Abstract
Chemotherapeutic agents have been the mainstay of cancer therapy for years. However, their effectiveness has been limited by toxicities they impart on normal cells. Staurosporine (ST) has been shown to arrest normal, but not breast cancer, cells in G1. Therefore, ST may become a chemoprotective agent, arresting normal cells while allowing tumor cells to enter cell cycle phases where they are sensitive to chemotherapeutic agents. Understanding the mechanism of ST-mediated G1 arrest may allow for a beneficial chemoprotective treatment strategy for patients. We utilized 76NE6 (pRb+/p53−), 76NF2V (pRb+/p53+) and 76NE7 (pRb−/P53+) non-tumorigenic human mammary epithelial cell lines to understand the role of the Rb and p53 pathways in ST-directed G1 arrest. CDK4 was downregulated by ST in Rb+ cells, but its presence could not reverse the arrest, neither did its stable downregulation alter ST-mediated cellular response. ST-mediated G1 arrest required pRb, which in turn initiated a cascade of events leading to inhibition of CDK4. Further assessment of this pathway revealed that Chk1 expression and activity were required for the Rb-dependent arrest. For example, pRb+ cells with small interfering RNA to Chk1 had approximately 60% less cells in G1 phase compared with controls and pRb− cells do not arrest upon ST. Furthermore, Chk1 expression facilitates the release of the Rb+ cells from G1 arrest. Collectively, our data suggest that pRb cooperates with Chk1 to mediate a G1 arrest only in pRb+ cells. The elucidation of this pathway can help identify novel agents to protect cancer patients against the debilitating effects of chemotherapy.
Introduction
The side effects of chemotherapy have long been documented (1,2). Chemotherapeutic agents target the cell cycle, and while they are thought to have a greater effect on tumor compared with normal cells, they are unable to differentiate whether the proliferating cells are normal or tumor. Therefore, the toxic effects of these drugs are not limited solely to tumor cells and cause numerous side effects to normal proliferating cells in the body. The severity of the side effects experienced by a patient can lower their maximum tolerated dose thereby altering the amount given and the effectiveness of the therapy.
The ability to protect normal cells from the harmful effects of chemotherapy while maintaining the sensitivity of tumor cells would increase the dose of a chemotherapeutic agent a patient could tolerate. In a study using polyoma virus transformed baby hamster kidney cells, streptovitacin A and caffeine were able to reversibly protect cells from the lethal effects of hydroxyurea, colcemid or arabinonucleoside by arresting the cells during the G1 phase of the cell cycle (3). More recently, two inhibitors [staurosporine (ST) and 7-hydroxystaurosporine (UCN-01)] have shown to reversibly arrest normal, but not breast tumor, cells in G1 phase of the cell cycle (4,5). Further study revealed that ST inhibits a wide range of other protein kinases such as protein kinase A (PKA) and phosphorylase kinase (6), cdc2 and c-Fgr (7) and v-Src (8) at low nanomolar ranges. At these low concentrations, ST is able to preferentially arrest normal proliferating cells in G0/G1, protecting them from lethal doses of the chemotherapeutic agent camptothecin. Furthermore, the cell cycle arrest of normal cells was reversible (5). ST acts on the G1 checkpoint by decreasing CDK4 protein and activity levels at concentrations as low as 0.5nM (9) and by lowering the CDK2 activity levels through increasing the binding of CDK2 to p27 (5). These actions of ST are Rb dependent and p53 independent (5).
The direct target of ST is unknown. We have shown previously that CDK4 is modulated by ST, and we suspect that the direct target is upstream of CDK4. Chk1 is a kinase upstream of Rb and CDK4, which was initially discovered in fission yeast and has been linked to the DNA damage pathway (10). Loss of Chk1 rendered cells sensitive to unligated DNA and ultraviolet radiation, revealing that this protein was necessary for cell cycle arrest in response to DNA damage. In addition to its role in DNA damage response, Chk1 is important in cell cycle progression. When activated, Chk1 phosphorylates Cdc25a on threonine 507 and serine 178 facilitating Cdc25a and 14-3-3 binding (11). The result is the nuclear export of Cdc25a (12) preventing it from acting as a phosphatase (13). As a phosphatase, Cdc25a removes the inhibitory phosphate group placed by wee1/myt1 on various CDKs (14) allowing for cell cycle progression. Based on the critical roles that both pRb and Chk1 play in cell cycle progression, we examined whether these two proteins could directly mediate the growth inhibitory action of ST in human mammary cells.
Materials and methods
Cell lines and culture conditions
The immortalized 76NE6 and 76NE7 cell lines, obtained from Dr V. Band (University of Nebraska Medical Center, Omaha, NE), were generated and maintained in culture as described previously (15–17). 76NF2V immortalized cell line was generated by transfecting the mortal 76N cells with the F2V mutant variant of HPV E6 gene which rendered the cells immortalized but with a functional and wild-type p53 (18). All immortalized cell lines were maintained in culture conditions as described earlier (15,19). HEK 293 cells utilized for adenovirus amplification were cultured in Dulbecco’s modified Eagle’s high-glucose medium (HyClone, Logan, UT) with 10mM N-2-hydroxyethylpiperazine-N-2-ethanesulfonic acid, 10% fetal calf serum (HyClone) and 1× penicillin–streptomycin–glutamine (Gibco/Invitrogen, Carslbad, CA). All cells were kept in a humidified incubator at 37ºC with 6.5% CO2 and were maintained at 70% or lower confluency at all times. All cells were tested for mycoplasmal contamination, and their identity was verified by karyotype analysis. Serum was purchased from HyClone Laboratories (Logan, Utah), and cell culture medium was from Life Technologies/Gibco BRL (Rockville, MD). ST was purchased from Roche Molecular Biochemicals (Indianapolis, IN). All cell culture and flow cytometry reagents were from Sigma–Aldrich (St Louis, MO), unless otherwise noted.
Cell cycle analysis
For determination of cell cycle distribution following ST treatment, DNA content was measured by flow cytometry using cells that were harvested and stained with propidium iodide (PI). Briefly, 24h after plating cells on 150mM plates, the media was removed and the cells were treated with ST or fresh media (control) for 48h. The cells were then harvested, and 3×106 cells were washed with cold phosphate-buffered saline (PBS). For PI staining, cell pellets were then fixed overnight with 70% ethanol. Cells were then washed in PBS and stained with a solution of 10 μg/ml PI and 20 μg/ml ribonuclease A in PBTB (PBS with 0.5% Tween-20 and 0.5% bovine serum albumin). Twenty-four hours after staining, 1×106 cells were filtered and allowed to incubate at 37°C for 30min. Cell cycle distribution was determined using a FACscalibur (Becton Dickinson, Franklin Lakes, NJ), flow cytometry machine with Cell Quest and Modfit software (Verity Software House, Topsham, ME). A diploid model was used for analysis as well as gates to remove debris and aggregates. The program’s “fit” option was selected to calculate relative percentages of cell cycle distribution. Differences were assessed for significance using a Student’s t-test.
Adenoviruses
Adenovirus to CDK4 was obtained from Dr J. Nevins (Duke University, Durham, NC). Adenoviral luciferase (LUC) was described previously (20), and adenoviruses to the wild-type and dominant negative Chk1 were obtained from Dr H. Piwnica-Worms (Washington University in St Louis School of Medicine, St Louis, MO) (11). Transgenes for luciferase, E2F1, CDK4, Chk1 wild-type (wt) and Chk1 dominant negative (dn) were expressed using a replication incompetent adenovirus type 5 containing E1 and E3 gene deletions downstream of a cytomegalovirus promoter. Viruses were first amplified utilizing the packaging cell line AD-293 (Stratagene-Agilent Technologies, Santa Clara, CA), and were then purified by centrifugation at 176 000g in a CsCl gradient at 4°C for 18h. The optical density at 260nm after particle lysis in 0.5% sodium dodecyl sulfate/PBS at 37°C for 15min was used to determine the total particles (vp) (21,22). OD260×1.1×1012 vp/ml was used to calculate titers (21). Cells (2×106) were plated on 100mm plates for infections. Twenty-four hours following seeding, cells were washed first with sterile PBS and then infected with adenoviruses diluted in 4ml of media. Two hours after infection, media was removed and exchanged with fresh culture media. A multiplicity of infection (MOI) of 750–1500 was used to optimally infect all cell lines. Western blot analysis was utilized to determine transgene expression.
Western blot analysis
Western blot analysis was performed as described by Rao et al. (23). Briefly, 50 µg of protein was loaded onto a sodium dodecyl sulfate–polyacrylamide gel electrophoresis denaturing gel and subjected to electrophoresis. The protein was then transferred overnight at 35 mV at 4°C onto a polyvinylidene diflouride membrane (NEN Life Sciences Products, Boston, MA). Blots were blocked in BLOTTO [20nM Tris, 0.05% Tween (pH 7.6) and 5% non-fat dried milk] overnight at 4°C. After blots were washed, they were incubated in primary antibodies for 2h. Primary antibodies used were against Rb and phospho-Rb (Cell Signaling, Danvers, MA), CDK4 and CDK2 (Transduction Laboratories, Lexington, KY), Chk1 and Chk2 (Santa Cruz Biochemicals, Santa Cruz, CA) all at 1 μg/ml and actin (Chemicon, Temecula, CA) at 0.1 μg/ml. Densitometry was performed using ImageQuant TL (GE Healthcare Biosciences, Pittsburgh, PA).
Immunoprecipitation and kinase assays
Immunoprecipitation (IP) and kinase assays were performed as described previously (23) with following modifications. For CDK4 and Chk1 kinase assays, 250 µg of cell extracts per IP condition was utilized with either 5 μl of CDK4 antibody or 4 μl of Chk1 antibody coupled to Sepharose A beads (Amersham Biosciences AB, Uppsala, Sweden). Immunoprecipitates for CDK4 kinase assays were incubated with a kinase assay buffer containing 5 µCi of [32P] adenosine triphosphate (ATP), 60 µM cold ATP and 2 µg of GST-Rb (Santa Cruz Biochemicals, Santa Cruz, CA) in the presence or absence of 10 µM PD0332991 (Selleckchem.com, Houston, TX) a specific CDK4/6 inhibitor at a 30 µl final volume for 30min at 37°C. Immunoprecipitates for Chk1 kinase assays were incubated with a kinase assay buffer containing 5 µCi of [32P] ATP, 60 µM cold ATP and 0.2 µg of GST-CDC25A (Sigma–Aldrich, St Louis, MO) at a 30 µl final volume for 30min at 37°C. The reaction products were analyzed on 13% sodium dodecyl sulfate–polyacrylamide gel electrophoresis gels. Finally, the gels were stained, destained, dried and exposed to X-ray film. Quantification was performed using a Typhoon phosphorimager (GE Healthcare Biosciences). For the IP followed by western blot analysis, 250 µg of cell extracts per IP condition was utilized with either 5 μl of CDK4 antibody or 4 μl of Chk1 antibody coupled to Sepharose A beads (Amersham Biosciences AB, Uppsala, Sweden). After immunoprecipitates were washed, they were loaded onto 13% gels, subjected to electrophoresis, transferred to polyvinylidene diflouride membrane, blocked and incubated with antibodies as with westerns.
Lentiviral shRNA-mediated silencing
Stable expression of short hairpin RNAs (shRNAs) against CDK4 was achieved in MCF-7 cells via use of the shRNAs in PGIP2-puro (Thermo Scientific, Waltham MA). Three such cloned shRNAs were cotransfected into 293 T cells along with packing plasmids (pCMV delta R8.2) and envelope plasmid (VSV-G) using Fugene reagents according to the manufacturer’s instructions for 2 days. The virus particles containing shRNA-CDK4 or control non-target shRNA were used to infect MCF-7 cells. The infected cells were selected with puromycin at 2 μg/ml.
Small interfering RNA transfections
All three cell lines (76NE6, 76NE7 and 76NF2V) were transfected transiently with pRb small interfering RNA (siRNA) (#16810), Chk1 predesigned siRNA oligos (#1330003) or scrambled siRNA (#12395-1000F, all from Ambion, Austin, TX) following manufacturer’s directions. Briefly, 2×106 cells were plated per well in a six-well plate for 6–12h. Eight microliters of the transfection reagent siPORT Amine (#4502 Ambion) with 30nM of Rb, Chk1 or scrambled siRNA was used per transfection. Following incubation, 200 μl of each mixture was added to cells and then kept at 37°C for 24h. After removing transfection reagents, cells were treated with either vehicle control (PBS) or 2nM of ST for 24h and then harvested for analysis.
Results
Decreased CDK4 levels are associated with ST-induced cell cycle arrest
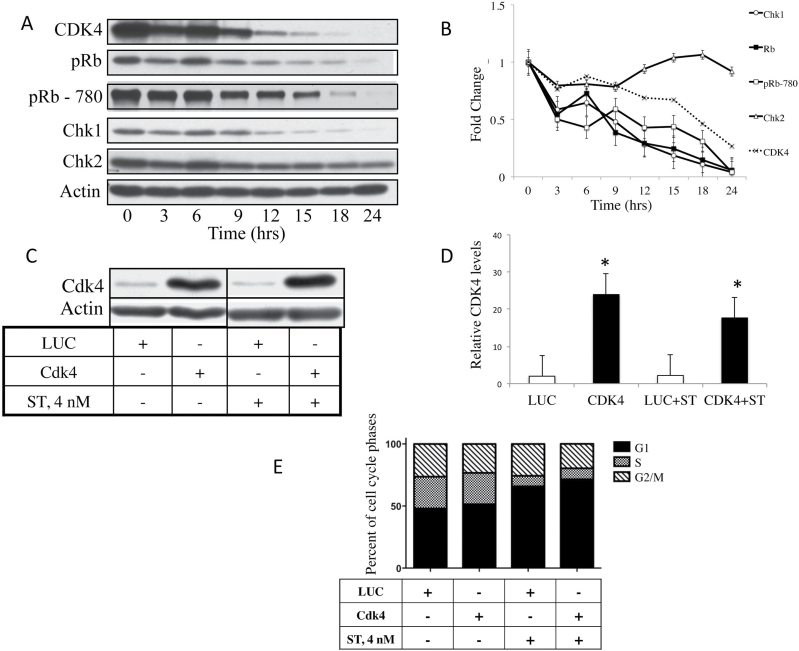
We have shown previously that ST modulates CDK4 protein expression. To understand the effect of ST on CDK4, we examined the kinetics of CDK4 expression with respect to the upstream and downstream mediators in response to ST treatment. 76NE6, human mammary epithelial cells, were treated with a low concentration of ST (i.e. 4nM), which was shown previously to result in G1 arrest (9). Cells were harvested at 3–6h intervals following treatment and subjected to western blot analysis with CDK4, pRb, phospho-Rb (S780), Chk1 and Chk2 (Figure 1A). The results revealed that in response to ST treatment, the levels of CDK4, pRb, pRb phosphorylated at serine 780 (CDK4/cyclin D dependent) and Chk1 decreased compared with untreated controls, whereas Chk2 levels remained unchanged (Figure 1B). These results suggest that the mechanism by which ST causes a G1 arrest could be through downregulation of kinases that act upstream of pRb, such as CDK4.
Fig. 1.
ST-mediated cell cycle arrest coincides with, but is not dependent on, decreased CDK4 levels. 76NE6 cells were treated with 4nM ST and harvested every 3h for (A) western blot analysis of CDK4, pRb, phospho-Rb (S780, CDK4/cyclin D dependent), Chk1 and Chk2. Blots representative of the two trials are shown. (B) Densitometry was performed on the western blot and the values were normalized to the zero time point and graphed. Bars represent the standard error. Adenovirus was used to overexpress CDK4 or an LUC control in 76NE6 cells. Cells were treated with 4nM ST and lysates were collected for (C) western blots, which were then analyzed by (D) densitometry. Each experiment was performed twice (*P < 0.05). (E) The cell cycle distribution of the infected cells was assessed by PI staining and flow cytometry of three independent experiments.
ST-mediated cell cycle arrest is not dependent on decreased CDK4 expression
To investigate whether CDK4 downregulation is responsible for the ST-induced G1 arrest, CDK4 was overexpressed to determine whether the ability of ST to arrest the cells would be abrogated. CDK4 was overexpressed in the 76NE6 cells using 1000 MOI of adenovirus and the cells were subjected to cell cycle analysis after treatment with ST or PBS. The results show that adenoviral CDK4 infection resulted in an increase in CDK4 expression levels compared with LUC control infections (Figure 1C and D). Treatment of cells with ST following CDK4 overexpression did not result in any appreciable changes in CDK4 protein levels (Figure 1D). Despite CDK4 overexpression, the distribution of cells throughout the cell cycle did not change (P = 0.659) upon ST treatment, with the majority of cells in G1 phase (Figure 1E). These results suggest that the ST-mediated G1 arrest cannot be reversed by overexpression of CDK4, placing the target of ST upstream of CDK4.
CDK4 activity is decreased after ST treatment in Rb positive, but not Rb negative, cells
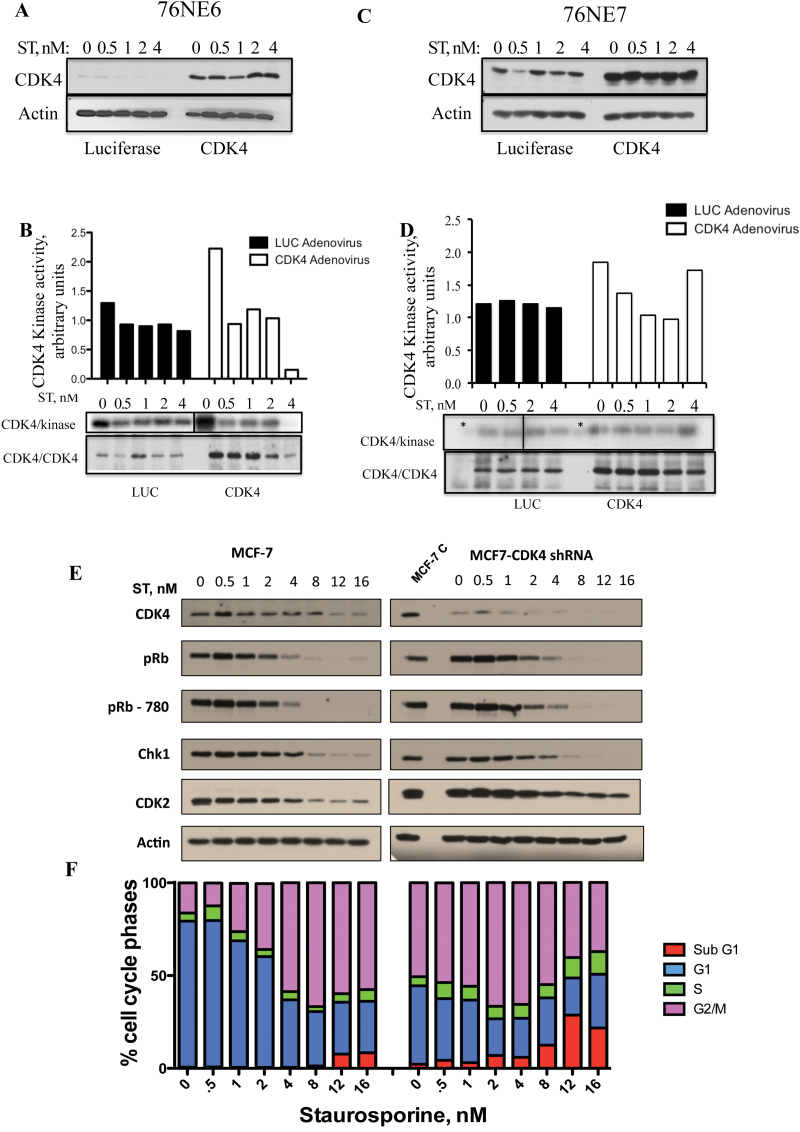
Infection of cells with Ad-CDK4 led to an increase in the protein expression, and this did not affect ST treatment in 76NE6 cells. To determine whether the activity of CDK4 is affected by ST treatment, 76NE6 (pRb positive) and 76NE7 (pRb negative) cells were infected with adenoviral CDK4 (or LUC control) and treated with ST. Cells were infected with an MOI of 1000 for 48h and then treated with a range of ST concentrations from 0 to 4nM. At 72h, cells were harvested and analyzed for CDK4 kinase activity using GST-Rb as a substrate (Figure 2B and D). The results revealed that along with the increased expression of CDK4 in 76NE6 cells after infection with the CDK adenovirus (Figure 2A), there was an increase in CDK4 activity level as compared with the LUC control (Figure 2B). The CDK4 activity decreased after treatment with increasing doses of ST. In contrast, the 76NE7 cells did not show a decrease in kinase activity even at the highest (4nM) concentration of ST (Figure 2D). The specificity of CDK4 kinase assay was assessed by including a specific CDK4/CDK6 inhibitor (10 µM PD0332991) in the kinase reaction mix (lanes marked by asterisk in CDK4/kinase autoradiogram in panel 2D). An IP of CDK4 followed by western blot confirmed that the CDK4 complex was equally pulled down in each condition (low in Luc control and high in CDK4 adenovirus in both cell lines) and that the kinase activity examined could be attributed to CDK4 (Figure 2B and D). To confirm if the phosphorylation of GST-Rb observed could be contributed specifically to the CDK4 kinase activity, stable clones of MCF-7 cells with CDK4 knocked down by shRNA were generated. These clones were treated with increasing concentrations of ST to assess the status of Rb phosphorylation. Western blots showed the decreased CDK4 expression in the clones with no significant changes in the other G1 regulatory proteins examined (Figure 2E). Specifically, there was little difference between the MCF-7 control cells and CDK4-shRNA cells in their ability to downregulate Rb and phospho-Rb (S780). Additionally, treatment of ST did not alter the pattern of cell cycle response in the control as compared with the CDK4-shRNA cells (Figure 2F). When CDK4 is downregulated, the cells tend to accumulate in G2/M cells in the absence of any drug (compare 0nM ST bar graph between the two cell lines in Figure 2F). Treatment of both cells with ST resulted in an accumulation of G2/M by 8nM followed by increase in sub-G1 population mainly in the CDK4-shRNA cells (Figure 2F). These data suggest that, while CDK4 activity is modulated by ST (Figure 2B), there is another protein upstream of CDK4 that is directly targeted by ST in Rb positive cells as CDK4 overexpression or downregulation does not alter the decrease in Rb levels imposed by ST.
Fig. 2.
Modulation of CDK4 by ST is pRb dependent. (A) 76NE6 and (C) 76NE7 cells were infected with adenoviral vectors containing 1000 MOI of CDK4 or LUC for 48h and were then treated with a range (0–4nM) of ST concentrations for an additional 24h. The cells were harvested and protein lysates were collected and subjected to western blot analysis with CDK4. CDK4 was immunoprecipitated from the lysates and tested for kinase activity against GST-Rb (B and D). Blots are shown in the bottom panel with densitometric values graphed above. The specificity of the CDK4 kinase assay was assessed using a CDK4/6 inhibitor, PD0332991, in the lanes marked with an asterisk (panel D*). The background GST-Rb kinase band in the PD0332991 lanes was subtracted from the other lanes to generate the densitometric values presented in panel D. The cell lysate from each cell line was also subjected to IP with CDK4 followed by western blot to show the amount of total protein subjected to the kinase assay (panel B and D, bottom panel). MCF-7 control and shRNA-CDK4 cells were treated with increasing concentrations of ST for 24h, cells were harvested and subjected to (E) western blot and (F) flow cytometry. To show the degree of downregulation rendered by shRNA-CDK4, an untreated aliquot of MCF-7 control cells was loaded on the same gel as the ST-treated MCF-7 CDK4-shRNA gel.
Chk1 is inhibited by ST treatment in Rb positive cells
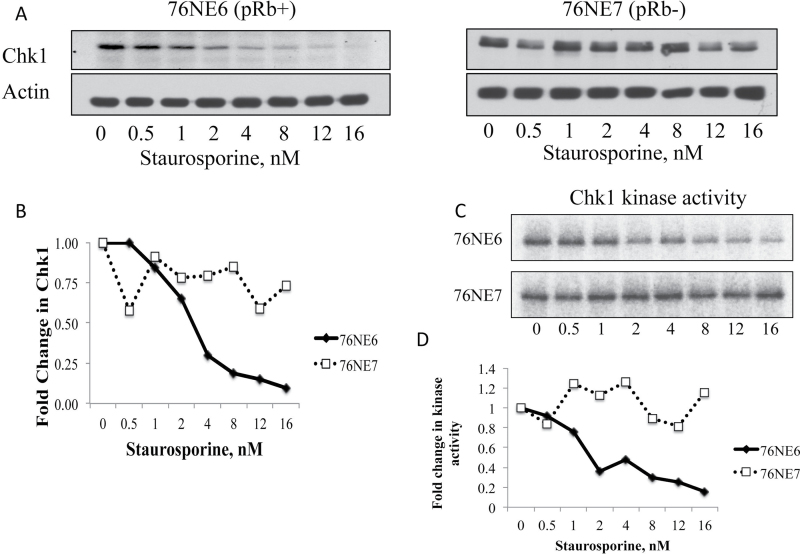
There are several proteins upstream of CDK4 that could be responsible for cell cycle arrest observed after ST treatment. Chk1 was chosen to examine because its promoter region contains E2F binding sites (24). E2F regulates transcription of proteins important for the G1 checkpoint. Additionally, it has been shown previously that Chk1 kinase activity is altered by ST treatment (25). Thus, we examined levels of Chk1 protein expression in both 76NE6 and 76NE7 cells in response to treatment with increasing concentrations of ST (Figure 3A). In the 76NE6 cells, Chk1 levels started decreasing with as little as 0.5nM of ST, reached a minimum at 2nM and stabilized with concentrations between 4 and 16nM. Densitometric analysis revealed that Chk1 protein levels decreased by 75% following 4nM of ST treatment in 76NE6 cells, without any negligible decrease in 76NE7 cells (Figure 3B). In concordance with data showing stable CDK4 activity in 76NE7 cells despite ST treatment (Figure 2), the Chk1 protein levels also remained unaffected by ST treatment (Figure 3A and B). Therefore, Chk1 protein levels are modulated preferentially in Rb+ versus Rb− cell lines, suggesting it may be a possible target of ST treatment.
Fig. 3.
ST inhibits Chk1 in pRb+, but not Rb−, cells. Chk1 expression was measured by (A) western blot in 76NE6 and 76NE7 cells that were treated with increasing concentrations (0–16nM) of ST. (B) Values from densitometric analysis of three independent trials were normalized to the untreated controls and graphed as fold change. (C) Chk1 was immunoprecipitated from the ST-treated 76NE6 and 76NE7 cells and the kinase activity was measured against GST-CDC25a. (D) The densitometry from the average of two independently performed kinase assay blots was graphed.
To determine whether the function of Chk1 is also affected predominantly in Rb+ compared with Rb− cells by ST, its kinase activity was measured following treatment of cells with ST (Figure 3C). The activity of Chk1 was measured using GST-CDC25A as a substrate. 76NE7 cells showed unremarkable changes in the kinase activity of Chk1 with the range of ST treatments (P < 0.01; Figure 3C and D), which was expected, as there was little change in the expression of Chk1 (Figure 3A and B). In comparison, 76NE6 cells showed significantly decreased kinase activity in response to ST treatment (Figure 3C and D). These data provide evidence that ST affects Rb positive cells preferentially over Rb negative by downregulating Chk1 protein and kinase activity levels.
pRb is required for ST-mediated G1 arrest
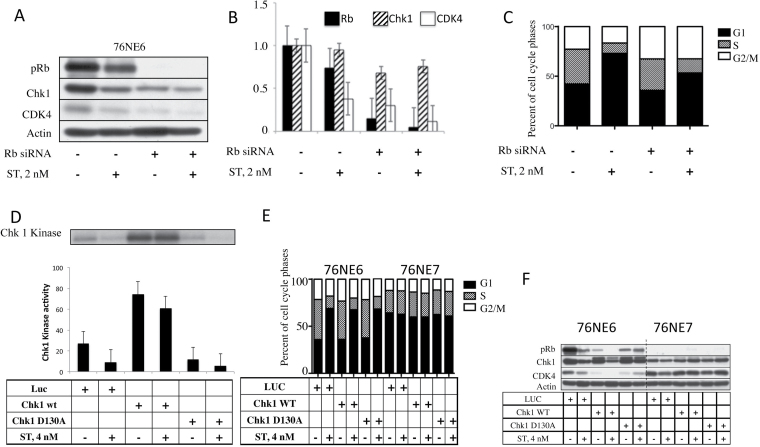
Our data suggest that Chk1 is affected by ST in a pRb-dependent manner. Therefore, we hypothesized that pRb and Chk1 are essential for the G1 arrest that was observed following ST treatment (Figure 1). To first test the dependence of the ST arrest on pRb, 76NE6 cells were transfected transiently with 30nM of siRNA against Rb for 24h and then treated with 2nM ST. Cell lysates were examined for the presence of pRb, Chk1 and CDK4 (Figure 4A and B) and cells were further assessed for their cell cycle distribution (Figure 4C). As expected, Rb levels in 76NE6 cells decreased by an average of 26% when treated with 2nM of ST (Figure 4B). Additionally, CDK4 levels decreased by 62% during ST treatment when Rb was not downregulated. However, there was no significant change in either of these proteins when Rb was silenced (Figure 4A and B). Analysis of the cell cycle distribution revealed that the presence of pRb allowed the arrest of cells in G1 phase (Figure 4C). However, when Rb expression was knocked down, there was a decrease in the percentage of cells in G1 phase after ST treatment (P = 0.018) and an increase in cells distributed throughout the cell cycle. These results indicate that pRb is necessary for a complete G1 arrest in response to ST.
Fig. 4.
ST requires pRb to induce G1 arrest and cannot be reversed when Chk1 is expressed. 76NE6 cells were incubated with 30nM siRNA against Rb for 24h and then treated with 2nM ST and subjected to (A) western blot followed by (B) densitometry. Each experiment was set up twice, and the bar graph shows the average of the two trials. (C) Flow cytometry using PI staining was used to assess the cell cycle distribution of cells. The graph shows the average of three trials. 76NE6 cells were infected with adenoviral LUC or Chk1 and treated with 4nM ST. (D) Cell lysates were immunoprecipitated for Chk1 to measure the kinase activity against GST-Cdc25a. The average of two quantifications by phosphorimaging is shown. (E) Flow cytometry was performed on PI-stained 76NE6 and 76NE7 cells to determine the percentage of cells in each cell cycle phase after infection with either Chk1 or LUC adenovirus and ST treatment. (F) Western blots were used to assess the expression of pRb, Chk1 and CDK4 after Chk1 overexpression and ST treatment.
Chk1 expression is not sufficient to reverse the ST-mediated G1 arrest
Chk1 is upstream of pRb, and we have shown that both are downregulated by ST in pRb+ cells. To understand the role of Chk1 in ST-induced G1 arrest, we overexpressed Chk1 to determine whether it could abrogate the G1 arrest whether Rb was present or not. Both 76NE6 and 76NE7 cell lines were infected with adenoviral LUC, wild-type (wt) Chk1 or Chk1 D130A (a Chk1 with mutations at both Ala 130 and Asp130, which causes the Chk1 kinase to become an inactive, dominant negative form) followed by treatment with 4nM of ST. Kinase activity of Chk1 was ascertained using GST-Cdc25a as a substrate (Figure 4D). When 76NE6 cells were infected with either LUC control or Chk1 D130A, there was a 66% decrease in Chk1-associated kinase activity following treatment with 4nM of ST, similar to levels of activity seen with the Chk1 D130A. In contrast, only a 19% decrease in Chk1 activity level was observed when wild-type Chk1 was overexpressed (Figure 4D). This suggests that Chk1 overexpression can partially abrogate the ST-mediated G1 arrest. Next, cell cycle distribution was analyzed with respect to the changes in protein expression observed with wild-type or Chk1 D130A expression to determine if Chk1 overexpression is sufficient to reverse the G1 arrest (Figure 4E and F). In the 76NE7 cells that do not express pRb, there was no change in Chk1 or CDK4 levels and no modulation of the cell cycle distribution following ST treatment, even when the Chk1 was overexpressed (Figure 4E). On the other hand, all the three proteins examined (pRb, Chk1 and CDK4) decreased following ST administration in the LUC control. When Chk1 was overexpressed, the pRb and CDK4 levels were decreased and were undetectable after treatment with 4nM of ST. However, when the Chk1 D130A adenovirus was used, there was a decrease in the Chk1 and pRb protein levels, but these were not affected by ST treatment. When cell cycle distribution was assessed in the 76NE6 cells, G1 arrest persisted after ST treatment despite overexpression of wild-type or Chk1 D130A (Figure 4E and F). These results suggest that while ST-mediated G1 arrest is associated with decreased Chk1 expression and kinase activity, Chk1 overexpression is not sufficient to prevent ST-mediated G1 arrest.
Chk1 is necessary for G1 arrest
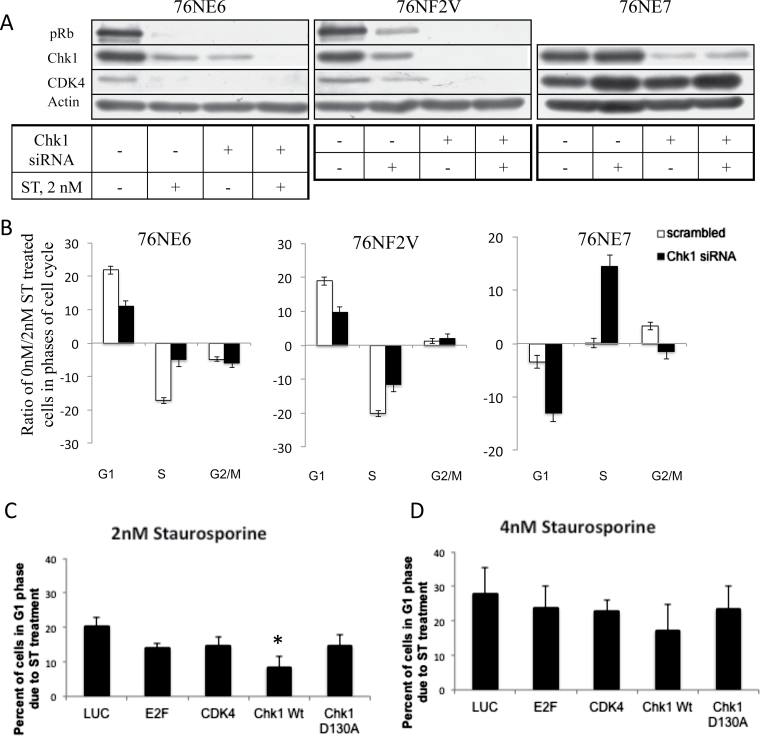
Though Chk1 was not sufficient to reverse the ST-mediated G1 arrest, it is still not known whether the presence of Chk1 is essential for the arrest to occur. We have shown (Figure 4) that pRb is required for ST-mediated G1 arrest. Therefore, to determine whether Chk1 and Rb are both essential for ST to achieve G1 arrest in cells, Chk1 was silenced in a panel of cells with different pRb status that were treated with ST. 76NE6 (Rb+) and 76NE7 (pRb−) cells were transfected with Chk1 siRNA for 24h preceding treatment with 2nM ST. In addition to these two cell lines, we also used 76NF2V as they have functional pRb and p53 and suitable as a positive control, since Chk1 is capable of phosphorylating p53 (26). With Chk1 present, 76NE6 and 76NF2V cells lost both pRb and CDK4 expression following treatment with ST (Figure 5A). Following silencing of Chk1, neither pRb nor CDK4 expression were detected whether or not the cells were treated with ST. However, in contrast to the 76NE6 and 76NF2V cells, silencing of Chk1 in the 76NE7 cells did not modulate CDK4 levels despite ST treatment (Figure 5A). Using flow cytometric measurement (Figure 5B), the ratio of cells in the cell cycle phases of Chk1-silenced and ST-treated cells revealed that downregulation of Chk1 hinders the ability of cells to arrest in G1. Specifically, the percentage of cells in G1 phase following ST treatment when Chk1 is silenced is reduced in both 76NE6 (21.86% with Chk1 versus 10.98% without) and 76NF2V (18.93% versus 9.71%) cells. Additionally, there is a decrease in the percentage of cells found in S phase of 76NF2V and 76NE6 cells (−20.11% versus −11.64% and −17.15% and −5.05%, respectively) with silenced Chk1 following ST treatment (Figure 5B). These results indicate that fewer cells are arresting in G1 phase when Chk1 is silenced (Figure 5B). In contrast, 76NE7 cells, which do not arrest upon treatment with ST, have a drastic increase in the percentage of cells in G1 when Chk1 is downregulated (−12.98% versus −3.39%). Additionally, there is a corresponding increase in S phase from 0.13% to 14.45% when Chk1 is knocked down. These results indicate that Chk1, the upstream effector of pRb and CDK4, is necessary for ST-mediated arrest of cells in G1, even in Rb− cells.
Fig. 5.
Chk1 is required for ST-mediated G1 arrest and helps cells recover from the arrest. 76NE6 (pRb+/p53−), 76F2V (pRb+/p53+) and 76NE7 (pRb−/p53+) cells were treated with siRNA to Chk1 and treated with 2nM ST. (A) Western blots were performed to assess pRb, Chk1 and CDK4 expression. (B) The percentage of cells in each phase of the cell cycle was assessed in cells treated with siRNA against Chk1 or a scrambled control using PI staining and flow cytometry. The percentage of cells in G1, S and G2/M phases of the cell cycle after treatment with 2nM ST compared with 0nM ST is shown as a ratio. The results of duplicate trials were graphed. 76NE6 cells were treated with (C) 2nM or (D) 4nM for 24h to arrest cells in G1. Cells were then allowed a 48h recovery period in the presence of adenoviral LUC, E2F, CDK4, Chk1 (wild-type) or Chk1 D130A (dominant negative). Flow cytometry was performed to assess the percentage of cells in G1 phase of the cell cycle of three independent experiments. Bars show the standard error, *P < 0.05.
Chk1 facilitates the release of cells from ST-induced G1 arrest
Because downregulation of Chk1 before treatment of cells with ST reduces the ability of cells to arrest in G1 in respond to ST treatment, we next asked if overexpression of Chk1 in cells that are already arrested in G1 (by ST) would facilitate the release of cells from G1 arrest. To address this question, we used several different key regulators of the G1 to S phase transition (i.e. Chk1, E2F or CDK4) to assess if overexpression of any would enable the release of 76NE6 cells from G1 arrest following ST treatment. For these experiments, cells were treated with ST (2–4nM) for 24h, washed and allowed to recover for 48h with the adenoviral infection of LUC, E2F, CDK4, Chk1 wt or Chk1 D130A as shown in the schema (Supplementary Figure 1, available at Carcinogenesis Online). Following treatment with 2nM ST and 48h of recovery, there were still 20% more cells in G1 phase compared with cells without ST treatment (Figure 5C). Infection of 76NE6 cells with E2F, CDK4 or Chk1 D130A adenoviruses resulted in a 14% increase in cells in G1 compared with untreated controls, following 48h of recovery. However, when the cells were infected with Chk1 adenovirus during the recovery period, the majority of the cells exited G1 phase (only 7% remained in G1 phase compared with 0nM), indicating that these cells have recovered from their ST-induced G1 arrest. The Chk1-mediated recovery of the 76NE6 cells from G1 arrest was also observed after 4nM ST treatment but was not as efficient as with 2nM ST (Figure 5D). After 48h of recovery from 4nM ST treatment, 28% of cells remained in G1 phase. E2F, CDK4 and Chk1 D130A infection resulted in about 23% of cells retained in G1, whereas 17% of Chk1 infected cells were retained in G1 phase. These data suggest that Chk1 is facilitating the release of cells from an ST-induced G1 arrest.
Chk1 facilitates release from G1 arrest through activation of the Rb pathway
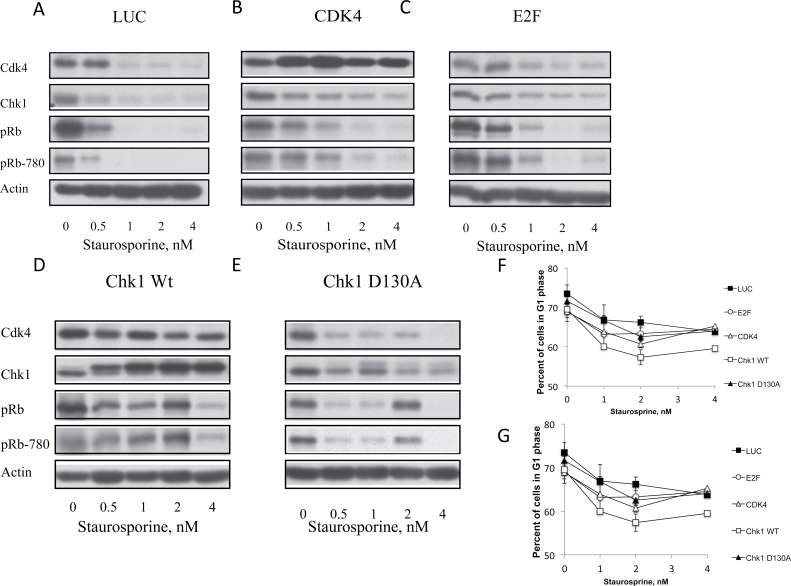
To further determine the mechanisms underlying the Chk1-mediated release of cells from G1 arrest, the expression levels of key regulators of G1-S transition (i.e. CDK4, Chk1, Rb and Rb-780) were analyzed in the adenoviral infected 76NE6 cells. Figure 6A shows that in the control cells (LUC) the expression of Chk1, Rb and Rb-780 all decrease with 0.5nM ST treatment, whereas CDK4 levels decrease after 1nM ST treatment (Figure 6A). CDK4 infection caused increased CDK4 and a decrease in Chk1, Rb and Rb-780 at 0.5nM, similar to the LUC control (Figure 6B). E2F overexpression increases the amount of ST that is needed to cause the decrease in Chk1, Rb and Rb-780 as the expression of these proteins was downregulated by 1nM as opposed to 0.5nM ST (Figure 6C). In the Chk1 wild-type overexpressing cells that recovered from ST-mediated G1 arrest (Figure 5), CDK4 expression remained unaffected by the ST treatment (Figure 6D). Additionally, there was an increase in Rb and Rb-780 expression at 2nM of ST while both of these proteins are decreased at 4nM. This could be involved in the Chk1-induced release of cells from ST arrest, as the levels of Rb and Rb-780 were undetectable in the LUC control cells. When Chk1 kinase is functionally inactive (Chk1 D130A), there is less Chk1, Rb, Rb-780 and CDK4 as compared with the Chk1 wt overexpressing cells (Figure 6E). Therefore, when 76NE6 cells are recovering from G1 arrest, there is an increase in the expression of CDK4, Rb and Rb-780, all of which are integral in allowing cells to progress through G1 phase.
Fig. 6.
The Rb pathway is activated in Chk1 facilitated release of ST arrest. 76NF2V and 76NE7 cells were arrested in G1 with 24h of treatment with increasing doses of ST. Cells were then infected with adenovirus to express (A) LUC, (B) CDK4, (C) E2F, (D) wt Chk1 or (E) Chk1 D130A (dominant negative Chk1) while cells recovered from G1 arrest and were then subjected to western blot analysis of CDK4, Chk1, pRb and phosphorylated pRb (pRb-780). The average percentage of (F) 76NF2V and (G) 76NE7 cells in G1 phase from each treatment group of two experiments was graphed. Bars show standard error.
To confirm that the Rb pathway is necessary for the recovery of cells from ST-mediated G1 arrest, 76NF2V (Rb+) and 76NE7 (Rb−) cell lines were infected with the adenoviruses used above in the 76NE6 cell lines at an MOI of 1500 for 48h following 24h of increasing concentrations of ST treatment. Based on our data, we predicted that Chk1 overexpression should assist 76NF2V, but not 76NE7 cells in G1 arrest recovery because of the presence of a functional pRb. Chk1wt overexpression in 76NF2V cells allowed for a partial recovery from G1 arrest. Approximately 55% of Chk1 expressing cells were in G1 phase after 2nM and 4nM ST treatment compared with 70% of uninfected cells after ST treatment (Figure 6F). There was no significant change in cell recovery seen in the 76NE7 cell lines (Figure 6G). These results indicate that a functional Rb is required for Chk1 overexpression to affect recovery of cells arrested in G1.
Discussion
The preferential protection of normal versus tumor cells by temporary cell cycle arrest would prevent normal cells from experiencing the toxic effects of chemotherapy. Previous studies have shown that various agents can be chemoprotective such as caffeine and streptovitacin A (3), ST (5) and UCN-01 (4) by arresting normal cells at different checkpoints. Checkpoints exist so that when cells are exposed to unfavorable growth conditions, they will arrest. However, tumor cells undergo genetic perturbations, which target the checkpoint controls and allow for unrestricted and continuous growth. There are two key components that a chemoprotective agent must meet. First, it has to target normal cells preferentially while tumor cells are unaffected. Targeting cell cycle checkpoints fits this criterion. Second, normal cells must be able to recover from growth arrest for the chemoprotective agent to be effective. ST was utilized in this study and meets both of these requirements (4,5,9).
ST causes arrest at various cell cycle checkpoints depending on concentration and cell type. For example, cells with an intact G1 checkpoint will preferentially arrest in G1 at low (<10nM) and G2/M at higher concentrations (>30nM) (27–30). In this study, concentrations in the lower range were used to induce and study cell cycle arrest in G1 phase of human mammary epithelial cells. Previous studies from our laboratory showed that 76NE6 cells arrest in G1 phase at low concentrations of ST (9). Hereby examining the G1 to S phase transition of three isogenic human mammary cell lines that differ in their ability to express both p53 and pRb (76NF2V), only pRb (76NE6) or only p53 (76NE7), we were able to identify the key regulators of this checkpoint that are modulated by ST. We determined that on treatment of 76NE6 cells with low concentration of ST, both pRb and Chk1 levels decreased at 12h followed shortly by CDK4 protein levels at 15h of ST treatment (Figure 1). Therefore, it was hypothesized that overexpression of CDK4 would abrogate the ST-mediated G1 arrest. However, while CDK4 was overexpressed, 76NE6 cells still arrested in G1 phase (Figure 1). Assessment of CDK4 kinase levels in Rb+ and Rb− cells revealed a decreased activity in Rb+, but not Rb− cells at the low concentrations of ST (Figure 2). Because ST downregulates CDK4 in 76NE6 cells, but the presence of CDK4 does not alter the arrest, these data raised the possibility that a protein upstream of CDK4 is directly targeted by ST to induce arrest. Chk1 was a logical choice because of its role upstream of the pRb/CDK4 pathway. Not only does Chk1 phosphorylate pRb (31), but there are also E2F binding domains located within the promoter region of Chk1, suggesting that phosphorylation of pRb releases E2F to transactivate Chk1 during G1 to S transition (24). Therefore, Chk1 levels and activity were analyzed over a range of ST concentrations, and in 76NE6 cells (pRB+), there was a decrease in protein levels and kinase activity at low concentrations while 76NE7 cells (pRb−) had Chk1 levels that remained unaffected by ST treatment (Figure 3). The decrease in Chk1 kinase activity levels has been seen with both ST and UCN-01 previously (25). Rb siRNA experiments confirmed the necessary role of pRb in the ST mechanism (Figure 4).
Similar to the CDK4 experiments, Chk1 was overexpressed in both 76NE6 and 76NE7 cell lines, and it was hypothesized that this could abrogate G1 arrest following ST treatment. While the reduction in kinase levels was abrogated with Chk1 overexpression, no change in cell cycle distribution occurred (Figure 4). Notably, when the Chk1 dominant negative adenovirus was utilized, pRb levels and CDK4 levels did not decrease with 4nM of ST indicating that an active Chk1 is important for ST to mediate G1 arrest. Silencing Chk1 did inhibit Rb+ cells from entering G1 arrest and this was independent of p53 status (Figure 5). It was therefore determined that both pRb and Chk1 are necessary for ST to induce cells to arrest in G1. However, for this to be useful in a therapeutic setting, the cells must be able to recover from G1 arrest. The expression of Chk1 following ST-induced G1 arrest facilitated the release of Rb+ cells from cell cycle arrest (Figure 6). These studies raise a novel mode of action for ST that is schematically presented in Supplementary Figure 2, available at Carcinogenesis Online. Because pRb is essential to ST-mediated G1 arrest, 76NE7 cells were able to continue progressing through the cell cycle unperturbed by the ST treatment. CDK4 overexpression did not alter the effects of ST; meanwhile, Chk1 siRNA decreased the ability of ST to mediate G1 arrest. Additionally, Chk1 overexpression facilitates cells with a functional pRb in recovering from G1 arrest following ST treatment. This novel effect of Chk1 has not been shown previously and reveals the importance of both Chk1 and pRb in ST-mediated G1 arrest. However, there are several examples of the cross talk between pRb and Chk1. First, sequential phosphorylation of pRb by CDKs is necessary for E2F to be released and cell cycle progression to take place (32). When Chk1 is activated by cell stress, it can block the phosphorylation of pRb by cyclin/CDK complexes by sequestering Cdc25a and inhibition of activation of the cyclin/CDK complexes. Additionally, following DNA damage Chk1 (or Chk2) phosphorylates pRb at the Ser612 residue resulting in increased binding of pRb and E2F1 and thereby preventing cell cycle progression (31). Not only is Chk1 able to regulate transcription factors in the pRb pathway, but several E2F1 binding sites are also located in the promoter region of Chk1 allowing modulation of this protein by the pRb pathway (24). The ATP-binding pocket of Chk1 is the site where both ST and UCN-01 bind to in vitro at low concentrations resulting in the inhibition of Chk1 kinase activity (25,33). Collectively, these studies suggest that targeting this pathway has the potential to preferentially and reversibly arrest normal cells that have an intact pRb/Chk1 pathway during the cell cycle and protect them from the harmful effects of chemotherapy. Tumor cells, which often possess a deregulated pRb pathway, will be non-responsive to such protection strategy.
By understanding the mechanisms of G1 arrest in normal cells, it may give us the tools necessary to determine whether a patient will benefit from a chemoprotective agent followed by a chemotherapeutic agent. For example, the ideal situation would be to administer a low dose of ST to protect normal cells of a patient who harbors an Rb− tumor, treat the tumor with an appropriate chemotherapy and then rely on the Chk1 in normal cells or even induce Chk1 expression to recover the normal cell proliferation.
Supplementary material
Supplementary Figures 1 and 2 can be found at http://carcin.oxfordjournals.org/
Funding
National Institutes of Health through MD Anderson’s Cancer Center Support Grant (CA016672, 5R01CA087548-10 to K.K.); the Susan G. Komen for the Cure (KG100521 to K.K.H.).
Conflict of Interest Statement: None declared.
Supplementary Material
Glossary
Abbreviations:
- ATP
adenosine triphosphate
- IP
immunoprecipitation
- MOI
multiplicity of infection
- PBS
phosphate-buffered saline
- PI
propidium iodide
- shRNA
short hairpin RNA
- siRNA
small interfering RNA
- ST
staurosporine.
References
- 1. Moss R.W. (1995). Questioning Chemotherapy. Equinox Press, Brooklyn, NY [Google Scholar]
- 2. Partridge A.H., et al. (2001). Side effects of chemotherapy and combined chemohormonal therapy in women with early-stage breast cancer. J. Natl Cancer Inst. Monogr., 30, 135–142 [DOI] [PubMed] [Google Scholar]
- 3. Pardee A.B., et al. (1975). Selective killing of transformed baby hamster kidney (BHK) cells. Proc. Natl Acad. Sci. USA, 72, 4994–4998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Chen X., et al. (1999). UCN-01-mediated G1 arrest in normal but not tumor breast cells is pRb-dependent and p53-independent. Oncogene, 18, 5691–5702 [PubMed] [Google Scholar]
- 5. Chen X., et al. (2000). Protection of normal proliferating cells against chemotherapy by staurosporine-mediated, selective, and reversible G(1) arrest. J. Natl Cancer Inst., 92, 1999–2008 [DOI] [PubMed] [Google Scholar]
- 6. Meyer T., et al. (1989). A derivative of staurosporine (CGP 41 251) shows selectivity for protein kinase C inhibition and in vitro anti-proliferative as well as in vivo anti-tumor activity. Int. J. Cancer, 43, 851–856 [DOI] [PubMed] [Google Scholar]
- 7. Meggio F., et al. (1995). Different susceptibility of protein kinases to staurosporine inhibition. Kinetic studies and molecular bases for the resistance of protein kinase CK2. Eur. J. Biochem., 234, 317–322 [DOI] [PubMed] [Google Scholar]
- 8. Nakano H., et al. (1987). Staurosporine inhibits tyrosine-specific protein kinase activity of Rous sarcoma virus transforming protein p60. J. Antibiot. (Tokyo)., 40, 706–708 [DOI] [PubMed] [Google Scholar]
- 9. McGahren-Murray M., et al. (2006). The differential staurosporine-mediated G1 arrest in normal versus tumor cells is dependent on the retinoblastoma protein. Cancer Res., 66, 9744–9753 [DOI] [PubMed] [Google Scholar]
- 10. Walworth N., et al. (1993). Fission yeast chk1 protein kinase links the rad checkpoint pathway to cdc2. Nature, 363, 368–371 [DOI] [PubMed] [Google Scholar]
- 11. Chen M.S., et al. (2003). Chk1 kinase negatively regulates mitotic function of Cdc25A phosphatase through 14-3-3 binding. Mol. Cell. Biol., 23, 7488–7497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lopez-Girona A., et al. (1999). Nuclear localization of Cdc25 is regulated by DNA damage and a 14-3-3 protein. Nature, 397, 172–175 [DOI] [PubMed] [Google Scholar]
- 13. Jinno S., et al. (1994). Cdc25A is a novel phosphatase functioning early in the cell cycle. EMBO J., 13, 1549–1556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Tessema M., et al. (2004). Cell cycle and no end. Virchows Arch., 444, 313–323 [DOI] [PubMed] [Google Scholar]
- 15. Band V., et al. (1990). Human papilloma virus DNAs immortalize normal human mammary epithelial cells and reduce their growth factor requirements. Proc. Natl Acad. Sci. USA, 87, 463–467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Band V., et al. (1991). Loss of p53 protein in human papillomavirus type 16 E6-immortalized human mammary epithelial cells. J. Virol., 65, 6671–6676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ratsch S.B. (2001). Multiple genetic changes are required for efficient immortalization of different subtypes of normal human mammary epithelial cells. Radiat. Res., 155 (1, Part 2), 143–150 [DOI] [PubMed] [Google Scholar]
- 18. Liu Y., et al. (1999). Multiple functions of human papillomavirus type 16 E6 contribute to the immortalization of mammary epithelial cells. J. Virol., 73, 7297–7307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Keyomarsi K., et al. (1993). Redundant cyclin overexpression and gene amplification in breast cancer cells. Proc. Natl Acad. Sci. USA, 90, 1112–1116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hunt K.K., et al. (1997). Adenovirus-mediated overexpression of the transcription factor E2F-1 induces apoptosis in human breast and ovarian carcinoma cell lines and does not require p53. Cancer Res., 57, 4722–4726 [PubMed] [Google Scholar]
- 21. Maizel J.V., Jr, et al. (1968). Evidence for differences in size and composition of the poliovirus-specific polypeptides in infected HeLa cells. Virology, 36, 48–54 [DOI] [PubMed] [Google Scholar]
- 22. Murakami P., et al. (1999). Quantitation of adenovirus DNA and virus particles with the PicoGreen fluorescent Dye. Anal. Biochem., 274, 283–288 [DOI] [PubMed] [Google Scholar]
- 23. Rao S., et al. (1998). Lovastatin mediated G1 arrest in normal and tumor breast cells is through inhibition of CDK2 activity and redistribution of p21 and p27, independent of p53. Oncogene, 17, 2393–2402 [DOI] [PubMed] [Google Scholar]
- 24. Carrassa L., et al. (2003). Characterization of the 5’ flanking region of the human Chk1 gene: identification of E2F1 functional sites. Cell Cycle, 2, 604–609 [PubMed] [Google Scholar]
- 25. Jackson J.R., et al. (2000). An indolocarbazole inhibitor of human checkpoint kinase (Chk1) abrogates cell cycle arrest caused by DNA damage. Cancer Res., 60, 566–572 [PubMed] [Google Scholar]
- 26. Bartek J. (2001). Mammalian G1- and S-phase checkpoints in response to DNA damage. Curr. Opin. Cell Biol., 13, 738–747 [DOI] [PubMed] [Google Scholar]
- 27. Ha M.W., et al. (2004). Effect of staurosporine on cycle of human gastric cancer cells. World J. Gastroenterol., 10, 161–166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Schnier J.B., et al. (1996). G1 arrest and down-regulation of cyclin E/cyclin-dependent kinase 2 by the protein kinase inhibitor staurosporine are dependent on the retinoblastoma protein in the bladder carcinoma cell line 5637. Proc. Natl Acad. Sci. USA, 93, 5941–5946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Yamasaki F., et al. (2003). Staurosporine-induced apoptosis is independent of p16 and p21 and achieved via arrest at G2/M and at G1 in U251MG human glioma cell line. Cancer Chemother. Pharmacol., 51, 271–283 [DOI] [PubMed] [Google Scholar]
- 30. Zhou W., et al. (2002). Staurosporine-induced G(1) arrest in cancer cells depends on an intact pRB but is independent of p16 status. Cancer Lett., 183, 103–107 [DOI] [PubMed] [Google Scholar]
- 31. Inoue Y., et al. (2007). Phosphorylation of pRB at Ser612 by Chk1/2 leads to a complex between pRB and E2F-1 after DNA damage. EMBO J., 26, 2083–2093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Lipinski M.M., et al. (1999). The retinoblastoma gene family in differentiation and development. Oncogene, 18, 7873–7882 [DOI] [PubMed] [Google Scholar]
- 33. Zhao H., et al. (2001). ATR-mediated checkpoint pathways regulate phosphorylation and activation of human Chk1. Mol. Cell. Biol., 21, 4129–4139 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.