Abstract
Although the infiltration of mesenchymal stem (stromal) cells (MSCs) into different tumors is widely recognized in animal models, the question whether these MSCs have a positive or negative effect on disease progression remains unanswered. The aim of this study is to investigate whether human hepatocellular carcinoma (HCC) harbors MSCs and whether these MSCs affect tumor growth. We observed that cells capable of differentiation into both adipocyte and osteocyte lineages and expressing MSC markers can be cultured from surgically resected HCC tissues. In situ staining of human HCC tissues with a STRO-1 antibody showed that the tumor and tumor-stromal region are significantly enriched with candidate MSCs compared with adjacent tissue (n = 12, P < 0.01). In mice, coengraftment of a human HCC cell line (Huh7) with MSCs resulted in substantially larger tumors compared with paired engraftment of Huh7 alone (n = 8, P < 0.01). Consistently, coculturing Huh7 with irradiated MSCs significantly increased the number and the size of colonies formed. This enhancement of Huh7 colony formation was also observed by treatment of MSC-conditioned medium (MSC-CM), suggesting that secreted trophic factors contribute to the growth-promoting effects. Genome-wide gene expression array and pathway analysis confirmed the upregulation of cell growth and proliferation-related processes and downregulation of cell death-related pathways by treatment of MSC-CM in Huh7 cells. In conclusion, these results show that MSCs are enriched in human HCC tumor compartment and could exert trophic effects on tumor cells. Thus, targeting of HCC tumor MSCs may represent a new avenue for therapeutic intervention.
Introduction
Liver cancer is one of the most devastating malignancies. Hepatocellular carcinoma (HCC) accounts for >90% of primary liver malignancies and is the third leading cause of cancer-related death worldwide. Most cases of HCC are found in patients with cirrhosis caused by chronic hepatitis B (HBV) or C (HCV) infection (1). It develops in particular when chronic infection with HBV or HCV repeatedly causes the body’s immune system to attack liver cells, followed by repetitive damage of the cell cycle, which leads to mistakes during its repair and in turn leads to carcinogenesis (2). For the majority of advanced HCC cases, curative treatments are not possible and the prognosis is dismal because of underlying cirrhosis and the poor tumor response to standard chemotherapy (3). For patients with advanced disease, representing the majority of patients at diagnosis, the only option includes sorafenib (Nexavar), an oral multikinase inhibitor, which increases patient survival by ~3 months (4). Evidently, new therapeutic options are urgently needed for advanced or metastatic HCC.
Remodeling of the liver microenvironment is a hallmark in the pathogenesis of liver cancer (5). In cancer, the microenvironment, which is also referred to as stroma, undergoes drastic changes, including the recruitment and the activation of stromal cells and the remodeling of the extracellular matrix. Coevolution of tumor cells with their microenvironment during tumorigenesis suggests that tumor–stroma cross talk may probably influence the phenotype of tumor cells and may provide a selective pressure for tumor initiation, progression and metastasis (6). In addition, the liver provides a distinct immunological environment and the ultimate effects of this environment on cancer progression may differ in the liver compared with the same in other organs (7).
Mesenchymal stem (stromal) cells (MSCs) were initially identified as a heterogeneous population of stromal cells in the bone marrow (BM) that support hematopoietic stem cells (8). Further studies demonstrated that MSCs possess multilineage differentiation potential, can exert anti-inflammatory function, have immunomodulatory properties and influence other cells through the production of paracrine factors (9). MSCs attract attention as a possible cell-based therapy, especially in immune-related diseases and >300 trials have been registered (January 2013, clinicaltrials.gov). The role of MSCs in pathogenesis has been less well studied. Recent evidence has come forward in various preclinical models that MSCs can migrate into certain types of tumors and using MSCs as an anticancer drug or for gene delivery has also been proposed (10,11). The role of MSCs in cancer development, however, remains unclear. Several studies indicated that MSCs restrain cancer growth (12–14), whereas other studies have shown that MSCs are able to promote tumor progression and metastasis in experimental cancer models (15–18). Thus, it remains largely elusive whether MSCs have a beneficial or detrimental role in the cancerous process (19) and experimentation with MSCs directly obtained from human cancer is deemed necessary to obtain answers here.
Previously, we have identified a resident population of MSCs that are phenotypically and functionally similar to BM MSCs within the human adult liver (20). This raises obvious questions as to the potential role of these cells in liver cancer. In this study, we demonstrate that human HCC indeed harbors MSCs. Furthermore, these HCC-derived MSCs are highly trophic for tumor growth and therefore represent an interesting target for novel therapy.
Materials and methods
Patients
For culturing MSCs, tissue samples from seven individuals who were eligible for surgical resection of HCC were collected. Paired fresh liver tumor and tumor-free liver tissue at the maximum distance from the tumor were used. For immunohistochemical staining of MSC markers, paraffin-embedded patient HCC (n = 12) tissues were collected from the tissue bank at the Erasmus MC, Rotterdam (Supplementary Table 1, available at Carcinogenesis Online). The use of patient materials was approved by the medical ethical committee of Erasmus MC (Medisch Ethische Toetsings Commissie Erasmus MC (21)).
Isolation and culture of MSCs
Single-cell suspensions from adjacent liver tissue and tumor were obtained by tissue digestion. Briefly, fresh tissue was cut into small pieces and digested with 0.5mg/ml of collagenase (Sigma–Aldrich, St Louis, MO) and 0.1mg/ml of DNase I (Roche, Indianapolis, IN) for 30 min at 37°C. Cell suspensions were filtered through cell strainers and mononuclear cells were obtained by Ficoll density-gradient centrifugation. Cells were cultured in Alpha Dulbecco’s modified Eagle’s medium (Alpha DMEM; Lonza, Verviers, Belgium) supplemented with 10% fetal bovine serum (Hyclone, Logan, UT), 100 IU/ml penicillin and 100 μg/ml streptomycin. Adjacent liver and tumor tissues were also cut into small pieces for culturing MSCs in 12-well plates. Tissues were cultured in Alpha DMEM medium as described earlier. If MSCs emerged after 2–3 weeks, the tissues were removed and MSCs were subcultured in conditions as described above.
Flow cytometry
Cells were stained for 30min at 4°C with directly labeled mouse monoclonal antibodies directed against human CD13-PECy7, CD34-APC, CD45-PERCP, HLA-I-APC (all BD Biosciences), CD73-PE, CD166-PE (BD Pharma, San Jose, CA) and CD105-FITC (R&D Systems, Abingdon, UK) for human MSCs and rat antibodies directed against mouse CD90 and CD105 (R&D Systems, Abingdon, UK) for mouse MSCs. Flow cytometric analysis was performed using the FACSCanto II (BD Biosciences) and 10 000 events were collected for analysis, which was performed using FlowJo software.
Adipogenic and osteogenic differentiation
For adipogenic differentiation, MSCs were cultured for 3 weeks in DMEM supplemented with 10% fetal bovine serum, 1 μM dexamethasone, 500 μM isobutyl methyl xanthine, 5 μg/ml insulin and 60 μM indomethacin (Sigma–Aldrich). Oil Red O (Sigma–Aldrich) staining was used for the detection of adipocytes. For osteogenic differentiation, cells were cultured for 3 weeks in DMEM with 10% fetal bovine serum supplemented with 0.2mM ascorbic acid, 100nM dexamethasone and 10mM β-glycerol phosphate (Sigma–Aldrich). Alizarin Red S (Sigma–Aldrich) staining was performed to detect deposited calcium phosphates.
Colony-forming assay of Huh7 cells
MSCs were cultured in 12-well plates to reach ≈30% confluence. After 24 h, MSCs were irradiated with 4 Gy of 60Co gamma radiation. Subsequently, 2000 Huh7 cells, a validated human HCC cell line (22), were added to the wells and were cultured in Alpha DMEM medium.
The colony-forming assay was also performed in Huh7 cells treated with MSC-conditioned medium (MSC-CM). MSC-CM was prepared by culturing MSCs to 70–90% confluence. CM medium was collected after 48 h of culture. As control, colony-forming assay was performed with Huh7 cells only. Huh7 colonies were counterstained with hematoxylin and eosin after 2 weeks. The colony numbers were counted and their sizes were measured by microscope.
Western blot analysis
Cell suspensions were lysed in lysis buffer (130 mM Tris–HCl, pH 8, 20% glycerol, 4.6% sodium dodecylsulfate, 0.02% Bromophenol Blue, 2% dithiothreitol) and boiled at 95ºC for 5 min. Twenty-five microliters of lysates were electrophoretically separated by 8% sodium dodecylsulfate–polyacrylamide gel electrophoresis gels (Invitrogen), transferred onto nitrocellulose membranes and the membranes were incubated with the following primary antibodies: STRO-1 (Invitrogen) and CD146 (Abcam). The immune complexes were detected using horseradish peroxidase-linked anti-mouse or anti-rabbit conjugates as appropriate (DAKO, Denmark) and visualized using an enhanced chemiluminescence detection system (Amersham Biosciences, Amersham, UK).
Immunohistochemistry
Paraffin-embedded liver tumor tissue slides were deparaffinized in xylene, rehydrated in graded alcohols, and rinsed once in phosphate-buffered saline plus Tween 0.05%. For antigen retrieval, slides were boiled in Tris–ethylenediaminetetraacetic acid (pH 9.0) for 10min; 1.5% H2O2 was used to block endogenous peroxidase for 10min at room temperature. The slides were incubated in 5% milk blocking solution, followed by overnight incubation with mouse monoclonal antibody STRO-1 (Invitrogen) at a concentration ratio of 1 to 200 and then counterstained with hematoxylin. As negative control, the primary antibody was replaced by phosphate-buffered saline; the positive controls were taken from other slides that had successfully stained before. STRO-1 staining was scored by two independent observers. The protocol for CD146 staining is similar as mentioned above.
HCC xenograft tumor in NOD/SCID mice
HCC xenograft tumor model in NOD/SCID mice was established as described previously (23). Eight mice, aged 6–8 weeks, were subcutaneously engrafted with human hepatoma Huh7 cells (1 × 106) with or without MSCs (1 × 106) into the lower left or right flank, respectively. Seven mice were injected with tumor-derived MSCs and one was injected with MSCs from adjacent liver tissue. Huh7 cells were labeled with the luciferase reporter gene in three out of the eight mice, as described previously (19). Luciferase activity was measured by an IVIS camera (Caliper Life Sciences) in living animals. Data were analyzed with Living Image 4.0 software.
At Day 19 or 21 postengraftment, mice were killed and the tumors were harvested, imaged and weighed. Part of the tumor was fixed with formalin and embedded in paraffin for histology evaluation or immunohistochemistry. The use of animals was approved by the institutional animal ethics committee (Dier Experimenten Commissie).
Genome-wide gene expression analysis
The total RNA of three independent Huh7 cell line cultures treated or untreated with MSC-CM was used for genome-wide microarray analysis with the Affymetrix GeneChip HuGene 1.0 ST.v1 array (Affymetrix, Santa Clara, CA) according to the manufacturer’s procedures. Transcript-level expression measures were generated with the robust multiarray average procedure, as implemented in the Affymetrix Gene Expression Console, and probe set annotations were retrieved from NetAffx with the same software. Principal-component analysis, forest plot and pathway analysis were performed with Partek (Partek, St Louis, MO).
Statistical analysis
Statistical analysis was performed using the paired non-parametric test, the unpaired non-parametric Mann–Whitney test or paired t-test using GraphPad InStat software (GraphPad Software, San Diego). P values <0.05 were considered statistically significant.
Results
Obtaining MSCs by culturing resected human HCC tissues
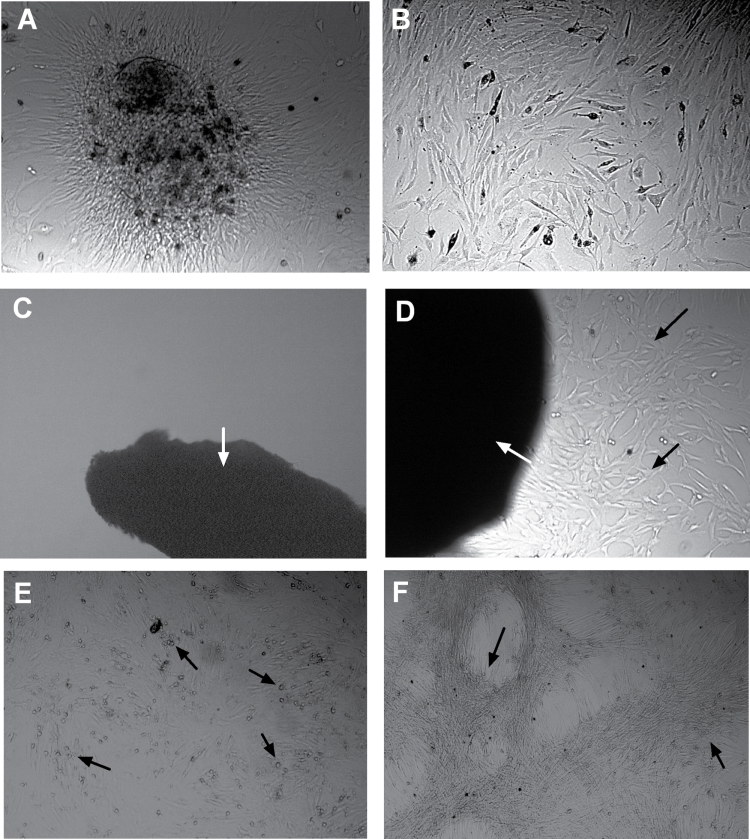
Following surgical resection, patient HCC tissues were subjected to collagenase digestion, followed by the MSC-specific culture protocol validated previously (20). This protocol yielded ample colonies with an apparent MSC morphology (Figure 1A and B). Furthermore, this stringent MSC culture protocol was successful in six out of seven HCC cases, but only in two of six cases when applied to adjacent non-transformed liver tissue, which suggests a possible enrichment of MSCs in HCC. An even more stringent protocol failed to yield MSCs from normal liver (Figure 1C) but still yielded MSCs from HCC tumors (Figure 1D). To confirm that these cells represent ‘bona fide’ MSCs, we assessed the capacity of these cells to yield multilineage progeny. As evident from Figure 1E and F, the cells had the capacity for both adipogenic and osteogenic differentiation. Furthermore, fluorescence-activated cell-sorting analysis of their antigenic profiles confirmed that these cells are positive for the common mesenchymal markers CD13, CD73, CD105 and CD166 and are negative for the common hematopoietic markers CD34 and CD45 (Figure 1G).
Fig. 1.
Culture and characterization of MSCs from human HCC tissues. After collagenase digestion of surgical resected human HCC tissues and culturing, colonized cell clusters appeared (A) and these cells could rapidly grow and expand by subculture, showing typical fibroblast-like morphology (B). Using another method of culturing tiny tissue specimens, MSC-like cells could not be obtained from adjacent liver tissues (C) but only could be obtained from tumor tissues (D). (E) Adipogenic differentiation of liver carcinoma-derived MSCs, detected by Oil Red O staining for lipid droplets (arrow). (F) Osteogenic differentiation of these cells was evaluated by detection of deposited calcium phosphates using Alizarin Red S staining (arrow). (G) Fluorescence-activated cell sorting and staining confirmed that these cells are positive for the common mesenchymal markers CD13, CD73, CD105 and CD166 and are negative for the common hematopoietic markers CD34 and CD45. The percentages of cells positive for tumor-derived MSCs are presented as mean ± SD (n = 6).
Enrichment of STRO-1 positive cells in human HCC
STRO-1 is the best-known MSC marker (24,25), in particular for in vivo immunohistochemical staining of candidate MSCs (11). Western blotting analysis showed that STRO-1 protein is abundantly expressed in HCC-derived MSCs (cell culture expanded), such as BM- or liver-derived MSCs, whereas it is hardly detectable in whole-cell lysates of liver tumor or adjacent liver tissue (Supplementary Figure 1A, available at Carcinogenesis Online). STRO-1 protein expression was further confirmed by immunohistochemical staining of cultured tumor MSCs (Supplementary Figure 1B, available at Carcinogenesis Online).
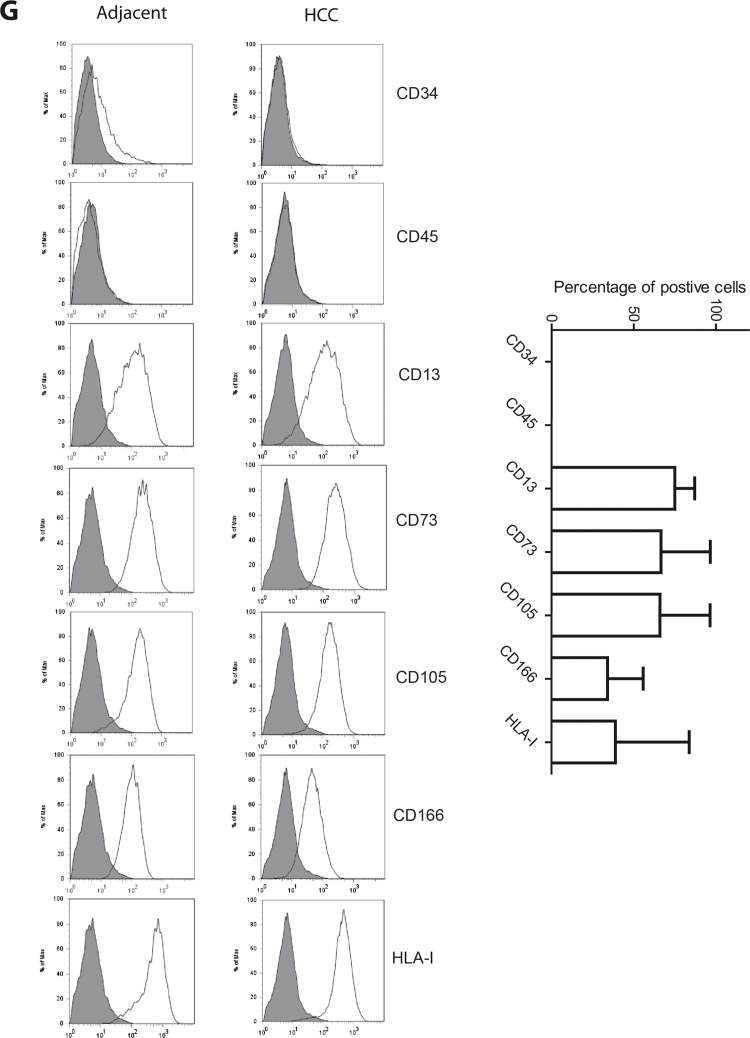
Thus, we chose to employ a STRO-1 antiserum to quantify the candidate MSCs in HCC and to compare the cell numbers with those in the adjacent tissue. For this study, 12 cases of confirmed HCC were obtained for immunohistochemical investigation of both candidate MSC number and histospatial distribution. STRO-1-positive cells were readily observed in both tissues adjacent to the cancer and within the tumor (Figure 2A). In the normal region, STRO-1-positive cells were mainly located in the liver sinusoid or veins. The frequency of STRO-1-positive cells in adjacent tissue appears low (mean ± SD: 11 ± 8 positive cells per view, n = 12), except for livers with extensive inflammation (Supplementary Figure 2, available at Carcinogenesis Online). Morphometric analysis of the samples confirmed that the tumor stroma is significantly enriched with STRO-1-positive cells compared with adjacent non-transformed tissue (Figure 2B). We conclude that HCC is enriched with candidate MSCs, suggesting active sequestration of these cells by the tumor and a possible role of MSCs in HCC tumorigenesis.
Fig. 2.
In situ localization of STRO-1-positive cells in paraffin-embedded patient HCC tissues. (A) Distribution of STRO-1 cells in the adjacent, tumor and tumor-stromal regions in HCC tissues. (B) STRO-1-positive cells are significantly enriched in the tumor, in particular the tumor-stromal region, compared with the adjacent area in HCC tissues (n = 12, *P < 0.05, **P < 0.01).
Human hepatoma cell formed tumors in mice are infiltrated with MSC-like cells
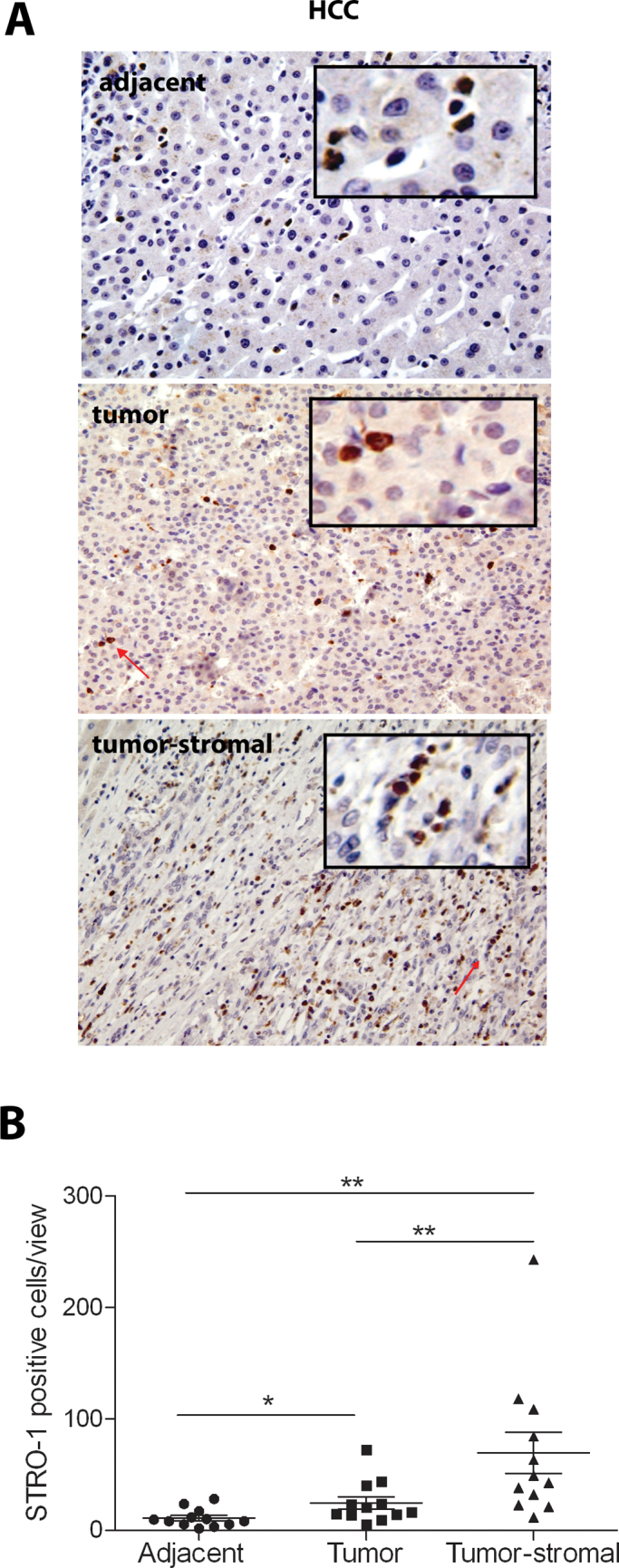
To further understand the enrichment of MSCs in human HCC, we evaluated whether MSCs can infiltrate into HCC tumors that are formed in immunodeficient mice by engraftment of human HCC cell line (1 × 106 Huh7 cells). After subcutaneous injection of Huh7 cells into NOD/SCID mice, solid tumors were formed within a period of 2–4 weeks. When Huh7 cells were labeled with luciferase reporter gene, the solid tumor under the skin could be visualized by IVIS camera (Figure 3A). Subsequently, the tumors were harvested, digested and cultured in vitro. After 3–7 days’ culture, Huh7 cells were colonized (Figure 3B, indicated by grey arrow) but, surprisingly, these were surrounded by fibroblast-like cells (Figure 3B, indicated by white arrow). All the tumors obtained from the six mice contained substantial numbers of these cells shown by cell culture expansion. Fluorescence-activated cell-sorting analysis using anti-mouse antibodies against typical MSC markers CD90 (Figure 3C) and CD105 (Figure 3D) estimated ~1% (mean of two batches) of cells to be positive. This suggests that human HCC tumors may actively attract MSCs and can transcend the species barrier. Subsequently, we initiated experimentation to address the potential role of these MSCs in HCC progression.
Fig. 3.
Infiltration of MSCs into human hepatoma cell-derived solid tumors in mice. (A) Subcutaneous engraftment of human hepatoma Huh7 cells was able to form solid tumors in mice. By luciferase labeling of Huh7 cells, the tumors can be visualized by IVIS cameras in living animals. (B) Cell culture of digested tumors resulted in colonized Huh7 cells (grey arrow) surrounded by fibroblast-like cells (white arrow; n = 6). Fluorescence-activated cell-sorting analysis using anti-mouse antibodies against the typical MSC markers CD90 (C) and CD105 (D) showed ~1% of cells to be positive (n = 2).
Tumor MSCs enhance colony unit formation and growth of hepatoma cells through secretion of trophic factors
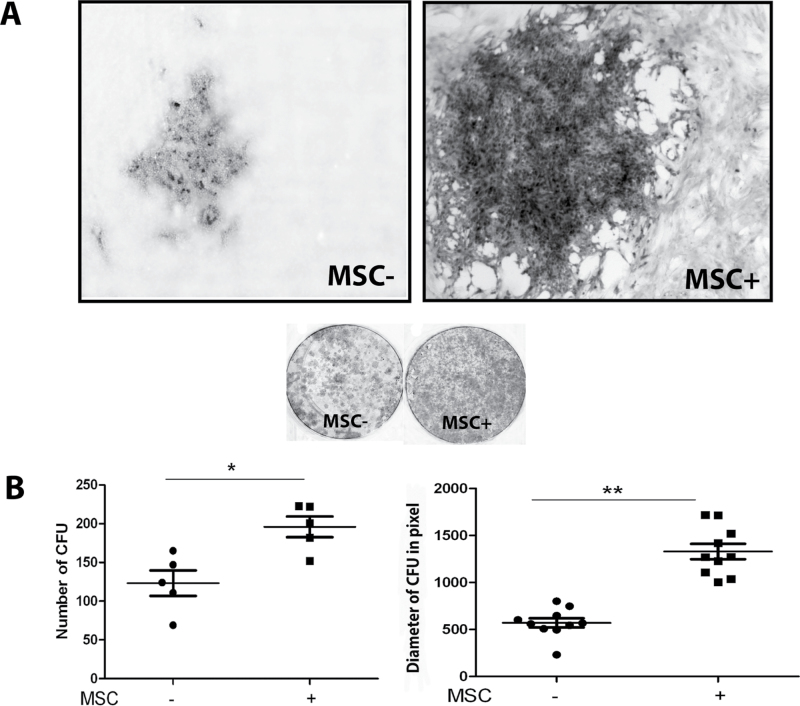
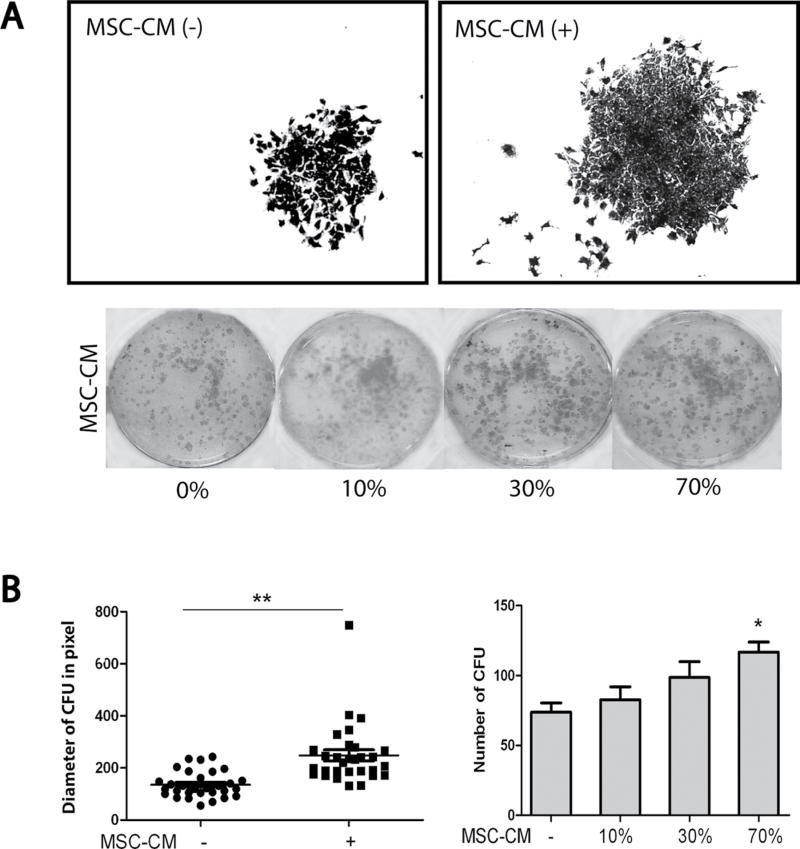
To explore the possible effects of MSCs on HCC, we performed coculture experiments, in which Huh7 cells were grown in the presence or absence of irradiated HCC-derived MSCs (Figure 4A). Coculture with MSCs significantly increased the number (mean ± SD: 196±29 versus 123±36 colonies per 2000 Huh7 cells, respectively, n = 5, P < 0.05) and the size (1329±258 versus 570±155 pixels, respectively, n = 10, P < 0.01) of Huh7-formed colonies (Figure 4B). To investigate whether the effects of MSCs in this model system are mediated by cell-to-cell contact or through paracrine mechanisms, the experiment was also performed using MSC-CM. It showed that such conditioned medium increased both the number and size of the Huh7 colonies (Figure 5A and B), demonstrating that trophic factors of MSCs constitute a powerful stimulus to support tumor cell growth.
Fig. 4.
MSCs promote colony formation and growth of hepatoma cells. (A) Coculturing 2000 Huh7 cells in 12-well plates with irradiated MSCs increased the size and the number of colonies formed. (B) The number of colonies formed was 196±29 (mean ± SD) in Huh7 cocultured with MSCs versus 123±36 in Huh7 alone (n = 5, *P < 0.05). The average size was 1329±258 pixels versus 570±155 pixels (n = 10, **P < 0.01).
Fig. 5.
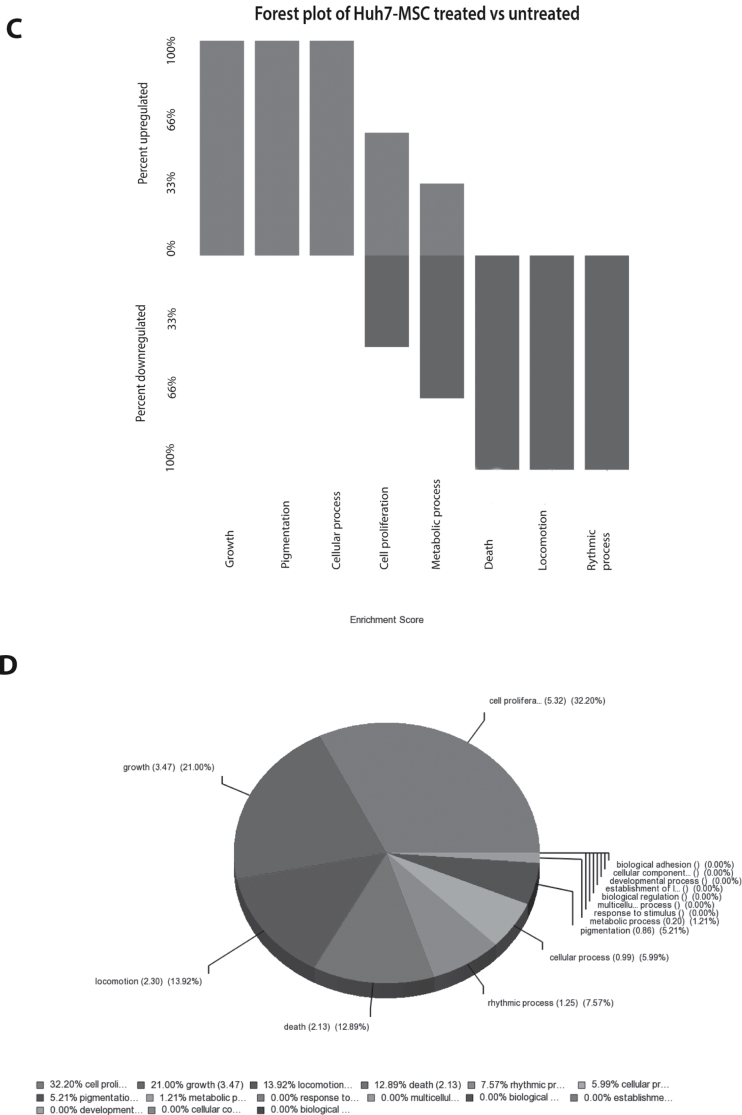
The trophic factors secreted by MSCs promote colony formation and growth of hepatoma cells. (A) Huh7 treated with MSC-conditioned medium resulted in formation of more and larger colonies. (B) The size and the number of colonies were significantly increased by treatment with MSC-CM. Forest plot (C) and gene set enrichment analysis (D) of genome-wide gene expression array confirmed both the upregulation of cell growth and proliferation-related processes and downregulation of cell death-related pathways by treatment of Huh7 cells with MSC-CM. *P < 0.05; **P < 0.01.
To map the molecular regulation of Huh7 cells by MSC trophic factors, genome-wide expression arrays were performed on Huh7 cells treated with (n = 3) or without (n = 3) MSC-CM . Principal-component analysis of genome-wide expression profiles separated both treatment groups into two clusters, reflecting the effects of MSC-CM treatment (Supplementary Figure 3, available at Carcinogenesis Online). Furthermore, forest plot (Figure 5C) and gene set enrichment analysis (Figure 5D) confirmed the upregulation of cell growth and proliferation-related processes and downregulation of cell death-related pathways by treatment of MSC-CM in Huh7 cells. These data further highlight the powerful trophic action of MSCs on liver cancer growth.
MSCs promote tumor growth in mice
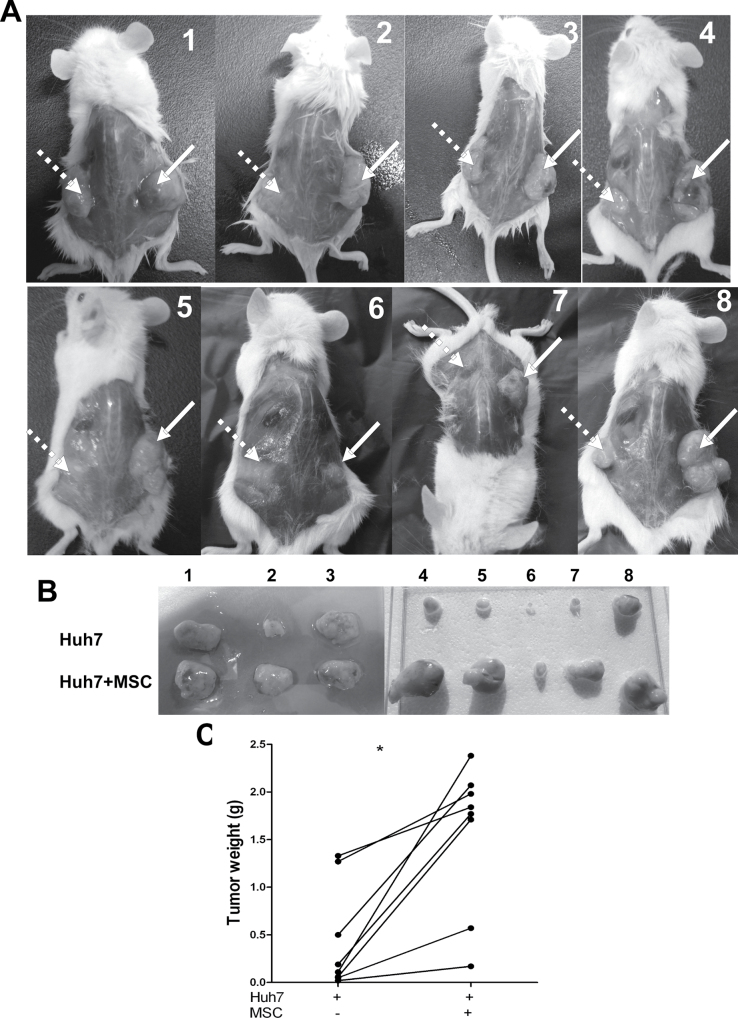
The in vitro studies described above strongly support the notion that MSCs can enhance tumor growth. To prove it, Huh7 cells with or without MSCs were subcutaneously injected into NOD/SCID mice at the left or right side of the same mouse. Among eight mice, three mice were injected with luciferase gene-labeled Huh7 cells. Therefore, the formation of tumors involved could be visualized in living animals by IVIS camera (Supplementary Figure 4, available at Carcinogenesis Online). After engraftment for 19–21 days, mice were killed for analysis of the solid tumors formed. As shown in Figure 6A, the tumors from the coinjected side (Huh7 with MSCs) were markedly bigger than those formed on the other side. This is consistent with the observation that the weight of the tumors coinjected with MSCs is significantly higher. The tumor weight was 1.56±0.27g (mean ± SEM) in the coengraftment group versus 0.44±0.19g in the Huh7-alone group (n = 8, P < 0.01; Figure 6B). Thus, cancer-derived MSCs can support tumor growth to a large extent.
Fig. 6.
MSCs promote tumor growth in mice. (A and B) Coengraftment of Huh7 with MSCs in mice resulted in larger tumors (right part) than engraftment of Huh7 alone (left part). (C) The tumor weight was 1.56±0.27g (mean ± SEM) in the coengraftment group versus 0.44±0.19g in the Huh7 alone group (n = 8, *P < 0.01).
Discussion
It is well recognized that the biology and pathology of cancer can only be understood by investigating individual specialized cell types and their cross talk within the tumor microenvironment (6). Recent studies have shown a possible involvement of MSCs as an important cellular element within the tumor microenvironment (26). In this study, we add to the existing knowledge by showing that STRO-1-positive MSCs are present in HCC at levels that are clearly higher than those observed in the surrounding tissue. Culturing these cells from resection material and subsequent investigation of their differentiation potential and cell surface marker repertoire confirmed the MSC status of these cells. The most straightforward interpretation of these results is that HCC actively recruits MSCs. This notion was supported by xenograft experiments, which shows that human HCC model cell line may actively recruit murine MSCs. We speculate that HCCs are subject to selection pressure that favors the acquisition of MSC-attracting properties. Although the mechanisms by which MSCs infiltrate into HCC are probably complicated, a potential key factor in this process could be hepatocyte growth factor, well known to be a potent chemoattractant for MSCs (27), which is produced at high levels by most HCC cell lines (28). Furthermore, human hepatocyte growth factor is active in mice and can thus transcend the species barrier, as we observed the presence of mouse MSCs in human HCC cell line-formed tumors in mice (Figure 3). Other cytokines too probably contribute to HCC-dependent recruitment of MSCs (29). The observation that HCCs are under apparent selection pressure to recruit MSCs into the tumor environment already provides a first hint of the importance of these cells for HCC growth.
There is a lively debate in the literature whether MSCs exert a pro- or anticancer action (19). Several studies reported antitumor effects (12–14), whereas others demonstrated tumor-promoting effects (15–17) of MSCs, depending on the particular cancer model and the methodologies applied. Our observation that HCCs are enriched with MSCs points to an important pro-oncogenic action. In addition, in this study, we show that tumor-associated MSCs produce trophic effects on HCCs through the production of soluble factors. Genome-wide gene expression profiles confirmed the upregulation of cell growth and proliferation-related processes and downregulation of cell death-related pathways by treatment of MSC-CM in hepatoma cells. Finally, coengraftment of human HCC-associated MSCs substantially promoted tumor growth in a xenograft model of HCC. Thus, for at least HCCs, the role of MSCs in the cancer process seems unequivocally pro-oncogenic. MSCs secrete paracrine factors, including a variety of growth factors that are known to influence tumor proliferation, migration and angiogenesis (19), which may explain the tumor support by MSCs observed in the present study. Although MSCs have been reported to support the tumor vasculature, directly by differentiating into pericytes and perhaps endothelial cells (30) and through indirect mechanisms by secreting vasculogenic growth factors (31), immunohistochemical staining and western blotting analysis of CD146 (angiogenesis marker) in the tumors formed in mice, however, showed no clear difference between the groups engrafted with or without MSCs (Supplementary Figure 5, available at Carcinogenesis Online). The effects of MSCs on HCC thus do not seem to involve improved vascularization and the effects seen on colony growth are more dominant. Interleukin 6 (IL-6), one of the cytokines secreted by MSCs, has been shown to promote formation of colorectal tumors in mice (32). We confirmed the secretion of IL-6 by MSCs using proteomic analysis of the secretome of MSCs (data not shown). However, neither adding exogenous IL-6 (Supplementary Figure 6, available at Carcinogenesis Online) nor neutralizing MSC-produced IL-6 (Supplementary Figure 7, available at Carcinogenesis Online) by antibody affected colony formation of Huh7 cells. These results excluded the involvement of IL-6 in our models. Conceivably, the pathways by which MSCs affect tumor growth are rather complicated as MSCs secrete >500 proteins as revealed by our proteomic analysis (data not shown). Innovative and high-throughput technologies are probably required to further elucidate this system of biological interaction between MSCs and tumor cells.
In addition, other mechanisms such as exosomes (or microvesicles) produced by MSCs may also be involved in this process. Several studies have demonstrated that MSCs can secrete exosomes that can result in a cell-to-cell transfer of messenger RNA, microRNA and proteins (33). Exosomes derived from BM MSCs have been shown to facilitate multiple myeloma progression (34) and to promote gastric carcinoma growth (35), whereas others have shown that exosomes from BM MSCs are able to inhibit growth of glioma (36), hepatoma, Kaposi’s sarcoma or ovarian tumor in animal models (37). However, the exact roles and mechanisms of MSC-produced exosomes in tumor biology remain largely elusive. Immunomodulation, another important feature of MSCs, could also have a drastic influence on the tumor microenvironment (38), although the current tumor models (mainly xenograft models in immunodeficient mice) are not able to properly evaluate these effects. Extensive studies have demonstrated the expression of several Toll-like receptors by MSCs (39), which are known to be critically linked with innate and adaptive immunity. It has been described that the activation of certain Toll-like receptors can polarize MSCs to switch from a predominantly immune-suppressive MSC2 (Toll-like receptor3-primed) to a proinflammatory MSC1 (TRL4-primed) phenotype (40,41). Furthermore, another study has shown that MSC1-based therapy attenuates tumor growth, whereas MSC2 treatment promotes tumor growth and metastasis (42). Thus, the immunomodulatory property of MSCs deserves more attention in tumor biology.
Many clinical applications of MSCs are proposed, either as therapeutic agents in their own right (immunomodulating and favoring outcome in transplantation and autoimmune medicine, for instance) or as anticancer drug or gene vehicles. The present study argues for caution. Clinical application of MSCs for treating liver diseases is currently being investigated in efforts to harness the hepatic differentiation potential, anti-inflammatory function and immunomodulatory properties of these cells. An early study involved the infusion of autologous BM cells, which include the MSC population, for treating decompensated liver cirrhotic patients, including HBV- and HCV-infected patients (43). More recently, using ex vivo cell-expanded MSCs, either hepatic differentiated or undifferentiated MSCs were used to treat liver cirrhotic patients (10,44). In addition, MSCs were also used for immunomodulation therapy of patients after liver transplantation (45). Such studies almost unavoidably involve patients who are positive for HBV or HCV. These infections are, however, not only important drivers of cirrhosis but are also important for developing HCC (46). Of note, HCC is an important indication for liver transplantation (47), but liver transplant patients also have increased incidence of developing de novo cancer (48). Despite the short-term safety reported by these clinical trials, the findings of the present study advise caution in the application of MSCs in such patients and call for vigilant surveillance in patients with high risk of developing HCC but already treated with MSCs.
In summary, this study demonstrated that HCCs are enriched with MSCs, which in turn provide trophic support for tumor growth. These results shed new light on the cross talk between MSCs and liver cancer cells and caution the therapeutic application of MSCs for liver cancer and other liver diseases with high risk of developing malignancy. Conceivably, targeting tumor MSCs may represent an innovative therapeutic approach against liver cancer.
Supplementary material
Supplementary Table 1 and Figures 1–7 can be found at http://carcin.oxfordjournals.org/
Funding
Netherlands Organization for Scientific Research (VENI- 916-13-032 to Q.P.).
Conflict of Interest Statement: None declared.
Supplementary Material
Glossary
Abbreviations:
- BM
bone marrow
- HBV
hepatitis B virus
- DMEM
Dulbecco’s modified Eagle’s medium
- HCC
human hepatocellular carcinoma
- HCV
hepatitis C virus
- IL-6
interleukin-6
- MSC
mesenchymal stem cell
- MSC-CM
MSC-conditioned medium.
References
- 1. Aquino J.B., et al. (2010). Mesenchymal stem cells as therapeutic tools and gene carriers in liver fibrosis and hepatocellular carcinoma. Gene Ther., 17, 692–708 [DOI] [PubMed] [Google Scholar]
- 2. Chen X.C., et al. (2006). Prophylaxis against carcinogenesis in three kinds of unestablished tumor models via IL12-gene-engineered MSCs. Carcinogenesis, 27, 2434–2441 [DOI] [PubMed] [Google Scholar]
- 3. Matar P., et al. (2009). Immunotherapy for liver tumors: present status and future prospects. J. Biomed. Sci., 16, 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Llovet J.M., et al. (2008). Molecular targeted therapies in hepatocellular carcinoma. Hepatology, 48, 1312–1327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Théret N., et al. (2001). Increased extracellular matrix remodeling is associated with tumor progression in human hepatocellular carcinomas. Hepatology, 34, 82–88 [DOI] [PubMed] [Google Scholar]
- 6. Hanahan D., et al. (2011). Hallmarks of cancer: the next generation. Cell, 144, 646–674 [DOI] [PubMed] [Google Scholar]
- 7. Holz L.E., et al. (2008). Liver tolerance and the manipulation of immune outcomes. Inflamm. Allergy Drug Targets, 7, 6–18 [DOI] [PubMed] [Google Scholar]
- 8. Friedenstein A.J., et al. (1974). Precursors for fibroblasts in different populations of hematopoietic cells as detected by the in vitro colony assay method. Exp. Hematol., 2, 83–92 [PubMed] [Google Scholar]
- 9. Keating A. (2012). Mesenchymal stromal cells: new directions. Cell Stem Cell, 10, 709–716 [DOI] [PubMed] [Google Scholar]
- 10. Gao Z., et al. (2013). Mesenchymal stem cells: a potential targeted-delivery vehicle for anti-cancer drug, loaded nanoparticles. Nanomedicine., 9, 174–184 [DOI] [PubMed] [Google Scholar]
- 11. Dai L.J., et al. (2011). Potential implications of mesenchymal stem cells in cancer therapy. Cancer Lett, 305, 8–20 [DOI] [PubMed] [Google Scholar]
- 12. Khakoo A.Y., et al. (2006). Human mesenchymal stem cells exert potent antitumorigenic effects in a model of Kaposi’s sarcoma. J. Exp. Med., 203, 1235–1247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Qiao L., et al. (2008). Suppression of tumorigenesis by human mesenchymal stem cells in a hepatoma model. Cell Res., 18, 500–507 [DOI] [PubMed] [Google Scholar]
- 14. Zhu Y., et al. (2009). Human mesenchymal stem cells inhibit cancer cell proliferation by secreting DKK-1. Leukemia, 23, 925–933 [DOI] [PubMed] [Google Scholar]
- 15. Karnoub A.E., et al. (2007). Mesenchymal stem cells within tumour stroma promote breast cancer metastasis. Nature, 449, 557–563 [DOI] [PubMed] [Google Scholar]
- 16. Galiè M., et al. (2008). Mesenchymal stem cells share molecular signature with mesenchymal tumor cells and favor early tumor growth in syngeneic mice. Oncogene, 27, 2542–2551 [DOI] [PubMed] [Google Scholar]
- 17. Kucerova L., et al. (2010). Tumor cell behaviour modulation by mesenchymal stromal cells. Mol. Cancer, 9, 129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Yan X.L., et al. (2013). Hepatocellular carcinoma-associated mesenchymal stem cells promote hepatocarcinoma progression: role of the S100A4-miR155-SOCS1-MMP9 axis. Hepatology,10.1002/hep.26257 [DOI] [PubMed] [Google Scholar]
- 19. Klopp A.H., et al. (2011). Concise review: Dissecting a discrepancy in the literature: do mesenchymal stem cells support or suppress tumor growth? Stem Cells, 29, 11–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Pan Q., et al. (2011). Mobilization of hepatic mesenchymal stem cells from human liver grafts. Liver Transpl., 17, 596–609 [DOI] [PubMed] [Google Scholar]
- 21. Pedroza-Gonzalez A., et al. (2013). Activated tumor-infiltrating CD4+ regulatory T cells restrain antitumor immunity in patients with primary or metastatic liver cancer. Hepatology, 57, 183–194 [DOI] [PubMed] [Google Scholar]
- 22. Lin Y., et al. (1995). Differential in situ hybridization for determination of mutational specific expression of the p53 gene in human hepatoma cell lines. Pathology, 27, 191–196 [DOI] [PubMed] [Google Scholar]
- 23. Pan Q., et al. (2008). Synergistic antitumor activity of XIAP-shRNA and TRAIL expressed by oncolytic adenoviruses in experimental HCC. Acta Oncol., 47, 135–144 [DOI] [PubMed] [Google Scholar]
- 24. Kolf C.M., et al. (2007). Mesenchymal stromal cells. Biology of adult mesenchymal stem cells: regulation of niche, self-renewal and differentiation. Arthritis Res. Ther., 9, 204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ning H., et al. (2011). Mesenchymal stem cell marker Stro-1 is a 75 kd endothelial antigen. Biochem. Biophys. Res. Commun., 413, 353–357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. D’Souza N., et al. (2012). MSC and tumors: homing, differentiation and secretion influence the therapeutic potentials. Adv Biochem Eng Biotechnol.,10.1007/10_2012_150 [DOI] [PubMed] [Google Scholar]
- 27. Vogel S., et al. (2013). Migration of mesenchymal stem cells towards glioblastoma cells depends on hepatocyte-growth factor and is enhanced by aminolaevulinic acid-mediated photodynamic treatment. Biochem. Biophys. Res. Commun., 431, 428–432 [DOI] [PubMed] [Google Scholar]
- 28. Ogunwobi O.O., et al. (2011). Hepatocyte growth factor upregulation promotes carcinogenesis and epithelial-mesenchymal transition in hepatocellular carcinoma via Akt and COX-2 pathways. Clin. Exp. Metastasis, 28, 721–731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Senst C., et al. (2013). Prospective dual role of mesenchymal stem cells in breast tumor microenvironment. Breast Cancer Res. Treat., 137, 69–79 [DOI] [PubMed] [Google Scholar]
- 30. Al-Khaldi A., et al. (2003). Postnatal bone marrow stromal cells elicit a potent VEGF-dependent neoangiogenic response in vivo . Gene Ther., 10, 621–629 [DOI] [PubMed] [Google Scholar]
- 31. Roorda B.D., et al. (2009). Bone marrow-derived cells and tumor growth: contribution of bone marrow-derived cells to tumor micro-environments with special focus on mesenchymal stem cells. Crit. Rev. Oncol. Hematol., 69, 187–198 [DOI] [PubMed] [Google Scholar]
- 32. Tsai K.S., et al. (2011). Mesenchymal stem cells promote formation of colorectal tumors in mice. Gastroenterology., 141, 1046–1056 [DOI] [PubMed] [Google Scholar]
- 33. Biancone L., et al. (2012). Therapeutic potential of mesenchymal stem cell-derived microvesicles. Nephrol. Dial. Transplant., 27, 3037–3042 [DOI] [PubMed] [Google Scholar]
- 34. Roccaro A.M., et al. (2013). BM mesenchymal stromal cell-derived exosomes facilitate multiple myeloma progression. J. Clin. Invest., 123, 1542–1555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Zhu W., et al. (2012). Exosomes derived from human bone marrow mesenchymal stem cells promote tumor growth in vivo . Cancer Lett., 315, 28–37 [DOI] [PubMed] [Google Scholar]
- 36. Katakowski M., et al. (2013). Exosomes from marrow stromal cells expressing miR-146b inhibit glioma growth. Cancer Lett., 335, 201–204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Bruno S., et al. (2013). Microvesicles derived from human bone marrow mesenchymal stem cells inhibit tumor growth. Stem Cells Dev., 22, 758–771 [DOI] [PubMed] [Google Scholar]
- 38. Hass R., et al. (2012). Mesenchymal stem cells as all-round supporters in a normal and neoplastic microenvironment. Cell Commun. Signal, 10, 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Raicevic G., et al. (2010). Inflammation modifies the pattern and the function of Toll-like receptors expressed by human mesenchymal stromal cells. Hum. Immunol, 71, 235–244 [DOI] [PubMed] [Google Scholar]
- 40. Waterman R.S., et al. (2010). A new mesenchymal stem cell (MSC) paradigm: polarization into a pro-inflammatory MSC1 or an Immunosuppressive MSC2 phenotype. PLoS One, 5, e10088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Dayan V., et al. (2011). Mesenchymal stromal cells mediate a switch to alternatively activated monocytes/macrophages after acute myocardial infarction. Basic Res. Cardiol., 106, 1299–1310 [DOI] [PubMed] [Google Scholar]
- 42. Waterman R.S., et al. (2012). Mesenchymal stem cell 1 (MSC1)-based therapy attenuates tumor growth whereas MSC2-treatment promotes tumor growth and metastasis. PLoS One, 7, e45590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Terai S., et al. (2006). Improved liver function in patients with liver cirrhosis after autologous bone marrow cell infusion therapy. Stem Cells, 24, 2292–2298 [DOI] [PubMed] [Google Scholar]
- 44. El-Ansary M., et al. (2012). Phase II trial: undifferentiated versus differentiated autologous mesenchymal stem cells transplantation in Egyptian patients with HCV induced liver cirrhosis. Stem Cell Rev., 8, 972–981 [DOI] [PubMed] [Google Scholar]
- 45. Popp F.C., et al. (2011). Safety and feasibility of third-party multipotent adult progenitor cells for immunomodulation therapy after liver transplantation–a phase I study (MISOT-I). J. Transl. Med., 9, 124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. El-Serag H.B. (2012). Epidemiology of viral hepatitis and hepatocellular carcinoma. Gastroenterology, 142, 1264–1273.e1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Samuel D., et al. (2011). Toward optimizing the indications for orthotopic liver transplantation in hepatocellular carcinoma. Liver Transpl., 17 suppl. 2), S6–S13 [DOI] [PubMed] [Google Scholar]
- 48. Tjon A.S., et al. (2010). Increased incidence of early de novo cancer in liver graft recipients treated with cyclosporine: an association with C2 monitoring and recipient age. Liver Transpl., 16, 837–846 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.