Abstract
Background
The use of nanoparticles (NPs) in technological applications is rapidly expanding, but the potential health effects associated with NP exposure are still largely unknown. Given epidemiological evidence indicating an association between inhaled ambient ultrafine particles and increased risk of cardiovascular disease morbidity and mortality, it has been suggested that exposure to NPs via inhalation may induce similar cardiovascular responses.
Methods
Male C57BL/6 mice were exposed via whole-body inhalation to either filtered air (FA) or nickel hydroxide (NH) NPs (100, 150, or 900 μg/m3) for 1, 3, or 5 consecutive days (5 h/day). At 24-h post-exposure, vascular function in response to a vasoconstrictor, phenylephrine (PE), and a vasodilator, acetylcholine (ACh), was measured in the carotid artery.
Results
Carotid arteries from mice exposed to all concentrations of NH-NPs showed statistically significant differences in graded doses of PE-induced contractile responses compared with those from FA mice. Similarly, vessels from NH-NP-exposed mice also demonstrated impaired vasorelaxation following graded doses of ACh as compared with FA mice.
Conclusions
These results suggest that short-term exposure to NH-NPs can induce acute endothelial disruption and alter vasoconstriction and vasorelaxation. These findings are consistent with other studies assessing vascular tone and function in the aorta, coronary, and mesenteric vessels from mice exposed to motor vehicular exhaust and concentrated ambient particles.
Keywords: Vascular function, nanoparticles, nickel, carotid artery, whole-body inhalation, mice
Introduction
Epidemiological studies have demonstrated an association between cardiovascular diseases (CVD) and exposure to ambient air pollution of various sizes and sources (Mills et al., 2009; Brook et al., 2010). Consequently, it is an ongoing public health concern to identify the specific pollution sources that are associated with the observed adverse health effects (Brook, 2008). Ultrafine or nanosized particles in particular have been cited as the main culprit (Delfino et al., 2005; Schulz et al., 2005). The market for products containing NPs, such as cosmetics, energy, medications, and nutritional supplements, continues to grow, placing occupational workers at an increasing risk. By 2015, nanotechnology will have a $1 trillion impact on the global economy as well as on the workforce (Department of Health and Human Services, Centers for Disease Control & National Institute for Occupational Safety and Health, 2008). With an industry growing at exponential rates, the researchers investigating these harmful effects struggle to keep workers and consumers safe.
Toxicological studies in mouse and human cells have shown that exposures to ambient ultrafine particles are associated with significant increases in cardiopulmonary responses, such as inflammation (Kleinman et al., 2008), altered lung function (Peters et al., 1997; Knaapen et al., 2004; Gong et al., 2008), blood pressure, heart rate variability, DNA oxidation, and blood coagulation (Araujo et al., 2008), decreased peak expiratory flow (Pekkanen et al., 1997), systemic oxidative stress (Kang et al., 2010), and an association to atherosclerosis and CVD mortality (Wichmann et al., 2000; Araujo & Nel, 2009). However, as mentioned earlier, with the increase in the rapidly growing field of nanotechnology, there is additional concern about potential exposure to particles in the ultrafine range, that is nanoparticles (NP).
While ambient ultrafine particles (UFP) and NPs have similar characteristics, size (<0.1 μm), large surface area, and are able to cross biological barriers, there are notable differences between the two. UFP are chemically complex mixtures that are inadvertently generated, whereas NPs are mostly homogeneous in their composition and intentionally generated for the use in novel applications. Exposures to NPs, especially those containing transition metals like nickel, magnesium, and vanadium, have been associated with increased respiratory and cardiovascular morbidity and mortality (Schulz et al., 2005, Elder & Oberdorster, 2006; Gojova et al., 2007). Nickel, for example, due to its corrosion resistance and energy conductance properties, is of particular interest in nanotechnology. More specifically, nickel has been a key component in electrical chemical applications, batteries, catalysts, coinage, foundry products, and plating (Vidotti et al., 2009). Increasing usage of this material creates increasing opportunity for potential occupational exposures. Research conducted in our laboratory recently demonstrated that inhalation exposures of NPs, specifically nickel hydroxide (NH) NP, elicited pulmonary inflammation in mice (Gillespie et al., 2010). From the same NH-NP exposure, an increase in gene expression of inflammatory markers in cardiovascular tissue of mice was also observed (Kang et al., 2010).
While research findings from UFP studies can serve as a database for predicting potential effects of NP exposure, the differences between the two and their exact mechanisms of damage warrant attention to validate research investigating the potential toxicity of NPs. Growing evidence suggests that particles and/or their soluble components infiltrate the systemic circulation and modulate vascular tone (Oberdorster et al., 2002; Elder & Oberdorster, 2006; Wallenborn et al., 2007; Kennedy et al., 2009). Therefore, the aim of the study was to investigate if NH-NP exposures can alter vascular tone and reactivity by impairing arterial vasoconstriction and vasorelaxation in the carotid artery. We investigated the vascular response of NH-NP exposure. Vascular responses in the carotid artery (diameters of 400 μm) are of particular interest since the carotid artery is the conduit vessel that delivers blood from the heart to the brain.
Materials and methods
Animals
Male C57BL/6 mice (12 week old, body weight 20–25 g) were obtained from Taconic Farms (Germantown, NY) and housed in an Association for Assessment and Accreditation of Laboratory Animal Care (AAALAC) accredited facility in Tuxedo, NY. Animals were kept on normal 12 h light/dark cycles and received food and water ad libitum, except during the exposure period. After a 2-week acclimation period, mice were exposed in whole-body inhalation chambers to either filtered air (FA) or NH-NPs. Details about the exposure chambers have been previously described elsewhere (Maciejczyk et al. 2005). All procedures were conducted in compliance with New York University’s guidelines for ethical animal research.
Inhalation chamber studies
Mice were exposed to NH-NPs in a whole-body exposure chamber for 5 h/day for either 1, 3, or 5 days to mass concentrations of 100, 150, and 900 μg/m3 (gravimetrical values: 124, 142.6, 923 μg/m3 respectively; Table 1). Exposure durations were selected to mimic the beginning, mid, and the end of an occupational work-week and designed to estimate worker exposures. Concentrations were selected based on the Occupational Safety and Health Administration permissible exposure limit (OSHA PE; 1 mg Ni/m3); 100–150 μg/m3 being roughly 10% of the limit and 900 μg/m3 being the upper end of the limit. In order to determine Ni lung and blood content and PE- and ACh-induced vascular reactions in the carotid artery, mice were euthanized with an overdose of ketamine hydrochloride (30 mg/kg) and sodium pentobarbital (150–200 mg/kg) via an intraperitoneal injection 24 h post-final exposure. Mice from the control group were treated in a similar manner with FA substituted for NH-NPs (n = 3–6 per exposure group).
Table 1.
Effect of various exposures of NH-NP on carotid artery contraction and relaxation grouped by exposure duration, approximate and actual concentrations and number of animals per exposure group (mean ± SE).
| Approx. Conc. (μg/m3) | Actual Conc. (μg/m3) | PE Log EC50 (M ± SE) | Max Contraction (mN ± SE) | PE Doses (M) | ACh Log ED50 (M ± SE) | Max Relaxation (mN ± SE) | ACh Doses (M) |
|---|---|---|---|---|---|---|---|
| 1-Day exposure | |||||||
| FA (n = 3) | — | −6.51 ± 0.04 | 91.4 ± 2.07 | — | −7.26 ± 0.19 | 12.9 ± 6.8 | — |
| 150 (n = 4) | 142.6 | −6.24 ± 0.05* | 87.2 ± 2.06 | 10−7.5–10−6.5 | −7.08 ± 0.15 | 32.6 ± 4.8** | >10−5 |
| 3-Day exposure | |||||||
| FA (n = 3) | — | −7.06 ± 0.08 | 95.0 ± 3.52 | — | −7.15 ± 0.20 | 17.2 ± 7.80 | — |
| 150 (n = 6) | 142.6 | −6.74 ± 0.08 | 74.2 ± 5.36* | >10−7.5 | −6.96 ± 0.15 | 32.8 ± 3.84* | >10−7 |
| 900 (n = 4) | 923.2 | −6.27 ± 0.07* | 65.4 ± 5.02** | >10−7.5 | −6.97 ± 0.20 | 47.2 ± 4.29*** | >10−5.5 |
| 5-Day exposure | |||||||
| FA (n = 3) | — | −7.04 ± 0.11 | 99.7 ± 3.48 | — | −7.00 ± 0.28 | 6.82 ± 3.89 | — |
| 100 (n = 6) | 124 | −6.48 ± 0.07* | 97.2 ± 4.67 | 10−7.5–10−6.5 | −7.62 ± 0.08 | 33.4 ± 7.57*** | >10−7.5 |
mN = millinewton; M = Molar.
FA = Filter air; NH-NP = Nickel Hydroxide Nanoparticles.
P ≤ 0.05,
P ≤ 0.01,
P ≤ 0.001.
Exposure system and NP characterization
Extensive details of the exposure system utilized in this study and the methods of NH-NP characterization have been described by our group elsewhere (Gillespie et al., 2010). In brief, NH-NPs were produced by opposing metallic nickel electrodes (99.995% purity; ESPI, Ashland, OR) in an ultrapure argon chamber using a Palas® GmbH arc furnace (Model GFG-1000, Karlsruhe, Germany). Ultrapure oxygen was added to filtered dilution air in order to keep the exposure atmosphere at 20% oxygen. NPs were characterized by transmission electron microscopy (TEM), Scanning Mobility Particle Sizer (SMPS), X-ray fluorescence (XRF), and photoelectron spectroscopy (XPS) (Gillespie et al., 2010).
Nickel tissue content
At 24 h post-exposure, right ventricle puncture was used to collect whole blood in a 2-mL centrifuge tube containing ethylenediaminetetraacetic acid (EDTA). Lungs from each mouse were removed and weighed. Harvested lung tissue was wet-ashed and nickel content was determined by graphite furnace atomic absorption (AA) spectroscopy (GF95; Thermo Scientific, Waltham, MA) using a five-point calibration curve constructed from certified nickel reference standards (Fisher Scientific, Pittsburgh, PA). More detailed methods of Ni elemental content analysis have been previously described by Gillespie et al. (2010).
Vascular function
Immediately following sacrifice, 2-mm segments of the carotid artery were isolated from each mouse and suspended on a four-channel wire myograph (multi-Myograph; 610 M, Danish Myo Technology, Aarhus N, Denmark) in individual organ chambers baths filled with physiological salt solution (PSS) buffer (pH 7.4). The chambers were kept at 37°C and continuously aerated with 5% carbon dioxide in oxygen at 37°C. Mounted vessels were allowed to equilibrate for at least 1 h to a resting basal pressure of 120 mmHg before being subjected to graded doses of agonists. Each experiment was initiated by precontracting the vessels with 60 mM of non-specific depolarizing agent K+-PSS, followed by contracting the vessels with 120 mM K+-PSS. Shortly after, the vessels were washed and allowed to equilibrate to their resting state before beginning procedures for graded dose responses.
Grades dose response
Phenylephrine (PE), a vasoconstrictor agonist, was added in graded doses (from concentrations of 1 nM to 3 μM) to evaluate vascular function (Sigma-Aldrich, St. Louis, MO). PE responses were expressed as a percentage of the peak response to 120 mM of potassium chloride (KCl). Vessels were then thoroughly washed and allowed to return to their basal state. After the vessels were washed and became equilibrated, vasorelaxation was tested by precontracting the segments with 1 μM PE and subsequently relaxing them with increasing concentrations (1 nM to 3 μM) of acetylcholine (ACh), an endothelium-dependent agonist (Sigma-Aldrich, St. Louis, MO). ACh results were expressed as a percentage of precontraction by PE (1 μM).
Statistics
Statistical significance of nickel content in tissues (AA analysis) was determined using a Student’s t-test. Maximal contraction or relaxation data from vascular response was fitted to a sigmoidal dose–response curves using nonlinear regression analysis and expressed as mean ± standard error (SE) unless otherwise noted. Statistical significance of treatment- related vascular responses was determined by one-way ANOVA followed by Bonferroni’s multiple comparison post hoc analysis for samples of unequal sizes and P < 0.05 was considered significant. All statistical analyses were performed using Graphpad Prism software (V4.0, Graphpad Software Inc., San Diego, CA).
Results
Particles
Using TEM analysis and electrostatic classification, Gillespie et al. (2010) reported that primary NPs generated had an approximate count mean diameter (CMD) of 5 nm and the agglomerated particles had a CMD of 40 nm, with an overall average geometric standard deviation of 1.50. Further, XPS determined that both the core and surface of the generated NH-NP to be comprised of nickel (II) hydroxide (Ni(OH)2) (Gillespie et al., 2010).
Nickel tissue content
Levels of Ni found in the blood of exposed mice were below the detection limit; however, as expected there were significant differences in the amount of Ni found in the lungs of exposed mice as compared with their FA counterparts (P < 0.01).
Vascular function
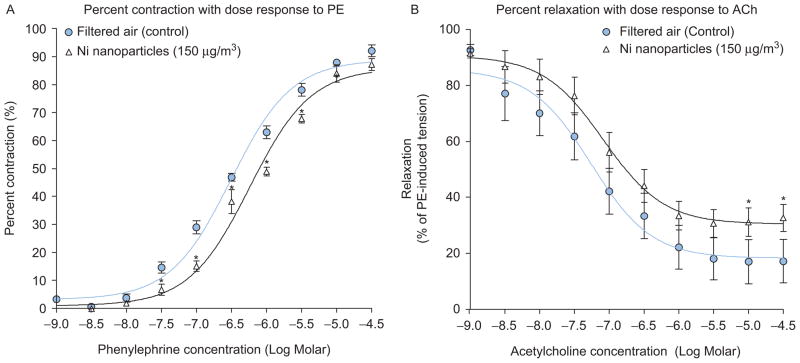
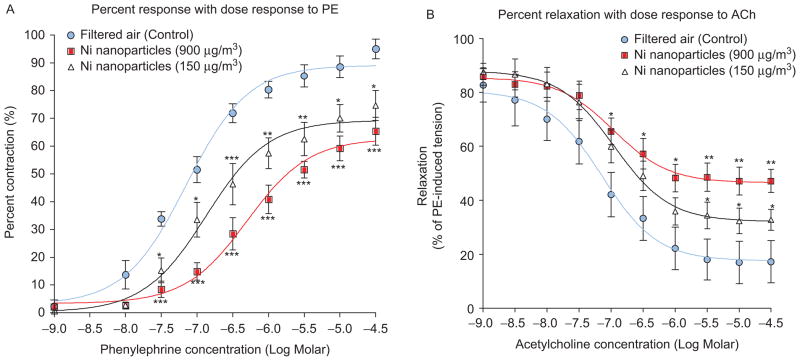
Since constrictive responses to 120 mM KCl did not differ significantly among groups, PE-induced responses were standardized using the 120 mM KCl responses (Figure 1A). Further, no significant differences were observed in the percentage of PE-induced precontraction between all groups and were therefore used to normalize ACh-mediated responses (Figure 1B). Carotid arteries from NH-NP-exposed mice showed significant impairment in PE-induced vasoconstriction and decreased ACh-induced vasorelaxation in comparison with FA vessels (Figures 2–4). Results are reported by each NH-NP exposure (in days) as follows (Table 1):
Figure 1.

(A) Administration of 120 mM KCl yielded no significant differences among groups. (B) No significant differences were observed with phenylephrine (PE)-induced maximum constriction (millinewton, mN) between all groups.
Figure 2.
Vascular responses 24 h post 1 day FA or NH-NP exposure of 150 μg/m3 to graded dose of (A) vasoconstrictor PE; (B) vasodilator ACh (vessels PE pre-contraction 10−6 M; *P < 0.05, **P < 0.01, ***P < 0.001).
Figure 4.
Vascular responses 24 h post 5 day FA or NH-NP of 100 μg/m3 to graded dose of (A) vasoconstrictor PE; (B) vasodilator ACh (vessels PE pre- contraction 10−6 M; *P < 0.05, **P < 0.01, ***P < 0.001).
1 Day: Significant decreases in percent response were seen in carotid arteries exposed for 1 day to NH-NP (150 μg/m3); specifically, significant differences in vasoconstriction were seen at PE doses of 10−7.5 through 10−5.5 M (Figure 2A). ACh doses greater than 10−5 M also yielded significantly reduced relaxation compared with FA controls (Figure 2B).
3 Days: Three-day exposures to NH-NP concentrations of 150 and 900 μg/m3 resulted in PE-induced contractions that were significantly less than contractions from control vessels at PE doses >10−7.5 (Figure 3A). Significantly different ACh-induced response occurred at doses >10−7 M compared with control ACh-induced responses (Figure 3B).
5 Days: Five-day exposures to NH-NP at concentrations of 100 μg/m3 resulted in a significant decrease in carotid artery responsiveness to graded doses of PE (10−7.5 to 10−6.5 M; Figure 4A). Similarly, the same 5-day exposures yielded impairment in ACh-induced vasorelaxation at all doses >10−7.5 M in pre PE-contracted carotid arteries from NH-NP-exposed mice (Figure 4B).
Figure 3.
Vascular mediated response as a result of 3-day exposures to NH-NP concentrations (150 μg/m3, 900 μg/m3, or FA); (A) vasoconstrictor PE; (B) vasodilator ACh (vessels PE pre-contraction 10−6 M; *P < 0.05, **P < 0.01, ***P < 0.001).
Discussion
It is important to understand how NPs, and particularly Ni, elicit biological effects. Ni NPs are highly favorable for use as material sources of chemical energy, such as in rechargeable batteries (Gálvez et al., 2010) and in medical technologies (Li et al., 2010). Our results complement those from other in vivo animal studies reporting that inhalation exposure of environmental and other model particles are associated with impairment of endothelial function (Sun et al., 2005; Nurkiewicz et al., 2006; Upadhyay et al., 2008; LeBlanc et al., 2009). Specifically, Upadhyay et al. (2008) found that low levels of inhaled ultrafine carbon particles induce systemic effects leading to spikes in blood pressure and decreases in heart rate variability in spontaneous hypertensive rats. Based on their findings, they suggest that ultrafine carbon exposures can cause cardiovascular and pulmonary impairment in sensitive populations via endothelial mechanisms (Upadhyay et al., 2008). Similarly Sun et al. (2005) demonstrated that subchronic inhalation exposure to PM2.5, even at low concentrations, potentiates atherosclerosis and alters vasomotor tone in aorta from an apolipoprotein-deficient E−/− mouse model (Sun et al., 2005). Another study found that microvessels in the sinotrapezius muscle from rats exposed to inhaled TiO2 NPs exhibited impaired vasorelaxation when compared with vessels from rats exposed to FA (Nurkiewicz et al., 2008). Further, after an inhalation exposure also using TiO2 NPs as a test material, LeBlanc et al. (2009) documented a blunted vasodilation response in the coronary arterioles of rats (LeBlanc et al., 2009). These observations all demonstrate acute exposures to nanomaterial have the potential to alter vasoreactivity.
We observed that short-term inhalation exposures designed to mimic beginning, mid, and end of an occupational work-week (1, 3, and 5 days) with occupationally relevant concentrations of NH-NPs are associated with acute impaired endothelial vasoconstriction and vasorelaxation in carotid arteries of mice, suggesting that mechanisms in both smooth muscle and endothelial layers of the vessel may be involved. Specifically vessels from both the 3-day high (900 μg/m3) and low (150 μg/m3) NH-NP exposures inflicted vascular function in comparison with the function of the FA vessels. The vasocontraction response post 5-day low (100 μg/m3) NH-NP did not significantly differ from the 3-day low (150 μg/m3); however, there appeared to be cumulative effect in the vasorelaxation effect, implying possible endothelial damage at even low levels of exposure. ACh, via its receptor on endothelial cells, promotes the formation of endothelium-derived relaxing factor, which regulates vessel tone. When this receptor has been missed by damage or denudation, the vessels lose the ability of vasorelaxation (Cheng & Kang, 1999). The impairment in vasorelaxation in our study mimicked results from studies of denuded vessels (Cheng & Kang, 1999), perhaps implying carotid arteries from our NH-NP-exposed mice may have had a damaged endothelial layer.
Disruption in endothelial contraction/dilation abilities may lead to a cascade of events that are associated with the development of a variety of disease pathologies: hypertension, atherosclerosis, and myocardial infarction (Heitzer et al., 2001). Based on our observation of impaired vascular responses, we posit that there was a direct impact on endothelium function and impaired vascular responsiveness post-inhalation of NH-NPs. It is possible that NH-NP exposures create a proinflammatory cascade releasing considerable amounts of reactive oxygen species (ROS) and vascular mediators (cytokines and chemokines), depleting nitric oxide (NO) (Miller et al., 2009). Impaired vasculature could result in inadequate blood circulation and insufficient blood supply delivered to the respected tissue. The changes that occurred in the carotid artery observed in our study may have significant impact to the central nervous system and propagate to the other organ systems.
Various limitations exists in our study. One in particular is that we only assessed vascular function using arterial tone response as the main endpoint in one tissue type. We plan to expand to other markers of vascular health (iNOS, eNOS activity, and glutathione levels) in the future. Levels of oxidative and inflammatory genes, cytokine, and protein expression will also be assessed. Impaired vascular response can be either due to a lack of NO or a dysfunction of the smooth muscle cells. Therefore, future studies are also needed to investigate the role of NO and vascular function using sodium nitroprusside (SNP) and endothelin-1 (ET-1) as vasodialators in addition to ACh. Further, our data is limited to fairly short-exposure durations and only 24 h post last exposure endpoints were evaluated. Longer exposures, dose and time course of responses would help to delineate the development of NP-induced vascular dysfunction.
Conclusion
Our findings of endothelial dysfunction due to NH-NP inhalation suggest that the adverse cardiovascular effects of Ni nanosized particles or the soluble components could alter endothelium function, hence, elucidating vascular effects. In turn, this may adversely affect the endothelial layer in other tissues, thereby increasing circulating vascular mediators (inflammatory cytokines and vascular adhesion molecules), equating a reduction in vascular tone, arterial compliance, and increases central arterial pressure and left ventricular workload. Therefore, any vasculature impairment (e.g. arterial noncompliance or stiffening) can ultimately decrease the blood volume to the brain increasing risk for stroke or a variety of other CVDs.
Acknowledgments
We would like to thank Dr. Matthew Campen for his guidance and support.
Footnotes
Declaration of interest
This study was supported by R01ES015495 (Chen, LC), NIH Fellowship: F31 ES018236 (Cuevas, AK), CIHR-DRA (Liberda, EN), Center Grant ES00260.
References
- Araujo JA, Barajas B, Kleinman M, Wang X, Bennett BJ, Gong KW, Navab M, Harkema J, Sioutas C, Lusis AJ, Nel AE. Ambient particulate pollutants in the ultrafine range promote early atherosclerosis and systemic oxidative stress. Circ Res. 2008;102:589–596. doi: 10.1161/CIRCRESAHA.107.164970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araujo JA, Nel AE. Particulate matter and atherosclerosis: role of particle size, composition and oxidative stress. Part Fibre Toxicol. 2009;6:24. doi: 10.1186/1743-8977-6-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brook RD. Cardiovascular effects of air pollution. Clin Sci. 2008;115:175–187. doi: 10.1042/CS20070444. [DOI] [PubMed] [Google Scholar]
- Brook RD, Rajagopalan S, Pope CA, III, Brook JR, Bhatnagar A, Diez-Roux AV, Holguin F, Hong Y, Luepker RV, Mittleman MA, Peters A, Siscovick D, Smith SC, Jr, Whitsel L, Kaufman JD on behalf of the American Heart Association Council on Epidemiology and Prevention, Council on the Kidney in Cardiovascular Disease, and Council on Nutrition, Physical Activity and Metabolism. Particulate matter air pollution and cardiovascular disease: an update to the scientific statement from the american heart association. Circulation. 2010;121:2331–2378. doi: 10.1161/CIR.0b013e3181dbece1. [DOI] [PubMed] [Google Scholar]
- Cheng YW, Kang JJ. Inhibition of agonist-induced vasocontraction and impairment of endothelium-dependent vasorelaxation by extract of motorcycle exhaust particles in vitro. J Toxicol Environ Health. 1999;57(Part A):75–87. doi: 10.1080/009841099157791. [DOI] [PubMed] [Google Scholar]
- Delfino R, Sioutas C, Malik S. Potential role of ultrafine particles in associations between airborne particle mass and cardiovascular health. Environ Health Perspect. 2005;113:934–46. doi: 10.1289/ehp.7938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Department of Health and Human Services, Centers for Disease Control & National Institute for Occupational Safety and Health. Strategic Plan for NIOSH Nanotechnology Research and Guidance: Filling the Knowledge Gaps. The National Institute for Occupational Safety and Health; 2008. [Google Scholar]
- Elder A, Oberdorster G. Translocation and effects of ultrafine particles outside of the lung. Clin Occup Environ Med. 2006;5:785–796. doi: 10.1016/j.coem.2006.07.003. [DOI] [PubMed] [Google Scholar]
- Gálvez N, Valero E, Ceolin M, Trasobares S, López-Haro M, Calvino JJ, Domínguez-Vera JM. A bioinspired approach to the synthesis of bimetallic CoNi nanoparticles. Inorg Chem. 2010;49:1705–1711. doi: 10.1021/ic902128g. [DOI] [PubMed] [Google Scholar]
- Gillespie PA, Kang GS, Elder A, Gelein R, Chen L, Moreira AL, Koberstein J, Tchou-Wong K, Gordon T, Chen LC. Pulmonary response after exposure to inhaled nickel hydroxide nanoparticles: short and long-term studies in mice. Nanotoxicology. 2010;4:106–119. doi: 10.3109/17435390903470101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gojova A, Guo B, Kota RS, Rutledge JC, Kennedy IM, Barakat AI. Induction of inflammation in vascular endothelial cells by metal oxide nanoparticles: effect of particle composition. Environ Health Perspect. 2007;115:403–409. doi: 10.1289/ehp.8497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong H, Jr, Linn WS, Clark KW, Anderson KR, Sioutas C, Alexis NE, Cascio WE, Devlin RB. Exposures of healthy and asthmatic volunteers to concentrated ambient ultrafine particles in Los Angeles. Inhal Toxicol. 2008;20:533–545. doi: 10.1080/08958370801911340. [DOI] [PubMed] [Google Scholar]
- Heitzer T, Schlinzig T, Krohn K, Meinertz T, Munzel T. Endothelial dysfunction, oxidative stress, and risk of cardiovascular events in patients with coronary artery disease. Circulation. 2001;104:2673–2678. doi: 10.1161/hc4601.099485. [DOI] [PubMed] [Google Scholar]
- Kang GS, Soh YF, Kofidis T, Lee CN. Five-year experience with congenital cardiac surgery at National University Heart Centre, Singapore. Singapore Med J. 2010;51:570–575. [PubMed] [Google Scholar]
- Kennedy I, Wilson D, Barakat A. Uptake and inflammatory effects of nanoparticles in a human vascular endothelial cell line. Research report. 2009;136:3. [PubMed] [Google Scholar]
- Kleinman MT, Araujo JA, Nel A, Sioutas C, Campbell A, Cong PQ, Li H, Bondy SC. Inhaled ultrafine particulate matter affects CNS inflammatory processes and may act via MAP kinase signaling pathways. Toxicol Lett. 2008;178:127–130. doi: 10.1016/j.toxlet.2008.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knaapen AM, Borm PJ, Albrecht C, Schins RP. Inhaled particles and lung cancer. Part A: mechanisms. Int J Cancer. 2004;109:799–809. doi: 10.1002/ijc.11708. [DOI] [PubMed] [Google Scholar]
- LeBlanc AJ, Cumpston JL, Chen BT, Frazer D, Castranova V, Nurkiewicz TR. Nanoparticle inhalation impairs endothelium-dependent vasodilation in subepicardial arterioles. J Toxicol Environ Health Part A. 2009;72:1576–1584. doi: 10.1080/15287390903232467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q, Zhang H, Chen Q, Quan N. Existence of seven human IL-1R1 promoters. J Inflamm Res. 2010;2010:17–24. doi: 10.2147/JIR.S7095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maciejczyk P, Zhong M, Li Q, Xiong J, Nadziejko C, Chen LC. Effects of subchronic exposures to concentrated ambient particles (CAPs) in mice. II. The design of a CAPs exposure system for biometric telemetry monitoring. Inhal Toxicol. 2005;17:189–197. doi: 10.1080/08958370590912743. [DOI] [PubMed] [Google Scholar]
- Miller MR, Borthwick SJ, Shaw CA, McLean SG, McClure D, Mills NL, Duffin R, Donaldson K, Megson IL, Hadoke PW, Newby DE. Direct impairment of vascular function by diesel exhaust particulate through reduced bioavailability of endothelium-derived nitric oxide induced by superoxide free radicals. Environ Health Perspect. 2009;117:611–616. doi: 10.1289/ehp.0800235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills NL, Donaldson K, Hadoke PW, Boon NA, MacNee W, Cassee FR, Sandström T, Blomberg A, Newby DE. Adverse cardiovascular effects of air pollution. Nat Rev Cardiol. 2009;6:36–44. doi: 10.1038/ncpcardio1399. [DOI] [PubMed] [Google Scholar]
- Nurkiewicz TR, Porter DW, Barger M, Millecchia L, Rao KM, Marvar PJ, Hubbs AF, Castranova V, Boegehold MA. Systemic microvascular dysfunction and inflammation after pulmonary particulate matter exposure. Environ Health Perspect. 2006;114:412–419. doi: 10.1289/ehp.8413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nurkiewicz TR, Porter DW, Hubbs AF, Cumpston JL, Chen BT, Frazer DG, Castranova V. Nanoparticle inhalation augments particle-dependent systemic microvascular dysfunction. Part Fibre Toxicol. 2008;5:1. doi: 10.1186/1743-8977-5-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberdorster G, Sharp Z, Atudorei V, Elder A, Gelein R, Lunts A, Kreyling W, Cox C. Extrapulmonary translocation of ultrafine carbon particles following whole-body inhalation exposure of rats. J Toxicol Environ Health. 2002;65(Part A):1531–1543. doi: 10.1080/00984100290071658. [DOI] [PubMed] [Google Scholar]
- Pekkanen J, Timonen KL, Ruuskanen J, Reponen A, Mirme A. Effects of ultrafine and fine particles in urban air on peak expiratory flow among children with asthmatic symptoms. Environ Res. 1997;74:24–33. doi: 10.1006/enrs.1997.3750. [DOI] [PubMed] [Google Scholar]
- Peters A, Wichmann HE, Tuch T, Heinrich J, Heyder J. Respiratory effects are associated with the number of ultrafine particles. Am J Respir Crit Care Med. 1997;155:1376–1383. doi: 10.1164/ajrccm.155.4.9105082. [DOI] [PubMed] [Google Scholar]
- Schulz H, Harder V, Ibald-Mulli A, Khandoga A, Koenig W, Krombach F, Radykewicz R, Stampfl A, Thorand B, Peters A. Cardiovascular effects of fine and ultrafine particles. J Aerosol Med. 2005;18:1–22. doi: 10.1089/jam.2005.18.1. [DOI] [PubMed] [Google Scholar]
- Sun Q, Wang A, Jin X, Natanzon A, Duquaine D, Brook RD, Aguinaldo JG, Fayad ZA, Fuster V, Lippmann M, Chen LC, Rajagopalan S. Longterm air pollution exposure and acceleration of atherosclerosis and vascular inflammation in an animal model. JAMA. 2005;294:3003–3010. doi: 10.1001/jama.294.23.3003. [DOI] [PubMed] [Google Scholar]
- Upadhyay S, Stoeger T, Harder V, Thomas RF, Schladweiler MC, Semmler-Behnke M, Takenaka S, Karg E, Reitmeir P, Bader M, Stampfl A, Kodavanti UP, Schulz H. Exposure to ultrafine carbon particles at levels below detectable pulmonary inflammation affects cardiovascular performance in spontaneously hypertensive rats. Part Fibre Toxicol. 2008;5:19. doi: 10.1186/1743-8977-5-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vidotti M, Salvador RP, Córdoba de Torresi SI. Synthesis and characterization of stable Co and Cd doped nickel hydroxide nanoparticles for electrochemical applications. Ultrason Sonochem. 2009;16:35–40. doi: 10.1016/j.ultsonch.2008.07.006. [DOI] [PubMed] [Google Scholar]
- Wallenborn JG, McGee JK, Schladweiler MC, Ledbetter AD, Kodavanti UP. Systemic translocation of particulate matter-associated metals following a single intratracheal instillation in rats. J Toxicol Sci. 2007;98:231–239. doi: 10.1093/toxsci/kfm088. [DOI] [PubMed] [Google Scholar]
- Wichmann HE, Spix C, Tuch T, Wolke G, Peters A, Heinrich J, Kreyling WG, Heyder J. Daily mortality and fine and ultrafine particles in Erfurt, Germany part I: role of particle number and particle mass. Res Rep (Health Effects Inst) 2000;98:5–86. discussion 87–94. [PubMed] [Google Scholar]