Abstract
Objective
To learn the attitudes and concerns of the local community on participating in research, infant feeding practices, and maternal nutrition in order to inform the design of a clinical trial in Lilongwe, Malawi on the safety and efficacy of antiretroviral and nutrition interventions to reduce postnatal transmission of HIV.
Design
Formative research methods were used, including semi-structured interviews, focus group discussions, home observations, and taste trials. Data were collected, analyzed, and incorporated into the protocol within three months.
Results
Participants were supportive of the clinical trial, although their overall understanding of research was limited. Mothers agreed that infants’ blood could be drawn by venipuncture, yet concern was raised about the amount of blood proposed to be collected from both infants and mothers. Data demonstrated that rapid breastfeeding cessation would be difficult and malnutrition could be a risk if infants were weaned early. Mothers selected a maternal supplement suitable for use in the clinical trial.
Conclusions
The protocol was rapidly modified to achieve cultural acceptability while maintaining study objectives. Without the formative research, several significant areas would have been undetected and may have jeopardized the implementation of the trial. Additional research was carried out to develop a meaningful informed consent process, the amount of blood collected was reduced to acceptable levels, and the protocol was modified to reduce the risk of malnutrition. Researchers who conduct clinical trials are encouraged to incorporate formative research into their protocol design to ensure participant understanding of the research, to safeguard participants, and to increase feasibility and acceptance of the clinical research in the community.
Keywords: Qualitative research, ethics, clinical trial, HIV, Africa
Introduction
Overview
Formative research is the systematic collection of information to inform an intervention, research, or policy. While formative research has been used to inform clinical trial protocols [1,2], the importance of such research and examples of study designs are not often published in the scientific literature. In this paper, we describe a formative research study involving semi-structured interviews, focus group discussions, home observations, and trials of nutrition supplements and practices to inform a clinical trial protocol on interventions to improve maternal nutrition and reduce postnatal transmission of HIV. Data collection and preliminary analysis were completed within three months and findings were immediately incorporated into the protocol. Comprehensive analyses followed to inform data collection instruments and counseling materials. Through examples, we will demonstrate the value of such research for improving the design of clinical trials.
Background
The Breastfeeding, Antiretroviral, and Nutrition (BAN) study
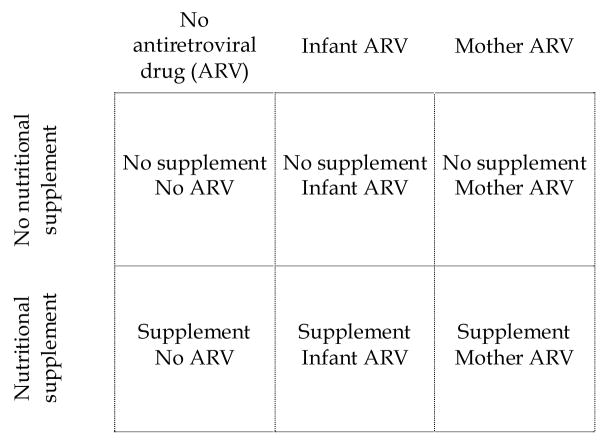
The BAN study is an un-blinded clinical trial in Lilongwe, Malawi, on the safety and efficacy of antiretroviral and nutritional interventions to reduce mother-to-child transmission of HIV during breastfeeding [3, 4]. The objectives are to determine the benefit of nutritional supplementation given to mothers during breastfeeding, the benefit and safety of antiretroviral medications given either to infants or to their mothers to prevent HIV transmission during breastfeeding, and the feasibility of exclusive breastfeeding followed by early, rapid breastfeeding cessation to prevent late postnatal HIV transmission. The study has a randomized 2-by-3 factorial design where mothers receive or do not receive a daily nutritional supplement, and mothers, infants, or neither receives daily antiretroviral prophylaxis for 24 to 28 weeks during the breastfeeding period (Figure 1). The trial is conducted in an urban area hospital setting and participants are individually randomized as individual participants are unlikely to know each other. Healthy breastfeeding mothers with a CD4 count >200 cells/μL and hemoglobin ≥ 7 g/dL and their HIV-negative newborns are eligible to enroll. All mothers receive infant feeding counseling and are advised to stop breastfeeding completely between 24 to 28 weeks post delivery. Enrollment began in March 2004.
Figure 1.
BAN study design
Infant feeding and nutrition considerations
Previous research has suggested that mother-to-child transmission of HIV during breastfeeding is higher when mothers practice mixed feeding (providing solids and liquids in addition to breast milk) compared to exclusive breastfeeding [5, 6], and the risk of HIV transmission continues as long as the mother breastfeeds [7, 8]. While early cessation of breastfeeding reduces the risk of HIV transmission, it may increase the likelihood of malnutrition and malnutrition-associated mortality if nutritionally adequate replacement foods are not available and regularly consumed [9]. Mothers in many parts of Africa, however, lack the resources necessary to purchase replacement foods regularly, face stigma and discrimination if they choose not to breastfeed, and have limited access to clean water that is needed to prepare replacement foods safely. For these reasons, WHO currently recommends exclusive breastfeeding for the first months of life and discontinuation of breastfeeding as soon as replacement feeding is acceptable, feasible, affordable, sustainable and safe for all HIV-positive mothers who chose to breastfeed [10].
In sub-Saharan Africa, neither exclusive breastfeeding nor early breastfeeding cessation are commonly practiced [11] and the non-HIV risks associated with early cessation are not known. The median duration of exclusive breastfeeding among mothers in Malawi is 2 months, and 54% of infants continue to breastfeed at 24 months [12]. While some mothers may be familiar with rapid breastfeeding cessation for reasons such as pregnancy or illness, rarely, if ever, does it occur before 28 weeks [13]. Malnutrition rates are unacceptably high in Malawi, with 49% of children less than 5 years suffering from stunting (low height-for-age) [12]. High rates of malnutrition are due in part to annual food shortages that sometimes reach crisis proportions. Breast milk is a major source of infant nutrition from birth through the first two years and locally available weaning foods are of poor nutritional quality, with low energy and micronutrient density [14]. Thus, early breastfeeding cessation would likely increase the likelihood of severe adverse events such as malnutrition and malnutrition-associated mortality if nutritionally adequate replacement foods were not regularly available and consumed [9,15].
Formative research objectives
With this background in mind, the formative research had three primary objectives: (1) to understand local infant feeding practices and to explore the feasibility of modifying them to prevent postnatal transmission of HIV; (2) to explore issues related to participation in the BAN clinical trial, such as attitudes toward participation in the study, being randomized to different interventions, and the collection of blood and other body fluid samples; and (3) to design a nutrition supplement for breastfeeding mothers.
The formative research was approved by the institutional review boards at the Centers for Disease Control and Prevention and University of North Carolina at Chapel Hill, and the National Health Science Research Committee in Malawi. All participants provided a written informed consent.
Methods
During June and July 2002, 72 semi-structured interviews were conducted with 40 HIV-positive mothers. Mothers participated in up to three interviews each, covering study participation, infant feeding, and maternal nutrition issues. Semi-structured interviews were also conducted in the homes of 35 mothers of undisclosed HIV status. Mothers participated in three interviews each and topics included infant feeding, maternal nutrition and dietary intake, and observation of hygiene and sanitation conditions. All mothers had infants less than 1 year old. Data on maternal and infant dietary intakes were collected by using a modified 24-hour recall methodology developed by Ferguson et al. [16].
Mothers visited at home also participated in trials of four different nutrition supplements to assess their acceptability. The supplements tested included: (1) a nutritionally fortified maize-soy blend; (2) a nutritionally fortified sweet biscuit; (3) a nutritionally fortified unsweetened biscuit; and (4) vitamin-mineral sprinkles to mix with the staple food (maize). Mothers were given a 2-day supply of each supplement and the follow-up interview was scheduled within this time period to assess their experience with each product. Mothers were counseled on how to prepare and consume each supplement and were also advised not to share the supplement with other family members or friends.
Additionally, 12 focus group discussions were conducted with 77 pregnant women, grandmothers, and fathers, and 33 semi-structured interviews were conducted with health providers, community leaders, and traditional birth attendants; a few of these interviews were conducted prior to the primary data collection period. Details on the sample and interview topics are provided in Table 1.
Table 1.
Study sample, methods, topics explored, and number of participants
| Sample | Method | Topics | Number of participants |
|---|---|---|---|
| HIV-positive mothers of infants less than 1 year old (women participated in up to three separate interviews) | In-depth interview | Study participation issues, including
|
23 |
| In-depth interview | Infant nutrition issues, including
|
27 | |
| In-depth interview | Maternal nutrition issues, including
|
22 | |
| Mothers of undisclosed HIV status of infants less than 1 year old (women participated in three separate interviews) | Home visit interviews |
|
35 |
| Mothers of undisclosed HIV status of infants less than 1 year old | Home visit interviews | Additional taste testing of maternal nutrition supplements | 17 |
| Pregnant women | Focus group discussion |
|
4 groups total; 25 participants |
| Grandmothers of grandchildren less than 1 year old | Focus group discussion | 4 groups total; 26 participants | |
| Fathers who have infants less than 1 year old | Focus group discussion | 4 groups total; 26 participants | |
| Health providers | In-depth interview |
|
19 |
| Traditional birth attendants | In-depth interview |
|
7 |
| Community leaders | In-depth interview |
|
7 |
Local AIDS support organizations and nurses from health clinics identified eligible mothers. Participants were selected from several geographic areas using non-probability, purposeful sampling strategies [17].
Eleven experienced Malawian interviewers conducted all interviews, focus group discussions, home observations, and taste trials. Before data collection, the interviewers participated in a 2-week training course. Question guides were translated into the local language, Chichewa, and back-translated into English. All interviews and focus group discussions were audiotaped, simultaneously transcribed and translated verbatim into English by the interviewer directly after the interviews and focus group discussions were conducted, and then subsequently reviewed by investigators. A two-day workshop with the formative research team was held immediately after data collection to formulate specific recommendations for protocol modification, followed by a meeting with the clinical trial team to discuss and incorporate protocol changes based on the research findings.
Comprehensive analyses followed for other study purposes, such as to inform behavioral-related questions on data collection forms and to create messages for nutrition counseling. Content analysis identified cultural, social, and behavioral themes and patterns related to the research topics following the analytical methods described by Miles and Huberman [18]. We created deductive codes based on research questions and data-derived codes for key concepts that emerged from the data. Transcripts were coded by multiple investigators using QSR NUD*IST Vivo software. Each code was then examined in detail for sub-themes and patterns across the interviews, and the most essential points were identified. Here we present data from focus group discussions and interviews with HIV-positive mothers and mothers of undisclosed HIV status. Data from interviews with health care providers are described elsewhere [19].
Results
Infant feeding
Twenty-seven HIV-positive mothers and 35 mothers of undisclosed status were interviewed about infant feeding practices and nutrition; dietary recalls were collected for infants of mothers of undisclosed status. Although all of the 27 HIV-positive mothers interviewed were currently breastfeeding their infants, only 50% knew about exclusive breastfeeding and even fewer mothers had ever practiced it. Approximately 85% of the HIV-positive mothers said they gave water or thin porridge to their infants before the fourth month, and 60%of the 17 mothers of undisclosed HIV status who had an infant less than 6 months old had already introduced foods and/or liquids other than breast milk into their infants’ diets by the fourth month. One mother said, “Traditionally, we are told that if a child cries, it’s a sign of hunger. Because my baby was crying, I thought breast milk was not adequate, so I started giving him water at 3 months.”
All HIV-positive mothers acknowledged the nutritional importance of breast milk, but one-half asserted that infants less than 6 months old “cannot grow properly with breast milk only.” Several mothers also believed that mothers would need additional foods to produce enough breast milk to breastfeed their infants exclusively for 6 months. In addition, several commented that HIV intensifies the nutritional needs of breastfeeding mothers. One mother explained: “When you have the virus, there’s a need to eat different types of food. For a breastfeeding mother, she can be sharing her vitamins with the baby, thus paving the way for the virus to multiply in the mother.”
Contrary to the cultural norm of breastfeeding infants through the second year of life, HIV-positive mothers said they would be willing to discontinue breastfeeding at 6 months to reduce transmission of HIV, but most expressed concern about their ability to purchase replacement foods. One mother said, “Many mothers will not manage to get extra food for the baby, and, as a result the baby may be malnourished.” Due to their inability to afford additional foods for their infants, several HIV-positive mothers were certain that infants would become malnourished if they were no longer given breast milk. One mother said, “The baby will suffer and start losing weight because breast milk is taken away.” As a result, many mothers believed that a mother’s ability to stop breastfeeding at 6 months of age depended on the availability of replacement foods. This finding was also mentioned repeatedly in the focus group discussions. As explained by a pregnant woman: “It depends if adequate amounts of other foods are available. If food is available, the baby will be all right. But, if food is not available, the baby can become malnourished.”
Concerns about the possibility of malnutrition are well substantiated. Findings from the modified 24-hour dietary recalls, which were conducted among infants who were breastfeeding, indicated very low energy intakes (mean, 397 kcal/day) from foods other than breast milk, particularly during the 6-to-8 month period after birth. Thus, complete removal of breast milk from the diet during this period without access to adequate replacement foods might lead to weight loss and severe malnutrition, given that the total energy requirement for a healthy infant is about 615 kcal/per day at this age [14].
Energy intakes from foods other than breast milk were higher in infants who were 9-to-11 months old (mean, 737 kcal/day) but the quality of the diet was very poor, particularly in terms of micronutrients. For example, the average daily iron intakes were 1.8 and 3.3 mg/day for infants aged 6-to-8 and 9-to-11 months old, respectively. The recommended daily intake of iron is 22 mg/day during this age period [14].
Similar to the cultural norm for weaning practices, HIV-positive mothers stressed that infants should be weaned gradually (within a few weeks) rather than rapidly (within a few days). Rapid cessation was perceived by many as damaging to infants and cruel, and therefore few supported it as a cessation method for HIV-positive mothers who choose to discontinue breastfeeding at 6 months. Focus group participants also did not support rapid cessation of breastfeeding. One grandmother described why rapid cessation is not acceptable: “A baby can become thin if you don’t stop breastfeeding gradually. This happens because sometimes the baby can be refusing the other foods that are given to it.“ One man explained, “When infants are introduced to new foods that they are not used to, it may cause an upset stomach.” Concerns about possible stigma associated with early breastfeeding cessation were mentioned, but this issue was not specifically linked to HIV status.
Study participation issues
Willingness to participate and perceived benefits of research
Twenty-three HIV-positive mothers were interviewed about study participation issues. Of these, all said they would join the BAN study if they were eligible at the time of enrollment but their reasons for wanting to participate demonstrated a limited understanding of the research. For example, even though all the mothers appeared to have understood that the purpose of providing antiretroviral drugs was to prevent infants from getting HIV during breastfeeding, few recognized that the purpose of the research was to determine whether the drugs were indeed safe and efficacious for this use, an entirely different concept. As a result, most HIV-positive mothers strongly believed that the physicians involved in the research would give them only medicines that had already been proven safe and efficacious in preventing HIV transmission during breastfeeding. As described by one mother, “Because the medicines were tested, the doctors know that the medicines are helpful.” Another mother said, “These medicines were made so that they should be given to babies to prevent the spread of HIV, so they are not harmful.” Responses were also indicative of the strong trust HIV-positive mothers have in doctors: “If the medicine is given by doctors, that means they know that the medicine is helpful and can prevent the baby [from getting HIV].”
Similarly, because of their belief in the effectiveness of the medicines, responses also reflected the faith HIV-positive mothers placed in the power of medicine. Accordingly, all but two of the 23 HIV-positive mothers interviewed about study participation expected that infants would obtain a therapeutic benefit from the study medicines. One mother said, “Because the baby is receiving medications, there is no reason why the baby should get HIV.” Another mother said, “Mothers will think that the research wants to assist them so that their babies should grow healthy.” In addition, more than three-fourths of HIV-positive mothers mistakenly believed that another purpose of the drugs used in the research was to “prolong the mothers’ lives.” One mother said, “Mothers will want to take the medicine so that they do not develop AIDS quickly.” Moreover, 30% of 23 HIV-positive mothers interviewed suggested they might share their medicines with their husbands, who were presumed to be HIV-positive.
Understanding of randomization
HIV-positive mothers’ understanding of the rationale for randomization was limited. The majority did not understand the purpose of placing study participants in different study conditions when all were HIV-infected. Limited understanding of randomization was also found among focus group participants, as described by a male participant:
“To me, it’s bad to put [people into] groups. Let’s say all of us here have diarrhea. If one is given the cure for the diarrhea and others aren’t, are you going to be happy? You cannot be happy. All of us should be given the medicine to cure the diarrhea. The same [is true] with mothers [in this research]. All have HIV. All need to receive the same medicine. If there are drugs to protect mothers, every mother must be given [them].”
Some HIV-positive mothers believed allocation of participants to study conditions was determined by the participant’s medical needs and not by chance. In response to a question that explored the rationale for randomization of the nutrition intervention, one mother explained, “Participants will think that they are healthy, and that’s why they are not given the food supplements.” Yet, other mothers perceived that preferential treatment determined who would be assigned to each study condition, as described by another mother: “Women will be questioning why they have not received the supplements that [others] received. They will feel that there is favoritism.” Those who understood that allocation to study conditions was based on chance thought it was unfair that some participants would not receive antiretroviral drugs or nutritional supplements. These findings further demonstrate the belief of a therapeutic benefit from participation in research and illustrate the complexity of explaining the purpose of research that uses antiretrovirals as prophylaxis against transmission.
Acceptable amount of biological specimens
All 23 HIV-positive mothers said that venous blood could be drawn regularly from mothers for research purposes, and 21 mothers felt that infants’ blood could also be drawn by venipuncture. Despite their agreement, concerns were raised. One mother said, “People will definitely be suspicious when large amounts of blood are drawn.” Some feared that their blood would be sold. Furthermore, 52% of 23 HIV-positive mothers interviewed indicated that the proposed amount of blood (50 ml) to be drawn from mothers was too much, and 47% indicated that the proposed amount from infants (15 ml) was also too much. One mother said, “The mother will despair and think that the baby will fall sick or she will run short of blood, and she will be sick now and then.” Focus group participants also shared these beliefs. For example, one man said, “The mother is sick, and therefore she needs a lot of blood. It is important that only a little amount of blood be drawn from her.” As a result, we asked participants to identify suitable amounts of blood quantity that would be acceptable to be drawn at each study visit. Few concerns were raised about breast milk samples from mothers and urine samples from infants.
Maternal nutrition supplement
Twenty-two HIV-positive mothers and 35 mothers of undisclosed status were interviewed about their nutrition in general; mothers of undisclosed status participated in the taste trials. Data on maternal nutrition among the HIV-positive mothers are published elsewhere [20]. Here we describe data on the maternal nutrition supplement. Mothers preferred the maize soy blend and the sweet biscuit. Due to difficulties in product supply, an additional taste test of a nutritionally fortified peanut butter paste was conducted with 17 women at a later date, and this product was also found acceptable. Although mothers were amenable to daily consumption of any of these foods, it was also clear that supplement sharing would occur with all of the products. For example, 88% of the 17 mothers interviewed said they would consume the peanut butter-spread daily, yet 53% shared it with their neighbors, family members, and/or children. Of those who did not share the supplement, 38% said it would be very difficult not to share it in the future because, as told by one mother, “[in our culture], food has to be shared, no matter how small it is.” Another mother said, “My children are also malnourished. I would share the supplements with them so they can also have good health.” We explored methods to reduce sharing, such as providing a small bag of maize for the rest of the family. While many mothers presumed that this could offset the sharing of the maternal supplement, several others believed the maternal supplement would still be shared.
In the end, we chose to use the peanut butter spread because supply and quality of this supplement were easiest to ensure, particularly with respect to micronutrients. Based on the information provided by mothers, the supplement was named “Nutrition for Breastfeeding Mothers,” to minimize any stigma associated with its use in the context of the study and to possibly reduce sharing. To further offset supplement sharing, all families are provided with a small bag of maize from the study. Food is also provided to participants from the World Food Programme, including additional maize, oil, and beans. Maternal supplement consumption and sharing is measured during follow-up visits.
Discussion
The formative research allowed us to rapidly identify and modify aspects of the clinical trial protocol in response to local attitudes, concerns, and circumstances, and it helped us to determine important additional areas of inquiry. Concerns about the quality of local weaning diets, and the impact of early breastfeeding cessation and infant malnutrition and health were highlighted and quantified in this research. Although WHO recommends early breastfeeding cessation for HIV-positive mothers, there is little guidance available on safe infant feeding once breastfeeding ends. To reduce the risk of malnutrition and non-HIV related morbidity and mortality that inevitably would result from early cessation of breastfeeding, we modified our protocol to include the provision of a locally produced ready-to-use food for replacement feeding until the child is 1 year of age [21]. Because the cultural norm in Malawi is to gradually wean infants from breast milk, we found resistance to rapid breastfeeding cessation to be very strong. In response, we modified the protocol to recommend cessation starting at 6 months of age, with the complete cessation by 7 months of age. Cessation over a 1-month period adds a small risk of HIV-transmission, approximately 0.0028% per day of breastfeeding, on average, accordingly to a study in Kenya [22]. The protocol for maternal and infant ARV prophylaxis and maternal nutrition supplementation were therefore modified accordingly so that these interventions are continued to 7 months of age. Moreover, the infant feeding counseling and support provided to study mothers were intensified to facilitate understanding and adoption of new feeding practices. To respond to participants’ concerns about the amount of blood to be drawn from mothers and infants, the amount was reduced to a quantity that was suitable to mothers but still adequate for laboratory analyses.
We also discovered that our ability to obtain a meaningful informed consent from study participants would be difficult. It was very clear that HIV-positive mothers and the broader community did not recognize the experimental nature of medical research, and mothers did not fully understand the antiretroviral intervention. For these reasons, we designed and carried out additional formative research and a small intervention trial on informed consent to learn how to better explain medical research and the purpose of the BAN study. Results from these studies will be published elsewhere.
In summary, using rapid formative research methods, we were able to collect and analyze qualitative data to inform the clinical trial protocol within three months. The formative research did not prolong completion of the protocol, and approximately 5% of the clinical trial year 1 budget was used. We believe the modifications and additions made to the protocol on the basis of the formative research findings not only improved the design of the research and safeguarded the health of study participants, but will also increase the acceptance of the research within the community, especially among trial participants. We identified areas in the protocol that had to be modified to achieve cultural sensitivity and acceptability while maintaining study objectives. We also incorporated new interventions to minimize any unplanned negative impact of our study on infants’ nutritional health and conducted additional research to maximize understanding of the research during the informed consent process. Without the formative research, we would have been unaware of the significant issues that would have jeopardized the implementation of this research. We encourage researchers who conduct clinical trials to incorporate formative research into the protocol design to ensure participant understanding of the research, to safeguard participants, and to increase feasibility and acceptance of the clinical research among participants and in the community.
Acknowledgments
This work was funded by the Prevention Research Centers Special Interest Project SIP 13-01 U48-CCU409660-09 and SIP 26-04 U48-DP000059-01, Centers for Disease Control and Prevention; supported by the NIAID P30-AI50410 UNC Center for AIDS Research; DHHS/NIH/FIC 2-D43 Tw01039-06 AIDS International Training and Research Program; Elizabeth Glaser Pediatric AIDS Foundation Call to Action Award and International Leadership Award, UNICEF, World Food Programme, Abbott Laboratories, Boehringer-Ingelheim, BristolMyersSquibb Company, GlaxoSmithKline, and Roche Pharmaceuticals, and the Ministry of Health and Population of Malawi as well as the support of the patients.
Funding/Support: This work was funded by the Prevention Research Centers Special Interest Project SIP 13-01 U48-CCU409660-09 and SIP 26-04 U48-DP000059-01, Centers for Disease Control and Prevention; supported by the NIAID P30-AI50410 UNC Center for AIDS Research; DHHS/NIH/FIC 2-D43 Tw01039-06 AIDS International Training and Research Program; Elizabeth Glaser Pediatric AIDS Foundation Call to Action Award and International Leadership Award, UNICEF, World Food Programme, Abbott Laboratories, Boehringer-Ingelheim, BristolMyersSquibb Company, GlaxoSmithKline, and Roche Pharmaceuticals, and the Ministry of Health and Population of Malawi as well as the support of the patients.
Human participant protection statement: This research was approved by the institutional review boards at the University of North Carolina at Chapel Hill, the Centers for Disease Control and Prevention, and the Health Science Research Committee in Malawi. All participants provided their written informed consent.
Footnotes
BAN Study Team: Linda Adair, Yusuf Ahmed, Mounir Ait-Khaled, Sandra Albrecht, Kant Bangdiwala, Ronald Bayer, Margaret Bentley, Emily Bobrow, Brian Bramson, Sal Butera, Charles Chasela; Charity Chavula, Maggie Chigwenembe, Joseph Chimerang’ambe, David Chilongozi, Grace Chiudzu, Ann Cole, Amanda Corbett, Amy Corneli, Ann Duerr, Henry Eliya, Yvonne Owens Ferguson, Susan Fiscus, Shannon Galvin, Chad Heilig, Irving Hoffman, Elizabeth Hooten, Tien Hsiao, Stacy Hurst, Denise J. Jamieson, George Joaki, David Jones, Gift Kamanga, Portia Kamthunzi, Cecilia Kanyama, Angela Kashuba, Damson Kathyola, Peter Kazembe, Rod Knight, Athena Kourtis, Robert Krysiak, Edde Loeliger, Misheck Luhanga, Alice Maida, Maganizo Majawa, Francis Martinson, Douglas Mayers, Isabel Mayuni, Marita McDonough, Ceppie Merry, Agnes Moses, Wezi Msungama, Beatrice Mtimuni, Jane Muita, Charles Mwansambo, Gerald Mwapasa, Jacqueline R. Nkhoma, Megan Parker; Richard Pendame, Ellen Piwoz, Byron Raines, David Reddy, John Rublein, Mairin Ryan, Diane Shugars, Dorothy Sichali, Alice Soko, Jean-Marc Steens, Gerald Tegha, Martin Tembo, Roshan Thomas, Beth Tohill, Charles van der Horst, Esther Waalberg, Chifundo Zimba.
Previous publication: These data have not been published elsewhere. They were presented at the 10th Conference on Retroviruses and Opportunistic Infections in February 2003.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Coreil J, Losikoff P, Incu R, et al. Cultural feasibility studies in preparation for clinical trials to reduce maternal-infant HIV transmission in Haiti. AIDS Educ Prev. 1998;10:46–62. [PubMed] [Google Scholar]
- 2.Tolley EE, Severy LJ. Integrating Social and Behavioral Research into Microbicide Clinical Trials: Challenges and Opportunities. Am J Pubic Health. 2005;96:79–83. doi: 10.2105/AJPH.2004.043471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gaillard P, Fowler MG, Dabis F, et al. Use of antiretroviral drugs to prevent HIV-1 transmission through breast-feeding: from animal studies to randomized clinical trials. J Acquir Immune Defic Syndr Human Retrovirol. 2004;35:178–187. doi: 10.1097/00126334-200402010-00013. [DOI] [PubMed] [Google Scholar]
- 4.van der Horst C, Fiscus S, Piwoz E, et al. Prevention of mother to infant transmission of HIV through breastfeeding and reduction of morbidity and mortality of the breastfeeding mothers: a study in Malawi. Antiviral Ther. 2003;8 (Suppl 1):S477. [Google Scholar]
- 5.Coutsoudis A, Pillay K, Spooner E, et al. Influence of infant-feeding patters on early mother-to-child transmission of HIV-1 in Durban, South Africa: a prospective cohort study. The Lancet. 1999;354:471–476. doi: 10.1016/s0140-6736(99)01101-0. [DOI] [PubMed] [Google Scholar]
- 6.Iliff PJ, Piwoz EG, Tavengwa NV, et al. Early exclusive breastfeeding reduces the risk of postnatal HIV-1 transmission and increases HIV-free survival. AIDS. 2005;19:699–708. doi: 10.1097/01.aids.0000166093.16446.c9. [DOI] [PubMed] [Google Scholar]
- 7.The Breastfeeding and HIV International Transmission Study Group (BHITS) Late postnatal transmission of HIV-1 in breastfed children: An individual patient data meta-analysis. JID. 2004;189:2154–2166. doi: 10.1086/420834. [DOI] [PubMed] [Google Scholar]
- 8.Miotti PG, Taha TE, Kumwenda NI, et al. HIV transmission from breastfeeding: a study from Malawi. JAMA. 1999;282:744–749. doi: 10.1001/jama.282.8.744. [DOI] [PubMed] [Google Scholar]
- 9.Pelletier DL. The relationship between child anthropometry and mortality in developing countries: implications for policy, programs, and future research. J Nutr. 1994;124:2047S–2081S. doi: 10.1093/jn/124.suppl_10.2047S. [DOI] [PubMed] [Google Scholar]
- 10.WHO/UNICEF/UNFPA/UNAIDS. HIV and Infant Feeding: Guidelines for Decision-makers. 2003. [Google Scholar]
- 11.Haggerty P, Rutstein S. DHS Comparative Studies, Number 30. Calverton (MD): Macro International; Jun, 1999. Breastfeeding and Complementary Infant Feeding, and the Post-Partum Effects of Breastfeeding. [Google Scholar]
- 12.National Statistical Office and ORC Macro. Malawi Demographic and Health Survey 2000. Zomba (Malawi) and Calverton (MD): 2001. [Google Scholar]
- 13.Piwoz EG, Huffman SL, Lusk D, et al. Issues, Risks, and Challenges Associated With Early Breastfeeding Cessation to Reduce Postnatal Transmission of HIV in Africa. Washington, DC: Academy for Educational Development; Aug, 2001. [Google Scholar]
- 14.Brown KH, Dewey K, Allen L. WHO/NUT/98.1. Geneva: World Health Organization; 1998. Complementary Feeding of Young Children in Developing Countries: A Review of Current Scientific Knowledge. [Google Scholar]
- 15.Piwoz EG, Ross J, Humphrey J. Human immunodeficiency virus transmission during breastfeeding: knowledge, gaps, and challenges for the future. Adv Exp Med Biol. 2004;554:195–210. [PubMed] [Google Scholar]
- 16.Ferguson EL, Gadowsky SL, Huddle JM, et al. An interactive 24-h recall technique for assessing the adequacy of trace mineral intakes of rural Malawian women; its advantages and limitations. Eur J Clin Nutr. 1995;49:565–578. [PubMed] [Google Scholar]
- 17.Patton MQ. Qualitative Evaluation and Research Methods. 3. Newbury Park (CA): Sage Publications; 2002. [Google Scholar]
- 18.Miles MB, Huberman AM. Qualitative Data Analysis: An Expanded Sourcebook. 2. Thousand Oaks (CA): Sage Publications; 1994. [Google Scholar]
- 19.Piwoz E, Owens-Ferguson Y, Bentley ME, et al. Differences between international recommendations on breastfeeding in the presence of HIV and the attitudes and counselling messages of health workers in Lilongwe, Malawi. International Breastfeeding Journal. 2006;1:2. doi: 10.1186/1746-4358-1-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bentley ME, Corneli AL, Piwoz E, et al. Perceptions of the role of maternal nutrition in HIV-positive breastfeeding women in Malawi. J Nutr. 2005;135:945–949. doi: 10.1093/jn/135.4.945. [DOI] [PubMed] [Google Scholar]
- 21.Sandige H, Ndekha MJ, Briend A, et al. Home-based treatment of malnourished Malawian children with locally produced or imported ready-to-use food. J Pediatr Gastroenterol Nutr. 2004;39:141–146.20. doi: 10.1097/00005176-200408000-00003. [DOI] [PubMed] [Google Scholar]
- 22.Richardson BA, John-Stewart G, Hughes JP, et al. Breast milk infectivity in human immunodeficiency virus type-1 infected mothers. J Infect Dis. 2003;187:736–40. doi: 10.1086/374272. [DOI] [PMC free article] [PubMed] [Google Scholar]