Abstract
Objectives
The aim of the current study was to test the hypothesis that vascular and endothelial functional responses to acute mental stress are abnormal in patients with apical ballooning syndrome (ABS).
Background
Apical ballooning syndrome is a transient cardiomyopathy that occurs predominantly in post-menopausal women and may be triggered by acute mental stress. The mechanism for ABS is unknown.
Methods
Reactive hyperemia as a parameter of endothelial function and vascular responses to acute mental stress were measured using peripheral arterial tonometry (PAT) at baseline and following 3 acute mental stress tests in female patients with ABS (n = 12, at least 6 months after being hospitalized or diagnosed with ABS), post-menopausal female controls (n = 12), and female patients with myocardial infarction (MI) (n = 4). Plasma catecholamine levels were measured at baseline and following the 3 mental stress tests.
Results
Reactive hyperemia PAT scores following mental stress were significantly lower in patients with ABS compared with post-menopausal controls (p < 0.05). The PAT scores during mental stress were significantly lower in patients with ABS compared with patients with MI and post-menopausal controls (p < 0.05). There were no differences in PAT scores during acute mental stress in patients with MI versus post-menopausal controls. Furthermore, catecholamine levels were significantly increased in patients with ABS, compared with post-menopausal controls, following acute mental stress testing (p < 0.05).
Conclusions
There is increased vascular reactivity and decreased endothelial function in response to acute mental stress in patients with a prior episode of ABS. The findings implicate vasomotor dysfunction as a potential mechanism involved in the pathogenesis of this unique cardiomyopathy.
Keywords: endothelium, stress-induced cardiomyopathy, Tako-Tsubo cardiomyopathy, vasculature, women
Women are more likely than men to experience cardiovascular events and death in the absence of angiographic coronary obstruction. This phenomenon may be secondary to functional vascular abnormalities such as endothelial dysfunction and microvascular disease (1–3). Endothelial dysfunction has been associated with myocardial ischemia and is an independent risk factor for adverse cardiac events in patients without coronary artery disease. This effect is even more pronounced in women (4), underscoring the importance of endothelium and vascular function in regulating systemic hemodynamic responses and cardiovascular health (3,5,6).
Recently there has been increasing awareness of a unique acute cardiomyopathy occurring almost exclusively in post-menopausal women, known as apical ballooning syndrome (ABS), Tako-Tsubo cardiomyopathy, or stress cardiomyopathy (7–11). The mechanisms involved in the pathogenesis of ABS remain unknown; however, many patients with ABS provide a history of a preceding strong emotional or psychological trigger. Psychosocial stressors of daily life that trigger emotions are closely associated with the increased morbidity and mortality in women with cardiovascular disorders (12–15). The clinical presentation mimics acute myocardial infarction (MI) and is accompanied by reduced ejection fraction without obstructive coronary artery disease (7). We recently reported that there is a significant recurrence rate, which may indicate an underlying substrate that predisposes individuals to developing the syndrome (10). Thus, the aim of the study was to test the hypothesis that patients with ABS have impaired vascular responses and endothelial function in response to mental stress.
Methods
Study population
Female patients were recruited from among those previously admitted to our institution and diagnosed with ABS (10,16). The diagnosis of ABS required the fulfillment of the following Mayo diagnostic criteria (17): 1) transient akinesis, hypokinesis, or dyskinesis of the left ventricular mid segments with or without apical involvement, and the regional wall motion abnormalities extending beyond a single epicardial vascular distribution; 2) absence of obstructive coronary disease or angiographic evidence of acute plaque rupture; 3) new electrocardiography abnormalities at the time of the episode (either ST-segment elevation and/or T-wave inversion) or elevated cardiac troponin; and 4) absence of pheochromocytoma or myocarditis. We invited all patients admitted with ABS in the preceding 6 years by letter and recruited those who were willing to participate in the study. A control group of post-menopausal women was recruited by advertisements. The controls had no history of ABS or MI. A small control group (n = 4) of post-menopausal women with history of ST-segment elevation MI more than 6 months prior to the study, but no history of ABS, were recruited to show that any differences in responses to acute mental stress in patients with ABS were not due to the recent stress of being hospitalized. Unfortunately, we were unable to obtain plasma catecholamine levels from these patients.
The exclusion criteria for participants were history of liver or kidney disease and hormone replacement therapy in any group. Post-menopausal controls could not have any history of significant cardiovascular disease. The study was approved by the institutional review board. Written informed consent was obtained from each participant.
Experimental protocol
All studies were performed in a quiet, temperature-controlled room. Participants fasted for 4 hours before the study and abstained from coffee or tobacco use on the day of the examination. None of the participants used vasoactive medications within 24 h of the study. The study was conducted in the sitting position in a comfortable chair with armrests. A fitted blood pressure cuff was placed on one arm, and the finger cuffs of the EndoPAT 2000 device (Itamar Medical Inc. Ltd., Caesarea, Israel) were placed on the middle finger of each hand (18). Participants relaxed for 10 min before initiation of the protocol. Baseline blood pressure and heart rate were obtained via a digital automated blood pressure cuff (Omron Healthcare, Inc., Vernon Hills, Illinois). Double product was calculated as: systolic blood pressure × heart rate.
Peripheral arterial tonometry (PAT)
PAT signals were obtained using the EndoPAT 2000 device, as described previously (18–22). The EndoPAT 2000 was used specifically in this study because it is a Food and Drug Administration–approved noninvasive device, it allows continuous recording of the signal, and interpretation of the data is operator independent. The use of a control arm allowed the elimination of systemic interference of the test and independence from the participant’s knowledge or conscious control of the signals (18–22).
The finger probes consist of inflatable latex air cuffs connected by pneumatic tubes to an inflating device controlled through a computer algorithm. A constant counter pressure (predetermined by baseline diastolic blood pressure) was applied through the air cushions. This prevents venous pooling and thereby venoarteriolar reflex vasoconstriction while not occluding arterial blood flow. Pulsatile volume changes of the distal digit induces pressure alterations in the finger cuff, which are sensed by pressure transducers and transmitted to and recorded by the Endo-PAT 2000 device. A decrease in the arterial blood volume in the distal finger tip causes a decrease in pulsatile arterial column changes, reflected as a decrease in the measured PAT signal, and vice versa.
Reactive hyperemia protocol
Endothelial function was measured via a reactive hyperemia PAT index or score. The method has been previously described (18–22). Previous studies have demonstrated dependence of the reactive hyperemia PAT score on nitric oxide, confirming that reported reactive hyperemia PAT scores indeed reflect changes in nitric oxide–dependent endothelial function, rather than neurohormonal activation (23).
The experimental protocol included a 16-min reactive hyperemia test at baseline (5-min baseline period, 5-min arterial occlusion by blood pressure cuff, 6-min post-occlusion signal recording), followed by a 5-min rest period, the 3 mental stress tests, another 5-min rest period, and another 16-min reactive hyperemia test. Each of the 3 mental stress tests was 6 min in duration followed by 5 min of rest (6-min Stroop color-word test, 5-min rest, 6-min arithmetic test, 5-min rest, 6-min spiral omnibus test, 5-min rest). Blood pressure and heart rate measurements were taken 30 s before, 2 min into, and at the end of each 6-min mental stress task. The ratio of the PAT signal after cuff release compared with baseline was calculated through a computer algorithm automatically normalizing for baseline signal and indexed to the contralateral arm. The calculated ratio reflects the reactive hyperemia PAT score.
Mental stress protocol
The mental stress testing protocol was administered under the supervision of a licensed psychometrist. Following the first reactive hyperemia trial and a subsequent rest period, the three 6-min mental stress tasks were performed in random order. All tasks had intratest levels of varying difficulty and were externally paced to accentuate the induced mental stress (18,24). Blood pressure and heart rate measurements were taken 30 s before, 2 min into, and at the end of each 6-min mental stress task. Participants relaxed for 5 min between each mental stress task. At the end of the third and final mental stress task, the reactive hyperemia protocol was repeated.
The tasks consisted of a number-letter recall challenge of increasing length and complexity (spiral omnibus), number subtraction of increasing difficulty (subtracting 1 digit from 2-digit numbers up to subtracting 3 digits from 3-digit numbers), and computerized version of the Stroop word-color conflict. The Stroop word-color conflict test consisted of 3 colored words (red, blue, and green) displayed in random order on a computer screen. Each word could appear in either its own color (visually concordant) or in one of the other 2 colors (visually discordant). The participant’s task was to enunciate the color of the word, not the actual word itself. A rectangular box surrounded the stimulus color-word, and this task was also externally paced, with the participant having to keep pace with the advancing box.
Blood collection
Before the study, an 18-gauge intravenous catheter was placed in the control (nontest) arm of the participant for the duration of the study. A blood sample of 10 ml was drawn from the intravenous catheter before the start of the study and following the acute mental stress testing. Samples were sent to the Mayo Clinic central laboratory for measurement of plasma catecholamine levels using high-performance liquid chromatography (25). We were unable to obtain blood samples from MI controls.
Statistical analysis
Results are expressed as mathematical mean ± SD. Repeated-measures analysis of variance was used to assess differences between treatment groups and levels. Differences between groups were analyzed using unpaired t test. Within-group differences were analyzed using paired t tests. No adjustments for multiple comparisons were made. Statistical significance was accepted at p < 0.05.
Results
Baseline characteristics
A total of 12 patients with ABS and 12 age-matched post-menopausal controls were studied, as were a group of 4 post-menopausal women with a history of MI and no history of ABS. There were no significant differences in subject characteristics (age; body mass index; or plasma cholesterol, triglycerides, or lipid levels) or prevalence of cardiovascular risk factors (hypertension, diabetes mellitus, or smoking) among the 12 patients with ABS, 12 post-menopausal controls, and 4 MI controls (p > 0.05). There were no significant differences in subject characteristics (age; body mass index; or plasma cholesterol, triglycerides, or lipid levels) or prevalence of cardiovascular risk factors (hypertension, diabetes mellitus, or smoking) between the 12 patients with ABS and either the 4 MI or 12 post-menopausal control groups (p > 0.05). There were a significantly higher number of both patients with ABS and patients with MI taking beta-blockers, angiotensin-converting enzyme (ACE) inhibitors, and medications used to treat anxiety compared with post-menopausal controls (p < 0.05). There were no differences in the number of patients with ABS or MI taking any medications, including beta-blockers, ACE inhibitors, and statins.
Furthermore, there were no differences in participant characteristics, prevalence of cardiovascular risk factors, or survival between the 12 patients with ABS in this study and the Mayo Clinic database of more than 100 patients with ABS from which these 12 patients were recruited. The documented causes of the ABS event in the 12 patients with ABS were emotional stress (n = 6), physical stress (n = 2), or unknown (n = 4). The emotional stressors precipitating the ABS events were associated with death of a husband or other family member (n = 3), divorce (n = 1), claustrophobia associated with magnetic resonance imaging exam (n = 1), church fundraising (n = 1), or activation of a fire alarm (n = 1). Quartile time limits (first quartile, median, and third quartile) from date of diagnosis of ABS to study date in the 12 patients were 6.8, 15.2, and 19.4 months, respectively, and the mean ± SD was 16.8 ± 14.3 months. No participants were studied within 6 months of being hospitalized or diagnosed with ABS or MI.
Hemodynamic responses to mental stress
The baseline mean arterial pressures were 86 ± 10 mm Hg, 90 ± 11 mm Hg, and 88 ± 10 mm Hg for patients with MI, post-menopausal controls, and patients with ABS, respectively. The baseline heart rates were 60 ± 8 beats/min, 66 ± 9 beats/min, and 64 ± 11 beats/min for patients with MI, post-menopausal controls, and patients with ABS, respectively. The baseline double products were 7,300 ± 746, 8,185 ± 1,677, and 8,263 ± 1,930 for patients with MI, post-menopausal controls, and patients with ABS, respectively. There were no differences in baseline blood pressures, heart rates, or double product among groups (p > 0.05).
During acute mental stress testing, systolic, diastolic, and mean arterial blood pressures; heart rates; and double product increased significantly above baseline values in patients with MI, post-menopausal controls, and patients with ABS (p < 0.05), although these values were not different among groups (p > 0.29). During the Stroop color-word mental stress tests, the double products for the patients with MI, post-menopausal controls, and patients with ABS were 9,216 ± 1,494, 10,039 ± 2,300, and 10,037 ± 2,205, respectively (p > 0.29). During the arithmetic mental stress tests, the double products for the patients with MI, post-menopausal controls, and patients with ABS were 8,993 ± 2,441, 10,070 ± 1,959, and 10,003 ± 2,712, respectively (p > 0.45). During the spiral omnibus mental stress tests, the double products for the patients with MI, post-menopausal controls, and patients with ABS were 8,237 ± 1,318, 9,549 ± 2,145, and 9,699 ± 2,213, respectively (p > 0.31). The average percentage increases in double product during mental stress were 22 ± 9%, 30 ± 25%, and 26 ± 24% for the patients with MI, post-menopausal controls, and patients with ABS, respectively (p > 0.55 between groups).
Mental stress PAT scores
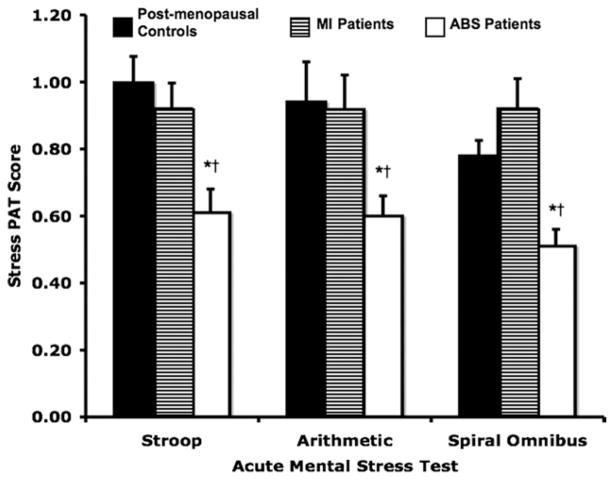
The peripheral vascular responses to mental stress, as measured by the ratio of stress to baseline PAT signal amplitude (PAT score), are shown in Figure 1. The PAT scores during each mental stress test were significantly lower in the patients with ABS compared with those in patients with MI or post-menopausal controls during each of the 3 tests (p < 0.05). There were no significant differences in PAT scores during mental stress testing between patients with MI and post-menopausal controls (p > 0.05). There was no correlation between the PAT score during mental stress testing and the time between the date of ABS diagnosis and the date of mental stress testing in patients with ABS (data not shown).
Figure 1. Stress PAT Scores.
Peripheral arterial tonometry (PAT) scores during acute mental stress testing (Stroop color-word, mental arithmetic, and spiral omnibus) for patients with apical ballooning syndrome (ABS), patients with myocardial infarction (MI), and post-menopausal controls. *p < 0.001 for patients with ABS versus post-menopausal controls. †p < 0.04 for patients with ABS versus patients with MI.
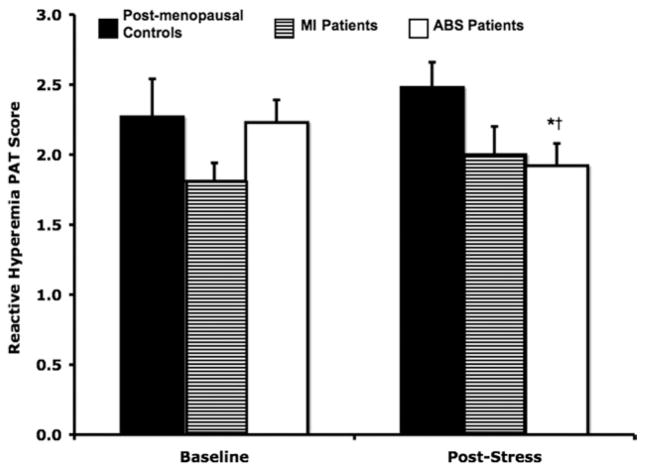
Reactive hyperemia PAT scores
Baseline and post-stress reactive hyperemia PAT scores, which represent endothelial function, are shown in Figure 2. Baseline reactive hyperemia PAT scores were significantly lower in the patients with MI (1.81 ± 0.26) compared with those in the post-menopausal controls (2.35 ± 0.49; p = 0.02), but there were no differences between either of these groups and the patients with ABS (2.23 ± 0.44; p > 0.07). The reactive hyperemia PAT scores after mental stress testing were significantly attenuated only in patients with ABS (1.92 ± 0.47; p = 0.02). There were no significant changes in reactive hyperemia PAT scores from baseline in either the patients with MI (p = 0.52) or post-menopausal controls (p = 0.26). Post-stress reactive hyperemia PAT scores were only significantly different between patients with ABS and post-menopausal controls (p < 0.03; 1.92 ± 0.47 vs. 2.48 ± 0.41, respectively).
Figure 2. Reactive Hyperemia PAT Scores.
Reactive hyperemia PAT scores following acute mental stress testing for patients with ABS, patients with MI, and post-menopausal controls. *p = 0.02 for baseline versus post-stress reactive hyperemia PAT scores in patients with ABS. †p = 0.03 for post-stress reactive hyperemia PAT scores in patients with ABS versus post-menopausal controls. Abbreviations as in Figure 1.
Plasma catecholamine levels
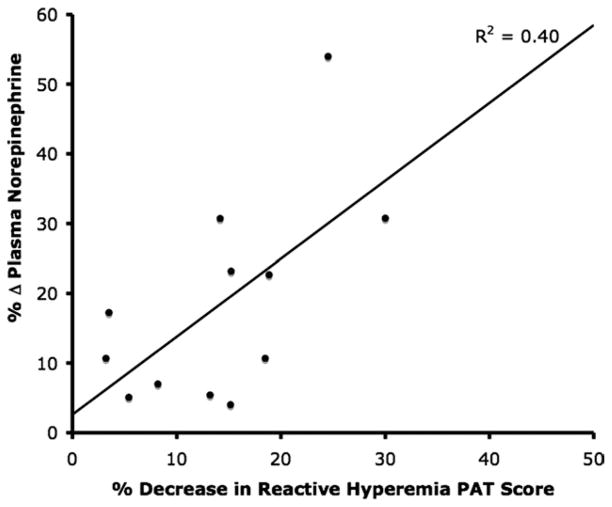
Mental stress–induced changes in plasma catecholamine levels for the patients with ABS and the post-menopausal controls are shown in Figure 3. There was no difference in plasma dopamine levels at baseline or after stress or baseline norepinephrine levels between post-menopausal controls and patients with ABS (415 ± 213 pg/ml vs. 463 +227 pg/ml; p = 0.89) (Fig. 3). There was no significant effect of mental stress testing on plasma dopamine, epinephrine, or norepinephrine levels in post-menopausal controls. On the other hand, mental stress significantly increased plasma epinephrine and norepinephrine levels among patients with ABS (p < 0.05). For the post-menopausal controls and patients with ABS, the plasma epinephrine levels were 29.4 ± 9.6 pg/ml vs. 19.7 ± 5.0 pg/ml, respectively (p = 0.04) (Fig. 3) and the plasma norepinephrine levels were 439 ± 244 pg/ml vs. 608 ± 219 pg/ml, respectively (p = 0.02) (Fig. 3). There was a significant correlation between the percentage change in plasma norepinephrine levels and percentage decrease in endothelial function, as manifested by reactive hyperemia PAT score, after acute mental stress testing in all subjects (R 2 = 0.40) (Fig. 4).
Figure 3. Plasma Catecholamine Levels.
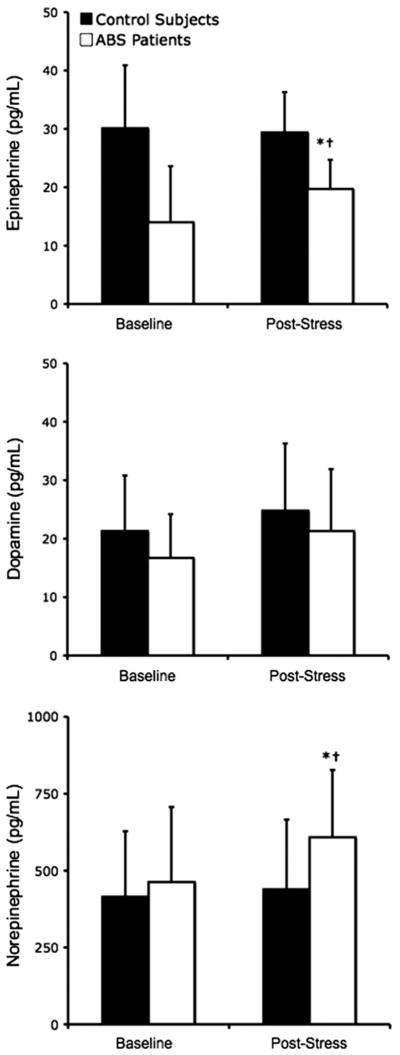
Plasma catecholamine levels at baseline and following acute mental stress testing for patients with apical ballooning syndrome (ABS) and post-menopausal control. *p < 0.05 for patients with ABS versus post-menopausal controls. †p < 0.05 for baseline versus after stress.
Figure 4. Correlation Between Reactive Hyperemia PAT Scores and Plasma Norepinephrine Levels.
Percent change in plasma norepinephrine levels versus percent decrease in reactive hyperemia peripheral arterial tonometry (PAT) scores following acute mental stress testing for patients with apical ballooning syndrome.
Discussion
The major and novel findings of the study were that women with a history of ABS demonstrated an impaired endothelium-dependent vasodilation, excessive vasoconstriction, and augmented sympathetic activation after experiencing acute mental stress compared with age-matched post-menopausal controls and patients with MI. The present study underscored the role of vascular reactivity in response to mental stress in women with a prior episode of ABS.
We observed that resting plasma catecholamine levels were not elevated in the patients. However, plasma epinephrine and norepinephrine levels were higher following mental stress in patients with ABS compared with controls. We found a significant correlation between the increase in norepinephrine levels and decrease in reactive hyperemia PAT scores following acute mental stress testing in the laboratory setting. This may implicate catecholamines in the abnormal vasomotion and endothelial dysfunction in response to mental stress in ABS.
The sample size was small, but we were able to detect a difference in reactive hyperemia PAT ratios following acute mental stress between patients with ABS and post-menopausal controls with a power of 0.97. We were able to detect a difference in plasma catecholamine levels between patients with ABS and post-menopausal controls with a power of 0.98. The study was conducted after an episode of ABS. However, the study was conducted at least 6 months following the event, and the mean duration of recruitment into the study was approximately 15 months from the transient event, excluding the potential impact of the acute event on the results and making it unlikely that there was persistent vasomotor impairment consequent to the acute event. There was no association between the length of time from the ABS event to the laboratory mental stress study day and the reactive hyperemia PAT scores or PAT mental stress scores.
We added a control group of post-menopausal women without history of ABS but with history of ST-segment elevation MI more than 6 months prior to study day. The augmented vasomotor responses to acute mental stress seen in the patients with ABS were not seen in the patients with MI. There was also no difference in PAT scores during mental stress testing between patients with MI and post-menopausal controls. Thus, the abnormal response to mental stress is a unique phenomenon to patients with a history of ABS.
Drug therapy regimens were different between the post-menopausal controls and patients with ABS but not between the patients with ABS and the patients with MI. A subanalysis of the patients with ABS taking beta-blockers or ACE inhibitors against those not taking these medications showed no differences in reactive hyperemia PAT scores, stress PAT ratios, or plasma catecholamine levels (p > 0.05) between these 2 subgroups. Along with the lack of difference we saw in the vascular and endothelial responses of the patients with MI compared with the post-menopausal group, this suggests that there were no significant medication-mediated effects on these results.
Although reactive hyperemia PAT scores may not be the gold standard for endothelial function, several studies have shown good correlation between these methods and more widely accepted methods of endothelial function testing, such as intra-arterial acetylcholine infusion and brachial artery Doppler ultrasound following reactive hyperemia (20–22).
Conclusions
Patients with ABS have abnormal vasoreactivity and sympathetic responses to acute mental stress testing in the laboratory setting. This is manifested as impaired endothelium-dependent dilation, excessive vasoconstriction, and catecholamine release. The current study supports a role for vascular reactivity as a potential mechanism and a marker for ABS in post-menopausal women. Studies with therapeutic interventions that modify vascular function and the sympathetic system would be of interest to establish whether recovery during an acute episode or recurrence rates may be modifiable.
Acknowledgments
This work was supported by Department of Defense grant W74V8H-05-1-0001, National Institutes of Health grant 1R01-HL092954-01A1, and the Mayo Foundation. Dr. Amir Lerman is on the advisory board at Itamar Medical.
The authors wish to thank Rebecca E. Nelson, CCRC, and M. Donna Felmlee-Devine, MS, for their assistance with these studies.
Abbreviations and Acronyms
- ABS
apical ballooning syndrome
- MI
myocardial infarction
- PAT
peripheral arterial tonometry
Footnotes
All other authors have reported that they have no relationships to disclose.
References
- 1.Redberg RF, Cannon RO, 3rd, Bairey Merz N, Lerman A, Reis SE, Sheps DS. Women’s Ischemic Syndrome Evaluation: current status and future research directions: report of the National Heart, Lung and Blood Institute workshop: October 2–4, 2002: section 2: stable ischemia: pathophysiology and gender differences. Circulation. 2004;109:e47–9. doi: 10.1161/01.CIR.0000116207.38349.FF. [DOI] [PubMed] [Google Scholar]
- 2.Wittstein IS, Thiemann DR, Lima JA, et al. Neurohumoral features of myocardial stunning due to sudden emotional stress. N Engl J Med. 2005;352:539–48. doi: 10.1056/NEJMoa043046. [DOI] [PubMed] [Google Scholar]
- 3.Zeiher AM, Drexler H, Wollschlager H, Just H. Endothelial dysfunction of the coronary microvasculature is associated with coronary blood flow regulation in patients with early atherosclerosis. Circulation. 1991;84:1984–92. doi: 10.1161/01.cir.84.5.1984. [DOI] [PubMed] [Google Scholar]
- 4.Shaw LJ, Bairey Merz CN, Pepine CJ, et al. Insights from the NHLBI-sponsored Women’s Ischemia Syndrome Evaluation (WISE) study: part I: gender differences in traditional and novel risk factors, symptom evaluation, and gender-optimized diagnostic strategies. J Am Coll Cardiol. 2006;47:S4–20. doi: 10.1016/j.jacc.2005.01.072. [DOI] [PubMed] [Google Scholar]
- 5.Suwaidi JA, Hamasaki S, Higano ST, Nishimura RA, Holmes DR, Jr, Lerman A. Long-term follow-up of patients with mild coronary artery disease and endothelial dysfunction. Circulation. 2000;101:948–54. doi: 10.1161/01.cir.101.9.948. [DOI] [PubMed] [Google Scholar]
- 6.Hasdai D, Gibbons RJ, Holmes DR, Jr, Higano ST, Lerman A. Coronary endothelial dysfunction in humans is associated with myocardial perfusion defects. Circulation. 1997;96:3390–5. doi: 10.1161/01.cir.96.10.3390. [DOI] [PubMed] [Google Scholar]
- 7.Tsuchihashi K, Ueshima K, Uchida T, et al. Transient left ventricular apical ballooning without coronary artery stenosis: a novel heart syndrome mimicking acute myocardial infarction. Angina Pectoris-Myocardial Infarction Investigations in Japan. J Am Coll Cardiol. 2001;38:11–8. doi: 10.1016/s0735-1097(01)01316-x. [DOI] [PubMed] [Google Scholar]
- 8.Bybee KA, Kara T, Prasad A, et al. Systematic review: transient left ventricular apical ballooning: a syndrome that mimics ST-segment elevation myocardial infarction. Ann Intern Med. 2004;141:858–65. doi: 10.7326/0003-4819-141-11-200412070-00010. [DOI] [PubMed] [Google Scholar]
- 9.Sharkey SW, Lesser JR, Zenovich AG, et al. Acute and reversible cardiomyopathy provoked by stress in women from the United States. Circulation. 2005;111:472–9. doi: 10.1161/01.CIR.0000153801.51470.EB. [DOI] [PubMed] [Google Scholar]
- 10.Elesber AA, Prasad A, Lennon RJ, Wright RS, Lerman A, Rihal CS. Four-year recurrence rate and prognosis of the apical ballooning syndrome. J Am Coll Cardiol. 2007;50:448–52. doi: 10.1016/j.jacc.2007.03.050. [DOI] [PubMed] [Google Scholar]
- 11.Merli E, Sutcliffe S, Gori M, Sutherland GG. Tako-Tsubo cardiomyopathy: new insights into the possible underlying pathophysiology. Eur J Echocardiogr. 2006;7:53–61. doi: 10.1016/j.euje.2005.08.003. [DOI] [PubMed] [Google Scholar]
- 12.Rozanski A, Bairey CN, Krantz DS, et al. Mental stress and the induction of silent myocardial ischemia in patients with coronary artery disease. N Engl J Med. 1988;318:1005–12. doi: 10.1056/NEJM198804213181601. [DOI] [PubMed] [Google Scholar]
- 13.Rozanski A, Blumenthal JA, Kaplan J. Impact of psychological factors on the pathogenesis of cardiovascular disease and implications for therapy. Circulation. 1999;99:2192–217. doi: 10.1161/01.cir.99.16.2192. [DOI] [PubMed] [Google Scholar]
- 14.Jiang W, Blumenthal JA, McNulty SE, et al. Myocardial ischemia induced by mental stress predicts a poorer prognosis in patients with coronary artery disease. J Am Coll Cardiol. 1995;25 (Suppl 1):88A. [Google Scholar]
- 15.Ueyama T, Senba E, Kasamatsu K, et al. Molecular mechanism of emotional stress-induced and catecholamine-induced heart attack. J Cardiovasc Pharmacol. 2003;41 (Suppl 1):S115–8. [PubMed] [Google Scholar]
- 16.Bybee KA, Murphy J, Prasad A, et al. Acute impairment of regional myocardial glucose uptake in the apical ballooning (TakoTsubo) syndrome. J Nucl Cardiol. 2006;13:244–50. doi: 10.1007/BF02971249. [DOI] [PubMed] [Google Scholar]
- 17.Prasad A, Lerman A, Rihal CS. Apical ballooning syndrome (Tako-Tsubo or stress cardiomyopathy): a mimic of acute myocardial infarction. Am Heart J. 2008;155:408–17. doi: 10.1016/j.ahj.2007.11.008. [DOI] [PubMed] [Google Scholar]
- 18.Martin EA, Tan SL, Macbride LR, Lavi S, Lerman LO, Lerman A. Sex differences in vascular and endothelial responses to acute mental stress. Clin Auton Res. 2008;18:339–45. doi: 10.1007/s10286-008-0497-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Goor DA, Sheffy J, Schnall RP, et al. Peripheral arterial tonometry: a diagnostic method for detection of myocardial ischemia induced during mental stress tests: a pilot study. Clin Cardiol. 2004;27:137–41. doi: 10.1002/clc.4960270307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bonetti PO, Pumper GM, Higano ST, Holmes DR, Jr, Kuvin JT, Lerman A. Noninvasive identification of patients with early coronary atherosclerosis by assessment of digital reactive hyperemia. J Am Coll Cardiol. 2004;44:2137–41. doi: 10.1016/j.jacc.2004.08.062. [DOI] [PubMed] [Google Scholar]
- 21.Kuvin JT, Patel AR, Sliney KA, et al. Assessment of peripheral vascular endothelial function with finger arterial pulse wave amplitude. Am Heart J. 2003;146:168–74. doi: 10.1016/S0002-8703(03)00094-2. [DOI] [PubMed] [Google Scholar]
- 22.Kuvin JT, Mammen A, Mooney P, Alsheikh-Ali AA, Karas RH. Assessment of peripheral vascular endothelial function in the ambulatory setting. Vasc Med. 2007;12:13–6. doi: 10.1177/1358863X06076227. [DOI] [PubMed] [Google Scholar]
- 23.Nohria A, Gerhard-Herman M, Creager MA, Hurley S, Mitra D, Ganz P. Role of nitric oxide in the regulation of digital pulse volume amplitude in humans. J Appl Physiol. 2006;101:545–8. doi: 10.1152/japplphysiol.01285.2005. [DOI] [PubMed] [Google Scholar]
- 24.Renaud P, Blondin JP. The stress of Stroop performance: physiological and emotional responses to color-word interference, task pacing, and pacing speed. Int J Psychophysiol. 1997;27:87–97. doi: 10.1016/s0167-8760(97)00049-4. [DOI] [PubMed] [Google Scholar]
- 25.Dimsdale JE, Moss J. Plasma catecholamines in stress and exercise. JAMA. 1980;243:340–2. [PubMed] [Google Scholar]