Abstract
It is well-known that stress can significantly impact learning; however, whether this effect facilitates or impairs the resultant memory depends on the characteristics of the stressor. Investigation of these dynamics can be confounded by the role of the stressor in motivating performance in a task. Positing a cohesive model of the effect of stress on learning and memory necessitates elucidating the consequences of stressful stimuli independently from task-specific functions. Therefore, the goal of this study was to examine the effect of manipulating a task-independent stressor (elevated light level) on short-term and long-term memory in the novel object recognition paradigm. Short-term memory was elicited in both low light and high light conditions, but long-term memory specifically required high light conditions during the acquisition phase (familiarization trial) and was independent of the light level during retrieval (test trial). Additionally, long-term memory appeared to be independent of stress-mediated glucocorticoid release, as both low and high light produced similar levels of plasma corticosterone, which further did not correlate with subsequent memory performance. Finally, both short-term and long-term memory showed no savings between repeated experiments suggesting that this novel object recognition paradigm may be useful for longitudinal studies, particularly when investigating treatments to stabilize or enhance weak memories in neurodegenerative diseases or during age-related cognitive decline.
Keywords: Corticosterone, memory consolidation, novel object recognition, longitudinal design, mice
1. Introduction
In humans, many factors impact the ability to acquire, consolidate, or retrieve memories, including the attention, motivation, anxiety or stress of the subject during the relevant event or experience (McGaugh, 2013). In rodents, however, it is difficult to precisely define or measure these (and other) psychological constructs; instead investigators must often rely on the manipulation of an external variable (such as an environmental parameter) and the measurement of an indirect output (such as behavioral performance). To further complicate matters, the effect of altering external stimuli on learning and memory is not straight-forward: several distinct characteristics including the duration, intensity, and learning phase in which it occurs (for example, consolidation versus retrieval) can all affect whether the resulting memory is enhanced or degraded.
Emotional arousal due to stress has been extensively studied in rodents and thus represents a useful framework in which to examine the complex interplay between different factors of emotionally arousing stimuli. Interestingly, stress has been shown to result in both facilitation and impairment of memory (Bartolomucci, de Biurrun, Czeh, van Kampen, and Fuchs, 2002; Conrad, LeDoux, Magarinos, and McEwen, 1999; Diamond, Park, Heman, and Rose, 1999; Holscher, 1999; Luine, Martinez, Villegas, Magarinos, and McEwen, 1996; Luine, Villegas, Martinez, and McEwen, 1994; Mather, 2007; Miracle, Brace, Huyck, Singler, and Wellman, 2006; Nishimura, Endo, and Kimura, 1999; Sandi, Loscertales, and Guaza, 1997; Shors, 2001; Song, Che, Min-Wei, Murakami, and Matsumoto, 2006). Several recent reviews (Joels, Pu, Wiegert, Oitzl, and Krugers, 2006; Kim and Diamond, 2002; Sandi and Pinelo-Nava, 2007) have helped considerably in reconciling these seemingly contradictory results by summarizing important characteristics that need to be taken into account when evaluating the effect of stress on learning and memory. For example, stress differentially affects particular types of memory: stress has been shown to simultaneously facilitate memory for emotionally arousing events, but impair memory for neutral events (Payne, Jackson, Hoscheidt, Ryan, Jacobs, and Nadel, 2007). Finally, there is a complex relationship (often referred to as an “inverted-U-shape”) between the intensity of a stressor and the effect on learning and memory: animals that experienced moderate stress (cooler water temperature) during spatial learning in the Morris water maze exhibited better memory than animals that experienced a less stressful condition (warmer water temperature) (Sandi et al., 1997). However, if the water temperature was lowered further (representing a more intense stressor), animals did not exhibit a corresponding enhancement of memory; in fact, memory was significantly impaired relative to the moderate stress group (Salehi, Cordero, and Sandi, 2010).
Exposure to bright light in an open area is thought to be stressful to rodents and produces anxiety-like behavior (Bert, Felicio, Fink, and Nasello, 2005); further, previous work has shown that altering light levels can disrupt learning and memory (Huang, Zhou, and Zhang, 2012; Pico and Davis, 1984; Roedel, Storch, Holsboer, and Ohl, 2006). However, we postulated that modulating light level may also be able to facilitate learning and memory, similar to the bidirectional effect observed with other stressors. In order to test this hypothesis, it was critical to choose a paradigm in which performance is not aversively motivated (for example, by shock delivery or water temperature, which are inherently stressful themselves). The novel object recognition paradigm is ideal for this purpose because it takes advantage of a rodent’s intrinsic exploratory drive and, at least below some threshold, ambient light level does not significantly impair exploration (Bats, Thoumas, Lordi, Tonon, Lalonde, and Caston, 2001). To perform this task, animals are first allowed to explore two identical objects during a familiarization trial. After a delay period (which can be varied to investigate short-term or long-term memory), they are exposed to one copy of the original object (“familiar”) and a new object (“novel”) in a test trial. Because rodents have an inherent preference for novelty, memory for the object from the familiarization trial is inferred if significantly more time is spent exploring the novel object relative to the familiar one (they must be able to remember the previously encountered familiar object to determine which object is “novel” during the test trial) (Bevins and Besheer, 2006; Dere, Huston, and De Souza Silva, 2007; Ennaceur, 2010).
Thus, by manipulating light levels during novel object recognition, we were able to examine the effect of modulating this emotionally arousing stimulus on learning and memory. We show that short-term memory could be reliably elicited regardless of light level, while long-term memory required elevated light levels during the familiarization trial and could not be elicited even with multiple familiarization sessions under low light conditions. Importantly, the light level during the test trial (when memory was being assessed) did not impact performance, suggesting that it was the formation of long-term memory (during the familiarization trial) that was critically dependent on the effects of elevated light. Interestingly, both low and high light conditions during familiarization produced significant elevations in plasma corticosterone concentration compared to baseline, but the formation of long-term memory did not correlate with corticosterone level. In combination with previous work, which reported memory impairments produced by modulating light levels (Huang et al., 2012), our results demonstrate that light level can bidirectionally modulate learning and serve to strengthen the information encoded such that a weak, short-term memory is converted into a robust, long-term memory.
2. Materials and methods
2.1 Animals
Stock C57BL/6 mice were obtained from Taconic Farms (Cambridge City, IN). To eliminate potential sex-related confounds in the interpretation of our results, only male mice were used for these experiments. Mice were group-housed in cages of 3-5 animals, maintained on a 14:10-hour light:dark cycle with ad libitum access to food and water. All procedures were performed in accordance with the University of Michigan Animal Care and Use Committee.
2.2 Novel object recognition
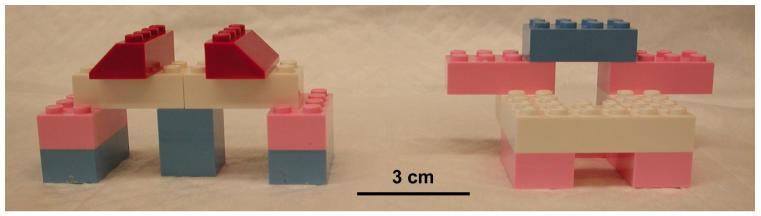
The arena used for all trials was a 17-gallon circular container made of white polyethylene, 42 cm high and 44.5 cm in diameter (Chem-Trainer, West Babylon, NY). The first day of each experiment consisted of 2-3 habituation trials (5 minutes each, 15-20 minutes apart) during which mice were exposed to the arena alone (no objects) in the training room. Twenty-four hours later, the experimental trials began, which consisted of a familiarization phase and a test phase separated by a variable delay period. During the familiarization phase (which consisted of 1 or 3 individual trials, as indicated), mice were placed in the arena which contained two copies of an object and allowed to freely explore (5 minutes per trial). After either a short (2 minutes) or long (24 hours) delay period, a test trial (5 minutes) was conducted; mice were returned to the arena which contained one of the original objects (“familiar”) and a new, different object (“novel”). The objects used in all experiments were custom made in-house from LEGOs® (see Fig. 1). These objects had been previously validated to ensure they would elicit substantial exploration (at least 30 seconds, on average) and that there was no inherent preference for either object. The object assignments (familiar or novel) and locations (left or right side of the arena) were counterbalanced within each experiment, as well as within subject for subsequent experiments. Objects were placed in the center of the arena approximately 10 cm from the arena wall and held in place with adhesive tack (such as Blu-Tack®). The arena and objects were cleaned between each trial with 70% ethanol. For all trials, background white noise (approximately 66 dB) was provided by an air purifier. The room was illuminated by indirect white light, the level of which (measured in the center of the arena) was defined as “low” (range: 2.7-3.3 lux) or “high” (range: 20.9-22.2 lux) as indicated for each experiment (all habituation trials were always conducted in low light). It should be noted here that these are relative terms; ambient light levels in our animal housing room are typically 400-500 lux. Therefore, the “high” light level in our experiments should be considered moderate in a general context, and the terms “low” or ~3 lux and “high” or ~21 lux are used for clarity in the text.
Figure 1. Objects used in novel object recognition experiments.
Objects were custom built in-house from Legos®. Pilot tests showed that objects which evoked the highest levels of exploration had two main features in common: 1) they were not significantly larger than the mouse (approximately 9 cm long, 4 cm wide, and 4 cm high); and 2) they contained multiple crevices amenable to nose-poke investigation. The objects shown were selected for use in subsequent experimental trials because they elicited relatively high exploration (at least 30 seconds total, on average) and there was no inherent preference for one object compared to the other.
Corticosterone assay
In a separate experiment, corticosterone (CORT) levels were also measured in mice that performed novel object recognition. Because CORT levels are generally lowest at the beginning of the light phase (Malisch, Breuner, Gomes, Chappell, and Garland, 2008; Ottenweller, Meier, Russo, and Frenzke, 1979), all experiments involving CORT (see Figure 9) were performed from 6 am to 10 am. Blood samples were collected from each mouse (via tail-vein bleed) at the following points: 1) two weeks prior to the novel object recognition experiment (to establish a baseline level); and 2) twenty minutes after the familiarization trial. The 20-minute post-familiarization time point was selected because pilot studies indicated that CORT levels peaked approximately 15-30 minutes after the “stressful” experience (being placed in the arena with the objects during the familiarization trial) before declining towards baseline levels. For the baseline measurement, mice were brought into the testing room in their home cages and then individually transferred to a plastic restrainer where tail-vein blood was collected (in low light conditions for all mice). For the post-familiarization measurement, mice were returned to their home cage after completing the 5-minute familiarization trial; 20 minutes later, each mouse was individually transferred to the plastic restrainer where tail-vein blood was again collected (in either low light or high light conditions, corresponding to the condition experienced during the familiarization trial). CORT levels were then assessed by radioimmunoassay using an ImmuChem 125I Corticosterone RIA kit (MP Biomedicals, Orangeburg, NY) according to manufacturer directions and as previously described (Burrows, Nakajima, Lesh, Goosens, Samuelson, Inui, Camper, and Seasholtz, 1998).
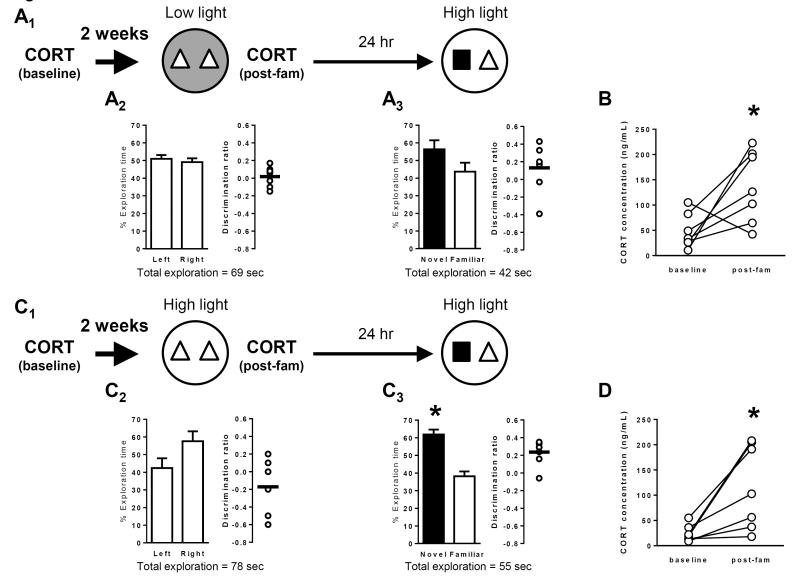
Figure 9. Long-term memory under high light conditions is not due to differential regulation of corticosterone.
Schematics depict experimental procedure, including collection of blood samples for subsequent corticosterone (CORT) measurements and conditions for novel object recognition. Dark grey arenas denote low light (3 lux) conditions and white arenas denote high light (21 lux) conditions. Bar graphs show exploration time (as a percent of the total) for each object (mean ± SEM; n = 7 (A) and n = 7 (B); * p < 0.05). Total exploration time for each trial is indicated below the graphs. Scatter dot plots show individual discrimination ratios (open circles) and the group mean (horizontal line); positive values (>0) reflect a preference for the left-side object (familiarization trial) or the novel object (test trial). Corresponding matched-pair plots show the baseline and post-familiarization (post-fam) CORT levels for each mouse in the experiment. (A) Long-term memory was assessed using a 24-hour delay between the familiarization trial, conducted in low light, and the test trial, conducted in high light (A1). There was no preference for object location (left or right side) during the familiarization trial (A2). Further, mice failed to exhibit a significant preference for the novel object during the test trial 24 hours later (A3), indicating an impairment of long-term memory for the familiar object. (B) Although mice in the low-light familiarization group failed to exhibit long-term memory for the familiar object, CORT levels after the familiarization trial were significantly increased relative to baseline. (C) Long-term memory was also assessed in a second group of mice with a 24-hour delay, except that the familiarization trial was conducted in high light (as before, the test trial was also conducted in high light; C1). Again, there was no preference for object location (left or right side) during the familiarization trial (C2). However, during the test trial 24 hours later, mice exhibited a preference for the novel object (C3), indicative of the formation of a long-term memory for the familiar object. (D) Experience in the familiarization trial under high light conditions also resulted in a significant increase in CORT levels compared to baseline. However, this level was not different between mice in the two groups (low light group, 134.4 ± 27 ng/mL; high light group, 117 ± 31 ng/mL; p = 0.65).
2.3 Analysis and Statistics
All trials were recorded with a CCD camera controlled by Limelight software (Actimetrics, Evanston, IL) and stored on a Dell computer. Behavior was hand-scored for all trials by a trained observer (substantiated for randomly selected trials by a second trained observer). Object exploration was defined as any time the mouse was within 2 cm and oriented towards the object or climbing on the object. Mice that did not have at least 10 seconds of total exploration on both the familiarization and test trials in a given experiment were excluded from subsequent analysis for that Data for novel object recognition are presented in two ways: 1) the percent exploration for each object (mean ±SEM), which was calculated as time spent exploring an object divided by total time spent exploring both objects together; and 2) a discrimination ratio between objects (individual points are plotted for each animal as well as the group mean), which was calculated as the difference in time spent exploring the objects divided by the total time spent exploring both objects together. For familiarization trials in which two copies of the same object were placed in the arena, the discrimination ratio was calculated for the left versus right object (positive discrimination ratios reflect a preference for the left-hand object); for test trials, the discrimination ratio was calculated for the novel versus familiar object (positive discrimination reflect a preference for the novel object). Data for CORT measurements are presented as match-pair plots (from baseline to post-familiarization) for individual mice. A paired Student’s t-test was used to determine significance within experiment for percent exploration or CORT concentration; a one-sample t-test versus a hypothetical value of 0 (indicative of chance performance with no preference for either object) was used to determine significance for the discrimination ratio. For comparisons between experiments, an unpaired Student’s t-test was performed on the discrimination ratios or CORT concentrations. To determine correlation between two parameters (either exploration time versus discrimination ratio or CORT concentration versus discrimination ratio), linear regression was used to find the best-fit line through all data points. The slope of this line was tested against a hypothetical line with a slope of 0 (no correlation). The alpha value for all statistical analyses was set at 0.05. Statistical calculations were performed with Prism5 software (GraphPad, La Jolla, CA).
3. Results
3.1 Low light conditions support the formation of short-term memory, which is unaffected by previous experience
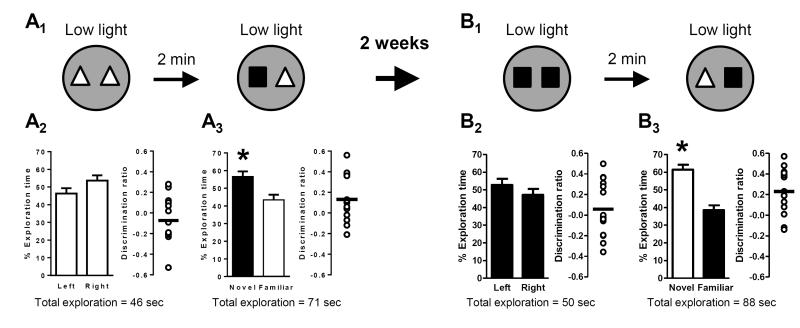
Naïve mice were first exposed to two identical objects in a familiarization trial under low light conditions (Fig. 2A1). During this trial, mice explored both objects equally, demonstrating no preference for the object on either side of the arena (Fig. 2A2). After a short (2-minute) delay, mice were returned to the arena for the test trial, again in low light conditions, where they were exposed to one of the same objects from the familiarization trial and one novel object. Mice preferentially explored the novel object, indicating that they had formed a short-term memory for familiar object (Fig. 2A3). After two weeks, this cohort of mice was tested in a second experiment under the same delay and light conditions (Fig. 2B1). Importantly, however, the identities of the familiar and novel objects were counterbalanced within subjects relative to the first experiment (the object previously assigned as “familiar” was assigned as “novel”). This experimental design tests whether short-term memory in the novel object recognition paradigm is subject to “savings” (that is, carry-over of information from a previous experience that can influence learning and memory during a subsequent experience): at the beginning of second test trial, mice had been exposed to both objects for the same amount of time (taking into account the familiarization trial in the first experiment, the test trial in the first experiment, and the familiarization trial in the second experiment). Therefore, if there were savings from the first experiment, both objects should have been equally familiar and mice should have had no preference for either object. On the other hand, if there were not savings, the second experiment should have been functionally independent; memory in the test trial would then have depended only on the immediately preceding familiarization trial and a preference for the object assigned as “novel” in the second experiment should have been exhibited. Again, there was no side preference for either object during the second familiarization trial (Fig. 2B2). After a short (2-minute) delay, mice exhibited a significant preference for the “novel” (as assigned for the second experiment) object during the test trial (Fig. 2B3), consistent with the formation of an independent short-term memory, with no savings from previous object exposure.
Figure 2. Low light conditions support the formation of short-term memory, which is independent of experience in a previous experiment.
Schematics depict experimental procedure; dark grey arenas denote low light (3 lux) conditions. Bar graphs show exploration time (as a percent of the total) for each object (mean ± SEM; n = 14 (A) or n = 15 (B); * p < 0.05). Total exploration time for each trial is indicated below the graphs. Scatter dot plots show individual discrimination ratios (open circles) and the group mean (horizontal line); positive values (>0) reflect a preference for the left-side object (familiarization trial) or the novel object (test trial). (A) In the first experiment, short-term memory in low light conditions was assessed using a 2-minute delay between the familiarization and test trials (A1). No preference for object location (left or right side) was exhibited during the familiarization trial (A2) but mice exhibited a significant preference for the novel object 2 minutes later during the test trial (A3), indicative of short-term memory for the familiar object. (B) After a 2-week interval, the experiment was repeated using the same delay and light conditions (B1). Importantly, however, the object assignments (familiar or novel) and locations (left or right side) were counterbalanced within subjects relative to the first experiment. Again there was no preference for object location (left or right side) during the familiarization trial (B2). During the test trial 2 minutes later, mice exhibited a significant preference for the novel object (B3), indicating that they formed a new short-term memory for the most recently experienced familiar object with no indication of savings from the previous exposure.
3.2 Long-term memory is not elicited under low light conditions
Next, we examined whether mice could also form long-term memory under low light conditions. Mice were assigned to one of two groups: 1) a short-term memory group (Fig. 3A1) that experienced a 2-minute delay between the familiarization and test trials (both in low light); or 2) a long-term memory group (Fig. 3B1) that experienced a 24-hour delay between the familiarization and test trials (both in low light). Neither group exhibited a side preference during the familiarization trial (Fig. 3A2 and 3B2). Similar to the previous experiment (compare to Fig. 2A3 and 2B3), mice in the 2-minute delay group exhibited a significant preference for the novel object during the test trial (Fig. 3A3), indicating that they formed a short-term memory for the familiar object. Conversely, mice in the 24-hour delay group failed to exhibit a preference for the novel object (Fig. 3B3), indicating that the formation of a long-term memory for the familiar object was impaired.
Figure 3. Long-term memory, unlike short-term memory, is impaired in low light conditions.
Schematics depict experimental procedure; dark grey arenas denote low light (3 lux) conditions. Bar graphs show exploration time (as a percent of the total) for each object (mean ± SEM; n = 15; * p < 0.05). Total exploration time for each trial is indicated below the graphs. Scatter dot plots show individual discrimination ratios (open circles) and the group mean (horizontal line); positive values (>0) reflect a preference for the left-side object (familiarization trial) or the novel object (test trial). (A) Short-term memory in low light conditions was assessed using a 2-minute delay between the familiarization and test trials (A1). There was no preference for object location (left or right side) during the familiarization trial (A2) but mice exhibited a significant preference for the novel object in the test trial 2 minutes later (A3), indicative of short-term memory for the familiar object. (B) Long-term memory in low light conditions was assessed using a 24-hour delay between the familiarization and test trials (B1). No preference for object location (left or right side) was exhibited during the familiarization trial (B2). Furthermore, mice failed to exhibit a preference for the novel object during the test trial 24 hours later (B3), indicating an impairment in long-term memory for the familiar object.
3.3 Further exposure to the familiar objects under low light conditions does not rescue long-term memory formation
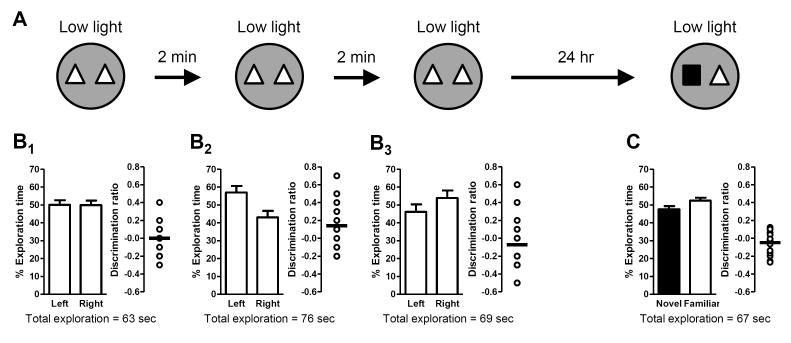
Extended or additional training can facilitate memory formation (Rescorla, 1988), and multiple familiarization trials have been used to increase learning in a spatial recognition task in mice (Oliveira, Hawk, Abel, and Havekes, 2010) and a novel object recognition task in rats (Albasser, Chapman, Amin, Iordanova, Vann, and Aggleton, 2010). Therefore, we investigated whether multiple exposures to the familiar objects could facilitate the formation of long-term memory under low light conditions. Initially, we modeled the experimental design on a massed training method; mice were exposed to the same pair of identical objects in three sequential familiarization trials (with a 2-minute break between trials during which mice were removed from the arena and returned to their home cage; Fig. 4A). There was no preference for the object on either side of the arena during any of the familiarization trials (Fig. 4B1-3). After a 24-hour delay, mice still failed to exhibit a preference for the novel object during the test trial (Fig. 4C), indicating that the increased exposure to the familiar objects was not sufficient to elicit long-term memory.
Figure 4. Massed training does not rescue long-term memory in low light conditions.
Schematic depicts experimental procedure; dark grey arenas denote low light (3 lux) conditions. Bar graphs show exploration time (as a percent of the total) for each object (mean ± SEM; n = 15). Total exploration time for each trial is indicated below the graphs. Scatter dot plots show individual discrimination ratios (open circles) and the group mean (horizontal line); positive values (>0) reflect a preference for the left-side object (familiarization trial) or the novel object (test trial). (A) Long-term memory in low light conditions was assessed 24 hours after mice were exposed to three successive familiarization trials (2 minutes apart; massed training). (B) There was no preference for object location (left or right side) in any of the familiarization trials (B1-3). (C) During the test trial (24 hours after the last familiarization trial), mice failed to exhibit a preference for the novel object, indicating that increased exposure to the familiar object did not facilitate formation of long-term memory.
Spaced training (multiple trials spread out over hours or days) has been shown to be more effective than massed training for facilitating memory (Fanselow and Tighe, 1988; Lattal, 1999). Thus, we next examined whether repeated familiarization trials, spaced 24 hours apart, would be effective in eliciting long-term memory under low light conditions. Mice were exposed to the same pair of identical objects in one familiarization trial each day for three successive days (Fig. 5A). In all familiarization trials, mice explored both objects equally, exhibiting no preference for the object located on either side of the arena (Fig. 5B1-3). Twenty-four hours after the final familiarization trial, a test trial was conducted. Again, mice failed to exhibit a preference for the novel object during the test trial (Fig. 5C), indicating that this additional exposure to the familiar objects was also not sufficient to elicit long-term memory under low light conditions.
Figure 5. Spaced training does not rescue long-term memory in low light conditions.
Schematic depicts experimental procedure; dark grey arenas denote low light (3 lux) conditions. Bar graphs show exploration time (as a percent of the total) for each object (mean ± SEM; n = 15). Total exploration time for each trial is indicated below the graphs. Scatter dot plots show individual discrimination ratios (open circles) and the group mean (horizontal line); positive values (>0) reflect a preference for the left-side object (familiarization trial) or the novel object (test trial). (A) Long-term memory in low light conditions was assessed 24 hours after mice were exposed to three separate familiarization trials (24 hours apart; spaced training). (B) There was no preference for object location (left or right side) in any of the familiarization trials (B1-3). (C) During the test trial (24 hours after the last familiarization trial), mice failed to exhibit a preference for the novel object, indicating that additional exposures to the familiar object did not facilitate formation of long-term memory.
3.4 High light conditions are required for the formation of long-term memory
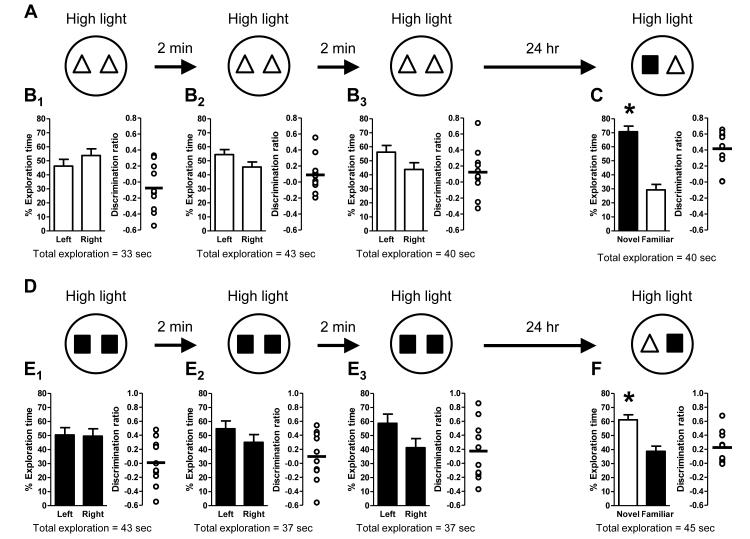
The intensity of a stressor can be a crucial parameter in determining the effect on learning and memory. Often, a very high intensity stressor is detrimental to learning and memory, while an intermediate intensity can be beneficial (Salehi et al., 2010; Sandi et al., 1997). We hypothesized that the lack of long-term memory under low light conditions may have been due to the lack of sufficient arousal during the familiarization and test trials and that moderately enhancing this arousal during the task could facilitate the formation of long-term memory. Because mice exhibit increased anxiety-like behavior in brightly lit open fields (Bats et al., 2001; Bert et al., 2005), we used a moderate increase in the ambient light level to elevate arousal during novel object recognition. Mice were split into two groups: 1) one that experienced a 2-minute delay between the familiarization and test trials (both in high light; Fig. 6A1); and 2) a group that experienced a 24-hour delay between the familiarization and test trials (both in high light; Fig. 6B1). Neither group exhibited a side preference during the familiarization trial (Fig. 6A2 and 6B2). Mice in the 2-minute delay group exhibited a significant preference for the novel object during the test trial (Fig. 6A3), indicating that they formed a short-term memory for the familiar object. Importantly, their preference for the novel object under high light conditions was not different than that exhibited under low light conditions (compare to Fig. 2A and Fig. 3A). Conversely, unlike mice in the 24-hour delay low light group (compare to Fig. 3B), mice in 24-hour delay high light group also exhibited a significant preference for the novel object (Fig. 6B3), indicating that high light conditions were required for the formation of a long-term memory for the familiar object.
Figure 6. High light conditions support the formation of both short-term and long-term memory.
Schematics depict experimental procedure; white arenas denote high light (21 lux) conditions. Bar graphs show exploration time (as a percent of the total) for each object (mean ± SEM; n = 20; * p < 0.05). Total exploration time for each trial is indicated below the graphs. Scatter dot plots show individual discrimination ratios (open circles) and the group mean (horizontal line); positive values (>0) reflect a preference for the left-side object (familiarization trial) or the novel object (test trial). (A) Short-term memory in high light conditions was assessed using a 2-minute delay between the familiarization and test trials (A1). There was no preference for object location (left or right side) during the familiarization trial (A2) but mice exhibited a significant preference for the novel object in the test trial 2 minutes later (A3), indicative of short-term memory for the familiar object. (B) Long-term memory in high light conditions was assessed using a 24-hour delay between the familiarization and test trials (B1). No preference for object location (left or right side) was exhibited during the familiarization trial (B2). However, 24 hours later during the test trial, mice exhibited a preference for the novel object (B3), indicative of long-term memory formation.
3.5 Additional exposure to the familiar objects under high light conditions does not further enhance long-term memory or result in savings between repeated experiments
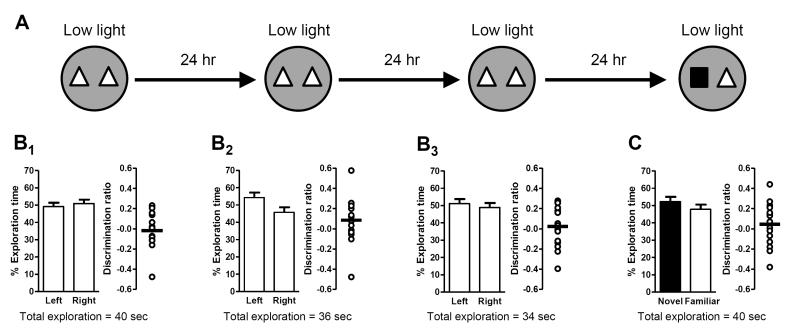
High light conditions were sufficient to elicit long-term memory (measured as a significant preference for the novel object during the test trial) after only one exposure to the familiar objects; however, we hypothesized that additional familiarization trials might further potentiate long-term memory. To test this hypothesis, mice were evaluated in a massed training experimental design where they were exposed to the same pair of identical objects in three sequential familiarization trials under high light conditions (with a 2-minute break between trials during which mice were removed from the arena and returned to their home cage; Fig. 7A). There was no preference for the object located on either side of the arena during any of the familiarization trials (Fig. 7B1-3). Twenty-four hours after the last familiarization trial, a test trial was performed in high light; mice exhibited a significant preference for the novel object (Fig. 7C), indicating they formed a long-term memory for the familiar object. However, this preference was not significantly different from that exhibited after only one familiarization trial in high light (compare to Fig. 6B), suggesting that additional exposure to the familiar objects did not further enhance long-term memory under these conditions.
Figure 7. Massed training in high light conditions does not further improve long-term memory, which is independent of experience in a previous experiment.
Schematics depict experimental procedure; white arenas denote high light (21 lux) conditions. Bar graphs show exploration time (as a percent of the total) for each object (mean ± SEM; n = 10; * p < 0.05). Total exploration time for each trial is indicated below the graphs. Scatter dot plots show individual discrimination ratios (open circles) and the group mean (horizontal line); positive values (>0) reflect a preference for the left-side object (familiarization trial) or the novel object (test trial). (A-C) Long-term memory in high light conditions was assessed 24 hours after mice were exposed to three successive familiarization trials (2 minutes apart; massed training) (A). There was no preference for object location (left or right side) during any of the familiarization trials (B1-3) but during the test trial (24 hours after the last familiarization trial), mice exhibited a significant preference for the novel object (C), indicative of long-term memory for the familiar object. In addition, the increased exposure to the familiar object did not further improve long-term memory relative to that observed after a single familiarization trial (compare to Figure 6B3). (D-F) After a 2-week interval, the experiment was repeated using the same design (D), except that the object assignments (familiar or novel) and locations (left or right side) were counterbalanced within subjects relative to the first experiment. Again there was no preference for object location (left or right side) during any of the familiarization trials (E1-3). In the test trial (24 hours after the last familiarization trial), mice exhibited a significant preference for the novel object (F), indicating that they formed a new long-term memory for the most recently experienced familiar object with no indication of savings from previous exposures.
Although there were no savings for short-term memory between subsequent experiments under low light conditions, it is possible that multiple trials in high light conditions could result in savings for long-term memory. To investigate this possibility, we repeated the massed familiarization experiment in high light after a 2-week rest period (Fig. 7D). As in the previous test of memory savings (see Fig. 2 and section 3.1 of Results), the identities of the familiar and novel objects were counterbalanced within subject between experiments. In this set of familiarization trials, there again was no side preference for either object (Fig 7E1-3). Twenty-four hours later, during the test trial, mice exhibited a significant preference only for the object assigned as novel in the second experiment (Fig. 7F), which indicates that mice formed an independent long-term memory. This experiment suggests that there were no savings from the familiarization trials in the first experiment and that previous experience does not interfere with subsequent long-term memory formation.
3.6 High light conditions during familiarization are required for long-term memory
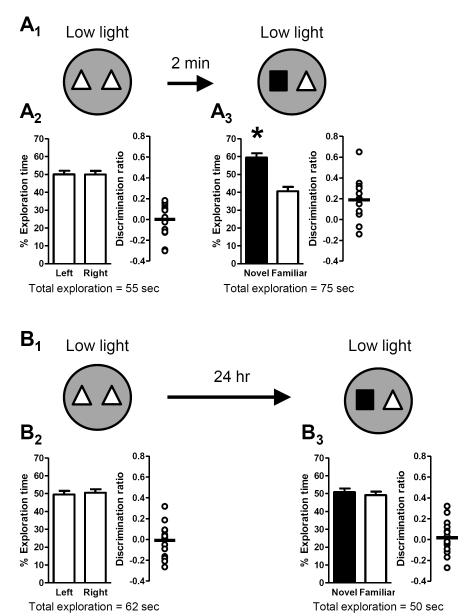
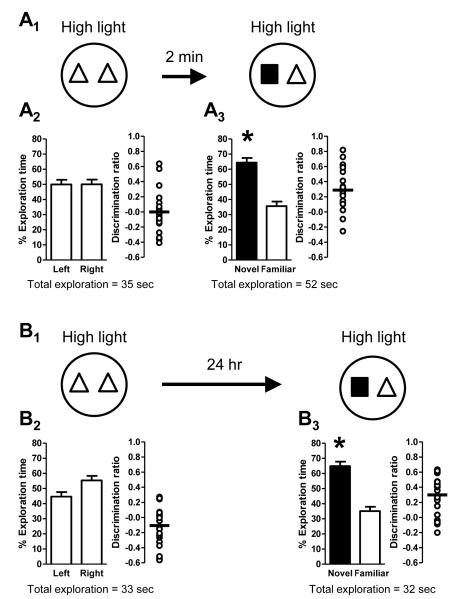
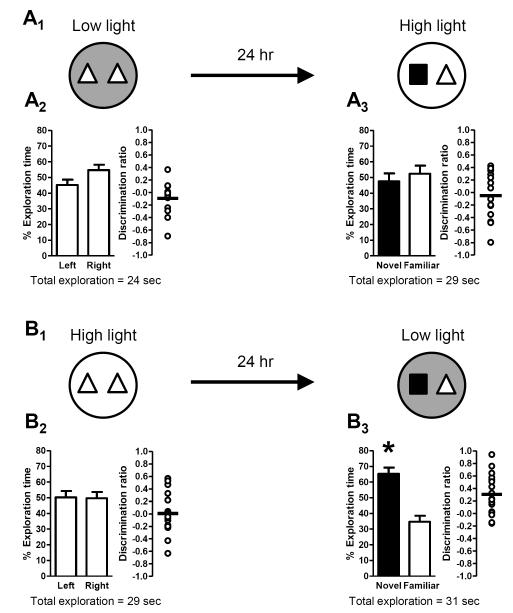
While our previous experiments demonstrated that high light conditions promoted the formation of long-term memory, they did not differentiate between the requirement for high light during acquisition (familiarization trial), retrieval (test trial), or both. To distinguish between these possibilities, mice were separated into two groups that were differentially exposed to low and high light conditions. In the first group, the familiarization trial was conducted under low light conditions, but the test trial (24 hours later) was conducted under high light conditions (Fig. 8A1). These mice did not exhibit a side preference during the familiarization trial (Fig. 8A2), and also did not exhibit a preference for the novel object during the test trial (Fig. 8A3), indicating the lack of a long-term memory for the familiar object. In the second group of mice, conditions were reversed: the familiarization trial was conducted under high light conditions while the test trial (24 hours later) was conducted under low light conditions (Fig. 8B1). Again, there was no side preference during the familiarization trial (Fig. 8B2). Importantly, however, mice exhibited a significant preference for the novel object during the test trial (Fig. 8A3), indicating that they had formed a long-term memory for the familiar object. Taken together, these data demonstrate that high light conditions are necessary during the familiarization trial but are not required during the test trial for long-term memory.
Figure 8. High light conditions during acquisition are required for the formation of long-term memory.
Schematics depict experimental procedure; dark grey arenas denote low light (3 lux) conditions and white arenas denote high light (21 lux) conditions. Bar graphs show exploration time (as a percent of the total) for each object (mean ± SEM; n = 14 (A) or n = 17 (B); * p < 0.05). Total exploration time for each trial is indicated below the graphs. Scatter dot plots show individual discrimination ratios (open circles) and the group mean (horizontal line); positive values (>0) reflect a preference for the left-side object (familiarization trial) or the novel object (test trial). (A) Long-term memory was assessed using a 24-hour delay between the familiarization trial, conducted in low light, and the test trial, conducted in high light (A1). There was no preference for object location (left or right side) during the familiarization trial (A2). Further, mice failed to exhibit a significant preference for the novel object during the test trial 24 hours later (A3), indicating an impairment of long-term memory for the familiar object. (B) Long-term memory was also assessed in a second group of mice with a 24-hour delay, except that the familiarization trial was conducted in high light while the test trial was conducted in low light (B1). Again, there was no preference for object location (left or right side) during the familiarization trial (B2). However, during the test trial 24 hours later, mice exhibited a preference for the novel object (B3), indicative of the formation of long-term memory for the familiar object.
(CORT) groups
4. Discussion
The results presented here demonstrate that light levels modulate the conversion of short-term to long-term memory in the novel object recognition paradigm. Long-term memory could not be elicited when the familiarization phase was carried out in low light conditions and this deficit could not be overcome by performing multiple familiarization trials. Instead, the formation of long-term memory required elevated light conditions during acquisition (familiarization trial) and was independent of light level during retrieval (test trial). On the other hand, the formation of short-term memory was reliably evoked under both low and high light conditions. Interestingly, the light-induced conversion of short-term to long-term memory did not correlate with changes in CORT concentration, suggesting that this form of memory consolidation does not require glucocorticoid activation. Additionally, repeated experience in the novel object recognition task did not degrade the ability to form subsequent, independent memories for a “novel” object.
Deficits in long-term memory formation are the hallmark of many mental illnesses and neurodegenerative disorders. Furthermore, even in healthy individuals, there is a decline in cognitive function as a result of the aging process. In addition to elucidating the mechanisms that underlie these impairments, there has recently been a growing interest in identifying interventions that can be used to enhance cognition in affected populations (Bibb, Mayford, Tsien, and Alberini, 2010). However, in order to accomplish this goal, it is necessary to employ behavioral tasks where both a deficit as well as an improvement in learning and memory can be detected. The low-light novel object recognition paradigm presented here would be useful for screening potential interventions that strengthen the low-light memory, converting it to a more robust, longer-lasting memory.
Another aspect of many diseases that affect learning and memory is that they are progressive in nature, starting with relatively mild deficits that worsen over time to ultimately result in severe impairments. Substantial progress has been made characterizing the course of cognitive decline using animal models to elucidate potential mechanisms that may be targeted to slow or reverse disease progression. However, many of these experiments rely on a cross-sectional design, with each animal being assessed at only one time point. While this has been a useful approach in many respects, it may obscure important information about the nature and time course of cognitive decline, particularly with reference to individual differences in disease progression. A task that could be employed longitudinally using a within-subjects design would have great utility in augmenting our understanding of these impairments. Our results demonstrate that both the “weak memory” (low light) and “strong memory” (high light) versions of the novel object recognition paradigm can be performed repeatedly with no savings between assessments that are at least two weeks apart. Therefore, this task may be useful in monitoring the cognitive abilities of cohorts of mice across time, particularly in models of neurodegenerative disease, such as Alzheimer’s disease, or over the lifespan to model cognitive aging.
While it is clear in our experiments that light levels modulate the conversion of short-term to long-term memory in the novel object recognition paradigm, the precise neurobiological mechanism(s) underlying this conversion remain unknown. Perhaps the most obvious candidate is hypothalamus-pituitary-adrenal (HPA) axis-mediated release of glucocorticoids (such as CORT) that can then act through their receptors to alter neuronal excitability, neuronal morphology, and gene transcription (de Kloet, Joels, and Holsboer, 2005). However, we found that CORT was not differentially elevated by exposure to low or high light during the familiarization trial, and, further, that CORT levels did not correlate with long-term memory consolidation. Based on these results, it seems unlikely that HPA axis-mediated release of CORT contributes substantially to the light-induced conversion of short-term memory to long-term memory under these conditions. Further, we only examined light-induced CORT elevation at one point (20 minutes post-familiarization, based on pilot data that suggested CORT concentrations were maximal at this time); instead, measuring CORT at multiple intervals may reveal critical differences that regulate long-term memory formation induced by exposure to high light during the familiarization trial. A final possibility is other signaling pathways may be engaged by activation of the HPA axis (independent of and thus not reflected in the CORT measurement) and/or by elevated light levels that ultimately regulate the consolidation of long-term memory. Indeed, neuromodulators such as serotonin, dopamine, and acetylcholine have all been implicated in long-term memory formation in mice (for example, see: Anagnostaras, Murphy, Hamilton, Mitchell, Rahnama, Nathanson, and Silva, 2003; Dai, Han, Tian, Cao, Xiu, Song, Huang, Xu, Ding, and Xu, 2008; De Jaeger, Cammarota, Prado, Izquierdo, Prado, and Pereira, 2013; Eriksson, Alvarsson, Stan, Zhang, Hascup, Hascup, Kehr, Gerhardt, Warner-Schmidt, Arango-Lievano, Kaplitt, Ogren, Greengard, and Svenningsson, 2012; Fadok, Darvas, Dickerson, and Palmiter, 2010; Nagai, Takuma, Kamei, Ito, Nakamichi, Ibi, Nakanishi, Murai, Mizoguchi, Nabeshima, and Yamada, 2007; Roozendaal, Okuda, Van der Zee, and McGaugh, 2006). Additional work will be required to determine the relative contribution of these (or other) signaling pathways to the cellular mechanisms that underlie the light-induced conversion of a weak short-term memory into a stable long-term memory in the novel object recognition paradigm.
Lastly, our data show that plasma CORT concentration was significantly elevated by experience in the familiarization trial (compared to baseline), regardless of light level. This is important because the novel object recognition task is often considered relatively “non-stressful” (Dere et al., 2007; Okuda, Roozendaal, and McGaugh, 2004; Sik, van Nieuwehuyzen, Prickaerts, and Blokland, 2003). More properly, it should be described as “not aversively-motivated” and investigators should be aware that, as defined by an elevation of CORT concentration, novel object recognition does represent a stressful experience for mice.
Supplementary Material
Acknowledgements
The authors would like to thank members of the Murphy lab, particularly Rachel Parent and Stephanie Jimenez Temme, for helpful discussions and critical reading of the manuscript. This work was supported by the National Institute on Aging (AG028488 to GGM and AG000114 to SJM).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Albasser MM, Chapman RJ, Amin E, Iordanova MD, Vann SD, Aggleton JP. New behavioral protocols to extend our knowledge of rodent object recognition memory. Learn Mem. 2010;17:407–419. doi: 10.1101/lm.1879610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anagnostaras SG, Murphy GG, Hamilton SE, Mitchell SL, Rahnama NP, Nathanson NM, Silva AJ. Selective cognitive dysfunction in acetylcholine M1 muscarinic receptor mutant mice. Nat Neurosci. 2003;6:51–58. doi: 10.1038/nn992. [DOI] [PubMed] [Google Scholar]
- Bartolomucci A, de Biurrun G, Czeh B, van Kampen M, Fuchs E. Selective enhancement of spatial learning under chronic psychosocial stress. Eur J Neurosci. 2002;15:1863–1866. doi: 10.1046/j.1460-9568.2002.02043.x. [DOI] [PubMed] [Google Scholar]
- Bats S, Thoumas JL, Lordi B, Tonon MC, Lalonde R, Caston J. The effects of a mild stressor on spontaneous alternation in mice. Behav Brain Res. 2001;118:11–15. doi: 10.1016/s0166-4328(00)00285-0. [DOI] [PubMed] [Google Scholar]
- Bert B, Felicio LF, Fink H, Nasello AG. The use of sudden darkness in mice: a behavioural and pharmacological approach. Psychopharmacology (Berl) 2005;179:846–853. doi: 10.1007/s00213-004-2107-0. [DOI] [PubMed] [Google Scholar]
- Bevins RA, Besheer J. Object recognition in rats and mice: a one-trial non-matching-to-sample learning task to study ‘recognition memory’. Nat Protoc. 2006;1:1306–1311. doi: 10.1038/nprot.2006.205. [DOI] [PubMed] [Google Scholar]
- Bibb JA, Mayford MR, Tsien JZ, Alberini CM. Cognition enhancement strategies. J Neurosci. 2010;30:14987–14992. doi: 10.1523/JNEUROSCI.4419-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burrows HL, Nakajima M, Lesh JS, Goosens KA, Samuelson LC, Inui A, Camper SA, Seasholtz AF. Excess corticotropin releasing hormone-binding protein in the hypothalamic-pituitary-adrenal axis in transgenic mice. J Clin Invest. 1998;101:1439–1447. doi: 10.1172/JCI1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conrad CD, LeDoux JE, Magarinos AM, McEwen BS. Repeated restraint stress facilitates fear conditioning independently of causing hippocampal CA3 dendritic atrophy. Behav Neurosci. 1999;113:902–913. doi: 10.1037//0735-7044.113.5.902. [DOI] [PubMed] [Google Scholar]
- Dai JX, Han HL, Tian M, Cao J, Xiu JB, Song NN, Huang Y, Xu TL, Ding YQ, Xu L. Enhanced contextual fear memory in central serotonin-deficient mice. Proc Natl Acad Sci U S A. 2008;105:11981–11986. doi: 10.1073/pnas.0801329105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Jaeger X, Cammarota M, Prado MA, Izquierdo I, Prado VF, Pereira GS. Decreased acetylcholine release delays the consolidation of object recognition memory. Behav Brain Res. 2013;238:62–68. doi: 10.1016/j.bbr.2012.10.016. [DOI] [PubMed] [Google Scholar]
- de Kloet ER, Joels M, Holsboer F. Stress and the brain: from adaptation to disease. Nat Rev Neurosci. 2005;6:463–475. doi: 10.1038/nrn1683. [DOI] [PubMed] [Google Scholar]
- de Quervain DJ, Roozendaal B, McGaugh JL. Stress and glucocorticoids impair retrieval of long-term spatial memory. Nature. 1998;394:787–790. doi: 10.1038/29542. [DOI] [PubMed] [Google Scholar]
- Dere E, Huston JP, De Souza Silva MA. The pharmacology, neuroanatomy and neurogenetics of one-trial object recognition in rodents. Neurosci Biobehav Rev. 2007;31:673–704. doi: 10.1016/j.neubiorev.2007.01.005. [DOI] [PubMed] [Google Scholar]
- Diamond DM, Park CR, Heman KL, Rose GM. Exposing rats to a predator impairs spatial working memory in the radial arm water maze. Hippocampus. 1999;9:542–552. doi: 10.1002/(SICI)1098-1063(1999)9:5<542::AID-HIPO8>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- Ennaceur A. One-trial object recognition in rats and mice: methodological and theoretical issues. Behav Brain Res. 2010;215:244–254. doi: 10.1016/j.bbr.2009.12.036. [DOI] [PubMed] [Google Scholar]
- Eriksson TM, Alvarsson A, Stan TL, Zhang X, Hascup KN, Hascup ER, Kehr J, Gerhardt GA, Warner-Schmidt J, Arango-Lievano M, Kaplitt MG, Ogren SO, Greengard P, Svenningsson P. Bidirectional regulation of emotional memory by 5-HT(1B) receptors involves hippocampal p11. Mol Psychiatry. 2012 doi: 10.1038/mp.2012.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fadok JP, Darvas M, Dickerson TM, Palmiter RD. Long-term memory for pavlovian fear conditioning requires dopamine in the nucleus accumbens and basolateral amygdala. PLoS One. 2010;5:e12751. doi: 10.1371/journal.pone.0012751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fanselow MS, Tighe TJ. Contextual conditioning with massed versus distributed unconditional stimuli in the absence of explicit conditional stimuli. J Exp Psychol Anim Behav Process. 1988;14:187–199. [PubMed] [Google Scholar]
- Holscher C. Stress impairs performance in spatial water maze learning tasks. Behav Brain Res. 1999;100:225–235. doi: 10.1016/s0166-4328(98)00134-x. [DOI] [PubMed] [Google Scholar]
- Huang Y, Zhou W, Zhang Y. Bright lighting conditions during testing increase thigmotaxis and impair water maze performance in BALB/c mice. Behav Brain Res. 2012;226:26–31. doi: 10.1016/j.bbr.2011.08.043. [DOI] [PubMed] [Google Scholar]
- Joels M. Corticosteroid actions in the hippocampus. J Neuroendocrinol. 2001;13:657–669. doi: 10.1046/j.1365-2826.2001.00688.x. [DOI] [PubMed] [Google Scholar]
- Joels M, Pu Z, Wiegert O, Oitzl MS, Krugers HJ. Learning under stress: how does it work? Trends Cogn Sci. 2006;10:152–158. doi: 10.1016/j.tics.2006.02.002. [DOI] [PubMed] [Google Scholar]
- Kim JJ, Diamond DM. The stressed hippocampus, synaptic plasticity and lost memories. Nat Rev Neurosci. 2002;3:453–462. doi: 10.1038/nrn849. [DOI] [PubMed] [Google Scholar]
- Lattal KM. Trial and intertrial durations in Pavlovian conditioning: issues of learning and performance. J Exp Psychol Anim Behav Process. 1999;25:433–450. doi: 10.1037/0097-7403.25.4.433. [DOI] [PubMed] [Google Scholar]
- Li S, Wang C, Wang W, Dong H, Hou P, Tang Y. Chronic mild stress impairs cognition in mice: from brain homeostasis to behavior. Life Sci. 2008;82:934–942. doi: 10.1016/j.lfs.2008.02.010. [DOI] [PubMed] [Google Scholar]
- Luine V, Martinez C, Villegas M, Magarinos AM, McEwen BS. Restraint stress reversibly enhances spatial memory performance. Physiol Behav. 1996;59:27–32. doi: 10.1016/0031-9384(95)02016-0. [DOI] [PubMed] [Google Scholar]
- Luine V, Villegas M, Martinez C, McEwen BS. Repeated stress causes reversible impairments of spatial memory performance. Brain Res. 1994;639:167–170. doi: 10.1016/0006-8993(94)91778-7. [DOI] [PubMed] [Google Scholar]
- Maccarrone M, Valverde O, Barbaccia ML, Castane A, Maldonado R, Ledent C, Parmentier M, Finazzi-Agro A. Age-related changes of anandamide metabolism in CB1 cannabinoid receptor knockout mice: correlation with behaviour. Eur J Neurosci. 2002;15:1178–1186. doi: 10.1046/j.1460-9568.2002.01957.x. [DOI] [PubMed] [Google Scholar]
- Malisch JL, Breuner CW, Gomes FR, Chappell MA, Garland T., Jr Circadian pattern of total and free corticosterone concentrations, corticosteroid-binding globulin, and physical activity in mice selectively bred for high voluntary wheel-running behavior. Gen Comp Endocrinol. 2008;156:210–217. doi: 10.1016/j.ygcen.2008.01.020. [DOI] [PubMed] [Google Scholar]
- Mather M. Emotional Arousal and Memory Binding An Object-Based Framework. Perspectives on Psychological Science. 2007;2:33–52. doi: 10.1111/j.1745-6916.2007.00028.x. [DOI] [PubMed] [Google Scholar]
- McGaugh JL. Making lasting memories: Remembering the significant. Proc Natl Acad Sci U S A. 2013 doi: 10.1073/pnas.1301209110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLay RN, Freeman SM, Zadina JE. Chronic corticosterone impairs memory performance in the Barnes maze. Physiol Behav. 1998;63:933–937. doi: 10.1016/s0031-9384(97)00529-5. [DOI] [PubMed] [Google Scholar]
- Miracle AD, Brace MF, Huyck KD, Singler SA, Wellman CL. Chronic stress impairs recall of extinction of conditioned fear. Neurobiol Learn Mem. 2006;85:213–218. doi: 10.1016/j.nlm.2005.10.005. [DOI] [PubMed] [Google Scholar]
- Nagai T, Takuma K, Kamei H, Ito Y, Nakamichi N, Ibi D, Nakanishi Y, Murai M, Mizoguchi H, Nabeshima T, Yamada K. Dopamine D1 receptors regulate protein synthesis-dependent long-term recognition memory via extracellular signal-regulated kinase 1/2 in the prefrontal cortex. Learn Mem. 2007;14:117–125. doi: 10.1101/lm.461407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimura J, Endo Y, Kimura F. A long-term stress exposure impairs maze learning performance in rats. Neurosci Lett. 1999;273:125–128. doi: 10.1016/s0304-3940(99)00645-x. [DOI] [PubMed] [Google Scholar]
- Okuda S, Roozendaal B, McGaugh JL. Glucocorticoid effects on object recognition memory require training-associated emotional arousal. Proc Natl Acad Sci U S A. 2004;101:853–858. doi: 10.1073/pnas.0307803100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliveira AM, Hawk JD, Abel T, Havekes R. Post-training reversible inactivation of the hippocampus enhances novel object recognition memory. Learn Mem. 2010;17:155–160. doi: 10.1101/lm.1625310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ottenweller JE, Meier AH, Russo AC, Frenzke ME. Circadian rhythms of plasma corticosterone binding activity in the rat and the mouse. Acta Endocrinol (Copenh) 1979;91:150–157. doi: 10.1530/acta.0.0910150. [DOI] [PubMed] [Google Scholar]
- Payne JD, Jackson ED, Hoscheidt S, Ryan L, Jacobs WJ, Nadel L. Stress administered prior to encoding impairs neutral but enhances emotional long-term episodic memories. Learn Mem. 2007;14:861–868. doi: 10.1101/lm.743507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pico RM, Davis JL. The radial maze performance of mice: assessing the dimensional requirements for serial order memory in animals. Behav Neural Biol. 1984;40:5–26. doi: 10.1016/s0163-1047(84)90134-1. [DOI] [PubMed] [Google Scholar]
- Rescorla RA. Behavioral studies of Pavlovian conditioning. Annu Rev Neurosci. 1988;11:329–352. doi: 10.1146/annurev.ne.11.030188.001553. [DOI] [PubMed] [Google Scholar]
- Roedel A, Storch C, Holsboer F, Ohl F. Effects of light or dark phase testing on behavioural and cognitive performance in DBA mice. Lab Anim. 2006;40:371–381. doi: 10.1258/002367706778476343. [DOI] [PubMed] [Google Scholar]
- Roozendaal B, Castello NA, Vedana G, Barsegyan A, McGaugh JL. Noradrenergic activation of the basolateral amygdala modulates consolidation of object recognition memory. Neurobiol Learn Mem. 2008;90:576–579. doi: 10.1016/j.nlm.2008.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roozendaal B, Okuda S, Van der Zee EA, McGaugh JL. Glucocorticoid enhancement of memory requires arousal-induced noradrenergic activation in the basolateral amygdala. Proc Natl Acad Sci U S A. 2006;103:6741–6746. doi: 10.1073/pnas.0601874103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salehi B, Cordero MI, Sandi C. Learning under stress: the inverted-U-shape function revisited. Learn Mem. 2010;17:522–530. doi: 10.1101/lm.1914110. [DOI] [PubMed] [Google Scholar]
- Sandi C, Loscertales M, Guaza C. Experience-dependent facilitating effect of corticosterone on spatial memory formation in the water maze. Eur J Neurosci. 1997;9:637–642. doi: 10.1111/j.1460-9568.1997.tb01412.x. [DOI] [PubMed] [Google Scholar]
- Sandi C, Pinelo-Nava MT. Stress and memory: behavioral effects and neurobiological mechanisms. Neural Plast. 2007;2007:78970. doi: 10.1155/2007/78970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shors TJ. Acute stress rapidly and persistently enhances memory formation in the male rat. Neurobiol Learn Mem. 2001;75:10–29. doi: 10.1006/nlme.1999.3956. [DOI] [PubMed] [Google Scholar]
- Shors TJ, Weiss C, Thompson RF. Stress-induced facilitation of classical conditioning. Science. 1992;257:537–539. doi: 10.1126/science.1636089. [DOI] [PubMed] [Google Scholar]
- Sik A, van Nieuwehuyzen P, Prickaerts J, Blokland A. Performance of different mouse strains in an object recognition task. Behav Brain Res. 2003;147:49–54. doi: 10.1016/s0166-4328(03)00117-7. [DOI] [PubMed] [Google Scholar]
- Song L, Che W, Min-Wei W, Murakami Y, Matsumoto K. Impairment of the spatial learning and memory induced by learned helplessness and chronic mild stress. Pharmacol Biochem Behav. 2006;83:186–193. doi: 10.1016/j.pbb.2006.01.004. [DOI] [PubMed] [Google Scholar]
- Voikar V, Polus A, Vasar E, Rauvala H. Long-term individual housing in C57BL/6J and DBA/2 mice: assessment of behavioral consequences. Genes Brain Behav. 2005;4:240–252. doi: 10.1111/j.1601-183X.2004.00106.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.