Abstract
Meibomian gland secretions (or meibum) are produced by holocrine meibomian glands and are secreted in melted form onto the ocular surface of humans and animals to form a protective tear film lipid layer (TFLL). Its protective effect strongly depends on the composition and, hence, thermotropic behavior of meibum. The goal of our study was to quantitatively evaluate the melting characteristics of human meibum and model lipid mixtures using differential scanning microcalorimetry. Standard calorimetric parameters, e.g. changes in calorimetric enthalpy, transition temperatures T(m), cooperativity of melting etc. were assessed. We found that thermotropic behavior of meibum resembled that of relatively simple mixtures of unsaturated wax esters, but showed a lower change in calorimetric enthalpy, which can be indicative of a looser packing of lipids in meibum compared with pure standards and their simple mixtures. The cooperativity of melting of meibomian lipids was comparable to that of an equimolar mixture of four oleic-acid based wax esters. We demonstrated that the phase transitions in meibum start at about 10 to 15 °C and end at 35-36 °C, with T(m) being about 30 °C. The highly asymmetrical shape of the thermotropic peak of meibum is important for the physiology and biophysics of TFLL.
Keywords: cooperativity, meibum, meibomian gland secretions, melting, phase transitions, microcalorimetry, lipids, wax esters
Introduction
The meibomian gland secretions (or meibum (Nicolaides et al., 1981)) are produced by holocrine meibomian glands that are located in the upper and lower eyelids of humans and many animals. Meibum is an integral part of the human tear film which covers the entire ocular surface of humans and animals alike. Meibum consists of a complex mixture of various lipids, and a variety of non-lipid compounds, e.g. proteins, peptides, salts, and other compounds. A wide range of lipids, such as wax esters (WE), cholesteryl esters, di- and triacylglycerols, (O-acyl)-ω-hydroxy fatty acids (OAHFA) and other di- and triesters, free cholesterol, free fatty acids, phospholipids, were identified in meibum (Butovich, 2011a; Chen et al., 2010; Lam et al., 2011). Amphiphilic lipids (such as OAHFA, diacylglycerols, free fatty acids, free cholesterol, etc.) are expected to form the lower sublayer, while nonpolar lipids form the upper portion that is on the surface of the tear film and is in contact with the air (Holly, 1973). This sandwiched structure is called the tear film lipid layer (TFLL). One of the acknowledged roles of TFLL is to slow down the evaporation of water from the ocular surface (Mishima and Maurice, 1961). Abnormal changes in the lipid composition of meibomian gland secretions can result in the tear film destabilization and increased evaporation rate, and can have detrimental changes on the melting and spreading characteristics of meibum.
Dry eye disease (DED) is one of such conditions. DED is a multifactorial disease of the tears and the ocular surface that results in symptoms of discomfort, visual disturbance, and the tear film instability. DED can, potentially, result in a serious damage to the ocular surface (2007a). Two different forms of DED are recognized: aqueous deficient dry eye and evaporated dry eye (Nelson et al., 2011). EDE (also defined as meibomian gland dysfunction, or MGD) is a chronic, diffuse abnormality of the meibomian glands, commonly characterized by terminal duct obstruction and/or qualitative and quantitative changes in the glandular secretion. The prevalence of MGD among DED patients appears to be high: MGD with glandular loss was a prominent feature in 61.1% with total meibomian gland loss being observed in 0.9% of the eyes in a Japanese study (Uchino et al., 2006), while an age-standardized prevalence of MGD was 56.3% in Singapore study (Siak et al., 2012). Recently, Lemp et al. reported that about 86% of DED patients evaluated in a multi-site study had signs of MGD, such as poor quality of meibum and the tear film instability, among others (Lemp et al., 2012). It is believed that one of the main reasons for the onset of EDE/MGD is the tear film destabilization possibly associated with changes in meibum lipid composition (Joffre et al., 2008).
Correlations between the dry eye syndrome and abnormalities of the tear film lipid layer have been studied using many techniques, among which various types of mass spectrometry have offered valuable information (Butovich, 2009, 2011a; Chen et al., 2010; Joffre et al., 2008; Lam et al., 2011; Saville et al., 2011; Shine and McCulley, 1993). It has been reported that meibum from MGD patients has shown significantly higher levels of branched-chain fatty acids and lower levels of saturated fatty acids, in particular lower levels of palmitic (C16:0) and stearic (C18:0) acids (Joffre et al., 2008; Joffre et al., 2009). The relative amount of cholesteryl esters increased in normal meibum with age and was 40% lower in MGD (Siak et al., 2012). It has been recognized that meibum of MGD patients is more solid than that of normal, non-DE controls (Arita et al., 2009). Borchman et al. recently confirmed those observations and reported that meibum of MGD patients had a 4 °C higher phase transition temperature than that of normal, age-matched controls (Borchman et al., 2011). Using elaborate spectroscopic experiments, the same authors also found that meibum of MGD patients contained relatively fewer cis–CH= and terminal −CH3 groups, which could increase its ordering, and, hence, lead to higher melting temperatures of abnormal meibum (Borchman et al., 2010). Reversible melting and crystallization of lipids is one of the basic phase transitions that lipids undergo in response to varying temperature. Therefore, in-depth studies of phase transitions in meibum could provide new and important insight on its biophysical and physiological properties, specifically with regard to the properties and functions of the tear film and TFLL.
The aims of this study was to characterize the phase transitions of lipids of human meibum and to compare those with individual standard wax esters and their mixtures, using differential scanning microcalorimetry (DSC).
Materials and Methods
Equipment and reagents
All the standard lipids used in this study were purchased from either Sigma Chemical Co. (St. Louis, MO) or Nu-Chek Prep. (Elysian, MN). Only HPLC or spectroscopy grade solvents from various manufacturers were used. A two-cell differential scanning VP-DSC microcalorimeter from GE/MicroCal (Piscataway, NJ) was used for microcalorimetric studies. A ThermoVac vacuum unit was also from GE/MicroCal. A Trace Ultra gas chromatograph equipped with an ITQ 1100 mass spectrometric detector from Thermo Electron (Waltham, MA) were used for GC-MS analyses of lipids.
Preparation and loading of standard lipids and their mixtures
Two different types of lipid samples loading procedures were tested. The first approach involved loading the dry lipids, while the second – loading their 10 mM chloroformic solutions. At the beginning of each experiment, both the sample cell and the control cell were washed with chloroform and then dried with a gentle stream of nitrogen at room temperature. Dry lipid samples were loaded directly into the DSC sample cell with a home-made insertion tool (“dry loading”). This approach did not involve any solvents, and the loaded sample was melted as is. “Wet loading”, on the other hand, was conducted by adding lipid solutions into the cell with a microsyringe. An aliquot of a lipid solution (typically, 25 or 50 μl of 10 mM stock solution of lipid in chloroform, i.e. 0.25 or 0.5 μmol of the analyte) was loaded into the cell using a microsyringe with a 10-cm needle (Hamilton, USA). Then the solvent was evaporated in a stream of nitrogen at room temperature for about 30 minutes and the cell was vacuumized for another 35 to 40 min using the ThermoVac. The dryness of the samples was critical for the success of the experiments – even small amounts of solvents, if left in the cell, affected the shapes of thermograms. Fortunately, the presence of traces of solvents could be easily spotted by evaluating the initial part of thermograms: if the solvent was present, the baseline deviated from a flat line, the melting curve broadened, and the intensity of the signal declined.
For individual pure lipids, a typical amount of loaded lipid was either 0.25 or 0.5 μmol per cell per experiment. For tested standard lipid mixtures, the amounts of individual components varied depending on the design of the experiments. Those amounts are indicated in the text and figure legends where appropriate. The sample solutions were stored in a −20°C freezer with no apparent deterioration during the course of the study.
To make lipid mixtures, stock solutions of individual compounds in chloroform were mixed in different molar ratios. The mixtures were loaded into the microcalorimeter as chloroformic solutions using a microsyringe as described above for individual lipid standards. The dry samples and stock solutions of individual lipids were stored at −20°C and were stable for many months.
Human meibum sample collection and loading
All the procedures were approved by the Institutional Review Board of the University of Texas Southwestern Medical Center and performed in accordance with the principles of the Declaration of Helsinki. Written informed consents were obtained. Two subjects (a male and a female; 50±2 years old) had no signs or symptoms of any ocular disease, nor were the subjects on hormonal therapy or anti-muscarinic medications. Collection of samples was conducted using a Zeiss surgery microscope under 7× magnification. Meibum was expressed only from lower eyelids. Before collecting samples, the donors’ eyelids were cleaned with a cotton swab and then meibum was expressed by soft-squeezing the eyelid with two cotton swabs on each side of the eyelid as illustrated earlier (Butovich, 2008).
The combined samples from the left and the right lower eyelids of a patient were dissolved in about 1 ml of chloroform placed in a HPLC-style glass vial. The dry, clean vial had been initially pre-weighed using an analytical balance. After the solvent has been evaporated to dryness, the vial with the sample was re-weighed to determine the weight of the collected sample. The average dry sample was dissolved in chloroform to give a 1 mg/ml solution. The samples then were kept at −20°C until use.
Typically, an aliquot of between 150 and 400 μg (or 150 to 400 μl of a 1 mg/ml solution) of human meibum was loaded into the sample cell. Meibum samples dissolved in chloroform were treated and analyzed as described above for solutions of standard lipids.
Sample measurement and data collection
DSC measurements were performed using a high sensitivity VP-DSC microcalorimeter. At least three individual samples of each standard compound or their mixtures were analyzed. At least three aliquots of each of meibum samples were analyzed. The scanning temperature range for standard lipids and lipid mixtures was set according to the literature data on the melting temperatures (Tm) of each specific standard lipid. Typically, the range was close to Tm ± 20 °C. The scan temperature range for human meibum was set to be from 3 °C to 40 °C. For pure lipids and lipid mixtures, the scan rates were programmed to be 10 °C/hour. The equilibration time before each successive scan of the same sample was 2 min. Thermograms (or “melting curves”) were recorded in cycles: for each sample run the scan program included at least three heating and three cooling stages that produced three thermograms.
Evaluation of microcalorimetric data
The DSC thermograms were evaluated in Origin software (v.7) as described in the MicroCal’s VP-DSC Tutorial Guide. At least three thermograms per sample recorded in cycles were analyzed. The first melting curve for a pure compound and the second curve for a lipid mixture were used in the analyses (see Results and Discussion sections below). Their melting temperatures Tm (also known as “midpoint temperatures”, “peak temperatures”, “transition midpoints”, or “melting points”), the widths of transition peaks (in degrees) at their half-heights (T1/2), the transition heat capacities (ΔCP), van’t Hoff enthalpy changes (ΔHv), calorimetric transition enthalpies (ΔHcal), cooperativity parameters σ, and the cooperative unit sizes n (Figure 1) were calculated where possible (see below) after the baseline correction. The parameters were calculated by averaging the data gained from several scans of the same compound or mixture. Data are presented as Mean ± Standard Error (M ± SE). The values of ΔHcal were determined either by integrating the experimental thermograms, after standard baseline correction procedures had been applied. The values of ΔHv were calculated either by using a non-2-state model (MN2State, from the MicroCal/Origin software package), or by using the following Equation 1 (Lewis et al., 2007):
| Eq. 1 |
where R is the universal gas constant (1.986 × 10-3 kCal × K-1 × mol-1). Also, from experimental thermograms, one can determine an important parameter which is called “cooperative unit size” [(Hinz and Sturtevant, 1972)] designated here as n. The cooperative unit size n is considered an estimate of the lowest number of molecules which form an independently melting cluster (or microcrystal) of lipid molecules within the sample. Mathematically, n equals ΔHv/ΔHcal (Hinz and Sturtevant, 1972; Lewis et al., 2007).
Figure 1.

A typical baseline-corrected DSC thermogram of a wax ester stearyl oleate and thermodynamic parameters computed from the data.
The quality of samples was monitored by using a combination of HPLC/MS and GC/MS techniques as described earlier (Butovich et al., 2012a; Butovich et al., 2012b). Tested human samples showed an average distribution of common lipids found in human meibum.
Results
Individual standard lipids and their transition temperatures Tm
Eleven authentic wax esters (lauryl laurate, myristyl laurate, myristyl myristate, palmityl laurate, palmityl palmitate, stearyl stearate, behenyl stearate, palmityl oleate, stearyl oleate, arachidyl oleate, and behenyl oleate) were selected and tested individually. Each lipid was analyzed using two different loading procedures – “dry loading” and “wet loading”. Individual lipids produced sharp and narrow endothermic peaks (or transitions) with Tm values that depended on the chemical structures of the lipids – the longer and the more saturated the lipid, the higher Tm (Figure 2, Panels A and B, and Table 1). Their baselines were smooth and flat, which facilitated the mathematical analysis and interpretation of the thermograms.
Figure 2.
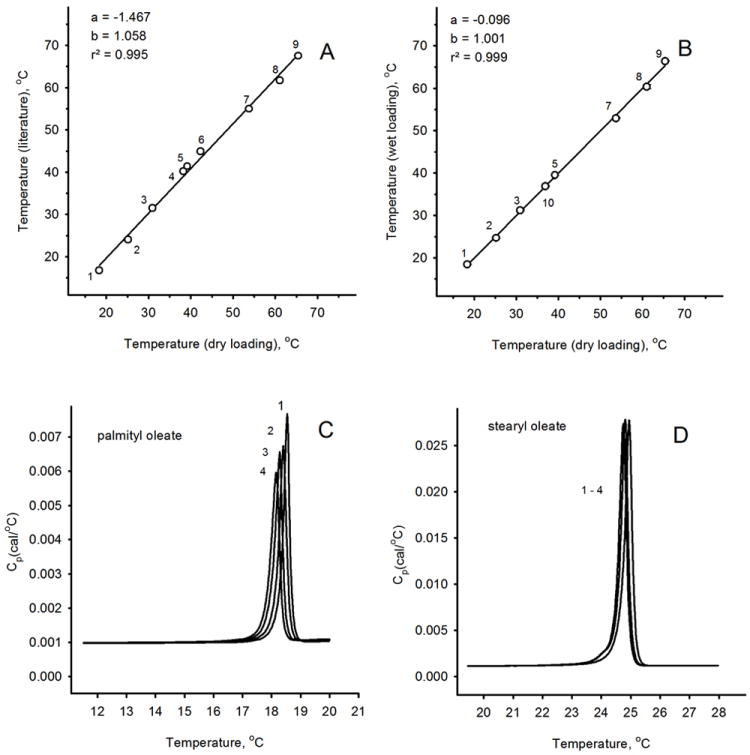
Validation of the experimental procedures.
Panel A. A correlation between the literature data on melting temperatures of standard wax esters and Tm values determined in experiments with dry-loaded samples.
Panel B. A correlation between the Tm values of standard wax esters determined using the “dry-loading” procedure and in experiments with chloroformic solutions of the analytes (“wet loading”).
Panel C. Raw, baseline-corrected thermograms of palmityl oleate obtained in four repetitive experiments are shown. Note an incremental, but unilateral, shift in Tm values toward lower temperatures with each repetitive cycle.
Panel D. Four repetitive raw, baseline-corrected thermograms of stearyl oleate are shown. The shift in Tm was much less noticeable than in experiments with palmityl oleate.
Table 1.
Properties of standard wax esters and meibum
| Wax ester | Structure, alcohol-acid | Molecular mass, Da | Tm (°C), literature | Ref. | Tm (VP-DSC), dry loading* | Tm (VP-DSC), wet loading* | ΔHcal, kCal/mole* | T1/2, °C | n |
|---|---|---|---|---|---|---|---|---|---|
| Palmityl oleate | 16:0-18:1 | 507 | 16.5-17.0 | (Iyengar, 1969) | 18.32±0.19 | 18.44±0.08 | 15.7 | 0.4 | 75 |
| Stearyl oleate | 18:0-18:1 | 535 | 23.8-24.2 | (Iyengar, 1969) | 25.17±0.01 | 24.68±0.18 | 20.1 | 0.4 | 60 |
| Arachidyl oleate | 20:0-18:1 | 563 | 31.5 | (Iyengar, 1969) | 30.94±0.28 | 31.19±0.09 | 15.0 | 0.4 | 110 |
| Behenyl oleate | 22:0-18:1 | 591 | n/a | n/a | 36.92±0.15 | 36.86±0.14 | 26.3 | 0.4 | 63 |
| Lauryl laurate | 12:0-12:0 | 369 | n/a | n/a | 28.61±0.10 | 28.02±0.11 | 14.2 | 1.2 | 64 |
| Myristyl laurate | 14:0-12:0 | 397 | 40-40.4 | (Iyengar, 1969) | 38.23±0.22 | 38.40±0.11 | 17.4 | 0.5 | 60 |
| Palmityl laurate | 16:0-12:0 | 425 | 41.2-41.6 | (Iyengar, 1969) | 39.16±0.11 | 39.49±0.05 | 16.9 | 0.3 | 102 |
| Myristyl myristate | 14:0-14:0 | 425 | 44.4-45.4 | (Iyengar, 1969) | 42.27±0.06 | 42.10±0.05 | 22.9 | 0.4 | 62 |
| Palmityl palmitate | 16:0-16:0 | 481 | 54-55 | (2007b; Patel, 2001) | 53.72±0.08 | 52.92±0.08 | 17.5 | 0.4 | 70 |
| Stearyl stearate | 18:0-18:0 | 537 | 61.6-61.9 | (Iyengar, 1969) | 61.03±0.21 | 60.35±0.48 | 32.2 | 0.5 | 51 |
| Behenyl stearate | 22:0-18:0 | 593 | 67.4-67.6 | (Iyengar, 1969) | 65.40±0.10 | 66.39±0.07 | 34.5 | 0.3 | 64 |
| Meibum | mixture | 700 (est.) | ~32 | (Butovich, 2008) | n/d | 30.20±0.60 | 6.4 ±1.3 | 9-12 | 8-13 |
Data presented as Mean ± SE; n/a – data not available; n/d – not determined
The reproducibility of the repetitive DSC analyses of two standard samples (namely, palmityl oleate and stearyl oleate) was tested by recording at least four thermograms of each sample in cycles (Figure 2, Panels C and D; “wet loading” procedure). The first thermograms for each of these wax esters produced Tm that were close to the results obtained using the “dry loading” procedure, and to the published melting points of wax esters (Figure 2, Panels A and B, Table 1). The values of Tm measured in our experiments and those published before showed a high degree of correlation (r2 values between 0.995 and 0.999). However, one needs to realize that the literature data on melting points (or ranges) of wax esters that we are using here were obtained in different laboratories using different batches of the compounds and different (often unspecified) analytical techniques. Moreover, a traditional technique of determining melting temperatures (or ranges) of lipids rely on determination of its “clearing point”, which is closer to the temperature which defines the end of the calorimetric peak rather than its Tm. Thus, in our project we relied mostly on our own data obtained microcalorimetrically for our own samples, and on their Tm, ΔHcal, ΔHv and cooperative unit sizes (n) specifically as the most well-defined and accurately measured or calculated analytical parameters.
The type of the sample loading procedure (“dry” vs. “wet”) did not alter the values of Tm: they were statistically indistinguishable. The latter indicated that the samples, regardless the experimental approach, had very similar, if not identical, properties, and did not decompose or alter chemically. These observations also justified the use of the “wet loading” procedure for samples that were difficult to handle in dry state because of their limited amounts, such as lipid mixtures and meibum.
The inter-sample reproducibility of Tm for different samples of the same compound was determined by testing at least 3 different samples of behenyl stearate three times each. The Tm of samples was found to be 65.4 ± 0.1 °C (sample variance 0.023).
However, repetitive cyclic melting of individual wax esters demonstrated a gradual and, typically, unidirectional shift of thermograms toward lower transition temperatures Tm accompanied by simultaneous broadening of the peaks for some lipids, but not for the others (Figure 2, Panels C and D). This change was observed regardless the type of the loading procedure. The magnitude of the shift depended on the nature of the lipid and the number of repetitive scans, and could vary from a few tenths of a degree to 1-3 °C. At this time, the nature of this phenomenon remains unknown. However, in microcalorimetric experiments multiple scans of the same sample traditionally are not performed, unless the goal of the experiment is to study stability of the sample, or to monitor a chemical reaction in the sample. Therefore, to determine if this shift was related to a chemical alteration of the analyte due to the experimental conditions, we recovered samples from the cell after completing the DSC experiments and analyzed them by GC/MS as described earlier (Butovich et al., 2012a). Importantly, no decomposition of the lipids was detected – chemically, the analytes remained intact (not shown). This conclusion was corroborated by calculating the calorimetric enthalpy (ΔHcal) of a standard wax ester – behenyl oleate: despite the shift in the values of Tm, the values of ΔHcal calculated from 13 successive thermograms remained virtually unchanged (ΔHcal = 27.4 ± 0.1 kCal/mol). Similarly tight distribution of ΔHcal, despite noted gradual and unidirectional shifts in the values of Tm (always toward lower Tm), was obtained for other tested compounds, such as behenyl stearate, arachidyl oleate, and palmityl laurate.
Next, it was observed that, in our hands, ΔHcal of all individually tested wax esters were close to each other: the mean enthalpy change ΔHcal was about 24.1 ± 5.6 kCal/mol for all compounds (Table 1), with the numbers for individual lipids varying from in a relatively narrow range of 17 to 34 kCal/mol.
Finally, all tested individual lipids melted abruptly, producing sharp transition peaks with T1/2 of 0.5 °C or so. This behavior indicated a cooperative nature of the melting process. By using the MN2State routine with just one transition temperature Tm, we were able to adequately model thermograms of individual lipids, and determined their Tm, ΔHcal, ΔHv and cooperative unit sizes (n)(as illustrated in Figure 1). We calculated n for all tested individual lipids (Table 1). Their cooperative unit sizes ranged from about 50 to about 100 with just a few outliers. No apparent correlation between the structures and cooperative unit size was found (Table 1): for all tested lipids, n was found to be 80.2 ± 35.3. However, as with Tm, the values of n for some compounds slightly decreased with each repetitive scan, indicating irreversible changes in their aggregation state during multiple melting/cooling cycles. Therefore, it was deemed reasonable to use the initial few thermograms as most useful for the purpose of calculating their Tm, ΔHcal, ΔHv and n.
Melting characteristics of mixtures of standard lipids
The thermotropic behavior of several mixtures of standard saturated and unsaturated wax esters was evaluated in cyclic melting/cooling experiments. As a rule of thumb, the first reproducible thermogram (typically, the second or the third) in each experiment were chosen to calculate the values of Tm and ΔHcal of the mixtures. The main reason for this choice is that the first melting/cooling cycle was considered to be of preparatory type for lipids to mix and equilibrate: the first thermograms were often very different from subsequent runs, and very irreproducible, while the following cycles gave much more coherent results (Figure 3).
Figure 3.
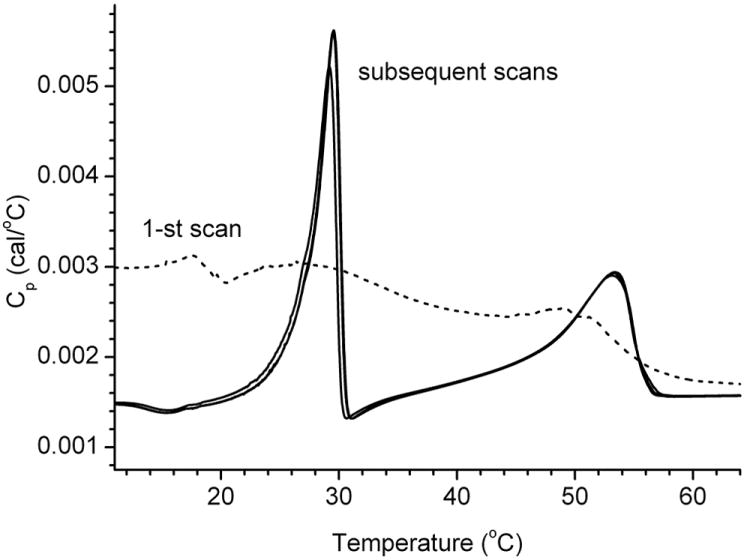
Thermograms of a four-component lipid mixture.
The mixture composition was as follows: arachidyl oleate:behenyl oleate:stearyl stearate:behenyl stearate = 1:1:0.5:0.5 (mol). The first melting/cooling cycle (labeled as 1-st scan) is considered to be a preparatory cycle during which the lipids mix, distribute, and assume their final shape inside the microcalorimeter’s cell. Note the bimodal nature of the thermogram. The ΔHcal of the peaks were comparable.
At first, simple equimolar binary mixtures were tested. A mixture of palmityl laurate and behenyl stearate systematically produced bimodal thermograms with two distinctively different Tm at about 37-38 and 60-61 °C (Figure 4). Note that the values of Tm of individual components of the mixture are 39.1 and 65.2 °C, respectively. Thus, a reproducible several-degree decline in melting temperatures of both lipids was observed. Even more interesting, their thermograms did not merge even after repetitive melting/cooling cycles, indicating that the compounds underwent phase transitions seemingly independently from each other, without creating a homogeneous mixture with intermediate properties. This behavior implied that these two wax esters coexisted as two virtually separate lipid phases. This was modeled using the MN2State model with two Tm of 37.8 and 60.2 °C (Figure 4).
Figure 4.

A sample thermogram of a binary mixture of palmityl laurate and behenyl stearate.
Solid line: experimental data; broken line: fitted curve. The curve-fitting procedure was performed using the ThermoCal/Origin software. Note the bimodal nature of the thermotropic transitions of the mixture. The ΔHcal of the peaks were close to each other. The thermograms were baseline-corrected.
Qualitatively similar behavior demonstrated a binary mixture of palmityl oleate and behenyl oleate – a bimodal pattern was observed with two clearly separate maxima at around 16 °C and 31 °C (not shown), again both being below the Tm of the corresponding individual components.
When a more complex mixture of four unsaturated wax esters – oleic acid esters of straight-chain C16:0, C18;0, C20:0, and C22:0 alcohols – was tested, a different pattern emerged (Figure 5, Panel A): their thermograms merged to produce just one, but very broad, transition peak. Importantly, the mixture started to melt between 10 and 14 °C – much lower than the Tm of its lowest-melting component – palmityl oleate (18.5 °C). The main transition reproducibly occurred at a very low Tm of 25 °C, and the melting was completed at about 27-28 °C, again much lower than the melting temperatures of pure arachidyl oleate (30.9 °C) and behenyl oleate (36.9 °C). An estimated T1/2 for this mixture was between 8 and 10 °C, while ΔHcal was calculated to be 16.2 kCal/mole. There is no doubt that the four tested lipids were able to mix with each other to form a more homogeneous phase than the one shown in Figure 4 for a binary mixture. Therefore, it seems that the presence of a sufficient number of components in the mixture with regularly distributed Tm (notice that an average step between Tm of tested waxes was about 6°C, Table 1) is needed for creating a relatively homogeneous mixture that undergoes smooth melting as a whole. Still, the resulting thermograms were highly asymmetric, which precluded using the non-2-state model with just one transition temperature for modeling, and necessitated their deconvolution.
Figure 5.
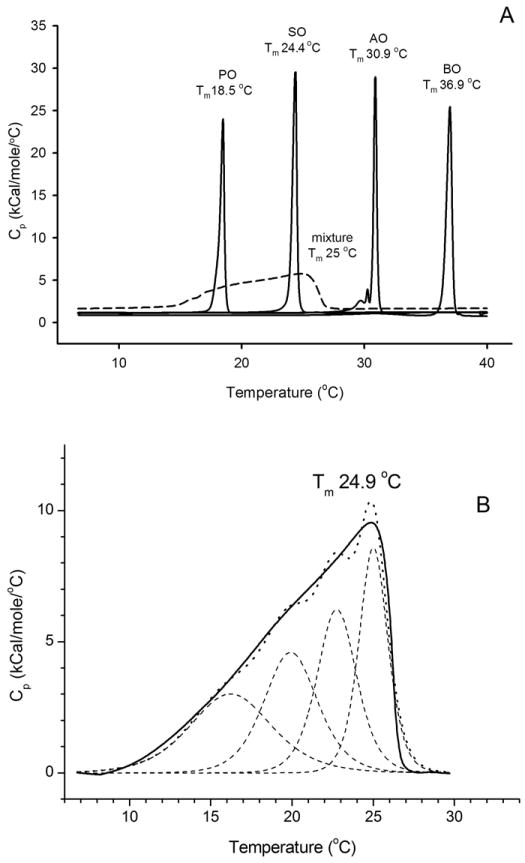
Effects of mixing of four unsaturated wax esters on their phase transition behavior.
Panel A. Raw thermograms of four unsaturated wax esters tested individually and as a four-component equimolar mixture. Tested lipids: palmityl oleate (PO), stearyl oleate (SO), arachidyl oleate (AO), and behenyl oleate (BO). Solid lines: individual wax esters; broken line: the mixture of four waxes. The thermograms were baseline-corrected.
Panel B. Deconvolution of a thermogram of the four-lipid mixture shown in Panel A. Note that at least four individually melting domains with comparable ΔHcal values are needed to adequately describe the experimental thermogram. For this particular mixture of four lipids, the following individual Tm were calculated: 16.2, 19.9, 22.8, and 25.0 °C. The value of T1/2 for the entire thermotropic peak was calculated to be about 8.4 min, Tm was 24.9 °C, while ΔHcal was 16 kCal/mole. Solid line: experimental data; broken line: deconvoluted peaks; dotted line: the sum of individual melting peaks.
The transitions peaks were deconvoluted to estimate the minimal number of individually melting components that are needed to adequately model the transition peaks of complex lipids mixtures similar to the one illustrated in Figure 5, Panel B. In our hands, to achieve a reasonable simulation, the minimal number of the components was three, or more.
A thermogram of another equimolar model mixture of four saturated waxes – palmityl laurate, palmityl palmitate, stearyl stearate, and behenyl stearate – is shown in Figure 6. The thermogram demonstrated a clearly bimodal behavior with two separate Tm, but required a model with at least four components with Tm of 35.2, 38.1, 50.3 and 53.8 °C to be adequately modeled. Notably, the carbon chain lengths of these lipids differed by four carbons, and the Tm values of the lowest melting and the highest melting individual components varied from about 39-40 °C to about 65-66 °C (i.e. were about 26 °C apart). Apparently, these differences were too large to allow these four wax esters to produce one merged transition peak.
Figure 6.
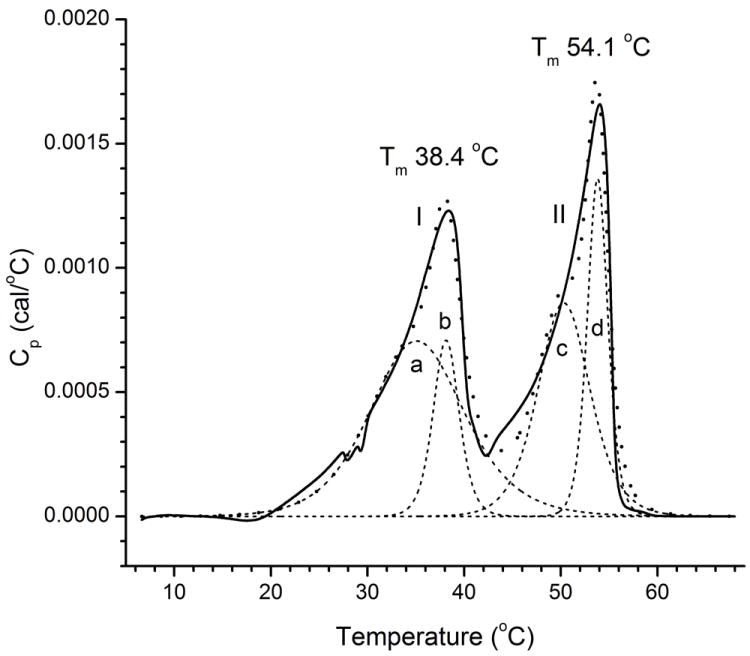
Effects of mixing of four saturated wax esters on their phase transition behavior.
Tested sample – an equimolar mixture of palmityl laurate, palmityl palmitate, stearyl stearate, and behenyl stearate. Note an approximately equal sizes of Peak I and Peak II. Solid line: experimental data; broken line: deconvoluted peaks I and II; dotted line: the sum of individual melting peaks a-d.
It was important to determine whether the properties of a wax ester mixture could be described as being additives of the properties of its components. In order to answer this question, the changes in enthalpies for four individual unsaturated wax esters were compared with ΔHcal of their equimolar mixture (Figure 7). It appeared that ΔHcal of the mixture was about 78% of the arithmetic sum of the enthalpy changes of its individual components. Considering a much broader shape of the thermographic peak of the mixture (and, correspondingly, larger errors in its integration) this difference can be considered to be relatively small, but reproducible.
Figure 7.
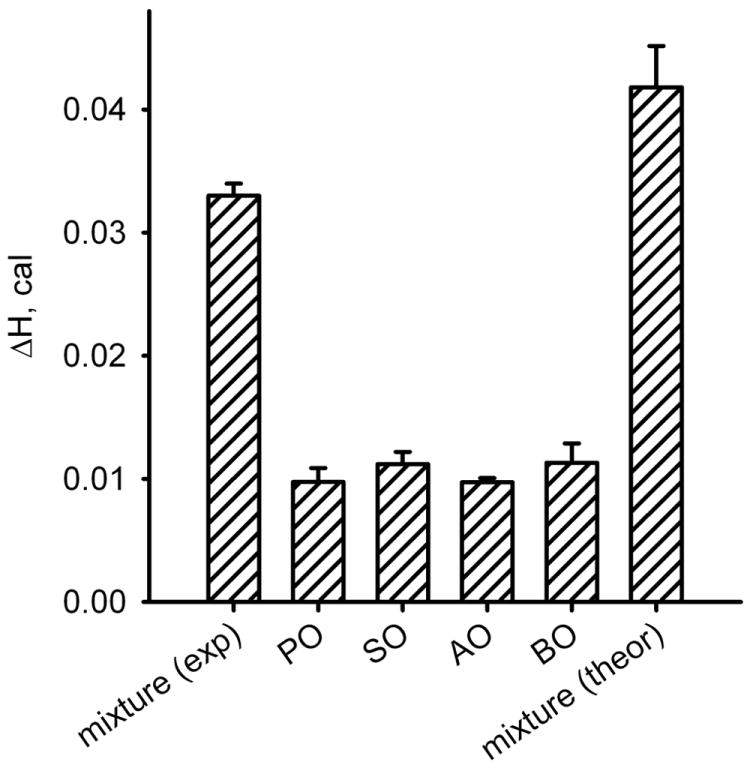
Effects of mixing of four unsaturated wax esters on ΔHcal.
Lipids were mixed in the equimolar ratio (0.5 μmol each). The experimental ΔHcal of the mixture (the left-most bar) was about 80% of the arithmetic sum of its individual components (the right-most bar).
The cooperative unit sizes (n) determined for lipid mixtures after deconvolution of thermograms, on the other hand, were much smaller than those for pure lipid standards and, typically, did not exceed n=10. While attempting to determine the apparent cooperativity unit size for the whole mixture by using Equation 1 without prior deconvolution of the thermogram, one need to realize that, because of the highly asymmetrical shape of the thermograms of mixtures, the resulting numbers should be treated only as rough estimates. However, the values of T1/2 (a parameter reliably measurable in an experiment) routinely were about 10 °C or so.
Human meibum
Meibum samples from two normal, non-dry eye donors – a male and a female – were studied as described above for lipid standards and their mixtures. Considering a very small size of non-pooled human samples (typically, between 0.2 and 1 mg each), the samples were loaded as chloroformic solutions.
The intra-sample reproducibility of the experiments was tested by running repetitive melting-cooling experiments of the same sample in cycles. From Figure 8, Panel A one can see that the repetitive thermograms were very reproducible. The thermotropic behavior of human meibum was qualitatively similar to the transitions observed in experiments with artificial lipid mixtures composed of oleic acid-based wax esters: transition peaks of meibum were wide and asymmetrical. Next, the thermograms were averaged using the MicroCal/Origin “Averaging multiple scans” routine, and baseline-corrected to produce a smoothed thermogram. The phase transitions of meibum samples from both the donors (Figure 8, Panel B) began at around 10 to 11 °C (this temperature is also called “effective starting temperatures of transition”), reached a maximum at Tm of about 30 °C, and ended at about 35 °C (also called “effective completion temperature of transition”, Te). The values of T1/2 (between 10 and 12 °C) were much higher than those of pure lipid standards, but on par with T1/2 of lipid mixtures. The thermotropic peaks could not be modeled using the MN2State equation with just one Tm: similarly to the oleic acid-based wax ester mixtures, the shapes of meibum thermograms necessitated deconvolution with, at least, three different Tm.
Figure 8.
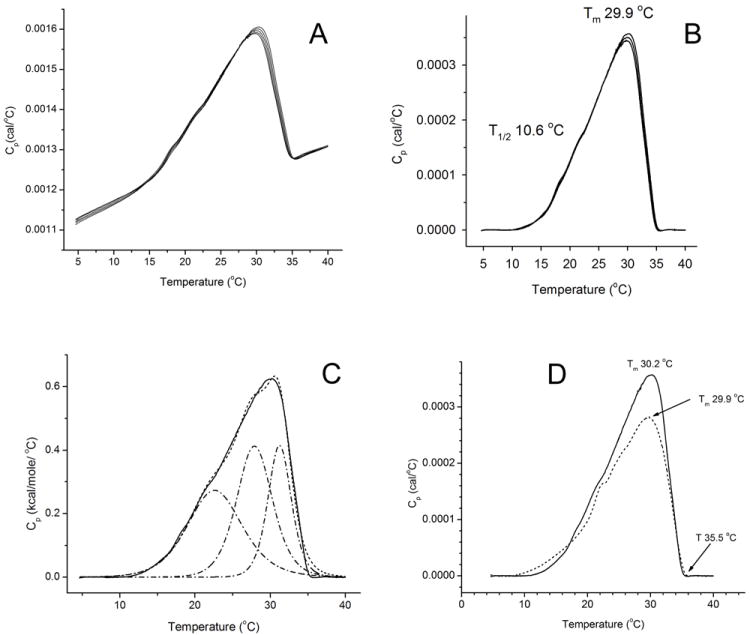
Thermotropic behavior of human meibum.
Panel A. Five repetitive raw thermograms of a sample of human male meibum. Approximately 0.4 mg of meibum was loaded.
Panel B. Repetitive raw thermograms of a sample of human meibum after baseline correction.
Panel C. A deconvoluted thermogram of a male meibum sample. At least three Tm were needed to adequately model the thermotropic behavior of human meibum. Cooperativity of melting of these three peaks, expressed as n, rose from 10 to 40 to 100 (from left to right). Note a striking resemblance of the meibum thermogram and a thermogram of a four-component mixture of unsaturated wax esters depicted in Figure 5, Panel B.
Panel D. Comparison of two human samples.
Both samples were loaded into the microcalorimeter in the amount of 0.4 mg (male, solid line; female, broken line). Note almost identical values of Tm (about 30 °C), T1/2 (10.0 ± 0.5 °C), and the completion temperature of transition (35.5 ± 0.5 °C) determined for both the samples.
Transition temperatures were found to be the same for the male and the female samples:the former produced a Tm of 30.3 ± 0.1 °C (Mean ± SE), while the later had a Tm of 30.1 ± 0.1 °C (four samples from each donor analyzed four or more times). Moreover, their ΔHcal were within 20% of each other (or 6.4 ± 1.3 kCal/mole, assuming an average molecular mass of meibomian lipids 700 Da), T1/2 were 11.2 ± 0.6 °C, while Te were 35 °C for the male, and 36 °C for the female samples. An mean value of an apparent cooperative size unit was estimated to be between 8 and 13.
Discussion
The goal of our project was to develop an experimental approach that would allow us to evaluate thermotropic properties of meibomian lipids. DSC is one of the most universal techniques that can be used to achieve this goal: the result of a DSC experiment is a thermogram which shows changes in the heat capacity of the entire sample as its temperature changes. From the thermogram, one can calculate a range of thermodynamic parameters that are important for understanding the phase transitions and properties of a sample at different temperatures, including the enthalpy of transition ΔH, transition temperature Tm, cooperativity of melting (n and σ) etc.
DSC is a well established technique that was developed in the early sixties of the 20th century (O’Neill, 1964). There are many types of DSC instruments currently available on the market, and multiple protocols related to lipid studies have been published (Huang and Li, 1999; Huang, 2001; Lewis et al., 2007). However, the high complexity of the meibum chemical composition, and low quantities of non-pooled human samples available for researchers, necessitated a systematic effort to design and validate DSC experiments with human meibomian lipids.
Our calorimetric experiments were to answer one of the most intriguing questions related to meibum studies: Why does meibum melt at relatively low physiological temperatures (between 20 and 40 °C; measured Tm 32-33 °C (Borchman et al., 2011; Butovich, 2008; Butovich et al., 2010) while being composed of extremely long-chain compounds, whose Tm are expected to be much higher than that (Butovich, 2011a)? As an illustration, consider that behenyl oleate – a natural component of meibum, whose Tm is about 37°C – is one of the shortest wax esters found there, with a typical oleic acid-based meibomian wax ester being two to five CH2 groups longer, and having, therefore, higher Tm. Cholesteryl esters, which constitute up to one-third of meibum, have even higher melting temperatures [between 70 and 85 for esters with C12:0 to C20:0 fatty acid chains (2007b)]. A range of still longer, and more complex, meibomian lipids – such as extremely long chain OAHFA (Butovich et al., 2009; Chen et al., 2010; Lam et al., 2011) and their cholesteryl esters (Butovich, 2011b; Butovich et al., 2011; Butovich et al., 2012b; Chen et al., 2010; Nicolaides et al., 1981) – are expected to melt at even higher temperatures. Unfortunately, these and many other common meibomian lipids are not available as pure standards, which prompted us to concentrate on synthetic analogs of meibomian lipids that are available in pure form from commercial suppliers (Table 1). In this pilot paper we focus on wax esters that represent, on average, at least 40 % of meibomian lipids (wt/wt).
First, we conducted preliminary calorimetric experiments with pure lipid standards to determine if their measured Tm match published data, and, in fact, no significant differences between the literature data and the values of Tm measured in our experiments were observed (Table 1 and Figure 2). Melting of individual wax esters, as expected, was highly cooperative: the thermotropic peaks of individual wax esters were typically tall, narrow, and symmetrical. Their shape allowed for the successful modeling of the transitions using a simple MN2State model with just one Tm for each lipid. The average cooperative unit size n for tested wax esters was found to be 80.2 ± 35.3 (Mean ± SD). We interpret this number as a lowest number of lipid molecules in an independently melting lipid cluster (or microcrystal) within the continuous lipid sample, the explanation which is in line with other publications on lipid melting (Hinz and Sturtevant, 1972; Lewis et al., 2007). Though never truly crystalline, the packing of lipid molecules within the cluster was tight and regular, as evidenced by their narrow T1/2 (typically, 0.5 °C or less). In an ideal case of infinite cooperativity, the T1/2 of the melting peak would have been approaching zero, and n would have been approaching the total number of lipid molecules in the sample.
Melting of lipid mixtures, on the other hand, was much less cooperative with apparent values of n being less than 10. The shapes of the peaks were very different from those of individual standards: the peaks were lower, broader and less symmetrical. To approximate the thermograms of lipid mixtures, they needed to be deconvoluted using a MN2State model with at least several melting components with different Tm, ΔH, and T1/2. However, a common trend was that to form a continuous and monotonous melting peak, the mixture had to have several (at least, four) components with close and regularly spaced Tm, meaning that their lengths should be changing in increments of two carbon atoms or less. If the number of components was three or less, or if their Tm were far apart from each other, bi- or even tri-modal thermograms were observed.
Typical thermograms of these types are shown in Figures 3 and 4). We consider these results to be an indication of the existence of two or more relatively poorly miscible lipid phases, rather than phase pre-transitions within one continuous ideally miscible lipid phase, primarily because of the comparable sizes (i.e., values of ΔHcal) of thermotropic peaks 1 and 2, which would most likely be different had they originated from some sort of a pre-transition that had occurred before the major solid (or gel)-to-liquid transition (Heimburg, 2000; Lewis et al., 2007; Rand, 1975). Notably, most if not all, poorly miscible lipid mixtures tested in this study showed at least two transitions peaks which were comparable to each other size-wise.
The best mixing was typically obtained if lipid mixtures were formed from several homologous wax esters with chain lengths differing by no more than two carbon atoms (such as those shown in Figure 5). Needless to say that evaluation of all possible combinations of all tested wax esters was impossible. Therefore, there could be situations when the thermograms of mixtures would deviate from this rule.
Importantly, mixtures of wax esters melted at temperatures noticeably lower than the Tm of their individual components. From our data, it appears that if two or more lipids were present in the sample, they could influence each other’s melting behavior by intercalating each other’s domains within the sample and making them less organized, which effectively decreased the degree of cooperativity of their melting. This conclusion is supported by two observations: 1) the much larger apparent values of T1/2 of the mixtures (e.g., 8 to 10 °C for a mixture of four oleic acid-based wax esters, Figure 5), and 2) relatively small (compared to pure standards) cooperative unit sizes n for their corresponding deconvoluted peaks, calculated as ΔHcal/ΔHv (about 5, 8, 11, and 15 for the four deconvoluted peaks with Tm values of 16, 20, 23, and 25 °C).
One peculiarity that remains to be fully understood and explained is our observation of the noticeable differences in the values of Tm (but, typically, not in ΔHcal) of the first and the subsequent thermograms for virtually every tested lipid sample. Sometimes, the shift in Tm did not exceed a few tenths of a degree, but from time to time it could exceed a few degrees of Celsius. A preliminary explanation of this phenomenon is that the lipid samples, which were allowed to repetitively melt and solidify in the microcalorimeter, could possibly change their physical shape in the cell, e.g. as a result of their spreading/thinning or irreversible changes in their aggregation state, which, in turn, could impact their Tm. However, once the samples had resumed their stable shapes after one or two initial melting/cooling cycles, the thermograms typically stabilized and their Tm became more reproducible. Note that no chemical decomposition of wax esters in the microcalorimeter after repetitive melting/cooling cycles was detected as tested by GC/MS as described earlier (Butovich et al., 2012a). With lipid mixtures, the first melting cycle routinely produced very complex and irreproducible thermograms similar to the one shown in Figure 3. We consider this to be a preparatory cycle during which different types of lipids, after they had been loaded into the DSC cell, undergo mixing or phase separation to assume their final shape.
Meibum samples (Figure 8), essentially, have demonstrated a behavior similar to the one of well-miscible wax esters. Never bi- or tri-modal, meibum thermograms were broad with T1/2 of 10 °C and above. To deconvolute these thermograms, one needed to use a model with at least three individually melting components. The accurate determination of the cooperative unit sizes for them is impossible because of their undetermined molecular weights and composition. However, if we assume an average molecular weight of meibum lipids to be about 700 Da (Butovich et al., 2010), then the apparent cooperativity unit size for the whole sample, estimated using Equation 1, is ~10, while for the three individual deconvoluted peaks the values of n ranged from 20 to 45 to 100 (Figure 8, Panel C, three deconvoluted peaks from left to right). The ΔHcal values for meibum (about 6 kCal/mole) were several times lower than those of the mixture of unsaturated wax esters (about 16 kCal/mole, Figure 5, Panel B). Three plausible effects can lower ΔHcal values for meibum compared to individual lipid standards and their simple mixtures. 1) Meibum is a much more diverse mixture of various lipids, which could impede formation of tightly packed lipid clusters due to better chances of intercalating each other’s domains. 2) In complex mixtures, formation of uniform, homogeneous lipid clusters formed from just one type of lipid could be suppressed because of the diffusion limitations (i.e., high viscosity of samples means slow diffusion of molecules). 3) Meibum has a substantial presence of branched lipids (Butovich et al., 2012a; Nicolaides et al., 1981), which generally melt at lower temperatures than their straight-chain counterparts, again due to their less tight packing.
Analyzed samples from two donors were shown to be similar. No major differences were observed between their samples collected on different occasions: their shapes, transition temperatures, effective starting and effective completion temperatures of transition, ΔHcal and apparent cooperativity of melting (in terms of T1/2 and n) were close.
The asymmetrical shape of the meibum’s melting curve with a relatively abrupt downward trend in its heat capacity Cp from about 30 °C and up is indicative of a highly cooperative melting of the sample in this temperature range (note the much higher value of n for the third deconvolution peak in Figure 8, Panel C), and may be important for the physiology of the tear film. Earlier, using a totally different experimental approach we demonstrated that the T1/2 of meibum was about 32 °C, with approximately 90% of meibum melted at 35 °C (Butovich et al., 2010). In recent studies, the mean eyelid temperature in healthy people with no ocular disorders was measured to be between 30.7 ± 1.2 °C (Pult et al., 2012), 32.2 ± 0.8 °C (Shih et al., 2010) and 33.4 ± 0.1 °C (Nagymihalyi et al., 2004), the mean corneal surface temperature was between 31.7 ± 0.9 °C (Shih et al., 2010), 32.3 ± 0.5 °C (Terada et al., 2004), and 34.8 ± 0.8 °C (Efron et al., 1989; Klamann et al., 2013). All these temperatures are falling between the Tm values of human meibum (~30 °C) and its effective completion temperature of transition (~35.5 ± 0.5 °C). Since meibomian lipids seem to complete undergoing this major phase transition at a normal bodily temperature, one can speculate that at physiologically relevant ocular surface temperatures of 30 to 35 °C meibum is always partly, but not completely, melted. This partly melted state of meibum can be characterized as liquid-crystalline state, while the state it assumes above 36 °C is disorganized and liquid [Butovich et al.; a manuscript in preparation]. The latter may facilitate excretion of meibum from the meibomian gland orifices, and its delivery onto the ocular surface. The higher cooperativity of meibum melting at higher temperatures (between 30 and 36 °C) than between 10 and 30 °C emphasizes the role of the eyelid and ocular surface temperatures in the physiology and biophysics of the tear film – even a slow change in those temperatures will have a measurable effect of the excretion of meibum from meibomian glands and on the properties of the meibomian lipid films.
The high susceptibility of meibum to changes in temperature, especially its rapid solidification upon lowering the temperature from 35 to 30 °C and below, may and, probably, does have a strong impact on the properties of the tear film and its outermost part – the tear film lipid layer – in vivo. Earlier we studied the effects of temperature on meibomian lipid films (Butovich et al., 2010), and concluded that their in-plane elasticity rises when their temperature falls. This makes the meibomian films rigid, and their spreading and restoration more difficult and slow, which could impact their protective properties. Thus, it seems to be important to correlate the main transition temperature Tm and the effective completion temperatures of transition – the two apparently most physiologically significant transition points in the thermograms of meibum – with changes in its composition in the relation to dry eye disease and other ocular pathologies. Experiments are underway to test this idea.
Highlights.
Meibomian gland secretions (meibum) are formed from a large number of various lipids
These lipids, if melted individually, have very high transition temperatures (Tm)
Also, they melt highly cooperatively with cooperativity unit size, n, being 50 to100
However, the meibum’s Tm is about 30 °C, while n is only about 10
Easy melting of meibomian lipids is important for their physiological functions
Acknowledgments
This project was supported by the National Institutes of Health grants R01EY019480, 3R01EY019480-01A1S1, and an unrestricted grant from Research to Prevent Blindness (New York, NY).
ABBREVIATIONS
- CE
cholesteryl ester
- DED
dry eye disease
- ΔHcal
calorimetric transition enthalpy
- ΔHv
van’t Hoff enthalpy change
- DSC
differential scanning calorimetry
- GC/MS
gas chromatography/mass spectrometry
- HPLC
high performance liquid chromatography
- MGD
meibomian gland dysfunction
- MS
mass spectrometry
- n
cooperative unit size
- OAHFA
(O-acyl)-ω-hydroxy fatty acid
- TFLL
tear film lipid layer
- Tm
melting temperature
- WE
wax ester
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Hua Lu, Email: hua.lu@utsouthwestern.edu.
Jadwiga C. Wojtowicz, Email: jadwiga.wojtowicz@utsouthwestern.edu.
References
- The definition and classification of dry eye disease: Report of the definition and classification subcommittee of the international dry eye workshop (2007) The ocular surface. 2007a;5:75–92. doi: 10.1016/s1542-0124(12)70081-2. [DOI] [PubMed] [Google Scholar]
- The lipid handbook. 3. CRC Press, Taylor & Francis Group; 2007b. [Google Scholar]
- Arita R, Itoh K, Maeda S, Maeda K, Furuta A, Fukuoka S, Tomidokoro A, Amano S. Proposed diagnostic criteria for obstructive meibomian gland dysfunction. Ophthalmology. 2009;116:2058–2063. e2051. doi: 10.1016/j.ophtha.2009.04.037. [DOI] [PubMed] [Google Scholar]
- Borchman D, Foulks GN, Yappert MC, Bell J, Wells E, Neravetla S, Greenstone V. Human meibum lipid conformation and thermodynamic changes with meibomian-gland dysfunction. Investigative ophthalmology & visual science. 2011;52:3805–3817. doi: 10.1167/iovs.10-6514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borchman D, Yappert MC, Foulks GN. Changes in human meibum lipid with meibomian gland dysfunction using principal component analysis. Experimental eye research. 2010;91:246–256. doi: 10.1016/j.exer.2010.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butovich IA. On the lipid composition of human meibum and tears: Comparative analysis of nonpolar lipids. Investigative ophthalmology & visual science. 2008;49:3779–3789. doi: 10.1167/iovs.08-1889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butovich IA. The meibomian puzzle: Combining pieces together. Progress in retinal and eye research. 2009;28:483–498. doi: 10.1016/j.preteyeres.2009.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butovich IA. Lipidomics of human meibomian gland secretions: Chemistry, biophysics, and physiological role of meibomian lipids. Progress in lipid research. 2011a;50:278–301. doi: 10.1016/j.plipres.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butovich IA. On the presence of (O-acyl)-omega-hydroxy fatty acids and of their esters in human meibomian gland secretions. Investigative ophthalmology & visual science. 2011b;52:639–641. doi: 10.1167/iovs.10-7028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butovich IA, Arciniega JC, Lu H, Molai M. Evaluation and quantitation of intact wax esters of human meibum by gas-liquid chromatography-ion trap mass spectrometry. Investigative ophthalmology & visual science. 2012a;53:3766–3781. doi: 10.1167/iovs.11-9333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butovich IA, Arciniega JC, Wojtowicz JC. Meibomian lipid films and the impact of temperature. Investigative ophthalmology & visual science. 2010;51:5508–5518. doi: 10.1167/iovs.10-5419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butovich IA, Borowiak AM, Eule JC. Comparative hplc-ms analysis of canine and human meibomian lipidomes: Many similarities, a few differences. Scientific reports. 2011;1:24. doi: 10.1038/srep00024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butovich IA, Lu H, McMahon A, Eule JC. Toward an animal model of the human tear film: Biochemical comparison of the mouse, canine, rabbit, and human meibomian lipidomes. Investigative ophthalmology & visual science. 2012b;53:6881–6896. doi: 10.1167/iovs.12-10516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butovich IA, Wojtowicz JC, Molai M. Human tear film and meibum. Very long chain wax esters and (o-acyl)-omega-hydroxy fatty acids of meibum. Journal of lipid research. 2009;50:2471–2485. doi: 10.1194/jlr.M900252-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Green-Church KB, Nichols KK. Shotgun lipidomic analysis of human meibomian gland secretions with electrospray ionization tandem mass spectrometry. Investigative ophthalmology & visual science. 2010;51:6220–6231. doi: 10.1167/iovs.10-5687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Efron N, Young G, Brennan NA. Ocular surface temperature. Current eye research. 1989;8:901–906. [PubMed] [Google Scholar]
- Heimburg T. A model for the lipid pretransition: Coupling of ripple formation with the chain-melting transition. Biophys J. 2000;78:1154–1165. doi: 10.1016/S0006-3495(00)76673-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinz HJ, Sturtevant JM. Calorimetric studies of dilute aqueous suspensions of bilayers formed from synthetic l- -lecithins. The Journal of biological chemistry. 1972;247:6071–6075. [PubMed] [Google Scholar]
- Holly FJ. Formation and rupture of the tear film. Experimental eye research. 1973;15:515–525. doi: 10.1016/0014-4835(73)90064-x. [DOI] [PubMed] [Google Scholar]
- Huang C, Li S. Calorimetric and molecular mechanics studies of the thermotropic phase behavior of membrane phospholipids. Biochimica et biophysica acta. 1999;1422:273–307. doi: 10.1016/s0005-2736(99)00099-1. [DOI] [PubMed] [Google Scholar]
- Huang CH. Mixed-chain phospholipids: Structures and chain-melting behavior. Lipids. 2001;36:1077–1097. doi: 10.1007/s11745-001-0818-1. [DOI] [PubMed] [Google Scholar]
- Iyengar BT, Schlenk H. Melting points of synthetic wax esters. Lipids. 1969;4:28–30. doi: 10.1007/BF02531790. [DOI] [PubMed] [Google Scholar]
- Joffre C, Souchier M, Gregoire S, Viau S, Bretillon L, Acar N, Bron AM, Creuzot-Garcher C. Differences in meibomian fatty acid composition in patients with meibomian gland dysfunction and aqueous-deficient dry eye. The British journal of ophthalmology. 2008;92:116–119. doi: 10.1136/bjo.2007.126144. [DOI] [PubMed] [Google Scholar]
- Joffre C, Souchier M, Leclere L, Buteau B, Gregoire S, Lizard G, Montange T, Acar N, Bron A, Creuzot-Garcher C, Diebold Y, Bretillon L. Branched-chain fatty acids, increased in tears of blepharitis patients, are not toxic for conjunctival cells. The British journal of ophthalmology. 2009;93:1391–1395. doi: 10.1136/bjo.2008.156356. [DOI] [PubMed] [Google Scholar]
- Klamann MK, Maier AK, Gonnermann J, Klein JP, Bertelmann E, Pleyer U. Ocular surface temperature gradient is increased in eyes with bacterial corneal ulcers. Ophthalmic research. 2013;49:52–56. doi: 10.1159/000343774. [DOI] [PubMed] [Google Scholar]
- Lam SM, Tong L, Yong SS, Li B, Chaurasia SS, Shui G, Wenk MR. Meibum lipid composition in asians with dry eye disease. PloS one. 2011;6:e24339. doi: 10.1371/journal.pone.0024339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemp MA, Crews LA, Bron AJ, Foulks GN, Sullivan BD. Distribution of aqueous-deficient and evaporative dry eye in a clinic-based patient cohort: A retrospective study. Cornea. 2012;31:472–478. doi: 10.1097/ICO.0b013e318225415a. [DOI] [PubMed] [Google Scholar]
- Lewis RN, Mannock DA, McElhaney RN. Differential scanning calorimetry in the study of lipid phase transitions in model and biological membranes: Practical considerations. Methods Mol Biol. 2007;400:171–195. doi: 10.1007/978-1-59745-519-0_12. [DOI] [PubMed] [Google Scholar]
- Mishima S, Maurice DM. The oily layer of the tear film and evaporation from the corneal surface. Experimental eye research. 1961;1:39–45. doi: 10.1016/s0014-4835(61)80006-7. [DOI] [PubMed] [Google Scholar]
- Nagymihalyi A, Dikstein S, Tiffany JM. The influence of eyelid temperature on the delivery of meibomian oil. Experimental eye research. 2004;78:367–370. doi: 10.1016/s0014-4835(03)00197-0. [DOI] [PubMed] [Google Scholar]
- Nelson JD, Shimazaki J, Benitez-del-Castillo JM, Craig JP, McCulley JP, Den S, Foulks GN. The international workshop on meibomian gland dysfunction: Report of the definition and classification subcommittee. Investigative ophthalmology & visual science. 2011;52:1930–1937. doi: 10.1167/iovs.10-6997b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicolaides N, Kaitaranta JK, Rawdah TN, Macy JI, Boswell FM, 3rd, Smith RE. Meibomian gland studies: Comparison of steer and human lipids. Investigative ophthalmology & visual science. 1981;20:522–536. [PubMed] [Google Scholar]
- O’Neill MJ. The analysis of a temperature-controlled scanning calorimeter. Anal Chem. 1964;36:1238–1245. [Google Scholar]
- Patel S, Nelson DR, Gibbs AG. Chemical and physical analyses of wax ester properties. J Insect Sci. 2001;1:1–7. doi: 10.1673/031.001.0401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pult H, Riede-Pult BH, Purslow C. A comparison of an eyelid-warming device to traditional compress therapy. Optometry and vision science : official publication of the American Academy of Optometry. 2012;89:E1035–1041. doi: 10.1097/OPX.0b013e31825c3479. [DOI] [PubMed] [Google Scholar]
- Rand R, Chapman D, Larsson K. Tilted hydrocarbon chains of dipalmitoyl lecithin become perpendicular to the bilayer before melting. Biophys J. 1975;15:1117–1124. doi: 10.1016/S0006-3495(75)85888-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saville JT, Zhao Z, Willcox MD, Ariyavidana MA, Blanksby SJ, Mitchell TW. Identification of phospholipids in human meibum by nano-electrospray ionisation tandem mass spectrometry. Experimental eye research. 2011;92:238–240. doi: 10.1016/j.exer.2010.12.012. [DOI] [PubMed] [Google Scholar]
- Shih SR, Li HY, Hsiao YL, Chang TC. The application of temperature measurement of the eyes by digital infrared thermal imaging as a prognostic factor of methylprednisolone pulse therapy for graves’ ophthalmopathy. Acta ophthalmologica. 2010;88:e154–159. doi: 10.1111/j.1755-3768.2010.01941.x. [DOI] [PubMed] [Google Scholar]
- Shine WE, McCulley JP. Role of wax ester fatty alcohols in chronic blepharitis. Investigative ophthalmology & visual science. 1993;34:3515–3521. [PubMed] [Google Scholar]
- Siak JJ, Tong L, Wong WL, Cajucom-Uy H, Rosman M, Saw SM, Wong TY. Prevalence and risk factors of meibomian gland dysfunction: The singapore malay eye study. Cornea. 2012;31:1223–1228. doi: 10.1097/ICO.0b013e31823f0977. [DOI] [PubMed] [Google Scholar]
- Terada O, Chiba K, Senoo T, Obara Y. ocular surface temperature of meibomia gland dysfunction patients and the melting point of meibomian gland secretions. Nippon Ganka Gakkai zasshi. 2004;108:690–693. [PubMed] [Google Scholar]
- Uchino M, Dogru M, Yagi Y, Goto E, Tomita M, Kon T, Saiki M, Matsumoto Y, Uchino Y, Yokoi N, Kinoshita S, Tsubota K. The features of dry eye disease in a japanese elderly population. Optometry and vision science : official publication of the American Academy of Optometry. 2006;83:797–802. doi: 10.1097/01.opx.0000232814.39651.fa. [DOI] [PubMed] [Google Scholar]


