Abstract
Established risk factors for pancreatic cancer, including tobacco smoking, chronic pancreatitis, obesity and type 2 diabetes, collectively account for less than half of all pancreatic cancer cases. Inflammation plays a key role in pancreatic carcinogenesis, but it is unclear what causes local inflammation, other than pancreatitis. Epidemiological data suggest that Helicobacter pylori may be a risk factor for pancreatic cancer, and more recently, data suggest that periodontal disease, and Porphyromonas gingivalis, a pathogen for periodontal disease, may also play a role in pancreatic carcinogenesis. Individuals with periodontal disease have elevated markers of systemic inflammation, and oral bacteria can disseminate into the blood, stomach, heart and even reach the brain. These infections may contribute to the progression of pancreatic cancer by acting jointly with other pancreatic cancer risk factors that impact the inflammation and immune response, such as smoking and obesity, and the ABO genetic variant, recently linked to pancreatic cancer through genome-wide association studies. The complex interplay between bacteria, host immune response and environmental factors has been examined closely in relation to gastric cancer, but new research suggests bacteria may be playing a role in other gastrointestinal cancers. This review will summarize the literature on epidemiological studies examining infections that have been linked to pancreatic cancer and propose mechanistic pathways that may tie infections to pancreatic cancer.
Introduction
In 2013, it was estimated that 45220 Americans will be diagnosed with pancreatic cancer and that 38460 Americans will die from this cancer, making it the most common fatal cancer after lung, colorectal and breast cancer (1). Pancreatic cancer is a rapidly fatal and devastating disease; only 26% of patients survive 1-year postdiagnosis and 6% survive 5 years (1). Symptoms for pancreatic cancer, such as weight loss or abdominal pain, are non-specific, and consequently more than half of patients are diagnosed at a distant stage of disease, where 5-year survival is 2% (1). Given that many patients are diagnosed late in the development of the disease, surgery is often not an option; treatment with radiation therapy or chemotherapy is mostly used for palliative care. Early detection of pancreatic cancer would provide the best opportunity to increase survival rates, but to date there are no biomarkers or screening tools available to be used on a population level. Understanding the etiology of pancreatic cancer is necessary to identify high-risk groups and to improve our ability to identify biomarkers of early detection.
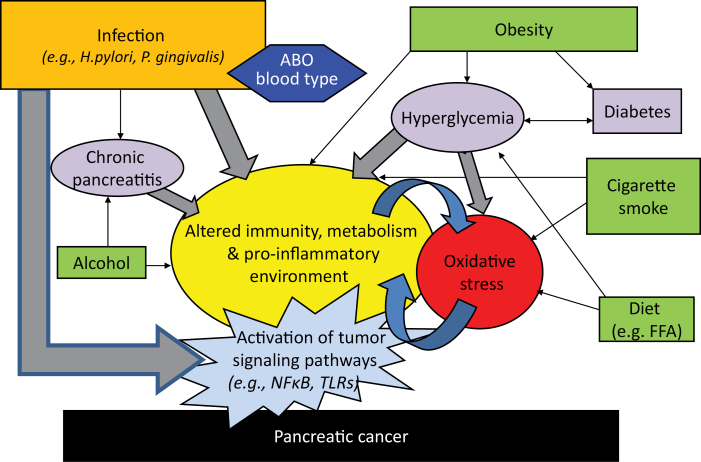
A growing number of studies suggest that there may be an underlying infectious component to pancreatic cancer etiology. However, unlike other cancers that have a known infectious cause, it is possible that infections that have been linked to pancreatic cancer impact tumor progression, and the tumor microenvironment, as opposed to having a direct impact on tumor initiation. In this review, I summarize the epidemiologic data that implicate various bacterial pathogens, and medical conditions linked to infections, to pancreatic carcinogenesis. In addition, I propose a mechanistic framework for their involvement in this cancer and interaction with other known risk factors (Figure 1).
Fig. 1.
Interplay between chronic bacterial infection, environmental and genetic factors and impact on pancreatic carcinogenesis.
Descriptive epidemiology and geographic trends
Examining patterns in incidence rates over time and across geographical regions can provide insights on potential causal risk factors that can subsequently be tested using more vigorous study designs. When it comes to infectious agents, geographical patterns in pathogen prevalence and cancer incidence rates have helped identify many associations that were later shown to be causal, such as hepatitis B and C viruses and liver cancer, schistosomiasis and bladder cancer, and Epstein–Barr virus and nasopharyngeal cancer. Changes in the prevalence of human papilloma virus have also been closely linked to changes in rates of cervical cancer. Geographic or time trends in pancreatic cancer rates have not yielded many clues into causal risk factors for this cancer. In the USA, male and female incidence rates for pancreatic cancer have been relatively stable for the past four decades (1). International variations are particularly difficult to interpret for pancreatic cancer given the large differences in diagnostic and detection capacities and also due to varying degrees of diagnosis confirmation across countries. Within the USA, regions with higher rates tend to align with higher smoking prevalence rates, especially for men. Nevertheless, lack of time trends and geographic patterns does not exclude the possibility that bacteria play a role in the etiology of pancreatic cancer.
Established environmental risk factors
Risk factors of pancreatic cancer account for <40% of all tumors (2); thus, efforts to improve our understanding of the environmental causes of this cancer are critical to making progress in prevention and early detection. Tobacco smoke is an established risk factor for pancreatic cancer; current smokers (and recent quitters—within past 5 years) have ~80% higher risk of pancreatic cancer than non-smokers (3). Obesity is now a well-accepted risk factor of pancreatic cancer given the overwhelming number of cohort studies that have consistently reported higher risk with elevated weight; in a pooled study of 14 prospective cohort studies, a 47% higher risk of pancreatic cancer was observed among obese individuals (body mass index > 30kg/m2) compared with those with a healthy body mass index (21–22.9kg/m2) (4). Similarly, a meta-analysis of 23 prospective cohort studies reported a 10% with each 5 unit increment in body mass index (5). Type 2 diabetes is a widely accepted risk factor for pancreatic cancer despite the long-standing concern that it is not causal (as diabetes type 2 can manifest as a consequence of the cancer). Pooled studies examining latency period have shown that a 50% increase in risk is observed among individuals who were diagnosed with type 2 diabetes 10 or more years prior to cancer diagnosis compared with those with no history of diabetes (6). Moreover, additional evidence for the role of diabetes comes from studies examining the relation between prediagnostic glucose levels and pancreatic cancer; elevated postload or fasting glucose levels have been consistently associated with a higher risk of pancreatic cancer in four cohort studies with 10–25 years of follow-up (7,8). In one study, the association between glucose levels (across non-diabetic ranges) and pancreatic cancer was stronger among cases whose blood had been collected 10 or more years prior to cancer diagnosis (8). Finally, chronic pancreatitis is an established risk factor for pancreatic cancer and is discussed in the next section; however, as it is a rare condition, it accounts for few cases of pancreatic cancer (<1.5%) (9).
Impact of known pancreatic cancer risk factors on immune response
The impact of smoking on the immune response is well established and includes altered innate immune response and suppressed humoral response (10,11), including decreasing immunoglobin G levels against oral bacteria (12). Excess adiposity has also been shown to alter immune response and decrease host defense to infection (13); mechanisms for increased risk of infections among obese individuals are still being elucidated. Finally, diabetes has been linked to altered immune response (14) and may act through Toll-like receptors (TLRs) directly to activate inflammatory pathways (15).
Role of inflammation
Inflammation plays a key role in pancreatic cancer (16); smoking and obesity, both established risk factors of pancreatic cancer, may increase risk by causing systemic inflammation. The best-established connection between local inflammation and pancreatic cancer comes from studies on pancreatitis (inflammation of the pancreas). Very high rates of pancreatic cancer have been observed in patients with chronic pancreatitis (17–19). Incidence rates of pancreatic cancer in patients with chronic pancreatitis were 160% higher than those observed in healthy populations; the risk remains high even after removing patients who developed cancer within the first 5 years after chronic pancreatitis diagnosis [standardized incidence ratio (SIR) = 14, 95% confidence interval (95% CI) = 8–23] (17). In case–control studies, however, where adjustment of other risk factors of pancreatic cancer is made, relative risks for pancreatitis are lower (after removing the first 2 years between pancreatitis diagnosis and cancer diagnosis—to avoid reverse causation and misdiagnosis). A pooled analysis of 10 studies with >5000 pancreatic cancer cases reported a <3-fold increase in risk [odds ratio (OR) = 2.71, 95% CI = 1.96–3.74], although the association was closer to 4-fold higher among those <65 years old (9). The most common cause of chronic pancreatitis is alcoholism, but other causes include biliary tract disease, hereditary and tropical pancreatitis. Associations observed with hereditary pancreatitis are even higher than those observed for chronic pancreatitis (20) and could be due to the fact that hereditary pancreatitis develops at a very early age (usually <21 years). Pancreatic cancer has also been associated with tropical pancreatitis, a condition that is also diagnosed at early ages (SIR = 100, 95% CI = 37–218, compared with the general population) (21).
Helicobacter pylori infection and peptic ulcers
Several epidemiologic studies reported positive associations between Helicobacter pylori and pancreatic cancer, raising the possibility that bacteria may play a role in the etiology of this cancer. The first report of an association between H. pylori and pancreatic cancer risk came from a case–control study with an extremely small control group (n = 27); a 2-fold increase in risk was observed (OR = 2.1, 95% CI = 1.09–4.05) (22). In the Alpha-Tocopherol, Beta-Carotene Cancer Prevention Study, a prospective cohort study of male smokers, men positive for H. pylori antibodies or CagA-positive strains had elevated risk of pancreatic cancer compared with men who were seronegative for those antibodies (OR = 1.87, 95% CI = 1.05–3.34; OR = 2.01, 95% CI = 1.09–3.70, respectively) (23). Since the first two studies, three additional observational studies published results on H. pylori and pancreatic cancer risk (24–26); none of these were significantly associated with risk, although positive associations were observed in two studies (25,26). A recent meta-analysis on this topic reported an overall 38% increase in risk of pancreatic cancer for H. pylori seropositivity (OR = 1.38, 95% CI = 1.08–1.75) (27).
A number of epidemiological studies have examined the relationship between peptic ulcers and risk of pancreatic cancer. Results from cohort studies with large number of pancreatic cancer cases and detailed information on type of peptic ulcers (i.e. gastric versus duodenal) observed positive associations with gastric ulcers, but not duodenal ulcers (28,29). Although H. pylori infections are associated with both types of peptic ulcers, gastric ulcers are associated with low acid production, whereas duodenal ulcers are associated with hyperacidity; consequently, nitrosamine levels are higher in individuals with gastric ulcers and may explain the association with pancreatic cancer risk. Low acidity, however, also allows for the colonization of other bacteria, which may provide an opportunity for oral bacteria to move into the stomach and the gut.
Periodontal disease
Periodontal disease is an inflammatory disease of the gums (gingivalis), which can advance and lead to gum recession, soft tissue damage, bone loss and tooth loss (severe periodontitis). As with many chronic diseases, periodontal disease has multiple risk factors, including smoking and diabetes, and several bacteria have been linked to the severity and progression of periodontitis (30). Of the bacteria believed to be pathogenic in periodontal disease, Porphyromonas gingivalis has been extensively studied due to its unique ability to evade the immune response (31).
Positive associations between tooth loss or periodontitis and pancreatic cancer risk have been reported in four separate cohort studies (32–35). In the Alpha-Tocopherol, Beta-Carotene Cancer Prevention Study cohort, a 63% elevation in pancreatic cancer risk was reported for individuals who were edentulous at baseline compared with those with 0–10 teeth missing, after adjusting for smoking and other risk factors (32), but no data were collected on periodontal disease in that study. In a second cohort study, the National Health and Nutrition Examination Survey (NHANES) I Epidemologic Follow-up Study, individuals with periodontitis at baseline had a higher risk of fatal pancreatic cancer [relative risk (RR) = 1.77, 95% CI = 0.85–1.85] compared with those with healthy peri odontium and edentulous individuals had a 90% elevation in fatal pancreatic cancer (RR = 1.90, 95% CI = 0.95–3.81), after controlling for age and gender (33). However, this study did not provide relative risks adjusted for smoking in the analysis of pancreatic cancer (as the analysis was an exploratory study on periodontal disease and cancer) (33). In a recent analysis of the NHANES III data, a 4-fold increase in risk of pancreatic cancer was observed among those with severe periodontitis, although this was not statistically significant, likely due to small sample size (RR = 4.56, 95% CI = 0.93–22.29) (35).
A strong positive association between periodontal disease and pancreatic cancer was reported in a prospective cohort study of male health professionals (Health Professionals Follow-up Study) (34). In the Health Professionals Follow-up Study, participants self-reported tooth loss and periodontal disease at baseline and were subsequently followed for 16 years. During that period, 216 cases of pancreatic cancer were newly diagnosed. After adjusting for age, smoking, diabetes, body mass index and a number of other dietary factors, men with periodontal disease had a 64% higher risk of pancreatic cancer compared with those reporting no periodontal disease. Among never smokers, a 2-fold increase in pancreatic cancer risk was observed (RR = 2.09, 95% CI = 1.18–3.71), ruling out the possibility that the overall association was confounded by smoking. Furthermore, the association was stronger among dentists (RR = 1.91, 95% CI = 1.31–2.78), who more accurately report history of periodontal disease (36).
Porphyromonas gingivalis infection
The association between antibodies to periodontal pathogens and risk of pancreatic cancer was examined in the large European Prospective Investigation into Cancer cohort (37). In this prospective cohort, blood samples were stored on >385 000 men and women at baseline (i.e. prior to disease). Using a nested case–control study design of 405 pancreatic cancer cases and 410 controls, a >2-fold increase in risk of pancreatic cancer was observed among those with high levels of antibodies to a pathogenic strain of P. gingivalis (OR = 2.38, 95% CI = 1.16–4.90, comparing >200ng/ml versus ≤200 ng/ml) (37), after adjusting for known risk factors.
In the NHANES cohort study, elevated antibodies to P.gingivalis [≥69.1 enzyme-linked immunosorbent assay unit (EU), compared with <69.1 EU] were associated with a 3-fold increase risk of orodigestive cancer mortality (RR = 3.03, 95% CI = 0.99–9.31). Interestingly, removing subjects with clinically apparent periodontal disease only decreased the association slightly (RR = 2.25, 95% CI = 1.23–4.14). A separate examination of P. gingivalis with pancreatic cancer mortality could not be conducted in that study due to insufficient case numbers (35).
Proposed biological mechanisms
Recent mice studies on bacteria and gastrointestinal cancers show that bacteria do not need to be present in the tissue where the tumor develops, nor do they need to cause inflammation in the tumor tissue, in order to promote carcinogenesis (38), providing a new perspective on the role of bacteria in cancer (39).
Previous studies have discussed the role of nitrosamines, which are known carcinogenic compounds, as a potential mechanism for the association between H. pylori infection and pancreatic carcinogenesis (40). Moreover, Risch (41) recently postulated that the increase in pancreatic risk observed with H. pylori seropositivity is due to the complex interaction between H. pylori colonization, ABO blood group type and exposure to N-nitrosamine from diet and smoking. Because these carcinogenic compounds are elevated in oral cavities of patients with gum disease (42,43), these mechanisms may also apply to the associations observed with periodontal disease; the level of nitrate reduction in the mouth is the major factor explaining interindividual differences in endogenous nitrosamine formation (43) and is therefore critical to overall exposure to nitrosamines, especially among non-smokers.
Alternatively, systemic changes in inflammatory biomarkers as a result of periodontal disease may play a role in pancreatic cancer. Associations between inflammatory biomarkers and periodontal disease have been noted in a number of studies. In a cross-sectional analysis of 468 men free of cancer, diabetes or cardiovascular disease, significantly higher levels of C-reactive protein, low-density lipids and tissue plasminogen activator were observed in participants with periodontitis compared with those with healthy gums (44). In a similar cross-sectional analysis of women, C-reactive protein, low-density lipids, intracellular adhesion molecule 1 and E-selectin were higher among 98 participants with periodontal disease (one or more sites with ≥5mm bone loss) compared with 175 periodontally healthy subjects after adjustment for major confounders (45).
It is plausible that the systemic inflammation and impact on the immune response do not require local inflammation for activation of carcinogenesis given the findings for Helicobacter hepaticus and liver cancer (38). In this scenario, bacteria would not be directly causal, but the inflammatory response from the presence and progression of periodontal disease would interact with preexisting conditions in the pancreas.
Activation of inflammatory pathways by local bacterial infection
Bacterial dissemination of oral bacteria to other body habitats has been documented for multiple organisms. In both humans and animal models, oral bacteria can disseminate from the gingival tissue into the circulation (blood or lymphatics) (46). Moreover, P. gingivalis, Aggregatibacter actinomycetemcomitans, Tannerella forsythia and Treponema denticola have been detected in atheromatous plaques of patients with atherosclerosis (47–49). Porphyromonas gingivalis can survive in blood and host tissue and has been found in heart, liver, kidney and spleen (46). It has been hypothesized that P. gingivalis, and other oral bacteria, may have a significant role in these diseases by causing inflammation and promoting tissue degenerative processes. Infection with P. gingivalis in mice models of athlerosclerosis (ApoE−/−) indicates that these bacteria can induce chronic inflammation and activate the innate immune response via the TLR2 and TLR4 (46). TLRs are a group of pathogen-associated molecular pattern receptors that play an important role in innate immune signaling in response to microbial infection. Porphyromonas gingivalis lipopolysaccharide (LPS) can specifically activate host response through both TLR2 and TLR4. In addition, TLRs have been shown to inhibit apoptosis and promote tumor growth and angiogenesis (50), and TLR signaling plays an important role in pancreatic tumors, thereby providing a potential mechanistic link between direct microbial stimulation and pancreatic carcinogenesis.
The TLR4/MyD88 signaling pathway has been implicated in pancreatic carcinogenesis. TLR4 is overexpressed in human pancreatic adenocarcinoma (51). In vitro, LPS was shown to increase the invasive ability of the pancreatic cancer cells through the TLR4 pathway, whereas blockade of TLR4 or MyD88 decreased the LPS-dependent increase in invasion (52). In mice models, LPS was been shown to accelerate pancreatic carcinogenesis through TLR4 activation, whereas TLR4 inhibition, through the MyD88-independent pathway, resulted in protection against tumor growth (53).
Other TLR may also be important in pancreatic cancer. TLR7 is expressed in human pancreatic cancer tissue but not human normal pancreatic tissue and is expressed within the tumor microenvironment (54), which supports tumor growth and promotes metastasis (55). Overexpression of TLR7 was observed in transgenic mice models and TLR7 ligation in these mice resulted in vigorous tumor growth 3 weeks postligation by inducing stromal expansion (54). Furthermore, TLR7 ligation resulted in a series of changes in oncogene expression in line with expected changes in pancreatic carcinogenesis, such as loss of PTEN expression and activation of PI3K/AKT pathway, and inhibition of TLR7 prevented malignant progression or stromal expansion.
Currently, it is not known whether bacterial infections in the pancreas, or in other parts of the body, have an impact on pancreatic stellate cells. However, given the important role of activated pancreatic stellate cells on the formation of a hypotoxic microenvironment and subsequent tumor development (56), it will be important to examine whether bacterial infections play a role in activation of pancreatic stellate cells.
Activation of tumor signaling pathways through infections in other organs
Increasing evidence suggest that micro-RNA (miRNA) plays an important role in carcinogenesis (57). miRNAs are single-stranded, non-coding short oligonucleotide sequences of ~23 bases that regulate gene expression at the posttranscriptional level (58). In mammals, miRNAs regulate genes by binding to messenger RNA targets in 3′ untranslated regions in conjunction with the RNA-induced silencing complex and either cleave the target messenger RNA or simply bind and inhibit translation (59). miRNAs are important modulators of the innate immune response (60).
Certain miRNAs are induced during the macrophage inflammatory response and have the ability to regulate host cell responses to pathogens. In addition, pathogens themselves may regulate miRNA expression; for example, P. gingivalis can induce the expression of several miRNA in gingival epithelial cells. miRNA impact networks that control innate and adaptive immunity and apoptosis by regulating signaling pathways such as the TLR (60,61), cytokine signaling pathways (60) and Stat3 (62). Recent studies suggest that cells can secrete miRNAs (63) and these can be delivered into recipient cells where they can impact gene expression (64,65), thereby opening the possibility that immune-related miRNAs might be traveling to the pancreatic tissue, even if the pathogens are located elsewhere.
Moreover, recent studies show that the presence of H.hepaticus in the gut can have far reaching impact on the host by triggering innate and T helper 1-type adaptive immunity and activating nuclear factor-kappaB networks in the liver (31). The proposed mechanisms for these observations include uptake of bacterial and/or luminal toxins into portal circulation, increased secretion of proinflammatory cytokines, increased exposure to oxidative stress, altered energy dynamics and disruption of complements and other blood proteins (38).
The role of ABO genetic susceptibility
Since the genome-wide association studies identification of single-nucleotide polymorphisms in the ABO locus as top hits for pancreatic cancer (66,67), its role in carcinogenesis has been examined more closely. Numerous studies demonstrate that individuals with the A or B blood type have higher risk of pancreatic cancer compared with those with blood type O (68–71). It is likely that the modified risk associated with the ABO genetic susceptibility is related to immunity; however, the exact role of the antigens in tumorigenesis is unclear. Several studies suggest that carriers of A or B alleles are at higher risk of hepatitis B virus infection (71) or H. pylori infection (26). In a recent genome-wide association studies examining genetic predisposition for soluble intracellular adhesion molecule 1 levels, an inflammatory adhesion receptor that facilitates leukocyte adhesion and migration across the endothelium, the ABO locus (9q34.2) was strongly associated with soluble intracellular adhesion molecule 1 concentrations, suggesting the histoblood group antigens play a regulatory role in the inflammation process (72). The established role of ABO histoblood groups in both the immune response and pancreatic cancer risk supports the notion that infections are likely to play a role in pancreatic cancer development; the challenge remains to understand their interaction and the mechanisms through which they may lead to carcinogenesis.
Summary
Given the mounting epidemiologic evidence suggesting that bacterial are associated with pancreatic cancer and that new mechanistic pathways between bacteria and onset of disease are being quickly unraveled, the role of bacterial infection on pancreatic carcinogenesis requires closer attention. The role of the immune response is critical, and known risk factors of pancreatic cancer, smoking, obesity and diabetes, have the ability to suppress innate and humoral immune response and open the door to opportunistic infections. In addition, bacteria may affect host immune defense and cause inflammatory responses, which may promote carcinogenesis. More research in this area will likely lead to a better understanding of this highly fatal cancer and may bring insight into mechanisms that may result in new opportunities for early detection and/or treatment development.
Funding
National Institutes of Health/National Cancer Institute (R21 CA139193).
Conflict of Interest Statement: None declared.
Glossary
Abbreviations:
- CI
confidence interval
- LPS
lipopolysaccharide
- miRNA
micro-RNA
- OR
odds ratio
- RR
relative risk
- TLR
Toll-like receptor.
References
- 1. American Cancer Society (2013). Cancer Facts & Figures 2013. American Cancer Society, Inc., Atlanta, GA: [Google Scholar]
- 2. Parkin D.M., et al. (2011). 16. The fraction of cancer attributable to lifestyle and environmental factors in the UK in 2010. Br. J. Cancer, 105 (suppl. 2), S77–S81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lynch S.M., et al. (2009). Cigarette smoking and pancreatic cancer: a pooled analysis from the pancreatic cancer cohort consortium. Am. J. Epidemiol., 170, 403–413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Genkinger J.M., et al. (2011). A pooled analysis of 14 cohort studies of anthropometric factors and pancreatic cancer risk. Int. J. Cancer, 129, 1708–1717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Aune D., et al. (2012). Body mass index, abdominal fatness and pancreatic cancer risk: a systematic review and non-linear dose-response meta-analysis of prospective studies. Ann. Oncol., 23, 843–852 [DOI] [PubMed] [Google Scholar]
- 6. Ben Q., et al. (2011). Diabetes mellitus and risk of pancreatic cancer: a meta-analysis of cohort studies. Eur. J. Cancer, 47, 1928–1937 [DOI] [PubMed] [Google Scholar]
- 7. Gapstur S.M., et al. (2000). Abnormal glucose metabolism and pancreatic cancer mortality. JAMA, 283, 2552–2558 [DOI] [PubMed] [Google Scholar]
- 8. Stolzenberg-Solomon R.Z., et al. (2005). Insulin, glucose, insulin resistance, and pancreatic cancer in male smokers. JAMA, 294, 2872–2878 [DOI] [PubMed] [Google Scholar]
- 9. Duell E.J., et al. (2012). Pancreatitis and pancreatic cancer risk: a pooled analysis in the International Pancreatic Cancer Case-Control Consortium (PanC4). Ann. Oncol., 23, 2964–2970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Arcavi L., et al. (2004). Cigarette smoking and infection. Arch. Intern. Med., 164, 2206–2216 [DOI] [PubMed] [Google Scholar]
- 11. Barbour S.E., et al. (1997). Tobacco and smoking: environmental factors that modify the host response (immune system) and have an impact on periodontal health. Crit. Rev. Oral Biol. Med., 8, 437–460 [DOI] [PubMed] [Google Scholar]
- 12. Vlachojannis C., et al. (2010). Determinants of serum IgG responses to periodontal bacteria in a nationally representative sample of US adults. J. Clin. Periodontol., 37, 685–696 [DOI] [PubMed] [Google Scholar]
- 13. Milner J.J., et al. (2012). The impact of obesity on the immune response to infection. Proc. Nutr. Soc., 71, 298–306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Knapp S. (2013). Diabetes and infection: is there a link? —A mini-review. Gerontology, 59, 99–104 [DOI] [PubMed] [Google Scholar]
- 15. Dasu M.R., et al. (2012). Toll-like receptors and diabetes: a therapeutic perspective. Clin. Sci. (Lond.), 122, 203–214 [DOI] [PubMed] [Google Scholar]
- 16. Greer J.B., et al. (2009). Inflammation and pancreatic cancer: an evidence-based review. Curr. Opin. Pharmacol., 9, 411–418 [DOI] [PubMed] [Google Scholar]
- 17. Lowenfels A.B., et al. (1993). Pancreatitis and the risk of pancreatic cancer. International Pancreatitis Study Group. N. Engl. J. Med., 328, 1433–1437 [DOI] [PubMed] [Google Scholar]
- 18. Malka D., et al. (2002). Risk of pancreatic adenocarcinoma in chronic pancreatitis. Gut., 51, 849–852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Talamini G., et al. (1999). Incidence of cancer in the course of chronic pancreatitis. Am. J. Gastroenterol., 94, 1253–1260 [DOI] [PubMed] [Google Scholar]
- 20. Lowenfels A.B., et al. (1997). Hereditary pancreatitis and the risk of pancreatic cancer. International Hereditary Pancreatitis Study Group. J. Natl Cancer Inst., 89, 442–446 [DOI] [PubMed] [Google Scholar]
- 21. Chari S.T., et al. (1994). Risk of pancreatic carcinoma in tropical calcifying pancreatitis: an epidemiologic study. Pancreas, 9, 62–66 [DOI] [PubMed] [Google Scholar]
- 22. Raderer M., et al. (1998). Association between Helicobacter pylori infection and pancreatic cancer. Oncology, 55, 16–19 [DOI] [PubMed] [Google Scholar]
- 23. Stolzenberg-Solomon R.Z., et al. (2001). Helicobacter pylori seropositivity as a risk factor for pancreatic cancer. J. Natl Cancer Inst., 93, 937–941 [DOI] [PubMed] [Google Scholar]
- 24. de Martel C., et al. (2008). Helicobacter pylori infection and development of pancreatic cancer. Cancer Epidemiol. Biomarkers Prev., 17, 1188–1194 [DOI] [PubMed] [Google Scholar]
- 25. Lindkvist B., et al. (2008). A prospective study of Helicobacter pylori in relation to the risk for pancreatic cancer. BMC Cancer, 8, 321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Risch H.A., et al. (2010). ABO blood group, Helicobacter pylori seropositivity, and risk of pancreatic cancer: a case-control study. J. Natl Cancer Inst., 102, 502–505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Trikudanathan G., et al. (2011). Association between Helicobacter pylori infection and pancreatic cancer. A cumulative meta-analysis. JOP, 12, 26–31 [PubMed] [Google Scholar]
- 28. Bao Y., et al. (2010). History of peptic ulcer disease and pancreatic cancer risk in men. Gastroenterology, 138, 541–549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Luo J., et al. (2007). The risk of pancreatic cancer in patients with gastric or duodenal ulcer disease. Int. J. Cancer, 120, 368–372 [DOI] [PubMed] [Google Scholar]
- 30. Papapanou P.N. (1996). Periodontal diseases: epidemiology. Ann. Periodontol., 1, 1–36 [DOI] [PubMed] [Google Scholar]
- 31. Tribble G.D., et al. (2013). Genetic diversity in the oral pathogen Porphyromonas gingivalis: molecular mechanisms and biological consequences. Future Microbiol., 8, 607–620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Stolzenberg-Solomon R.Z., et al. (2003). Tooth loss, pancreatic cancer, and Helicobacter pylori . Am. J. Clin. Nutr., 78, 176–181 [DOI] [PubMed] [Google Scholar]
- 33. Hujoel P.P., et al. (2003). An exploration of the periodontitis-cancer association. Ann. Epidemiol., 13, 312–316 [DOI] [PubMed] [Google Scholar]
- 34. Michaud D.S., et al. (2007). A prospective study of periodontal disease and pancreatic cancer in US male health professionals. J. Natl Cancer Inst., 99, 171–175 [DOI] [PubMed] [Google Scholar]
- 35. Ahn J., et al. (2012). Periodontal disease, Porphyromonas gingivalis serum antibody levels and orodigestive cancer mortality. Carcinogenesis, 33, 1055–1058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Joshipura K.J., et al. (1996). Validity of a self-reported periodontal disease measure. J. Public Health Dent., 56, 205–212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Michaud D.S., et al. (2012). Plasma antibodies to oral bacteria and risk of pancreatic cancer in a large European prospective cohort study. Gut. http://gut.bmj.com/content/early/2012/09/17/gutjnl-2012-303006.abstract (5 August 2013, date last accessed). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Fox J.G., et al. (2010). Gut microbes define liver cancer risk in mice exposed to chemical and viral transgenic hepatocarcinogens. Gut., 59, 88–97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Rogers A.B. (2011). Distance burning: how gut microbes promote extraintestinal cancers. Gut Microbes., 2, 52–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Risch H.A. (2003). Etiology of pancreatic cancer, with a hypothesis concerning the role of N-nitroso compounds and excess gastric acidity. J. Natl Cancer Inst., 95, 948–960 [DOI] [PubMed] [Google Scholar]
- 41. Risch H.A. (2012). Pancreatic cancer: Helicobacter pylori colonization, N-nitrosamine exposures, and ABO blood group. Mol. Carcinog., 51, 109–118 [DOI] [PubMed] [Google Scholar]
- 42. Nair J., et al. (1996). Endogenous formation of nitrosamines and oxidative DNA-damaging agents in tobacco users. Crit. Rev. Toxicol., 26, 149–161 [DOI] [PubMed] [Google Scholar]
- 43. Shapiro K.B., et al. (1991). Quantitative relationship between oral nitrate-reducing activity and the endogenous formation of N-nitrosoamino acids in humans. Food Chem. Toxicol., 29, 751–755 [DOI] [PubMed] [Google Scholar]
- 44. Joshipura K.J., et al. (2004). Periodontal disease and biomarkers related to cardiovascular disease. J. Dent. Res., 83, 151–155 [DOI] [PubMed] [Google Scholar]
- 45. Joshipura K., et al. (2003). Periodontal disease and biomarkers related to cardiovascular disease (abstract). J. Dent. Res., 82, B-247. [DOI] [PubMed] [Google Scholar]
- 46. Hayashi C., et al. (2010). Review: pathogen-induced inflammation at sites distant from oral infection: bacterial persistence and induction of cell-specific innate immune inflammatory pathways. Mol. Oral Microbiol., 25, 305–316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Padilla C., et al. (2006). Periodontal pathogens in atheromatous plaques isolated from patients with chronic periodontitis. J. Periodontal Res., 41, 350–353 [DOI] [PubMed] [Google Scholar]
- 48. Cavrini F., et al. (2005). Molecular detection of Treponema denticola and Porphyromonas gingivalis in carotid and aortic atheromatous plaques by FISH: report of two cases. J. Med. Microbiol., 54, 93–96 [DOI] [PubMed] [Google Scholar]
- 49. Haraszthy V.I., et al. (2000). Identification of periodontal pathogens in atheromatous plaques. J. Periodontol., 71, 1554–1560 [DOI] [PubMed] [Google Scholar]
- 50. Rakoff-Nahoum S., et al. (2009). Toll-like receptors and cancer. Nat. Rev. Cancer, 9, 57–63 [DOI] [PubMed] [Google Scholar]
- 51. Zhang J.J., et al. (2010). Expression and significance of TLR4 and HIF-1alpha in pancreatic ductal adenocarcinoma. World J. Gastroenterol., 16, 2881–2888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Ikebe M., et al. (2009). Lipopolysaccharide (LPS) increases the invasive ability of pancreatic cancer cells through the TLR4/MyD88 signaling pathway. J. Surg. Oncol., 100, 725–731 [DOI] [PubMed] [Google Scholar]
- 53. Ochi A., et al. (2012). MyD88 inhibition amplifies dendritic cell capacity to promote pancreatic carcinogenesis via Th2 cells. J. Exp. Med., 209, 1671–1687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Ochi A., et al. (2012). Toll-like receptor 7 regulates pancreatic carcinogenesis in mice and humans. J. Clin. Invest., 122, 4118–4129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Feig C., et al. (2012). The pancreas cancer microenvironment. Clin. Cancer Res., 18, 4266–4276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Tang D., et al. (2013). Persistent activation of pancreatic stellate cells creates a microenvironment favorable for the malignant behavior of pancreatic ductal adenocarcinoma. Int. J. Cancer, 132, 993–1003 [DOI] [PubMed] [Google Scholar]
- 57. Iorio M.V., et al. (2012). microRNA involvement in human cancer. Carcinogenesis, 33, 1126–1133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Bartel D.P. (2009). MicroRNAs: target recognition and regulatory functions. Cell, 136, 215–233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Esquela-Kerscher A., et al. (2006). Oncomirs —microRNAs with a role in cancer. Nat. Rev. Cancer, 6, 259–269 [DOI] [PubMed] [Google Scholar]
- 60. Virtue A., et al. (2012). MicroRNAs and Toll-like receptor/interleukin-1 receptor signaling. J. Hematol. Oncol., 5, 66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Benakanakere M.R., et al. (2009). Modulation of TLR2 protein expression by miR-105 in human oral keratinocytes. J. Biol. Chem., 284, 23107–23115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Moffatt C.E., et al. (2011). Porphyromonas gingivalis induction of microRNA-203 expression controls suppressor of cytokine signaling 3 in gingival epithelial cells. Infect. Immun., 79, 2632–2637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Valadi H., et al. (2007). Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat. Cell Biol., 9, 654–659 [DOI] [PubMed] [Google Scholar]
- 64. Rechavi O., et al. (2009). Cell contact-dependent acquisition of cellular and viral nonautonomously encoded small RNAs. Genes Dev., 23, 1971–1979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Zhang Y., et al. (2010). Secreted monocytic miR-150 enhances targeted endothelial cell migration. Mol. Cell, 39, 133–144 [DOI] [PubMed] [Google Scholar]
- 66. Amundadottir L., et al. (2009). Genome-wide association study identifies variants in the ABO locus associated with susceptibility to pancreatic cancer. Nat. Genet., 41, 986–990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Wei P., et al. (2012). Insights into pancreatic cancer etiology from pathway analysis of genome-wide association study data. PLoS One, 7, e46887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Wolpin B.M., et al. (2009). ABO blood group and the risk of pancreatic cancer. J. Natl Cancer Inst., 101, 424–431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Wolpin B.M., et al. (2010). Pancreatic cancer risk and ABO blood group alleles: results from the pancreatic cancer cohort consortium. Cancer Res., 70, 1015–1023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Wolpin B.M., et al. (2010). Variant ABO blood group alleles, secretor status, and risk of pancreatic cancer: results from the pancreatic cancer cohort consortium. Cancer Epidemiol. Biomarkers Prev., 19, 3140–3149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Wang D.S., et al. (2012). ABO blood group, hepatitis B viral infection and risk of pancreatic cancer. Int. J. Cancer, 131, 461–468 [DOI] [PubMed] [Google Scholar]
- 72. Paré G., et al. (2008). Novel association of ABO histo-blood group antigen with soluble ICAM-1: results of a genome-wide association study of 6,578 women. PLoS Genet., 4, e1000118. [DOI] [PMC free article] [PubMed] [Google Scholar]