Abstract
Background
Vertebral artery dissection (VAD) is an important cause of stroke in the young. VAD can present with a range of imaging findings. We sought to summarize the diagnostic value of various imaging findings in patients with symptomatic VAD.
Methods
We conducted a systematic review of observational studies, searching electronic databases (MEDLINE, EMBASE) for English-language manuscripts with >5 subjects with clinical or radiological features of VAD. Two independent reviewers selected studies for inclusion; a third adjudicated differences. Studies were assessed for methodological quality and imaging data were abstracted. Pooled proportions were calculated.
Results
Of 3996 citations, we screened 511 manuscripts and selected 75 studies describing 1,972 VAD patients. Most studies utilized conventional angiography or magnetic resonance angiography (MRA) to diagnose VAD; CT angiography (CTA) and Doppler ultrasonography were described less frequently. Imaging findings reported were vertebral artery stenosis (51%), string and pearls (48%), arterial dilation (37%), arterial occlusion (36%), and pseudoaneurysm, double lumen, and intimal flap (22% each). In cases where conventional angiography was the reference standard, CTA was more sensitive (100%) than either MRA (77%) or Doppler ultrasonography (71%) (p=0.001).
Conclusions
Imaging findings vary widely in patients with VAD, with no single radiographic sign present in the majority of VAD patients. Non-specific radiographic signs predominate. CTA probably has greater sensitivity for dissection than MRA or ultrasound relative to conventional angiography. Higher quality studies on imaging techniques and radiographic criteria in subjects with VAD are needed. Future studies should compare imaging techniques in well-defined, undifferentiated populations of clinical VAD suspects.
Keywords: vertebral artery dissection, neuroimaging, meta-analysis
INTRODUCTION
Vertebral artery dissection (VAD) is often a clinically elusive diagnosis, given a range of presentations depending on the presence or absence of precipitating factors, often nonspecific clinical symptoms, and varied symptomatology depending on where along the vertebral artery a dissection occurs. The diagnosis of VAD is complicated further by lack of cohesive imaging requirements in order to make the diagnosis.
Although conventional angiography is often considered the gold standard in the diagnosis of VAD, other noninvasive modalities are used with increasing frequency in evaluation of this condition. Many of the imaging findings are nonspecific, but still may be consistent with VAD.
The literature describing the radiographic findings in VAD is widely varied, with most studies consisting of case series or small observational studies. In this systematic review, we evaluated the reported imaging characteristics of VAD in studies of individuals with clinically diagnosed VAD in whom radiographic findings were reported.
METHODS
Search Strategy and Selection Criteria
Full search strategy, selection, and exclusion criteria are described in the accompanying manuscript on clinical characteristics of VAD. To summarize, we searched MEDLINE and EMBASE for English-language articles through February 2009, using text words and controlled vocabulary terms including: vertebral, vertebro-basilar, dissection, pseudoaneurysm, and other terms related to craniocervical dissection. We also performed a manual search of reference lists from eligible articles. All gathered literature was subject to title and abstract screening by two independent reviewers, and articles were selected using pre-determined criteria. We excluded studies with ≤5 patients. For evaluation of imaging characteristics, the same manuscripts were included as were selected for the clinical characteristics review, but data on radiologic features were extracted.
Assessment for methodological quality was performed using the same approach as for the clinical characteristics systematic review, based upon the Standards for Reporting Diagnostic Accuracy (STARD) Statement1 and other related criteria.2 We evaluated risk of bias based on compliance with these criteria. Quality of each manuscript was rated, as described in the accompanying manuscript.
Data Extraction and Synthesis
Details about data extraction are also provided in the accompanying manuscript. Potentially relevant radiologic features were determined based on clinical experience by the investigators prior to data extraction. Radiographic findings were considered either “direct” (i.e. double lumen, intimal flap (Figure 1)), based on previously used nomenclature,3 or “indirect” (i.e. arterial stenosis, occlusion (Figure 2, right vertebral artery), dilation, aneurysm or pseudoaneurysm, string and pearl sign) for VAD. A single author then extracted the data for these features for the manuscripts reporting on radiographic features. Data tables were created based on relevant radiographic features, and only those studies that were felt to be free of bias with regards to the particular feature were included in the estimates. For example, a study in which patients were recruited based on the presence of a certain radiological feature was not included in evaluation of the frequency of that particular feature. Potential bias was determined by two independent reviewers/data abstractors, and confirmed by another investigator when studies were pooled. Although we required a radiographically-confirmed diagnosis, included manuscripts were clinical series and not purely radiology-defined series of VAD.
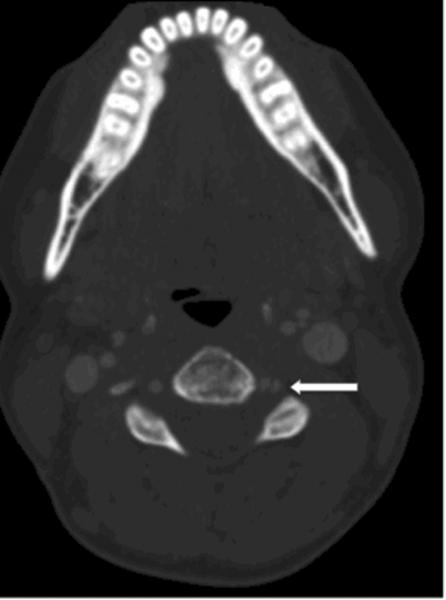
Figure 1.
CT Angiography showing left vertebral artery intimal flap (arrow) secondary to vertebral artery dissection.
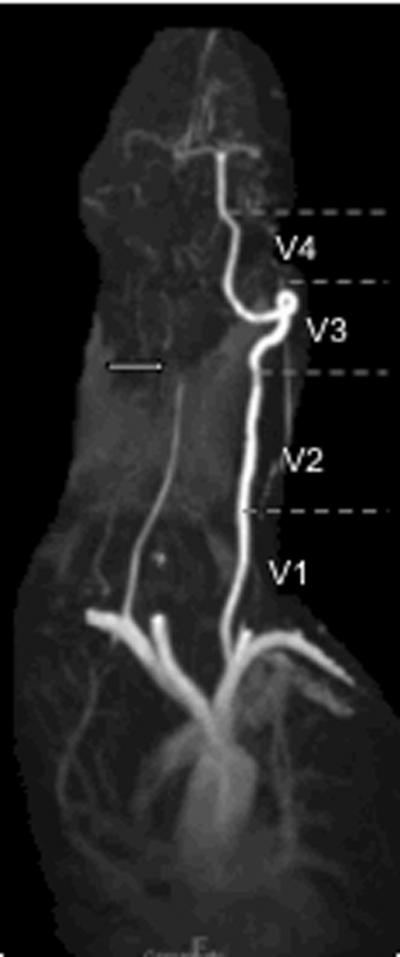
Figure 2.
MR Angiography of the neck showing right vertebral artery tapering occlusion (arrow), caused by vertebral artery dissection in the distal V2 segment. The V1 through V4 segments are indicated along the normal left vertebral artery.
Proportions were calculated, as were pooled standard errors, separately for each radiographic feature, using Microsoft Excel and Stata v8.0 for Macintosh (College Station, TX). No formal tests of heterogeneity were performed.
For analyses pertaining to imaging modality selected, we excluded studies for the calculation of pooled proportions where a “positive MRI” was described only because it showed a cerebral infarct. In these analyses, for an MRI to be considered “positive,” a scan had to have an abnormal finding of the vessels that led to the diagnosis of VAD. For the few studies in which other imaging modalities were directly compared to conventional angiography, we described the rates at which these modalities were positive, compared to conventional angiography.
RESULTS
Details of the included studies are described in the accompanying manuscript on clinical features of VAD. Of 3996 unique citations identified, 87.2% were excluded at the abstract level, and 511 full manuscripts were sought for full review. A total of 75 studies were included for final review and data abstraction, none of which were felt to meet criteria for high quality. About half of studies (48%) were rated as medium quality, while the remaining studies (52%) were considered low quality. These studies included a total of 1,972 individuals with VAD.
Radiologic features in the included manuscripts and their relative frequencies are summarized in Table 1. As demonstrated by the large standard errors, reported frequencies of these findings were quite inhomogeneous. Arterial stenosis was reported in 51% of individuals with VAD. At least one “direct” radiographic finding was present in 15%. Because imaging findings for individuals without arterial dissection were not reported in these reviewed studies, specificity of these imaging findings for the diagnosis of VAD could not be calculated.
Table 1.
Imaging characteristics of VAD.*
| Vessel Appearance | # of studies reporting on the finding |
Total # of subjects (N) |
Pooled proportion | Pooled SE |
|---|---|---|---|---|
| Arterial stenosis | 24 | 716 | 0.51 | 0.60 |
| String and pearls | 8 | 126 | 0.48 | 0.38 |
| Dilation of artery | 6 | 93 | 0.37 | 0.31 |
| Arterial occlusion | 22 | 679 | 0.36 | 0.54 |
| Aneurysm/ pseudoaneurysm |
19 | 569 | 0.22 | 0.46 |
| Double lumen | 11 | 232 | 0.22 | 0.35 |
| Intimal flap | 8 | 144 | 0.21 | 0.31 |
| Any “direct” finding (double lumen OR intimal flap) |
17 | 338 | 0.21† | 0.41 |
Clinically-defined cases with radiographic confirmation. Reported rates are pooled across studies reporting on presence/ absence of a given characteristic.
One study describing 22 VAD patients reported the frequency of these findings but did not report the overlap between double lumen and intimal flap (i.e., whether individual patients had only one or both findings). For this study, we conservatively estimated the frequency as the higher percentage of the two (e.g., double lumen in 59% and intimal flap in 86%;13 we counted 86% as having “any” direct finding).
Location of dissections
While most studies did not describe the location along the vertebral artery course of the dissection (Figure 2 Labels V1-V4), the proportions in those studies in which location along the vessel was provided were pooled. Dissections in the V1 section of the vessel were found slightly less frequently than other dissection sites (Table 2). Only those studies in which the number of dissections in each vessel segment was provided for at least 3 of the segments were included in these estimates.
Table 2.
Anatomic sites of VAD
| Segment of vertebral artery | Frequency (%) | Range(%) |
|---|---|---|
| V1 | 27.5 | 8-67 |
| V2 | 34.3 | 7-71 |
| V3 | 35.8 | 4-67 |
| V4 | 33.6 | 5-67 |
Imaging modality
Very few data were available comparing different imaging modalities to conventional angiography. In most cases, the reference standard for VAD diagnosis was a combination of imaging and clinical criteria (although details were usually not provided), and a non-invasive imaging technique was then compared to this overall clinico-radiographic diagnosis. Conventional angiography was the most frequently used imaging modality to confirm VAD (n=108 subjects). The relative sensitivity of each imaging approach for VAD is displayed in Table 3, as are the numbers of subjects across multiple studies in whom this information was provided. These results are stratified by whether a direct comparison to conventional angiography was made. Note that among patients in whom the clinico-radiographic diagnosis was made by MRI without MRA, the only radiographic sign reported was an infarct in the appropriate vascular territory.
Table 3.
Sensitivities of different imaging modalities for VAD.*
| Imaging compared to final clinical diagnosis | Imaging compared to diagnosis by conventional angiography | |||||||
|---|---|---|---|---|---|---|---|---|
| Imaging modality |
Studies | N | N (%) positive | p-value (sensitivity relative to CTA) |
Studies | N | N (%) positive | p-value (sensitivity relative to CTA) |
| CTA | 12, 14 | 32 | 32 (100%) | 12, 14 | 32 | 32 (100%) | ||
| MRI | 3, 15-18 | 81 | 50 (62%) | <0.0001 | None† | |||
| MRA | 17-20 | 34 | 28 (82%) | 0.01 | 18-20 | 22 | 17 (77%) | 0.004 |
| Duplex | 12, 21-25 | 99 | 78 (79%) | 0.005 | 12, 22, 24 | 28 | 20 (71%) | 0.001 |
Studies evaluating different imaging modalities, compared to clinical diagnosis and compared to conventional angiography. The first set of columns shows numbers of studies of the particular modality compared to clinical diagnosis, and the second set of columns reflects only those studies in which individuals had both conventional angiography and another imaging modality.
All studies comparing MRI to angiography only had infarcts on “positive” MRI’s, no vessel findings reported.
DISCUSSION
The observational literature describing imaging characteristics of VAD is heterogeneous and of limited quality. Studies reviewed took varied approaches to patient selection and imaging, used different or inadequately described image acquisition methods, failed to adequately define radiographic signs or measure their inter-rater reliability, and lacked appropriate control or comparison populations. Results were highly variable across studies, and it is impossible to discern whether these differences were due to sampling variation or bias in study design or reporting. Nevertheless, in this study we are able to provide aggregate estimates of the frequency of various imaging findings reported in VAD as well as pooled estimates of the relative sensitivity of various imaging modalities.
The most common imaging characteristic reported for VAD was nonspecific arterial stenosis in 51%, with only 15% of patients identified as having other patterns generally considered “direct” signs of VAD such as intimal flap or double lumen. Our sample is most likely biased towards more severe cases of VAD, so the true prevalence of intimal flap or double lumen may be even lower than our pooled estimate. The relative paucity of more “characteristic” findings compared to traditional teaching4 may reflect the fact that our systematic review excluded studies where subjects were recruited solely based on imaging characteristics. Also, some of the clinico-radiographic VAD diagnoses might have been incorrect (e.g., atherosclerosis rather than VAD), diluting the measured frequency of more classic findings. There was wide variation in reported signs across studies. The heterogeneity may partly result from random variation due to small sample size in most studies; nevertheless, it raises questions about whether image interpretation of various radiographic signs was consistent. Whether multiple radiographic findings in a given vessel or holistic interpretation of images across imaging modalities in the same patient contributed to the final diagnosis could not be assessed. None of the included studies assessed the specificity of any individual imaging sign for the diagnosis of VAD in an appropriate comparison population. The extent to which detecting or correctly interpreting any of these radiographic signs of VAD might have been dependent on training background or experience remains unknown, although common interpretive pitfalls5 and subtle variations6 have been described.
Although conventional angiography was the most frequently reported confirmatory imaging modality, it is possible that a form of publication bias played a role, leading to a greater likelihood of a report on imaging findings in VAD when the diagnosis was confirmed by what is generally considered to be the gold standard (i.e., conventional angiography). In current clinical practice, use of routine conventional angiography to “rule out” or “rule in” VAD is probably infrequent. This might lead to the exclusion of studies where conventional angiography was negative, but a dissection was seen using another technique, such as MRI of the neck with fat-suppression images, which could bias the results. Only a few studies offered direct comparisons between two or more imaging modalities. CTA had the highest sensitivity (100%) relative to conventional angiography, with MRA and duplex missing about one in five cases of VAD. The greater concordance between conventional angiography and CTA is probably not surprising given the mechanistic similarity of these two luminal imaging techniques. None of the included studies assessed the specificity of any imaging modality for the diagnosis of VAD in an appropriate comparison population.
The sensitivity of CTA for symptomatic VAD appears to be high. Although we found only two studies reporting on a total of 32 patients, there were no reported cases in which CTA was deemed falsely negative (i.e., a patient diagnosed with VAD clinically and with radiographic evidence by conventional angiography but in which the CTA was normal). Limited sample size, diagnostic misclassification, and patient selection may account for the apparent perfection, however, since there is reason to believe that luminal imaging techniques miss small dissections with intramural hematomas that do not reduce lumen diameter. A recent radiographic database study found six cases of VAD (5 symptomatic, 3 with stroke) where the CTA was normal save for increased wall thickness (“suboccipital rind” sign) beyond that seen in a radiographic control population.6 In that study, conventional angiography results were reported in only one patient and said to demonstrate a “relatively normal lumen;” the authors cautioned against the use of any exclusively lumen-diameter-based technique (including conventional angiography) to rule out VAD.6 One small series in trauma patients suggests CTA may have low sensitivity for detecting asymptomatic blunt vertebral injuries (3 of 8 cases missed) relative to conventional angiography,7 perhaps because of imaging artifacts near the base of skull.5 Occasional false negative CTA interpretations have been reported for various other technical reasons.5 Together, these results cast some doubt on the use of either conventional angiography or routine CTA as the definitive diagnostic gold standard in VAD. Nevertheless, given the relatively lower sensitivity of other non-invasive techniques, CTA may still be the preferred initial imaging test for screening patients with suspected VAD.
The relatively lower sensitivity of MRI and MRA for VAD found in this review must be interpreted with some caution, since imaging protocols may influence overall sensitivity. For example, MRI of the neck with fat suppression is believed to detect VAD with greater sensitivity than MRA alone, and optimized imaging protocols have been proposed.8, 9 Unfortunately, included studies generally did not provide details about sequences used for MRI and MRA imaging; suboptimal imaging protocols could have artificially reduced the measured sensitivity of the technique. Nevertheless, false negative MRI and MRA findings in dissection are well described.5 Whether newer techniques such as diffusion-weighted imaging10 or high-field MRI11 will improve the sensitivity for VAD detection remains unknown.
The overall specificity of any imaging technique for symptomatic VAD, including conventional angiography, remains virtually unstudied. False positive MRIs, MRAs, CTAs, and duplex scans have all been reported.5, 12 Whether findings of luminal imaging techniques alone should be considered confirmatory of VAD remains controversial. Some authors contend that only techniques which image the vessel wall and demonstrate intramural hematoma are definitive for dissection, since most forms of luminal stenosis are nonspecific with regard to underlying pathology.8
The primary limitations of this analysis are the modest quality and heterogeneity of the primary data. All included studies were rated as medium or low quality methodologically (described in the accompanying manuscript). Individual studies generally failed to provide clear VAD definitions, adequate descriptions of patient recruitment and imaging protocols, and consistent measures of image interpretation or qualifications of readers. It is therefore unknown how many clinico-radiographic diagnoses might have been incorrect and whether this sample is reasonably representative of all symptomatic VAD patients. Virtually all data types were reported with wide variation across studies. Since final clinico-radiographic diagnoses were used as the gold standard, and diagnostic standards differed across studies, the measured relative sensitivities of particular imaging modalities could be biased. In addition, because we demanded at least some form of radiographic confirmation for VAD in our review, it remains unknown whether some patients with true VAD might have completely normal (falsely negative) vascular imaging.
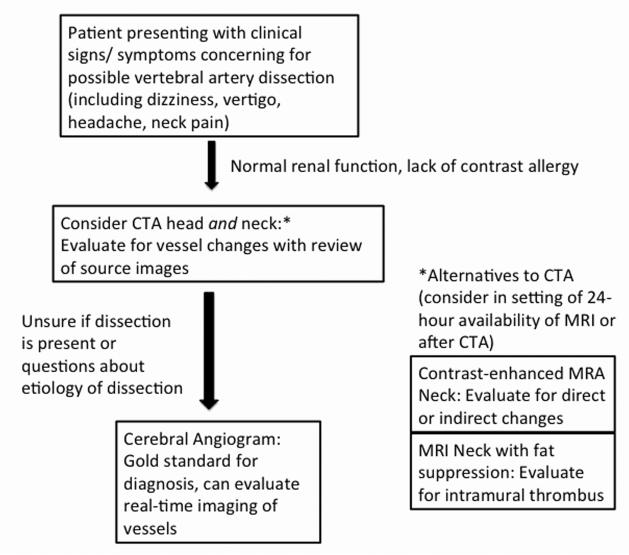
Despite these limitations, we feel that this analysis provides useful information about the relative sensitivity of different imaging techniques, emphasizing the potential role of CTA as the most promising, non-invasive alternative to conventional angiography in screening for VAD (Figure 3). It also indicates the relatively low frequency of certain “classic” radiographic findings, such as an intimal flap or double lumen; this emphasizes to the clinician that the lack of these findings should not eliminate the diagnosis of VAD as a possibility.
Figure 3.
Flow chart showing suggested diagnostic process in evaluating a patient with potential vertebral artery dissection. Imaging should be evaluated for “direct” findings (double lumen, intimal flap) or “indirect” findings (arterial stenosis, occlusion, dilation, aneurysm or pseudoaneurysm, string and pearl sign).
Conclusions
Future studies should compare imaging techniques in well-defined, undifferentiated populations of clinical VAD suspects (e.g., emergency department patients with dizziness or vertigo in association with head or neck pain). Imaging protocols should be standardized. Radiographic interpretation of pre-defined signs should be masked, and inter-rater reliability should be measured. Final clinico-radiographic diagnoses should be adjudicated by multi-disciplinary consensus according to clear criteria that also define the degree of diagnostic certainty. Such studies will permit robust measures of sensitivity, specificity, and likelihood ratios for each of the relevant diagnostic modalities in order to assess their potential clinical utility and offer more specific guidance to clinicians.
Acknowledgments
Funding Source: Supported by NIH (DNT: K23 RR17324-01).
Footnotes
Statistical Analysis: Conducted by Rebecca Gottesman, MD PhD
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bossuyt PM, Reitsma JB, Bruns DE, et al. Towards Complete and Accurate Reporting of Studies of Diagnostic Accuracy: The STARD Initiative. Clinical Chemistry. 2003;49:1–6. doi: 10.1373/49.1.1. [DOI] [PubMed] [Google Scholar]
- 2.Simel DL, Rennie D, Bossuyt PM. The STARD statement for reporting diagnostic accuracy studies: application to the history and physical examination. J Gen Intern Med. 2008;23:768–774. doi: 10.1007/s11606-008-0583-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hosoya T, Watanabe N, Yamaguchi K, et al. Intracranial vertebral artery dissection in Wallenberg syndrome. AJNR Am J Neuroradiol. 1994;15:1161–1165. [PMC free article] [PubMed] [Google Scholar]
- 4.Flis CM, Jager HR, Sidhu PS. Carotid and vertebral artery dissections: clinical aspects, imaging features and endovascular treatment. Eur Radiol. 2007;17:820–834. doi: 10.1007/s00330-006-0346-7. [DOI] [PubMed] [Google Scholar]
- 5.Provenzale JM, Sarikaya B, Hacein-Bey L, et al. Causes of misinterpretation of cross-sectional imaging studies for dissection of the craniocervical arteries. American Journal of Roentgenology. 2011;196:45–52. doi: 10.2214/AJR.10.5384. [DOI] [PubMed] [Google Scholar]
- 6.Lum C, Chakraborty S, Schlossmacher M, et al. Vertebral artery dissection with a normal-appearing lumen at multisection CT angiography: the importance of identifying wall hematoma. American Journal of Neuroradiology. 2009;30:787–792. doi: 10.3174/ajnr.A1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Malhotra AK, Camacho M, Ivatury RR, et al. Computed tomographic angiography for the diagnosis of blunt carotid/ vertebral artery injury. Annals of Surgery. 2007;246:632–642. doi: 10.1097/SLA.0b013e3181568cab. [DOI] [PubMed] [Google Scholar]
- 8.Oelerich M, Stogbauer F, Kurlemann G, et al. Craniocervical artery dissection: MR imaging and MR angiographic findings. Eur Radiol. 1999;9:1385–1391. doi: 10.1007/s003300050853. [DOI] [PubMed] [Google Scholar]
- 9.Shah GV, Quint DJ, Trobe JD. Magnetic resonance imaging of suspected cervicocranial arterial dissections. J Neuroophthalmol. 2004;24:315–318. doi: 10.1097/00041327-200412000-00009. [DOI] [PubMed] [Google Scholar]
- 10.Choi KD, Jo JW, Park KP, et al. Diffusion-weighted imaging of intramural hematoma in vertebral artery dissection. J Neurol Sci. 2007;253:81–84. doi: 10.1016/j.jns.2006.11.011. [DOI] [PubMed] [Google Scholar]
- 11.Swartz RH, Bhuta SS, Farb RI, et al. Intracranial arterial wall imaging using high-resolution 3-tesla contrast-enhanced MRI. Neurology. 2009;72:627–634. doi: 10.1212/01.wnl.0000342470.69739.b3. [DOI] [PubMed] [Google Scholar]
- 12.Pugliese F, Crusco F, Cardaioli G, et al. CT angiography versus colour-Doppler US in acute dissection of the vertebral artery. Radiol Med (Torino) 2007;112:435–443. doi: 10.1007/s11547-007-0152-6. [DOI] [PubMed] [Google Scholar]
- 13.Lee JS, Yong SW, Bang OY, et al. Comparison of spontaneous intracranial vertebral artery dissection with large artery disease. Arch Neurol. 2006;63:1738–1744. doi: 10.1001/archneur.63.12.1738. [DOI] [PubMed] [Google Scholar]
- 14.Chen CJ, Tseng YC, Lee TH, et al. Multisection CT angiography compared with catheter angiography in diagnosing vertebral artery dissection. AJNR Am J Neuroradiol. 2004;25:769–774. [PMC free article] [PubMed] [Google Scholar]
- 15.Leclerc X, Lucas C, Godefroy O, et al. Preliminary experience using contrast-enhanced MR angiography to assess vertebral artery structure for the follow-up of suspected dissection. AJNR Am J Neuroradiol. 1999;20:1482–1490. [PMC free article] [PubMed] [Google Scholar]
- 16.Hosoya T, Adachi M, Yamaguchi K, et al. Clinical and neuroradiological features of intracranial vertebrobasilar artery dissection. Stroke. 1999;30:1083–1090. doi: 10.1161/01.str.30.5.1083. [DOI] [PubMed] [Google Scholar]
- 17.Auer A, Felber S, Schmidauer C, et al. Magnetic resonance angiographic and clinical features of extracranial vertebral artery dissection. J Neurol Neurosurg Psychiatry. 1998;64:474–481. doi: 10.1136/jnnp.64.4.474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yoshimoto Y, Wakai S. Unruptured intracranial vertebral artery dissection. Clinical course and serial radiographic imagings. Stroke. 1997;28:370–374. doi: 10.1161/01.str.28.2.370. [DOI] [PubMed] [Google Scholar]
- 19.Lu CJ, Sun Y, Jeng JS, et al. Imaging in the diagnosis and follow-up evaluation of vertebral artery dissection. J Ultrasound Med. 2000;19:263–270. doi: 10.7863/jum.2000.19.4.263. [DOI] [PubMed] [Google Scholar]
- 20.Provenzale JM, Morgenlander JC, Gress D. Spontaneous vertebral dissection: clinical, conventional angiographic, CT, and MR findings. J Comput Assist Tomogr. 1996;20:185–193. doi: 10.1097/00004728-199603000-00004. [DOI] [PubMed] [Google Scholar]
- 21.Bartels E, Flugel KA. Evaluation of extracranial vertebral artery dissection with duplex color-flow imaging. Stroke. 1996;27:290–295. doi: 10.1161/01.str.27.2.290. [DOI] [PubMed] [Google Scholar]
- 22.Hoffmann M, Sacco RL, Chan S, et al. Noninvasive detection of vertebral artery dissection. Stroke. 1993;24:815–819. doi: 10.1161/01.str.24.6.815. [DOI] [PubMed] [Google Scholar]
- 23.Mas JL, Bousser MG, Hasboun D, et al. Extracranial vertebral artery dissections: a review of 13 cases. Stroke. 1987;18:1037–1047. doi: 10.1161/01.str.18.6.1037. [DOI] [PubMed] [Google Scholar]
- 24.Chiras J, Marciano S, Vega Molina J, et al. Spontaneous dissecting aneurysm of the extracranial vertebral artery (20 cases) Neuroradiology. 1985;27:327–333. doi: 10.1007/BF00339566. [DOI] [PubMed] [Google Scholar]
- 25.Wessels T, Mosso M, Krings T, et al. Extracranial and intracranial vertebral artery dissection: Long-term clinical and duplex sonographic follow-up. J Clin Ultrasound. 2008;36:472–479. doi: 10.1002/jcu.20511. [DOI] [PubMed] [Google Scholar]