyze how regional variations in WMH may impact brain structure and function. Using this method, we found that increasing extent of WMH was associated with expansion around the ventricular system [88] and that WMH affected frontal lobe metabolism independent of location [89]. This led to further studies that found differences in anatomical localization of WMH between normal aging, MCI and dementia [90] where rostral-caudal extension of WMH was associated with increasing degrees of cognitive impairment. This finding differed from the previously described periventricular location of WMH associated with vascular risk factors suggesting that vascular and degenerative processes may interact to cause white matter injury [90]. With further studies we also observed that there exists a subset of individuals with significant memory loss related to vascular brain injury [91], that MRI measures and vascular risk factors differ amongst subtypes of MCI [92] and that the WMH also affect cognitive trajectories measured over one to two years follow-up [93, 94]. By adding newer methods of diffusion tensor imaging (DTI), we further characterized differences in white matter integrity as they related to vascular and degenerative pathologies [95, 96] and found that anterior white matter injury was mediated by vascular risk factors, but posterior white matter injury was more strongly associated with degeneration. We have recently extended our observations on the biology of WMH formation by identifying a “penumbra” of at risk tissue surrounding WMH [97]. By combining DTI and FLAIR imaging, we found that white matter integrity declined in areas close to WMH suggesting that WMH are the extreme expression of more general injury in the vascular watershed region of the cerebral white matter. This new observation may be important to monitoring at risk brain tissue that may respond to clinical treatment.
More recently, I started a small program of cognitive neuroscience research in collaboration with Neuroscience and Psychology graduate students. In our first study, we used functional MRI to show that the extent of functional activation of dorsal lateral prefrontal cortex during working memory tasks in a group of older normal individuals was significantly and inversely associated with the extent of WMH volume [98]. These data suggested that WMH lead to functional disconnection of central executive processes necessary for working memory by injury to cerebral white matter tracks. These findings are consistent with my previous hypothesis [45] and some of my earlier work showing that WMH reduce frontal metabolism [32]. More recent work has focused further on other cognitive systems affected by WMH [99]. In this study we found that high WMH volumes in older individuals lead to impairments in cognitive control, reduced activation of dorsal lateral cortex and loss of correlations between dorsal lateral cortex and frontal and parietal targets. In addition, WMH were also associated with lack of suppression of the dorsal node of the default mode network, suggesting, like Alzheimer’s disease that there is an inverse relationship between suppression of the default mode during activation and performance [100]. These results suggest that at least some of the symptoms common to aging evidenced as subtle frontal lobe dysfunction [101] may result, in part, to the presence of asymptomatic vascular brain injury.
With increasing emphasis on genome wide association studies and evidence that brain and WMH volumes are heritable, I also collaborated with family based genetic association from the FHS [102], the Multi-institutional Research in Alzheimer Genetic Epidemiology (MIRAGE) study and community based studies as part of the Cohorts for Heart and Aging Research in Genomic Epidemiology (CHARGE) consortium. In these studies, we confirmed the heritability of WMH [103] and showed that heritability was high throughout most of the adult life span. We also performed linkage analysis [104] and found significant linkage with a locus on chromosome 4. Additional studies with MIRAGE identified pleotropic effects of the SORL1 gene using MRI as an endophenotype [105] that suggested two separate biological pathways (one through amyloid precursor metabolism and the other through lipid metabolism) by which SORL1 may lead to increased risk for late life dementia. New work with the CHARGE consortium has identified risk alleles for both WMH and asymptomatic MRI infarction [106, 107], but further work will be necessary to fully characterize the biology of these findings.
In summary, my focus on the impact of CVD on cognition has resulted in a number of important observations related to advancing age and cognitive impairment. First and foremost, MRI evidence of CVD-associated brain injury is common and likely begins in midlife. The symptoms of vascular brain injury are often subtle, but may falsely contribute to our current notion of cognitive weaknesses associated with advancing age. Importantly, however, vascular brain injury is clearly a substantial risk factor for clinically significant cognitive impairment such as MCI and dementia as well as increased mortality. While considerable evidence also suggests that vascular brain injury may be under genetic influence, vascular risk factors are treatable and have been associated with reduced likelihood of expressed dementia in preliminary studies [108]. Despite increased awareness of the impact on vascular disease and health individuals are often unaware of their vascular risk factor status, particularly at younger ages [37]. As an example, the United States is currently witnessing an obesity epidemic [109] and studies show that obesity is associated with multiple vascular disease and significantly increased risk for late-life dementia [110]. I strongly believe that clinically silent CVD results from poorly controlled risk factors and leads to late-life cognitive impairment. Aggressive public health policies which advocate healthy life-styles and the early treatment of hypertension and other vascular risk factors, therefore, will likely result in improved later-life cognitive health, substantially alleviating what is likely to become a huge public health burden [111].
ACKNOWLEGEMENTS
This research was supported by NIA grants P30 AG10129 and R01 AG021028.
REFERENCES
- 1.Kinsella K, Velkoff VA. An Aging World: 2001. Washington, D.C.: U.S Census Bureau; 2001. p. 190. [Google Scholar]
- 2.Werner CA. U.S.C. Bureau, editor. The Older Population: 2010. 2011
- 3.Cutler SJ, Grams AE. Correlates of self-reported everyday memory problems. Journal of Gerontology: Social Sciences. 1988;43:S82–S90. doi: 10.1093/geronj/43.3.s82. [DOI] [PubMed] [Google Scholar]
- 4.Wilson RS, Beckett LA, Barnes LL, Schneider JA, Bach J, Evans DA, Bennett DA. Individual differences in rates of change in cognitive abilities of older persons. Psychol Aging. 2002;17(2):179–193. [PubMed] [Google Scholar]
- 5.Hebert LE, Scherr PA, Bienias JL, Bennett DA, Evans DA. State-specific projections through 2025 of Alzheimer disease prevalence. Neurology. 2004;62(9):1645. doi: 10.1212/01.wnl.0000123018.01306.10. [DOI] [PubMed] [Google Scholar]
- 6.Ferri CP, Prince M, Brayne C, Brodaty H, Fratiglioni L, Ganguli M, Hall K, Hasegawa K, Hendrie H, Huang Y, Jorm A, Mathers C, Menezes PR, Rimmer E, Scazufca M. Global prevalence of dementia: a Delphi consensus study. Lancet. 2005;366(9503):2112–2117. doi: 10.1016/S0140-6736(05)67889-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ganguli M, Dodge HH, Chen P, Belle S, DeKosky ST. Ten-year incidence of dementia in a rural elderly US community population: the MoVIES Project. Neurology. 2000;54(5):1109–1116. doi: 10.1212/wnl.54.5.1109. [DOI] [PubMed] [Google Scholar]
- 8.Seshadri S, Beiser A, Kelly-Hayes M, Kase CS, Au R, Kannel WB, Wolf PA. The lifetime risk of stroke: estimates from the Framingham Study. Stroke. 2006;37(2):345–350. doi: 10.1161/01.STR.0000199613.38911.b2. [DOI] [PubMed] [Google Scholar]
- 9.Schneider JA, Arvanitakis Z, Bang W, Bennett DA. Mixed brain pathologies account for most dementia cases in community-dwelling older persons. Neurology. 2007;69(24):2197–2204. doi: 10.1212/01.wnl.0000271090.28148.24. [DOI] [PubMed] [Google Scholar]
- 10.Decarli C. Vascular factors in dementia: an overview. J Neurol Sci. 2004;226(1-2):19–23. doi: 10.1016/j.jns.2004.09.005. [DOI] [PubMed] [Google Scholar]
- 11.Decarli C, Massaro J, Harvey D, Hald J, Tullberg M, Au R, Beiser A, D'Agostino R, Wolf PA. Measures of brain morphology and infarction in the framingham heart study: establishing what is normal. Neurobiol Aging. 2005;26(4):491–510. doi: 10.1016/j.neurobiolaging.2004.05.004. [DOI] [PubMed] [Google Scholar]
- 12.Hachinski VC, Potter P, Merskey H. Leuko-araiosis: an ancient term for a new problem. Canadian Journal of Neurological Sciences. 1986;13(4 Suppl):533–534. doi: 10.1017/s0317167100037264. [DOI] [PubMed] [Google Scholar]
- 13.Steingart A, Lau K, Fox A, Diaz F, Fisman M, Hachinski V, Merskey H. The significance of white matter lucencies on CT scan in relation to cognitive impairment. Canadian Journal of Neurological Sciences. 1986;13(4 Suppl):383–384. doi: 10.1017/s0317167100036945. [DOI] [PubMed] [Google Scholar]
- 14.Hachinski VC, Potter P, Merskey H. Leuko-araiosis. Archives of Neurology. 1987;44(1):21–23. doi: 10.1001/archneur.1987.00520130013009. [DOI] [PubMed] [Google Scholar]
- 15.Inzitari D, Diaz F, Fox A, Hachinski VC, Steingart A, Lau C, Donald A, Wade J, Mulic H, Merskey H. Vascular risk factors and leuko-araiosis. Archives of Neurology. 1987;44(1):42–47. doi: 10.1001/archneur.1987.00520130034014. [DOI] [PubMed] [Google Scholar]
- 16.Steingart A, Hachinski VC, Lau C, Fox AJ, Diaz F, Cape R, Lee D, Inzitari D, Merskey H. Cognitive and neurologic findings in subjects with diffuse white matter lucencies on computed tomographic scan (leuko-araiosis) Archives of Neurology. 1987;44(1):32–35. doi: 10.1001/archneur.1987.00520130024012. [DOI] [PubMed] [Google Scholar]
- 17.Steingart A, Hachinski VC, Lau C, Fox AJ, Fox H, Lee D, Inzitari D, Merskey H. Cognitive and neurologic findings in demented patients with diffuse white matter lucencies on computed tomographic scan (leuko-araiosis) Archives of Neurology. 1987;44(1):36–39. doi: 10.1001/archneur.1987.00520130028013. [DOI] [PubMed] [Google Scholar]
- 18.Janota I, Mirsen TR, Hachinski VC, Lee DH, Merskey H. Neuropathologic correlates of leuko-araiosis [published erratum appears in Arch Neurol 1990 Mar;47(3)281] Archives of Neurology. 1989;46(10):1124–1128. doi: 10.1001/archneur.1989.00520460118023. [DOI] [PubMed] [Google Scholar]
- 19.Awad IA, Spetzler RF, Hodak JA, Awad CA, Carey R. Incidental subcortical lesions identified on magnetic resonance imaging in the elderly. I. Correlation with age and cerebrovascular risk factors. Stroke. 1986;17(6):1084–1089. doi: 10.1161/01.str.17.6.1084. [DOI] [PubMed] [Google Scholar]
- 20.Awad IA, Johnson PC, Spetzler RF, Hodak JA. Incidental subcortical lesions identified on magnetic resonance imaging in the elderly. II. Postmortem pathological correlations. Stroke. 1986;17(6):1090–1097. doi: 10.1161/01.str.17.6.1090. [DOI] [PubMed] [Google Scholar]
- 21.Lechner H, Schmidt R, Bertha G, Justich E, Offenbacher H, Schneider G. Nuclear magnetic resonance image white matter lesions and risk factors for stroke in normal individuals. Stroke. 1988;19(263-265) doi: 10.1161/01.str.19.2.263. [DOI] [PubMed] [Google Scholar]
- 22.Fazekas F. Magnetic resonance signal abnormaliaties in asymptomatic individuals: their incidence and functional correlates. Eur Neurol. 1989;29:164–168. doi: 10.1159/000116401. [DOI] [PubMed] [Google Scholar]
- 23.Fukuda H, Kobayashi S, Koide H, Yamaguchi S, Okada K, Shimode K, Tsunematsu T, Komatsu A. Age-related changes in cerebral white matter measured by computed cranial tomography. Computerized Medical Imaging and Graphics. 1990;14(1):79–84. doi: 10.1016/0895-6111(90)90143-y. [DOI] [PubMed] [Google Scholar]
- 24.Kobari M, Meyer JS, Ichijo M, Oravez WT. Leukoaraiosis: correlation of MR and CT findings with blood flow, atrophy, and cognition. Am J Neuroradiol. 1990;11:273–281. [PMC free article] [PubMed] [Google Scholar]
- 25.Mirsen TR, Lee DH, Wong CJ, Diaz JF, Fox AJ, Hachinski VC, Merskey H. Clinical correlates of white-matter changes on magnetic resonance imaging scans of the brain. Archives of Neurology. 1991;48(10):1015–1021. doi: 10.1001/archneur.1991.00530220031015. [DOI] [PubMed] [Google Scholar]
- 26.Schmidt R, Fazekas F, Offenbacher H, Lytwyn H, Blematl B, Niederkorn K, Horner S, Payer F, Freidl W. Magnetic resonance imaging white matter lesions and cognitive impairment in hypertensive individuals. Archives of Neurology. 1991;48(4):417–420. doi: 10.1001/archneur.1991.00530160087019. [DOI] [PubMed] [Google Scholar]
- 27.van Swieten JC, Geyskes GG, Derix MM, Peeck BM, Ramos LM, van Latum JC, van Gijn J. Hypertension in the elderly is associated with white matter lesions and cognitive decline. Annals of Neurology. 1991;30(6):825–830. doi: 10.1002/ana.410300612. [DOI] [PubMed] [Google Scholar]
- 28.Fazekas F, Niederkorn K, Schmidt R, Offenbacher H, Horner S, Bertha G, Lechner H. White matter signal abnormalities in normal individuals: correlation with carotid ultrasonography, cerebral blood flow measurements, and cerebrovascular risk factors. Stroke. 1988;19(10):1285–1288. doi: 10.1161/01.str.19.10.1285. [DOI] [PubMed] [Google Scholar]
- 29.DeCarli C, Maisog J, Murphy DG, Teichberg D, Rapoport SI, Horwitz B. Method for quantification of brain, ventricular, and subarachnoid CSF volumes from MR images. Journal of Computer Assisted Tomography. 1992;16(2):274–284. doi: 10.1097/00004728-199203000-00018. [DOI] [PubMed] [Google Scholar]
- 30.Murphy DG, DeCarli C, Schapiro MB, Rapoport SI, Horwitz B. Age-related differences in volumes of subcortical nuclei, brain matter, and cerebrospinal fluid in healthy men as measured with magnetic resonance imaging [published erratum appears in Arch Neurol 1994 Jan;51(1):60] Archives of Neurology. 1992;49(8):839–845. doi: 10.1001/archneur.1992.00530320063013. [DOI] [PubMed] [Google Scholar]
- 31.Salerno JA, Murphy DG, Horwitz B, DeCarli C, Haxby JV, Rapoport SI, Schapiro MB. Brain atrophy in hypertension. A volumetric magnetic resonance imaging study. Hypertension. 1992;20(3):340–348. doi: 10.1161/01.hyp.20.3.340. [DOI] [PubMed] [Google Scholar]
- 32.DeCarli C, Murphy DG, Tranh M, Grady CL, Haxby JV, Gillette JA, Salerno JA, Gonzales-Aviles A, Horwitz B, Rapoport SI, et al. The effect of white matter hyperintensity volume on brain structure, cognitive performance, and cerebral metabolism of glucose in 51 healthy adults. Neurology. 1995;45(11):2077–2084. doi: 10.1212/wnl.45.11.2077. [DOI] [PubMed] [Google Scholar]
- 33.Liao D, Cooper L, Cai J, Toole JF, Bryan NR, Hutchinson RG, Tyroler HA. Presence and severity of cerebral white matter lesions and hypertension, its treatment, and its control. The ARIC Study. Atherosclerosis Risk in Communities Study. Stroke. 1996;27(12):2262–2270. doi: 10.1161/01.str.27.12.2262. [DOI] [PubMed] [Google Scholar]
- 34.Liao D, Cooper L, Cai J, Toole J, Bryan N, Burke G, Shahar E, Nieto J, Mosley T, Heiss G. The prevalence and severity of white matter lesions, their relationship with age, ethnicity, gender, and cardiovascular disease risk factors: the ARIC Study. Neuroepidemiology. 1997;16(3):149–162. doi: 10.1159/000368814. [DOI] [PubMed] [Google Scholar]
- 35.Boone KB, Miller BL, Lesser IM, Mehringer CM, Hill-Gutierrez E, Goldberg MA, Berman NG. Neuropsychological correlates of white-matter lesions in healthy elderly subjects. A threshold effect. Arch Neurol. 1992;49(5):549–554. doi: 10.1001/archneur.1992.00530290141024. [DOI] [PubMed] [Google Scholar]
- 36.Aronow WS, Fleg JL, Pepine CJ, Artinian NT, Bakris G, Brown AS, Ferdinand KC, Forciea MA, Frishman WH, Jaigobin C, Kostis JB, Mancia G, Oparil S, Ortiz E, Reisin E, Rich MW, Schocken DD, Weber MA, Wesley DJ, Harrington RA. ACCF/AHA 2011 expert consensus document on hypertension in the elderly: a report of the American College of Cardiology Foundation Task Force on Clinical Expert Consensus Documents. Circulation. 2011;123(21):2434–2506. doi: 10.1161/CIR.0b013e31821daaf6. [DOI] [PubMed] [Google Scholar]
- 37.Lloyd-Jones D, Adams RJ, Brown TM, Carnethon M, Dai S, De Simone G, Ferguson TB, Ford E, Furie K, Gillespie C, Go A, Greenlund K, Haase N, Hailpern S, Ho PM, Howard V, Kissela B, Kittner S, Lackland D, Lisabeth L, Marelli A, McDermott MM, Meigs J, Mozaffarian D, Mussolino M, Nichol G, Roger VL, Rosamond W, Sacco R, Sorlie P, Thom T, Wasserthiel-Smoller S, Wong ND, Wylie-Rosett J. Heart disease and stroke statistics-2010 update: a report from the American Heart Association. Circulation. 2010;121(7):e46–e215. doi: 10.1161/CIRCULATIONAHA.109.192667. [DOI] [PubMed] [Google Scholar]
- 38.Swan GE, DeCarli C, Miller BL, Reed T, Wolf PA, Jack LM, Carmelli D. Association of midlife blood pressure to late-life cognitive decline and brain morphology. Neurology. 1998;51(4):986–993. doi: 10.1212/wnl.51.4.986. [DOI] [PubMed] [Google Scholar]
- 39.Carmelli D, Swan GE, Reed T, Wolf PA, Miller BL, DeCarli C. Midlife cardiovascular risk factors and brain morphology in identical older male twins [see comments] Neurology. 1999;52(6):1119–1124. doi: 10.1212/wnl.52.6.1119. [DOI] [PubMed] [Google Scholar]
- 40.DeCarli C, Miller BL, Swan GE, Reed T, Wolf PA, Garner J, Jack L, Carmelli D. Predictors of brain morphology for the men of the NHLBI twin study. Stroke. 1999;30(3):529–536. doi: 10.1161/01.str.30.3.529. [DOI] [PubMed] [Google Scholar]
- 41.DeCarli C, Reed T, Miller BL, Wolf PA, Swan GE, Carmelli D. Impact of apolipoprotein E epsilon4 and vascular disease on brain morphology in men from the NHLBI twin study. Stroke. 1999;30(8):1548–1553. doi: 10.1161/01.str.30.8.1548. [DOI] [PubMed] [Google Scholar]
- 42.Carmelli D, DeCarli C, Swan GE, Kelly-Hayes M, Wolf PA, Reed T, Guralnik JM. The joint effect of apolipoprotein E epsilon4 and MRI findings on lowerextremity function and decline in cognitive function. Journals of Gerontology. Series A, Biological Sciences and Medical Sciences. 2000;55(2):M103–M109. doi: 10.1093/gerona/55.2.m103. [DOI] [PubMed] [Google Scholar]
- 43.Swan GE, DeCarli C, Miller BL, Reed T, Wolf PA, Carmelli D. Biobehavioral characteristics of nondemented older adults with subclinical brain atrophy. Neurology. 2000;54(11):2108–2114. doi: 10.1212/wnl.54.11.2108. [DOI] [PubMed] [Google Scholar]
- 44.Launer LJ, Masaki K, Petrovich H, Foley D, Havlik RJ. The association between mid-life blood pressure levels and late-life cognitive function. The Honolulu-Asia Aging Study. JAMA. 1995;274(1846-1851) [PubMed] [Google Scholar]
- 45.DeCarli C, Miller BL, Swan GE, Reed T, Wolf PA, Carmelli D. Cerebrovascular and brain morphologic correlates of mild cognitive impairment in the National Heart, Lung, and Blood Institute Twin Study. Archives of Neurology. 2001;58(4):643–647. doi: 10.1001/archneur.58.4.643. [DOI] [PubMed] [Google Scholar]
- 46.Lopez OL, Jagust WJ, DeKosky ST, Becker JT, Fitzpatrick A, Dulberg C, Breitner J, Lyketsos C, Jones B, Kawas C, Carlson M, Kuller LH. Prevalence and classification of mild cognitive impairment in the cardiovascular health study cognition study: part 1. Arch Neurol. 2003;60(10):1385–1389. doi: 10.1001/archneur.60.10.1385. [DOI] [PubMed] [Google Scholar]
- 47.Lopez OL, Jagust WJ, Dulberg C, Becker JT, DeKosky ST, Fitzpatrick A, Breitner J, Lyketsos C, Jones B, Kawas C, Carlson M, Kuller LH. Risk factors for mild cognitive impairment in the cardiovascular health study cognition study: part 2. Arch Neurol. 2003;60(10):1394–1399. doi: 10.1001/archneur.60.10.1394. [DOI] [PubMed] [Google Scholar]
- 48.Carmelli D, DeCarli C, Swan GE, Jack LM, Reed T, Wolf PA, Miller BL. Evidence for genetic variance in white matter hyperintensity volume in normal elderly male twins. Stroke. 1998;29(6):1177–1181. doi: 10.1161/01.str.29.6.1177. [DOI] [PubMed] [Google Scholar]
- 49.Reed T, Kirkwood SC, DeCarli C, Swan GE, Miller BL, Wolf PA, Jack LM, Carmelli D. Relationship of family history scores for stroke and hypertension to quantitative measures of white-matter hyperintensities and stroke volume in elderly males. Neuroepidemiology. 2000;19(2):76–86. doi: 10.1159/000026242. [DOI] [PubMed] [Google Scholar]
- 50.Carmelli D, Reed T, DeCarli C. A bivariate genetic analysis of cerebral white matter hyperintensities and cognitive performance in elderly male twins. Neurobiol Aging. 2002;23(3):413–420. doi: 10.1016/s0197-4580(01)00336-0. [DOI] [PubMed] [Google Scholar]
- 51.Carmelli D, Swan GE, DeCarli C, Reed T. Quantitative genetic modeling of regional brain volumes and cognitive performance in older male twins. Biol Psychol. 2002;61(1-2):139–155. doi: 10.1016/s0301-0511(02)00056-x. [DOI] [PubMed] [Google Scholar]
- 52.Geschwind DH, Miller BL, DeCarli C, Carmelli D. Heritability of lobar brain volumes in twins supports genetic models of cerebral laterality and handedness. Proc Natl Acad Sci U S A. 2002;99(5):3176–3181. doi: 10.1073/pnas.052494999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lessov-Schlaggar CN, Hardin J, Decarli C, Krasnow RE, Reed T, Wolf PA, Swan GE, Carmelli D. Longitudinal genetic analysis of brain volumes in normal elderly male twins. Neurobiol Aging. 2012;33(4):636–644. doi: 10.1016/j.neurobiolaging.2010.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jeerakathil T, Wolf PA, Beiser A, Massaro J, Seshadri S, D'Agostino RB, DeCarli C. Stroke risk profile predicts white matter hyperintensity volume: the Framingham Study. Stroke. 2004;35(8):1857–1861. doi: 10.1161/01.STR.0000135226.53499.85. [DOI] [PubMed] [Google Scholar]
- 55.Seshadri S, Wolf PA, Beiser A, Elias MF, Au R, Kase CS, D'Agostino RB, DeCarli C. Stroke risk profile, brain volume, and cognitive function: the Framingham Offspring Study. Neurology. 2004;63(9):1591–1599. doi: 10.1212/01.wnl.0000142968.22691.70. [DOI] [PubMed] [Google Scholar]
- 56.Au R, Massaro JM, Wolf PA, Young ME, Beiser A, Seshadri S, D'Agostino RB, DeCarli C. Association of white matter hyperintensity volume with decreased cognitive functioning: the Framingham Heart Study. Arch Neurol. 2006;63(2):246–250. doi: 10.1001/archneur.63.2.246. [DOI] [PubMed] [Google Scholar]
- 57.Das RR, Seshadri S, Beiser AS, Kelly-Hayes M, Au R, Himali JJ, Kase CS, Benjamin EJ, Polak JF, O'Donnell CJ, Yoshita M, D'Agostino RB, Sr, DeCarli C, Wolf PA. Prevalence and correlates of silent cerebral infarcts in the Framingham offspring study. Stroke. 2008;39(11):2929–2935. doi: 10.1161/STROKEAHA.108.516575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Seshadri S, Wolf PA, Beiser AS, Selhub J, Au R, Jacques PF, Yoshita M, Rosenberg IH, D'Agostino RB, DeCarli C. Association of plasma total homocysteine levels with subclinical brain injury: cerebral volumes, white matter hyperintensity, and silent brain infarcts at volumetric magnetic resonance imaging in the Framingham Offspring Study. Arch Neurol. 2008;65(5):642–649. doi: 10.1001/archneur.65.5.642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lieb W, Beiser AS, Vasan RS, Tan ZS, Au R, Harris TB, Roubenoff R, Auerbach S, DeCarli C, Wolf PA, Seshadri S. Association of plasma leptin levels with incident Alzheimer disease and MRI measures of brain aging. Jama. 2009;302(23):2565–2572. doi: 10.1001/jama.2009.1836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Romero JR, Beiser A, Seshadri S, Benjamin EJ, Polak JF, Vasan RS, Au R, DeCarli C, Wolf PA. Carotid artery atherosclerosis, MRI indices of brain ischemia, aging, and cognitive impairment: the Framingham study. Stroke. 2009;40(5):1590–1596. doi: 10.1161/STROKEAHA.108.535245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Pikula A, Boger RH, Beiser AS, Maas R, DeCarli C, Schwedhelm E, Himali JJ, Schulze F, Au R, Kelly-Hayes M, Kase CS, Vasan RS, Wolf PA, Seshadri S. Association of plasma ADMA levels with MRI markers of vascular brain injury: Framingham offspring study. Stroke. 2009;40(9):2959–2964. doi: 10.1161/STROKEAHA.109.557116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Stavitsky K, Du Y, Seichepine D, Laudate TM, Beiser A, Seshadri S, Decarli C, Wolf PA, Au R. White matter hyperintensity and cognitive functioning in the racial and ethnic minority cohort of the Framingham Heart Study. Neuroepidemiology. 2010;35(2):117–122. doi: 10.1159/000313443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tan ZS, Beiser AS, Fox CS, Au R, Himali JJ, Debette S, Decarli C, Vasan RS, Wolf PA, Seshadri S. Association of metabolic dysregulation with volumetric brain magnetic resonance imaging and cognitive markers of subclinical brain aging in middleaged adults: the framingham offspring study. Diabetes Care. 2011;34(8):1766–1770. doi: 10.2337/dc11-0308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Debette S, Seshadri S, Beiser A, Au R, Himali JJ, Palumbo C, Wolf PA, Decarli C. Midlife vascular risk factor exposure accelerates structural brain aging and cognitive decline. Neurology. 2011;77(5):461–468. doi: 10.1212/WNL.0b013e318227b227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hebert LE, Scherr PA, Bienias JL, Bennett DA, Evans DA. Alzheimer disease in the US population: prevalence estimates using the 2000 census. Arch Neurol. 2003;60(8):1119–1122. doi: 10.1001/archneur.60.8.1119. [DOI] [PubMed] [Google Scholar]
- 66.Debette S, Beiser A, Decarli C, Au R, Himali JJ, Kelly-Hayes M, Romero JR, Kase CS, Wolf PA, Seshadri S. Association of MRI Markers of Vascular Brain Injury With Incident Stroke, Mild Cognitive Impairment, Dementia, and Mortality. The Framingham Offspring Study. Stroke. 2010 doi: 10.1161/STROKEAHA.109.570044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wright CB, Paik MC, Brown TR, Stabler SP, Allen RH, Sacco RL, Decarli C. Total Homocysteine Is Associated With White Matter Hyperintensity Volume. The Northern Manhattan Study. Stroke. 2005 doi: 10.1161/01.STR.0000165923.02318.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Khatri M, Wright CB, Nickolas TL, Yoshita M, Paik MC, Kranwinkel G, Sacco RL, DeCarli C. Chronic kidney disease is associated with white matter hyperintensity volume: the Northern Manhattan Study (NOMAS) Stroke. 2007;38(12):3121–3126. doi: 10.1161/STROKEAHA.107.493593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Brickman AM, Schupf N, Manly JJ, Luchsinger JA, Andrews H, Tang MX, Reitz C, Small SA, Mayeux R, DeCarli C, Brown TR. Brain morphology in older African Americans, Caribbean Hispanics, and whites from northern Manhattan. Arch Neurol. 2008;65(8):1053–1061. doi: 10.1001/archneur.65.8.1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Devanand DP, Tabert MH, Cuasay K, Manly JJ, Schupf N, Brickman AM, Andrews H, Brown TR, Decarli C, Mayeux R. Olfactory identification deficits and MCI in a multi-ethnic elderly community sample. Neurobiol Aging. 2008 doi: 10.1016/j.neurobiolaging.2008.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Prabhakaran S, Wright CB, Yoshita M, Delapaz R, Brown T, DeCarli C, Sacco RL. Prevalence and determinants of subclinical brain infarction: the Northern Manhattan Study. Neurology. 2008;70(6):425–430. doi: 10.1212/01.wnl.0000277521.66947.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wright CB, Festa JR, Paik MC, Schmiedigen A, Brown TR, Yoshita M, DeCarli C, Sacco R, Stern Y. White matter hyperintensities and subclinical infarction: associations with psychomotor speed and cognitive flexibility. Stroke. 2008;39(3):800–805. doi: 10.1161/STROKEAHA.107.484147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wright CB, Moon Y, Paik MC, Brown TR, Rabbani L, Yoshita M, DeCarli C, Sacco R, Elkind MS. Inflammatory biomarkers of vascular risk as correlates of leukoariosis. Stroke. 2009;40(11):3466–3471. doi: 10.1161/STROKEAHA.109.559567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Brickman AM, Siedlecki KL, Muraskin J, Manly JJ, Luchsinger JA, Yeung LK, Brown TR, Decarli C, Stern Y. White matter hyperintensities and cognition: Testing the reserve hypothesis. Neurobiol Aging. 2009 doi: 10.1016/j.neurobiolaging.2009.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Luchsinger JA, Brickman AM, Reitz C, Cho SJ, Schupf N, Manly JJ, Tang MX, Small SA, Mayeux R, DeCarli C, Brown TR. Subclinical cerebrovascular disease in mild cognitive impairment. Neurology. 2009;73(6):450–456. doi: 10.1212/WNL.0b013e3181b1636a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Reitz C, Brickman AM, Brown TR, Manly J, DeCarli C, Small SA, Mayeux R. Linking hippocampal structure and function to memory performance in an aging population. Arch Neurol. 2009;66(11):1385–1392. doi: 10.1001/archneurol.2009.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Aggarwal NT, Wilson RS, Bienias JL, De Jager PL, Bennett DA, Evans DA, DeCarli C. The association of magnetic resonance imaging measures with cognitive function in a biracial population sample. Arch Neurol. 2010;67(4):475–482. doi: 10.1001/archneurol.2010.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Brickman AM, Reitz C, Luchsinger JA, Manly JJ, Schupf N, Muraskin J, DeCarli C, Brown TR, Mayeux R. Long-term blood pressure fluctuation and cerebrovascular disease in an elderly cohort. Arch Neurol. 2010;67(5):564–569. doi: 10.1001/archneurol.2010.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Devanand DP, Tabert MH, Cuasay K, Manly JJ, Schupf N, Brickman AM, Andrews H, Brown TR, DeCarli C, Mayeux R. Olfactory identification deficits and MCI in a multi-ethnic elderly community sample. Neurobiol Aging. 2010;31(9):1593–1600. doi: 10.1016/j.neurobiolaging.2008.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Brickman AM, Siedlecki KL, Muraskin J, Manly JJ, Luchsinger JA, Yeung LK, Brown TR, Decarli C, Stern Y. White matter hyperintensities and cognition: Testing the reserve hypothesis. Neurobiol Aging. 2011;32(9):1588–1598. doi: 10.1016/j.neurobiolaging.2009.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Marcus J, Gardener H, Rundek T, Elkind MS, Sacco RL, Decarli C, Wright CB. Baseline and Longitudinal Increases in Diastolic Blood Pressure Are Associated With Greater White Matter Hyperintensity Volume: The Northern Manhattan Study. Stroke. 2011 doi: 10.1161/STROKEAHA.111.617571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Scarmeas N, Luchsinger JA, Stern Y, Gu Y, He J, DeCarli C, Brown T, Brickman AM. Mediterranean diet and magnetic resonance imaging-assessed cerebrovascular disease. Ann Neurol. 2011;69(2):257–268. doi: 10.1002/ana.22317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Willey JZ, Moon YP, Paik MC, Yoshita M, Decarli C, Sacco RL, Elkind MS, Wright CB. Lower prevalence of silent brain infarcts in the physically active: the Northern Manhattan Study. Neurology. 2011;76(24):2112–2118. doi: 10.1212/WNL.0b013e31821f4472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Hudson BI, Moon YP, Kalea AZ, Khatri M, Marquez C, Schmidt AM, Paik MC, Yoshita M, Sacco RL, DeCarli C, Wright CB, Elkind MS. Association of serum soluble receptor for advanced glycation end-products with subclinical cerebrovascular disease: the Northern Manhattan Study (NOMAS) Atherosclerosis. 2011;216(1):192–198. doi: 10.1016/j.atherosclerosis.2011.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.DeCarli C, Reed BR, Jagust W, Martinez O, Ortega M, Mungas D. Brain behavior relationships among African Americans, whites, and Hispanics. Alzheimer Dis Assoc Disord. 2008;22(4):382–391. doi: 10.1097/wad.0b013e318185e7fe. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Sacco RL, Boden-Albala B, Gan R, Chen X, Kargman DE, Shea S, Paik MC, Hauser WA. Stroke incidence among white, black, and Hispanic residents of an urban community: the Northern Manhattan Stroke Study. Am J Epidemiol. 1998;147(3):259–268. doi: 10.1093/oxfordjournals.aje.a009445. [DOI] [PubMed] [Google Scholar]
- 87.Manley JJ, M R. Ethnic and Racial Differences in Dementia and Alzheimer's Disease. In: NB A, Bulatao RA, Cohen B, editors. Critical Perspectives on Racial and Ethnic Differences in Health in Late Life. Washington D.C.: The National Academies Press; 2004. pp. 94–141. [PubMed] [Google Scholar]
- 88.DeCarli C, Fletcher E, Ramey V, Harvey D, Jagust WJ. Anatomical mapping of white matter hyperintensities (WMH): exploring the relationships between periventricular WMH, deep WMH, and total WMH burden. Stroke. 2005;36(1):50–55. doi: 10.1161/01.STR.0000150668.58689.f2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Tullberg M, Fletcher E, DeCarli C, Mungas D, Reed BR, Harvey DJ, Weiner MW, Chui HC, Jagust WJ. White matter lesions impair frontal lobe function regardless of their location. Neurology. 2004;63(2):246–253. doi: 10.1212/01.wnl.0000130530.55104.b5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Yoshita M, Fletcher E, Harvey D, Ortega M, Martinez O, Mungas DM, Reed BR, DeCarli CS. Extent and distribution of white matter hyperintensities in normal aging, MCI, and AD. Neurology. 2006;67(12):2192–2198. doi: 10.1212/01.wnl.0000249119.95747.1f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Nordahl CW, Ranganath C, Yonelinas AP, DeCarli C, Reed BR, Jagust WJ. Different mechanisms of episodic memory failure in mild cognitive impairment. Neuropsychologia. 2005;43(11):1688–1697. doi: 10.1016/j.neuropsychologia.2005.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.He J, Farias S, Martinez O, Reed B, Mungas D, Decarli C. Differences in brain volume, hippocampal volume, cerebrovascular risk factors, and apolipoprotein E4 among mild cognitive impairment subtypes. Arch Neurol. 2009;66(11):1393–1399. doi: 10.1001/archneurol.2009.252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Carmichael O, Mungas D, Beckett L, Harvey D, Tomaszewski Farias S, Reed B, Olichney J, Miller J, Decarli C. MRI predictors of cognitive change in a diverse and carefully characterized elderly population. Neurobiol Aging. 2010 doi: 10.1016/j.neurobiolaging.2010.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Carmichael O, Schwarz C, Drucker D, Fletcher E, Harvey D, Beckett L, Jack CRJ, Weiner M, DeCarli C, Initiative tADN. Longitudinal Changes in White Matter Disease and Cognition in the First Year of the Alzheimers Disease Neuroimaging Initiative. Arch Neruol. 2010 doi: 10.1001/archneurol.2010.284. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Lee DY, Fletcher E, Martinez O, Ortega M, Zozulya N, Kim J, Tran J, Buonocore M, Carmichael O, Decarli C. Regional pattern of white matter microstructural changes in normal aging, MCI, and AD. Neurology. 2009;73(21):1722–1728. doi: 10.1212/WNL.0b013e3181c33afb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Lee DY, Fletcher E, Martinez O, Zozulya N, Kim J, Tran J, Buonocore M, Carmichael O, DeCarli C. Vascular and degenerative processes differentially affect regional interhemispheric connections in normal aging, mild cognitive impairment, and Alzheimer’s disease. Stroke. 2010 doi: 10.1161/STROKEAHA.110.582163. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Maillard P, Fletcher E, Harvey D, Carmichael O, Reed B, Mungas D, DeCarli C. White matter hyperintensity penumbra. Stroke. 2011;42(7):1917–1922. doi: 10.1161/STROKEAHA.110.609768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Nordahl CW, Ranganath C, Yonelinas AP, Decarli C, Fletcher E, Jagust WJ. White matter changes compromise prefrontal cortex function in healthy elderly individuals. J Cogn Neurosci. 2006;18(3):418–429. doi: 10.1162/089892906775990552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Mayda AB, Westphal A, Carter CS, DeCarli C. Late life cognitive control deficits are accentuated by white matter disease burden. Brain. 2011;134(Pt 6):1673–1683. doi: 10.1093/brain/awr065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Vannini P, O'Brien J, O'Keefe K, Pihlajamaki M, Laviolette P, Sperling RA. What goes down must come up: role of the posteromedial cortices in encoding and retrieval. Cereb Cortex. 2011;21(1):22–34. doi: 10.1093/cercor/bhq051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.West RL. An application of prefrontal cortex function theory to cognitive aging. Psychol Bull. 1996;120(2):272–292. doi: 10.1037/0033-2909.120.2.272. [DOI] [PubMed] [Google Scholar]
- 102.Seshadri S, DeStefano AL, Au R, Massaro JM, Beiser AS, Kelly-Hayes M, Kase CS, D'Agostino RB, Sr, Decarli C, Atwood LD, Wolf PA. Genetic correlates of brain aging on MRI and cognitive test measures: a genome-wide association and linkage analysis in the Framingham Study. BMC Med Genet. 2007;8(Suppl 1):S15. doi: 10.1186/1471-2350-8-S1-S15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Atwood LD, Wolf PA, Heard-Costa NL, Massaro JM, Beiser A, D'Agostino RB, DeCarli C. Genetic variation in white matter hyperintensity volume in the Framingham Study. Stroke. 2004;35(7):1609–1613. doi: 10.1161/01.STR.0000129643.77045.10. [DOI] [PubMed] [Google Scholar]
- 104.DeStefano AL, Atwood LD, Massaro JM, Heard-Costa N, Beiser A, Au R, Wolf PA, DeCarli C. Genome-wide scan for white matter hyperintensity: the Framingham Heart Study. Stroke. 2006;37(1):77–81. doi: 10.1161/01.STR.0000196987.68770.b3. [DOI] [PubMed] [Google Scholar]
- 105.Cuenco TK, Lunetta KL, Baldwin CT, McKee AC, Guo J, Cupples LA, Green RC, St George-Hyslop PH, Chui H, DeCarli C, Farrer LA. Association of distinct variants in SORL1 with cerebrovascular and neurodegenerative changes related to Alzheimer disease. Arch Neurol. 2008;65(12):1640–1648. doi: 10.1001/archneur.65.12.1640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Debette S, Bis JC, Fornage M, Schmidt H, Ikram MA, Sigurdsson S, Heiss G, Struchalin M, Smith AV, van der Lugt A, DeCarli C, Lumley T, Knopman DS, Enzinger C, Eiriksdottir G, Koudstaal PJ, DeStefano AL, Psaty BM, Dufouil C, Catellier DJ, Fazekas F, Aspelund T, Aulchenko YS, Beiser A, Rotter JI, Tzourio C, Shibata DK, Tscherner M, Harris TB, Rivadeneira F, Atwood LD, Rice K, Gottesman RF, van Buchem MA, Uitterlinden AG, Kelly-Hayes M, Cushman M, Zhu Y, Boerwinkle E, Gudnason V, Hofman A, Romero JR, Lopez O, van Duijn CM, Au R, Heckbert SR, Wolf PA, Mosley TH, Seshadri S, Breteler MM, Schmidt R, Launer LJ, Longstreth WT., Jr Genome-wide association studies of MRI-defined brain infarcts: meta-analysis from the CHARGE Consortium. Stroke. 2010;41(2):210–217. doi: 10.1161/STROKEAHA.109.569194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Fornage M, Debette S, Bis JC, Schmidt H, Ikram MA, Dufouil C, Sigurdsson S, Lumley T, DeStefano AL, Fazekas F, Vrooman HA, Shibata DK, Maillard P, Zijdenbos A, Smith AV, Gudnason H, de Boer R, Cushman M, Mazoyer B, Heiss G, Vernooij MW, Enzinger C, Glazer NL, Beiser A, Knopman DS, Cavalieri M, Niessen WJ, Harris TB, Petrovic K, Lopez OL, Au R, Lambert JC, Hofman A, Gottesman RF, Garcia M, Heckbert SR, Atwood LD, Catellier DJ, Uitterlinden AG, Yang Q, Smith NL, Aspelund T, Romero JR, Rice K, Taylor KD, Nalls MA, Rotter JI, Sharrett R, van Duijn CM, Amouyel P, Wolf PA, Gudnason V, van der Lugt A, Boerwinkle E, Psaty BM, Seshadri S, Tzourio C, Breteler MM, Mosley TH, Schmidt R, Longstreth WT, DeCarli C, Launer LJ. Genome-wide association studies of cerebral white matter lesion burden: the CHARGE consortium. Ann Neurol. 2011;69(6):928–939. doi: 10.1002/ana.22403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Hanon O, Forette F. Prevention of dementia: lessons from SYST-EUR and PROGRESS. J Neurol Sci. 2004;226(1-2):71–74. doi: 10.1016/j.jns.2004.09.015. [DOI] [PubMed] [Google Scholar]
- 109.Lytle LA. Dealing with the childhood obesity epidemic: a public health approach. Abdom Imaging. 2012 doi: 10.1007/s00261-012-9861-y. [DOI] [PubMed] [Google Scholar]
- 110.Whitmer RA, Gunderson EP, Barrett-Connor E, Quesenberry CP, Jr, Yaffe K. Obesity in middle age and future risk of dementia: a 27 year longitudinal population based study. Bmj. 2005;330(7504):1360. doi: 10.1136/bmj.38446.466238.E0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Brookmeyer R, Gray S, Kawas C. Projections of Alzheimer's disease in the United States and the public health impact of delaying disease onset. Am J Public Health. 1998;88(9):1337–1342. doi: 10.2105/ajph.88.9.1337. [DOI] [PMC free article] [PubMed] [Google Scholar]