Abstract
Objective
To assess the relationship of lipoprotein subfractions to coronary heart disease (CHD).
Methods
Prospective 29.1 year follow-up of 1905 men measured for lipoprotein mass concentrations by analytic ultracentrifugation between 1954 and 1957. Vital status was determined for 97.2% of the cohort. Blinded physician medical record and death certificate review confirmed 179 CHD deaths. Follow-up questionnaires identified 182 nonfatal myocardial infarctions and 93 revascularization procedures from 1,346 (98.3%) of the surviving cohort and from the next-of-kin of 153 men who died.
Results
When adjusted for age, total incident CHD was inversely related to HDL2-mass (P=0.0001) and HDL3-mass (P=0.02), and concordantly related to LDL-mass (P<10−11), IDL-mass (P<10−7), and small (P<10−7) and large VLDL-mass concentrations (P=0.003). The hazard reduction per mg/dl of HDL was greater for HDL2-mass than HDL3-mass (P=0.04). The lowest quartiles of both HDL2-mass (P=0.007) and HDL3-mass (P=0.001) independently predicted total incident CHD when adjusted for traditional risk factors. Risk for premature CHD (≤ 65 years old) was significantly greater in men within the lowest HDL2 (P=0.03) and HDL3 quartiles (P=0.04) and having higher LDL-mass concentrations (P=0.001). Serum cholesterol’s relationship to incident CHD (P<10−8) was accounted for by adjustment for LDL-mass concentrations (adjusted P=0.90).
Conclusions
Lipoprotein subfractions differ in their relationship to CHD.
Keywords: Lipoprotein, high-density lipoproteins, risk factors, prevention
Lipoproteins include a heterogeneous mixture of particles that vary by size, density, and buoyancy [1]. Analytic ultracentrifugation was applied in the 1950’s to the separations of lipoprotein particles by their rates of migration in an intense centrifugal field, i.e. high-density lipoprotein 2 (HDL2, F1.203.5–9.0), HDL3 (F1.200–3.5), low-density lipoproteins (LDL, Sf0–12), intermediate-density lipoproteins (IDL, Sf12–20), small very-low-density lipoproteins (small VLDL, Sf20–100), and large VLDLs (Sf100–400), and remained in use through the end of the 20th century [2–4]. Analytic ultracentrifugation was, in essence, the gold standard against which other techniques were calibrated [5,6]. Although the instrument was further improved to measure lipoprotein mass concentrations within individual flotation intervals [7], the basic methodology and the aforementioned density intervals remained unchanged throughout its use.
Between 1955 and 1957 Gofman established a prospective study of Livermore Radiation Laboratory male employees [8]. After 10 years of follow-up they reported that when compared to mean serum concentrations of the total sample, the 38 men who developed clinical ischemic heart disease had 32% lower HDL2 (P<0.01), 8% lower HDL3 (P=0.02), 13% higher LDL (P<0.001), 23% higher IDL (P<0.001), and 21% higher small VLDL (P<0.01). Serum mass concentrations of larger VLDL were 14% greater in men with ischemic heart disease than the base population, but not significantly so [8]. Gofman et al. proposed that the relationships of ischemic heart disease to total cholesterol and the low- to- very-low-density lipoproteins diminished with increasing age, specifically after age 50. Livermore was the first prospective study to relate HDL subfractions to heart disease risk.
Although Gofman’s original photographic schlieren images were destroyed, the fortuitous discovery of the IBM punch cards provided a unique opportunity to assess the relationships of lipoprotein subfractions to the 29-year incidence of CHD in this historically important study. The providence of these data were confirmed by conversations and a computer data printout provided by Gofman, and sample log book maintained for the analytic ultracentrifuge at Donner laboratory. This paper uses survival analyses to assess the statistically independent relationships of incident CHD to both HDL2 and HDL3-mass concentrations, as well as the mass concentrations of the Sf0–400 low- to very-low density lipoproteins. The substantially greater number of incident cases, and the availability of multivariate techniques allow us to adequately address: 1) whether specific analytic ultracentrifuge lipoprotein mass measurements are significantly related to incident CHD; and 2) whether their ability to predict incident CHD is superior to serum total cholesterol measurements.
Methods
Design
The cohort consisted of male employees of the Livermore Radiation Laboratory who participated in the original follow-up study by Dr. John Gofman [8]. All of the men were free of prior ischemic heart disease at baseline. Qualification presumably corresponded to the criteria adopted by the Cooperative Study, i.e., disqualified for more than trace amounts of urine protein or sugar; diabetes mellitus; nephritis (except past history of pyelonephritis, nephrolithiasis, or loss of kidney); treatment with ACTH, cortisone, or related hormones; history of rheumatic heart disease; known congenital heart disease; syphilis or Buerger's disease.
Baseline measurements
Height, weight, blood pressure, and casual blood draws were obtained during employee annual medical physical examination. Cigarette consumption was determined by self report. Serum total cholesterol was assayed by a modification of the Abell method [9]. Corrected lipoprotein subfraction concentrations were obtained by analytic ultracentrifugation [3]. The 29-year follow-up study was approved by the committee for the Protection of Human Subjects, University of California, Berkeley.
Endpoint assignment
Criteria for fatal and nonfatal CHD were those previously employed for community surveillance of coronary heart disease [10]. Physician-diagnosis of CHD was made without knowledge of the cholesterol or lipoprotein concentrations. A preliminary diagnosis of fatal CHD was assigned when there was a death certificate indicating consistent underlying or immediate cause corresponding to ICD-9 410–414 (ICD-8 400–438). The 179 physician-determined CHD deaths included 24 fatal myocardial infarctions showing evidence of a nonfatal myocardial infarction from hospital records within 4 weeks of death or an autopsy diagnosis of acute myocardial infarction; 10 sudden coronary deaths with the decedent observed within one hour of their death with either no symptoms or symptoms compatible with acute myocardial infarction; and 50 fatal CHDs without documentation of myocardial infarction or sudden death. There were also 95 possible coronary heart disease deaths in which: 1) there was a death certificate with consistent underlying or immediate cause (ICD-9 410–414) but neither adequate preterminal documentation of an event, autopsy, previous myocardial infarction according to usual physician or hospital records, nor witness to the death; or 2) definite fatal myocardial infarction or CHD in the presence of another probably lethal, non-atherosclerotic process.
The surviving members of the cohort reported 137 hospitalizations for a heart attack, including 76 that were confirmed from retrieved hospital records. They also reported 82 coronary artery bypass grafts or angioplasties, 67 confirmed from hospital records. Next-of-kin reported 45 hospitalizations for a heart attack among those who had died, including 33 confirmed diagnoses from medical record review. The next-of-kin also reported 11 bypass grafts or angioplasties, 9 confirmed from hospital records. The survival analyses to follow are for two endpoints: 363 reported CHD representing both verified and unverified nonfatal myocardial infarctions and revascularization procedures, and physician diagnosis of definite and possible fatal CHD (234 prior to age 65 years), and 194 definite CHD representing confirmed hospital diagnoses for myocardial infarctions, confirmed revascularization procedures, and definite fatal myocardial infarction, sudden coronary death, and fatal CHD (137 prior to age 65 years).
Statistical analyses
Analyses were performed using JMP version 5.1 (SAS Institute, Cary NC). Stochastic imputation was used for missing covariates. Inclusion of the missing values did not noticeably alter the coefficients for lipoprotein mass concentrations as compared to deleting missing values (hazard ratios for lipoproteins, the principal outcome for this report, differed by <0.1%). The proportional hazards assumption was assessed by dividing the sample into <10 years follow-up, 10–20 year follow-up, and >20 year follow-up and testing whether the coefficients differed between age strata. There was no difference in the coefficients between the 10–20 year and the >20 year time intervals for cholesterol (P=0.89), HDL2 (P=0.60), HDL3 (P=0.07), LDL (P=0.63), IDL (P=0.76), or VLDL (P=0.32), however the coefficients did differ significantly for the 32 CHD cases occurring before the first 10 years versus later cases for total cholesterol (P=0.002) and LDL-mass (P=0.003), but not IDL (P=0.57), VLDL (P=0.71), HDL2 (P=0.43) or HDL3 (P=0.18). Therefore, the analyses for total cholesterol and LDL-mass concentrations were repeated excluding the 32 cases that occurred during the first 10 years of follow-up.
To test whether the per mg/dl increment in risk was the same for HDL2 and HDL3, we included both total HDL (HDL2+HDL3) and HDL2 simultaneously in the model, with the significance of the HDL2 coefficient serving to test whether their effects on CHD risk differed. Corresponding analyses were performed for LDL vs. IDL, and small VLDL vs. large VLDL.
Results
Baseline characteristics
Of the 1961 men originally cited by Gofman et al. [8], 56 records were found to be subsequent visits by the same subjects. Table 1 presents the baseline characteristics of the remaining 1905 men. They were generally young, with 31.1% under 30 years old, 55.1% between 30 and 44.9 years old, 13.8% between 45 and 59.9 years, and less than one percent 60 or older. The majority were of healthy weight as defined by BMI<25 kg/m2 (64.4%), with only about one-third moderately overweight (31.5%), and relatively few obese (4.1%). One-half smoked at baseline (49.4%). Only 7% were hypertensive (systolic BP ≥140 or diastolic blood pressure ≥90 mmHg), and 34% had serum total cholesterol ≥240 mg/dl.
Table 1.
Baseline characteristics and age-adjusted hazard ratios (95% confidence interval) for reported and definite total and premature incident CHD vs. individual risk factors during 29 years of follow-up.
| N | Mean±SD | Age-adjusted hazard ratio per packs/day, 10 mm Hg increment in blood pressure, kg/m2 increment in BMI, or 10 mg/dl increment in lipoprotein mass concentrations. |
||||
|---|---|---|---|---|---|---|
| Total CHD (i.e. all ages) | Premature CHD (i.e ≤65 years old) |
|||||
| All reported | Definite* | All reported | Definite* | |||
| Age (years) | 1905 | 35.15±8.56 | ||||
| Cigarettes (cigarettes/day) | 1692 | 8.89±10.69 | 1.42 (1.17, 1.72) P=0.0005 |
1.29 (0.98, 1.68) P=0.07 |
1.43 (1.12, 1.80) P=0.004 |
1.25 (0.90, 1.71) P=0.18 |
| BMI (kg/m2) | 1904 | 24.15±3.19 | 1.03 (0.99, 1.06) P=0.12 |
1.03 (0.99, 1.08) P=0.15 |
1.02 (0.97, 1.06) P=0.44 |
1.01 (0.95, 1.06) P=0.75 |
| Systolic blood pressure (mmHg) |
1896 | 120.35±12.36 | 1.20 (1.13, 1.27) P<10−7 |
1.20 (1.10, 1.29) P=0.0002 |
1.16 (1.07, 1.24) P=0.0008 |
1.15 (1.03, 1.26) P=0.02 |
| Diastolic blood pressure (mmHg) |
1895 | 70.77±9.06 | 1.26 (1.12, 1.42) P=0.0002 |
1.27 (1.08, 1.49) P=0.005 |
1.20 (1.04, 1.39) P=0.01 |
1.18 (0.97, 1.43) P=0.08 |
| Cholesterol (mg/dl) |
1864 | 223.96±45.43 | 1.08 (1.06, 1.11) P=10−10 |
1.09 (1.06, 1.12) P<10−7 |
1.09 (1.07, 1.13) P=10−9 |
1.10 (1.06, 1.14) P=10−6 |
| LDL (mg/dl) | 1905 | 352.36±86.56 | 1.05 (1.04, 1.07) P<10−11 |
1.05 (1.04, 1.07) P<10−7 |
1.06 (1.04, 1.07) P=10−11 |
1.05 (1.03, 1.07) P=10−6 |
| IDL (mg/dl) | 1905 | 49.12±23.37 | 1.13 (1.09,1.18) P<10−7 |
1.15 (1.09, 1.22) P<10−5 |
1.16 (1.10, 1.22) P=10−7 |
1.15 (1.07, 1.23) P=0.0001 |
| Small VLDL (mg/dl) |
1905 | 90.24±54.47 | 1.05 (1.03,1.06) P<10−7 |
1.06 (1.04, 1.07) P<10−7 |
1.04 (1.03, 1.06) P=10−5 |
1.05 (1.03, 1.07) P=0.0001 |
| Large VLDL (mg/dl) |
1905 | 50.36±66.35 | 1.02 (1.01,1.03) P=0.003 |
1.02 (1.01, 1.03) P=0.0003 |
1.02 (1.01, 1.03) P=0.005 |
1.03 (1.01, 1.04) P=0.001 |
| HDL3 (mg/dl) | 1905 | 220.29±43.39 | 0.97 (0.95, 1.00) P=0.02 |
0.97 (0.94, 1.01) P=0.12 |
0.94 (0.91, 0.97) P=0.0003 |
0.95 (0.91, 0.99) P=0.02 |
| HDL2 (mg/dl) | 1905 | 36.87±28.24 | 0.92 (0.88, 0.96) P=0.0001 |
0.94 (0.89, 0.99) P=0.03 |
0.91 (0.86, 0.96) P=0.0004 |
0.91 (0.85, 0.98) P=0.01 |
Includes only definite fatal myocardial infarction, sudden coronary death, and fatal coronary heart disease and confirmed diagnosis of nonfatal myocardial infarction from retrieved hospital record.
Twenty-nine of the 1905 men (1.52%) were lost to follow-up and had unknown vital status, and 505 (26.6%) had died by the time of the follow-up. Compared to those with known vital status (Table 1), those lost to follow-up were slightly heavier (difference±SE: 1.42 ± 0.60 kg/m2, P=0.02) and smoked more (0.33±0.10 cigarette packs/day, P=0.002), but did not differ with respect to age, blood pressure, total cholesterol, or any of the lipoprotein measurements (all P>0.21). Of the 1371 subjects known to be alive, histories of heart disease were obtained from follow-up questionnaires on 1346 subjects (98.3%), while 23 declined to provide follow-up surveys. Thus 97.2% of the men were known dead or had provided questionnaires on their history of CHD. Questionnaires reporting prior heart disease were also obtained from the next-of-kin of 153 men who had died. The mean±SD follow-up duration was 29.12±6.29 years.
Table 1 presents the age-adjusted hazard ratios for all 363 reported CHD and for 194 definite CHD, separately for total (i.e. all age) and for premature CHD (occurring before age 65). As expected, cigarette use and higher blood pressure predicted significantly greater CHD risk. Higher serum cholesterol levels also predicted greater CHD risk whereas, in this sample, BMI appeared to have little affect on risk. The hazard ratios were generally comparable for total and premature CHD. Restricting the analyses to definite CHD did not increase the magnitude of the hazard ratios, in fact, their significance diminished in accordance with their fewer events. Table 2 (model 1) shows that cigarette use, greater systolic blood pressure, and greater serum cholesterol concentrations independently predicted incident CHD.
Table 2.
Age-adjusted hazard ratios (95% confidence interval) for reported total and premature CHD (≤65 years old) vs. traditional risk factors and lipoprotein mass concentrations in 1876 men (excludes 29 men lost to follow-up).
| CHD ≤65 years old | All CHD | |||||
|---|---|---|---|---|---|---|
| Model 1 Subfractions excluded |
Model 2 HDL subfractions included |
Model 3 All subfractions included |
Model 1 Subfractions excluded |
Model 2 HDL subfractions included |
Model 3 All subfractions included |
|
| Cigarettes (packs/day) | 1.32 (1.04, 1.67) P=0.02 |
1.26 (0.99, 1.59) P=0.06 |
1.19 (0.93, 1.51) P=0.17 |
1.33 (1.10, 1.60) P=0.003 |
1.26 (1.04, 1.52) P=0.02 |
1.17 (0.96, 1.42) P=0.15 |
| BMI (kg/m2) | 0.99 (0.95, 1.04) P=0.73 |
0.97 (0.93, 1.01) P=0.19 |
0.96 (0.92, 1.01) P=0.11 |
1.00 (0.97, 1.04) P=0.92 |
0.99 (0.95, 1.02) P=0.44 |
0.98 (0.95, 1.02) P=0.27 |
| Systolic BP (per 10 mmHg) | 1.11 (1.00, 1.22) P=0.06 |
1.10 (0.99, 1.21) P=0.07 |
1.11 (0.99, 1.22) P=0.07 |
1.15 (1.06, 1.24) P=0.001 |
1.15 (1.06, 1.24) P=0.001 |
1.16 (1.06, 1.25) P=0.001 |
| Diastolic BP (per 10 mmHg) | 1.07 (0.89, 1.27) P=0.48 |
1.09 (0.91, 1.29) P=0.36 |
1.07 (0.90, 1.27) P=0.45 |
1.07 (0.93, 1.23) P=0.36 |
1.09 (0.94, 1.25) P=0.26 |
1.07 (0.92, 1.23) P=0.37 |
| Cholesterol (per 10 mg/dl) | 1.09 (1.06, 1.12) P<10−8 |
1.09 (1.06, 1.12) P<10−8 |
1.00 (0.95, 1.06) P=0.88 |
1.07 (1.05, 1.10) P<10−8 |
1.07 (1.05, 1.10) P<10−9 |
0.97 (0.93, 1.02) P=0.32 |
| LDL (per 10 mg/dl) | 1.05 (1.02, 1.08) P=0.001 |
1.06 (1.04, 1.09) P<10−6 |
||||
| IDL (per 10 mg/dl) | 0.99 (0.90, 1.07) P=0.74 |
0.95 (0.89, 1.02) P=0.16 |
||||
| Small VLDL (per 10 mg/dl) | 1.04 (0.99, 1.08) P=0.09 |
1.06 (1.03, 1.10) P=0.0003 |
||||
| Large VLDL (per 10 mg/dl) | 1.00 (0.97, 1.03) P=0.82 |
0.99 (0.97, 1.02) P=0.57 |
||||
| HDL3 (lowest quartile) | 1.49 (1.12, 1.97) P=0.007 |
1.36 (1.02, 1.81) P=0.04 |
1.48 (1.17, 1.86) P=0.001 |
1.29 (1.02, 1.63) P=0.03 |
||
| HDL2 (lowest quartile) | 1.61 (1.21, 2.11) P=0.001 |
1.39 (1.04, 1.87) P=0.03 |
1.38 (1.10, 1.73) P=0.006 |
1.13 (0.89, 1.44) P=0.31 |
||
The hazard ratios from the multivariate models are adjusted for the model’s other variables in addition to age.
Lipoprotein subfraction concentrations
Table 1 also presents the age-adjusted hazard ratios for serum lipoprotein subfraction concentrations. There is little difference in the hazard ratios for total reported CHD and definite CHD, except for the greater significance of the former. LDL, IDL, and VLDL were not found to be more significantly predictive of premature CHD than all CHDs irrespective of age. However, the cardioprotective influences of HDL2 and HDL3 were somewhat greater for premature CHD. With respect to the per mg/dl increment in lipoprotein mass concentration, the effect on age-adjusted CHD risk was significantly greater for small VLDL than large VLDL (P=0.0001), and for HDL2 than HDL3 (P=0.04), but not different between IDL than LDL (P=0.23, undisplayed analyses of total reported CHD regardless of age).
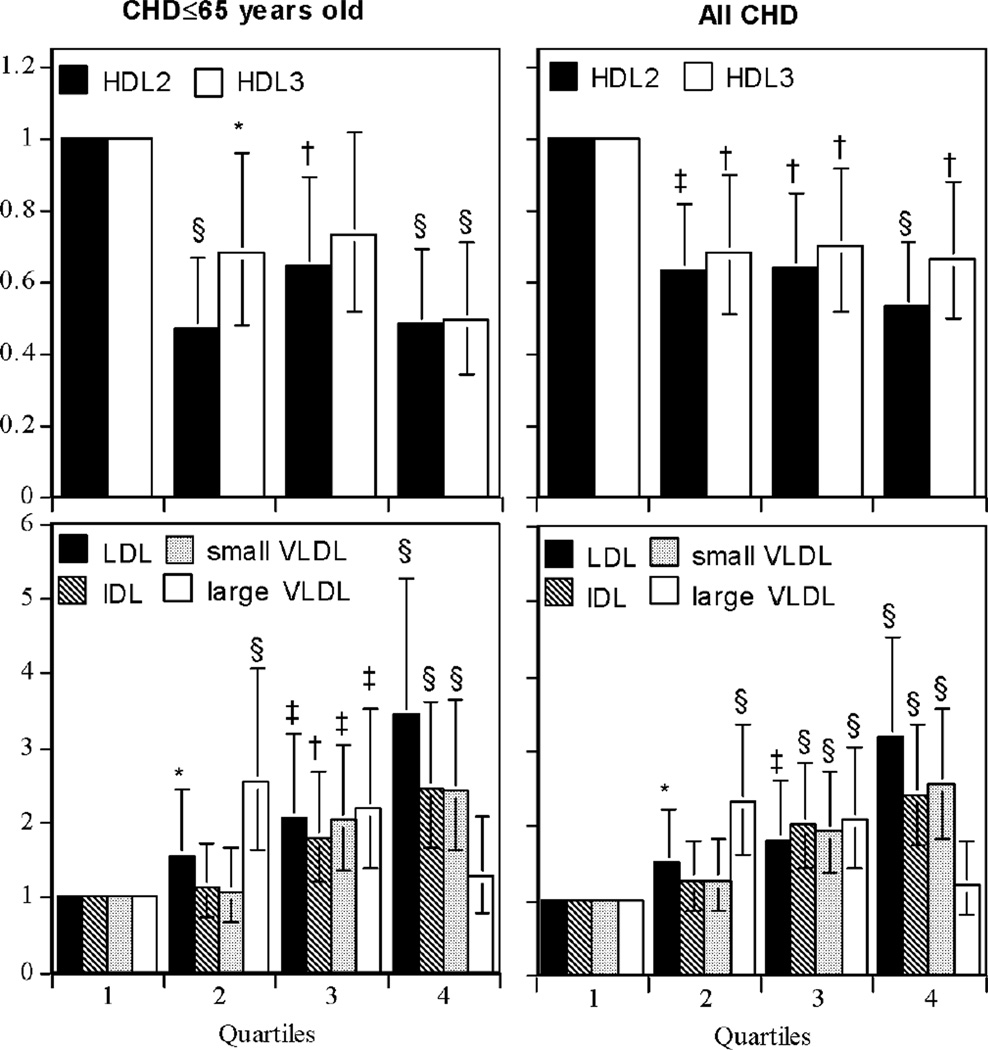
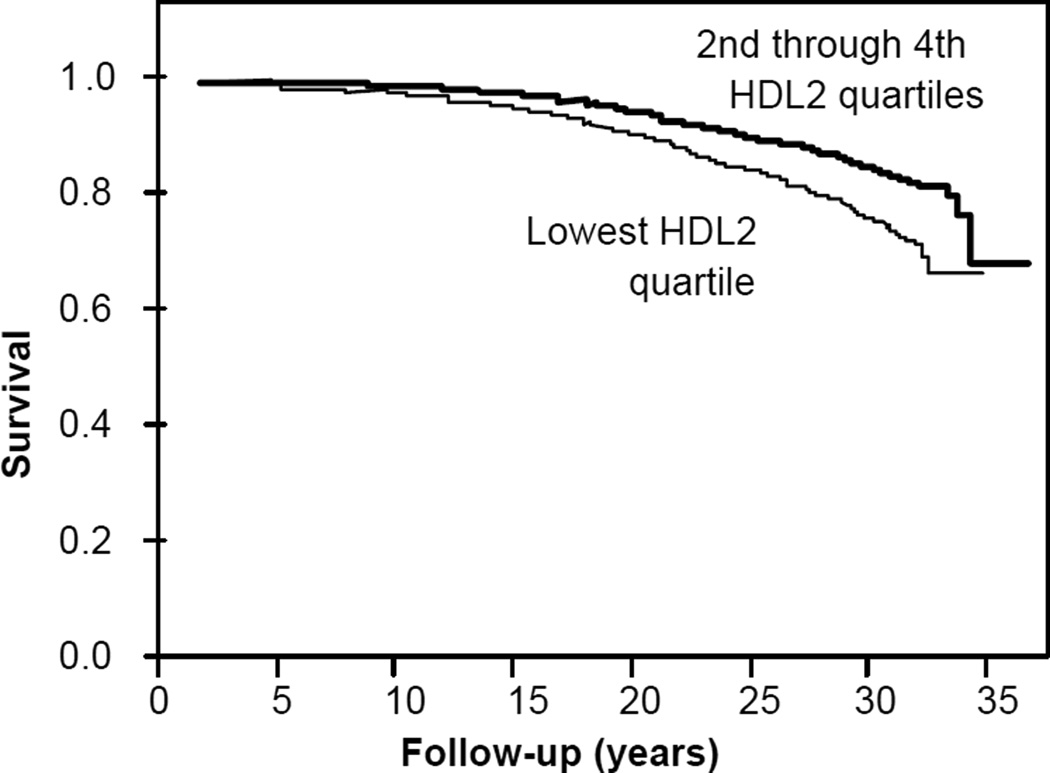
Figure 1 displays the hazard ratios for reported CHD by quartiles of lipoprotein mass concentrations. Increasing concentrations of LDL were associated with progressive increases in CHD risk, as were increases in IDL and small VLDL above their medians. Increasing concentrations of large VLDL were also associated with greater CHD risk except for the 4th quartile. In contrast, the majority of the risk reduction associated with greater HDL2 occurred between the first and second quartile, and appeared greater for premature than total CHD. Specifically, the age-adjusted hazard ratio for the 1st versus the 2nd through 4th HDL2 quartiles was 1.90 for premature CHD and 1.67 for total CHD (P<10−5 for both). The corresponding hazard ratios for HDL3 were 1.58 and 1.47, respectively (P=0.001 for both). Figure 2 displays the product limit survival curves for men in the lowest vs. 2nd through 4th quartiles of HDL2-mass concentrations.
Figure 1.
Hazard ratio for reported total and premature incident CHD during 29-year follow-up in 1876 men. There were 363 reported heart attacks, reported revascularization procedures, and physician diagnosed definite and possible fatal CHD, of which 234 occurred prior to age 65 years. Significance levels relative to the first quartile are coded * P<0.05; † P<0.01; ‡ P<0.001; and § P<0.0001.
Figure 2.
Product limit survival curves for men in the lowest vs. 2nd through 4th quartiles of HDL2-mass concentrations (P<0.0001 for difference).
Multivariate analyses of the subfractions are presented in Tables 2. When adjusted for traditional risk factors (Model 2), premature CHD risk was 60% greater for the lowest HDL2 quartile vs. higher values (P=0.001), and 49% greater for the lowest HDL3 quartile vs. higher values (P=0.007). The smaller hazard ratio for total vis-a-vis premature CHD presumably reflects a diminished importance of HDL2 as a risk factor in later events. Model 3 adds LDL, IDL and VLDL to the multivariate analyses, causing total cholesterol to be eliminated as a CHD risk factor. This was shown to be due to adjustment for LDL-mass concentrations rather than IDL or small VLDL. Specifically adjusting for small VLDL and IDL did not eliminate the significance of the cholesterol-CHD relationship (the cholesterol hazard ratio remains 1.05 at P=0.002) whereas adjusting for serum LDL-mass concentrations did (i.e. serum cholesterol’s relationship to incident CHD was reduced from P<10−8 to P=0.90, whereas LDL-mass concentrations remained a significant predictor for CHD, i.e., P<10−5, when adjusted for total cholesterol, analyses not displayed). In Table 2, HDL2 and HDL3 remain significant independent predictors of premature CHD when adjusted for other lipoproteins.
Exclusion of early events
The analyses of total cholesterol and LDL-mass concentrations were repeated excluding the first 10 years of follow-up because of the potential violation of the proportional hazards assumption of the survival analyses. The 32 excluded events represented less than nine percent of the total events. The hazard ratios were not much different from those presented in the tables for the total sample when adjusted for other non-lipoprotein risk factors (e.g., with early exclusions: the hazard ratios for total CHD were: 1.06 per 10 mg/dl of total cholesterol, 95% confidence interval 1.03 to 1.09, P<10−5; 1.04 per 10 mg/dl of LDL-mass concentrations, 95% confidence interval 1.03 to 1.06, P<10−9). The other analyses of Tables 2 were also little affected by the exclusion of the 32 events that occurred during the first 10 years of follow-up.
Logistic regression analyses
Finally we note that the aforementioned significant results were also confirmed by logistic regression analyses that do not assume proportional hazards, namely significantly age-adjusted lower odds for CHD with increasing LDL-mass (P=10−14), IDL-mass (P=10−9), small LDLmass (P=10−8), large VLDL-mass (P=0.0002), HDL2-mass (P=0.0002), and HDL3-mass (P=0.02); significantly greater age-adjusted odds per mg/dl increase in HDL2 than HDL3 (P=0.05), in small VLDL than large VLDL (P=0.002), but not per mg/dl increase in IDL than LDL (P=0.67); significant independent increases in the odds for premature CHD for the lowest quartiles of HDL2 and HDL3 when adjusted for traditional risk factors (P=0.001 and P=0.002, respectively) and the other lipoprotein mass concentrations (P=0.04 and P=0.01, respectively); and the elimination of the significance for total cholesterol when adjusted for LDL-mass (P<10−9 reduced to P=0.51 when adjusted) but not LDL-mass when adjusted for total cholesterol (P<10−12 reduced to P<0.0001 when adjusted) in predicting odds for CHD.
Discussion
HDL-cholesterol is now firmly established to lower CHD risk [11]. Although most published reports attribute the cardioprotective properties of HDL to HDL2, most also acknowledge that HDL3 may be inversely related to CHD risk as well. Most other prospective studies measure HDL-heterogeneity by precipitating HDL3-cholesterol and calculating HDL2-cholesterol as the difference from the total [12], which may be inadequate [13]. Currently, clinical evaluation of HDL focuses almost exclusively on the total HDL cholesterol without regard to the individual HDL subclasses [11].
Ten prospective studies to date have examined the relationship of HDL-subfractions to CHD [8,14–20]. The Livermore Study’s 29-year follow-up is the longest prospective study to date to examine the relationship of HDL subfractions to CHD and, except for the ARIC study [14], included the largest number of cases. The others involved 10 years of follow-up or less. CHD risk was found to be significantly associated with both HDL2- and HDL3-cholesterol in five studies [14–18], with HDL2- but not HDL3-cholesterol in one study [19], and with HDL3- but not HDL2-cholesterol in two studies [20]. Only one of the eight prospective studies showed that HDL2-cholesterol was significantly more predictive than total HDL-cholesterol [18], and only two showed that the cardioprotective properties of HDL2-cholesterol were significant when adjusted for HDL3-cholesterol [16,18]. More recently, NMR estimates of large HDL were shown to predict cardiovascular disease in 27,673 initially healthy women followed prospectively over 11 years, whereas smaller HDL did not [21]. In contrast, an earlier nested control study of patients treated with gemfibrozil or placebo suggested that CHD was reduced by increases in the particle concentration of small HDL as estimated by NMR [22]. Ion mobility estimates of a nested control study of 4368 subjects showed both small (P=0.01) and large HDL (P<0.001) predicted lower CHD risk [23].
Despite being based on only 38 cases, Gofman’s original conclusion that ischemic heart disease was inversely related to both HDL2 and HDL3 was upheld in the current analyses [8]. Moreover our analyses shows significantly longer age-adjusted survival per mg/dl increment in HDL2-mass than HDL3-mass, and in multivariate analyses we demonstratd independent cardioprotective benefits for both HDL-subfractions (Table 2, Model 2). Gofman et al. acknowledged in their 1966 follow-up that they were unable to assert whether the lower HDL2 and HDL3 they observed in cases were in excess of those expected from their inverse correlations with the low- to very low-density lipoproteins of Sf0–400 [8], whereas the current analyses showed that adjustment for VLDL did not eliminate the cardioprotective effects of HDL3 for total and premature CHD, or the cardioprotective effects of HDL2 for premature CHD. We also confirmed Gofman’s conclusion that incident CHD was inversely related to serum total cholesterol concentrations and systolic blood pressure, but not to diastolic blood pressure or excess body weight. Gofman reported that large VLDL was not significantly related to incident CHD, and although we found that CHD was less consistently related to large VLDL than small VLDL concentrations, this appeared to be primarily attributable to the lack of an association with the highest quartile of large VLDL (Figure 1), which we speculate could be due to postprandial contamination by chylomicrons. Except for HDL2, we did not observe that age strongly attenuates the effects of serum cholesterol or lipoprotein mass concentrations on incident CHD.
Prior studies suggest that measurements of HDL2-cholesterol and HDL3-cholesterol provide little clinical benefit over HDL-cholesterol in identifying persons at CHD risk [14–21]. However, when HDL-subfractions are separated by gradient gel electrophoresis rather than precipitation, lower HDL2b is consistently associated with angiographically defined arteriosclerosis [24–27]. Levy et al. reported that men with ≥50% luminal narrowing in 0, 1, 2, and 3 coronary vessels had mean HDL2b-mass concentrations of 15,10, 15, and 4 mg/dl respectively, HDL2a-mass concentrations of 59, 54, 55, and 33 mg/dl, respectively, and HDL3-mass concentrations was 169, 161, 159, and 150 mg/dl, respectively [28]. Levy et al. did not report whether any of the differences were statistically significant [28]. However, their results support the findings of Figure 1, i.e., that in contrast to a linear dose-response relationship, most of the differentiable risk is in a subset of individuals with low HDL2.
Our analyses also showed that adjustment for LDL-mass concentrations eliminated the significance of total cholesterol as a CHD risk factor (significance reduced from P<10−8 to P=0.90).
Limitations
There are important limitations to these analyses. The participants were almost exclusively younger white males, and caution is warranted in generalizing the results more broadly. However, the results are not necessarily irrelevant to the health concerns of minorities. The predictive value of most conventional risk factors for CHD, including the Framingham risk assessment, appears to be similar for African-Americans and Whites [29,30]. D'Agostino et al. showed that the Framingham sex-specific CHD prediction equations worked reasonably well in Black men and women, and in Japanese-American and Hispanic men and Native-American women after recalibration for different risk factor levels and disease rates [31]. We note that the lipoprotein measurements were nonfasting. However, the postprandial nonfasting condition is more representative of the usual metabolic state than fasting, and several studies have shown that nonfasting triglycerides are superior in predicting CHD risk over conventional fasting measurements [32,333]. In our analyses, the nonfasting state may have increased VLDL significance because it is more representative of the usual metabolic state, but also decreased significance due to greater measurement error. We also note that although the analytic ultracentrifuge is not currently used for the measurement of lipoprotein subfractions, there is a strong correspondence between lipoproteins separated by buoyancy, density, and particle size, such that lipoprotein subfractions measured analytic ultracentrifugation agree well with their electrophoresis or density gradient estimates [1,7].
In conclusion, our analyses demonstrate the importance of both HDL2 and HDL3 as independent CHD risk factors, and the greater CHD risk reduction per mg/dl of HDL2- than HDL3-mass concentration. Our analyses support Gofman’s 1966 conclusions that were based on univariate analyses of 38 cases.
Acknowledgment
We wish to thank Ms. Karen Vranizan, Mr. Eric Sneider, and Ms. Linda Parobeck who assisted with the follow-up, Dr. Max Biggs and Frederick Hatch who assisted in locating the initial data, and Dr. Ronald Krauss who assisted in the early development of the study.
Supported by grant AG72110 from the Institute of Aging.
Glossary
- HDL
high-density lipoprotein
- LDL
low-density lipoprotein
- IDL
intermediate-density lipoprotein
- VLDL
very-low-density lipoprotein
- CHD
coronary heart disease
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Musliner TA, Krauss RM. Lipoprotein subspecies and risk of coronary disease. Clin Chem. 1988;34:B78–B83. [PubMed] [Google Scholar]
- 2.Gofman JW, Lindgren F, Elliott H, Mantz W, Hewitt J, Herring V. The role of lipids and lipoproteins in atherosclerosis. Science. 1950;111:166–171. doi: 10.1126/science.111.2877.166. [DOI] [PubMed] [Google Scholar]
- 3.DeLalla OF, Gofman JW. Ultracentrifugal analysis of serum lipoproteins. Methods Biochem Anal. 1954;1:459–478. doi: 10.1002/9780470110171.ch16. [DOI] [PubMed] [Google Scholar]
- 4.Gofman JW. Serum lipoproteins and the evaluation of atherosclerosis. Ann N Y Acad Sci. 1956;64:590–595. doi: 10.1111/j.1749-6632.1956.tb36833.x. [DOI] [PubMed] [Google Scholar]
- 5.Lindgren FT, Silvers A, Jutaglr R, Layshot L, Bradley DD. A comparison of simplified methods for lipoprotein quantification using the analytic ultracentrifuge as a standard. Lipids. 1977;12:278–282. doi: 10.1007/BF02533347. [DOI] [PubMed] [Google Scholar]
- 6.Hulley SB, Cook SG, Wilson WS, Nichaman MZ, Hatch FT, Lindgren FT. Quantitation of serum lipoproteins by electrophoresis on agarose gel: standardization in lipoprotein concentration units (mg-100 ml) by comparison with analytical ultracentrifugation. J Lipid Res. 1971;12:420–433. [PubMed] [Google Scholar]
- 7.Kahlon TS, Glines LA, Lindgren FT. Analytic ultracentrifugation of plasma lipoproteins. Methods Enzymol. 1986;129:26–45. doi: 10.1016/0076-6879(86)29060-6. [DOI] [PubMed] [Google Scholar]
- 8.Gofman JW, Young W, Tandy R. Ischemic heart disease, atherosclerosis, and longevity. Circulation. 1966;34:679–697. doi: 10.1161/01.cir.34.4.679. [DOI] [PubMed] [Google Scholar]
- 9.Colman DM, McPhee AF. An improved method for determination of total serum cholesterol. Am J Clin Pathol. 1956;26:181–186. doi: 10.1093/ajcp/26.2_ts.181. [DOI] [PubMed] [Google Scholar]
- 10.Fortmann SP, Varady AN. Effects of a community-wide health education program on cardiovascular disease morbidity and mortality: the Stanford Five-City Project. Am J Epidemiol. 2000;152:316–323. doi: 10.1093/aje/152.4.316. [DOI] [PubMed] [Google Scholar]
- 11.Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. Executive Summary of The Third Report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, And Treatment of High Blood Cholesterol In Adults (Adult Treatment Panel III) JAMA. 2001;285:2486–2497. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 12.Warnick GR, Bengtsson B, Albers JJ. Quantitation of high-density lipoprotein subclasses after separation by dextran sulfate and Mg2+ precipitation. Clin Chem. 1982;28:1574. [PubMed] [Google Scholar]
- 13.Williams PT, Krauss RM, Vranizan KM, Stefanick ML, Wood PD, Lindgren FT. Associations of lipoproteins and apolipoproteins with gradient gel electrophoresis estimates of high density lipoprotein subfractions in men and women. Arterioscler Thromb. 1992;12:332–340. doi: 10.1161/01.atv.12.3.332. [DOI] [PubMed] [Google Scholar]
- 14.Sharrett AR, Ballantyne CM, Coady SA, et al. Coronary heart disease prediction from lipoprotein cholesterol levels, triglycerides, lipoprotein(a), apolipoproteins A–I and B, and HDL density subfractions: The Atherosclerosis Risk in Communities (ARIC) Study. Circulation. 2001;104:1108–1113. doi: 10.1161/hc3501.095214. [DOI] [PubMed] [Google Scholar]
- 15.Fujimoto WY, Bergstrom RW, Boyko EJ, et al. Visceral adiposity and incident coronary heart disease in Japanese-American men. The 10-year follow-up results of the Seattle Japanese-American Community Diabetes Study. Diabetes Care. 1999;22:1808–1812. doi: 10.2337/diacare.22.11.1808. [DOI] [PubMed] [Google Scholar]
- 16.Lamarche B, Moorjani S, Cantin B, Dagenais GR, Lupien PJ, Després JP. Associations of HDL2 and HDL3 subfractions with ischemic heart disease in men. Prospective results from the Québec Cardiovascular Study. Arterioscler Thromb Vasc Biol. 1997;17:1098–1105. doi: 10.1161/01.atv.17.6.1098. [DOI] [PubMed] [Google Scholar]
- 17.Stampfer MJ, Sacks FM, Salvini S, Willett WC, Hennekens CH. A prospective study of cholesterol, apolipoproteins, and the risk of myocardial infarction. N Engl J Med. 1991;325:373–381. doi: 10.1056/NEJM199108083250601. [DOI] [PubMed] [Google Scholar]
- 18.Salonen JT, Salonen R, Seppänen K, Rauramaa R, Tuomilehto J. HDL, HDL2, and HDL3 subfractions, and the risk of acute myocardial infarction. A prospective population study in eastern Finnish men. Circulation. 1991;84:129–139. doi: 10.1161/01.cir.84.1.129. [DOI] [PubMed] [Google Scholar]
- 19.Laakso M, Lehto S, Penttilä I, Pyörälä K. Lipids and lipoproteins predicting coronary heart disease mortality and morbidity in patients with non-insulin-dependent diabetes. Circulation. 1993;88:1421–1430. doi: 10.1161/01.cir.88.4.1421. [DOI] [PubMed] [Google Scholar]
- 20.Sweetnam PM, Bolton CH, Yarnell JW, et al. Associations of the HDL2 and HDL3 cholesterol subfractions with the development of ischemic heart disease in British men. The Caerphilly and Speedwell Collaborative Heart Disease Studies. Circulation. 1994;90:769–774. doi: 10.1161/01.cir.90.2.769. [DOI] [PubMed] [Google Scholar]
- 21.Mora S, Otvos JD, Rifai N, Rosenson RS, Buring JE, Ridker PM. Lipoprotein particle profiles by nuclear magnetic resonance compared with standard lipids and apolipoproteins in predicting incident cardiovascular disease in women. Circulation. 2009;119:931–939. doi: 10.1161/CIRCULATIONAHA.108.816181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Otvos JD, Collins D, Freedman DS, et al. Low-density lipoprotein and high-density lipoprotein particle subclasses predict coronary events and are favorably changed by gemfibrozil therapy in the Veterans Affairs High-Density Lipoprotein Intervention Trial. Circulation. 2006;113:1556–1563. doi: 10.1161/CIRCULATIONAHA.105.565135. [DOI] [PubMed] [Google Scholar]
- 23.Musunuru K, Orho-Melander M, Caulfield MP, et al. Ion mobility analysis of lipoprotein subfractions identifies three independent axes of cardiovascular risk. Arterioscler Thromb Vasc Biol. 2009;29:1975–1980. doi: 10.1161/ATVBAHA.109.190405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Johansson J, Olsson AG, Bergstrand L, et al. Lowering of HDL2b by probucol partly explains the failure of the drug to affect femoral atherosclerosis in subjects with hypercholesterolemia. A Probucol Quantitative Regression Swedish Trial (PQRST) Report. Arterioscler Throm Vasc Biol. 1995;15:1049–1056. doi: 10.1161/01.atv.15.8.1049. [DOI] [PubMed] [Google Scholar]
- 25.Johansson J, Carlson LA, Landou C, Hamsten A. High density lipoproteins and coronary atherosclerosis. A strong inverse relation with the largest particles is confined to normotriglyceridemic patients. Arterioscler Thromb. 1991;11:174–182. doi: 10.1161/01.atv.11.1.174. [DOI] [PubMed] [Google Scholar]
- 26.Tornvall P, Karpe F, Proudler A, et al. High-density lipoprotein: relations to metabolic parameters and severity of coronary artery disease. Metabolism. 1996;45:1375–1382. doi: 10.1016/s0026-0495(96)90118-3. [DOI] [PubMed] [Google Scholar]
- 27.Watanabe H, Söderlund S, Soro-Paavonen A, et al. Decreased high-density lipoprotein (HDL) particle size, prebeta-, and large HDL subspecies concentration in Finnish low-HDL families: relationship with intima-media thickness. Arterioscler Thromb Vasc Biol. 2006;26:897–902. doi: 10.1161/01.ATV.0000209577.04246.c0. [DOI] [PubMed] [Google Scholar]
- 28.Levy RI, Brensike JF, Epstein SE, et al. Effects of therapy with cholestyramine on progression of coronary arteriosclerosis: results of the NHLBI Type II Coronary Intervention Study. Circulation. 1984;69:313–324. doi: 10.1161/01.cir.69.2.313. [DOI] [PubMed] [Google Scholar]
- 29.NCEP ATP-III. Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. Executive Summary of The Third Report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, And Treatment of High Blood Cholesterol In Adults (Adult Treatment Panel III) JAMA. 2001;285:2486–2497. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 30.Cooper RS, Liao Y, Rotimi C. Is hypertension more severe among US. blacks, or is severe hypertension more common? Ann Epidemiol. 1996;6:173–180. doi: 10.1016/1047-2797(96)00009-9. [DOI] [PubMed] [Google Scholar]
- 31.D'Agostino RB, Sr, Grundy S, Sullivan LM, Wilson P CHD Risk Prediction Group. Validation of the Framingham coronary heart disease prediction scores: results of a multiple ethnic groups investigation. JAMA. 2001;28:180–187. doi: 10.1001/jama.286.2.180. [DOI] [PubMed] [Google Scholar]
- 32.Nordestrgaad BG, Benn M, Schnohr P, Tybjaerg-Hansen A. Nonfasting triglycerides and risk of myocardial infarction, ischemic heart disease, and death in men and women. JAMA. 2007;298:299–308. doi: 10.1001/jama.298.3.299. [DOI] [PubMed] [Google Scholar]
- 33.Bansal S, Buring JE, Rifai N, Mora S, Sacks FM, Ridker PM. Fasting compared with nonfasting triglycerides and risk of cardiovascular events in women. JAMA. 2007;298:309–316. doi: 10.1001/jama.298.3.309. [DOI] [PubMed] [Google Scholar]