Abstract
The investigation of novel anti-tumor agents that preferentially select for malignant cells with a tolerable toxicity level has been the focus of anti-cancer drug discovery. Our laboratories have previously reported that certain N-alkylthiolated β-lactams had DNA-damaging and apoptosis-inducing activity in various tumor lines but not in nontransformed cells. In the current study, we further delineated the effects of substitutions at C3 or N1 of the lactam ring for cell death-inducing capability with close attention paid to a discernible structure-activity relationship (SAR). We found that two β-lactam analogs (JG-5 and JG-19), both containing a branched-chain moiety at C3 of the lactam ring, exhibit potent apoptosis-inducing activity. Additionally, JG-5 exhibited superior in vitro biological activity over JG-19 owing to structural modifications made to substituents at the N1 and C3 positions of the lactam ring. Furthermore, the branched β-lactams were able to inhibit growth of mice bearing breast cancer xenografts, associated with induction of DNA damage and apoptosis in tumor tissues. Our results strongly warrant further investigation into these novel β-lactams as potential anti-cancer therapeutics.
Keywords: β-lactams, structure-activity relationship, apoptosis, DNA damage, tumor cell death
Introduction
Apoptosis (programmed cell death) is a natural, physiologic process that is critical in regulating the homeostatic cell number in a tightly controlled manner and deregulation of this process can lead to the pathogenesis of various diseases including cancer (1–3). From a morphological standpoint, apoptosis is characterized by cell shrinkage, DNA fragmentation and subsequent membrane blebbing and membrane-enclosed apoptotic bodies (3,4). Anti-cancer drugs, including chemotherapy, γ-irradiation and immunotherapy exert their effects predominantly by modulating key elements of the apoptosis cascade (5,6). However, many tumors possess defects in the apoptotic machinery and become resistant to standard cytotoxic regimens, posing serious clinical problems (7–9). Therefore, developing novel anti-cancer therapies that circumvent these limitations is of paramount importance.
The treatment of bacterial infections with β-lactam antibiotics has been the staple of treatment for over a half century. β-lactams such as penicillin represent a potent class of inhibitors of bacterial growth and various moieties have been isolated or synthesized since the discovery of penicillin (10). The discovery of penicillin was the impetus for the introduction and clinical use of various β-lactams, including cephalosporins, penems, carbapenems and monobactams (11). These β-lactam compounds function on bacterial cells by selectively disrupting cross-linking events during the final stage of bacterial cell wall synthesis, leading to subsequent cell lysis (12). These inhibitory properties are specific for bacteria which of course have the peptidoglycan cell wall, which are not found in mammalian tissue and thus do not affect human cell lines. Recently, however, our laboratories discovered a novel class of N-thiolated β-lactams (13,14) that rapidly induce DNA damage, inhibit DNA replication and induce apoptosis in a number of mammalian cell lines including human leukemic T cells, breast, prostate and head and neck (15–17). These surprising findings formed the basis of developing novel β-lactam analogs with various structural modifications in the hope of generating lead candidates that have selective tumor cell apoptosis-inducing activity.
In the current study, we screened a focused library of N-thiolated β-lactam analogs with substitutions made at either N1 or C3 of the lactam ring (Fig. 1), with close attention being paid to a possible structure-activity relationship. Our current study shows that manipulation and addition of carbon chain moieties at these two positions correlate directly to their cell death-inducing capability in vitro. Two β-lactam analogs, JG-5 and JG-19, were found to exhibit potent antiproliferative activity tested against human leukemic cell lines and that this effect directly correlates to its branched moiety at position 3 of the lactam ring structure. Furthermore, this cell death-inducing ability exerted by JG-5 and JG-19 is directly associated with apoptosis as indicated by cleavage of apoptosis-specific poly(ADP-ribose) polymerase (PARP) and staining of apoptotic nuclei. Additionally, the biological effect exhibited in vitro was emulated in a human breast cancer xenograft in mice. Our data strongly support the concept of investigating β-lactam antibiotics as a novel approach in the treatment of cancer.
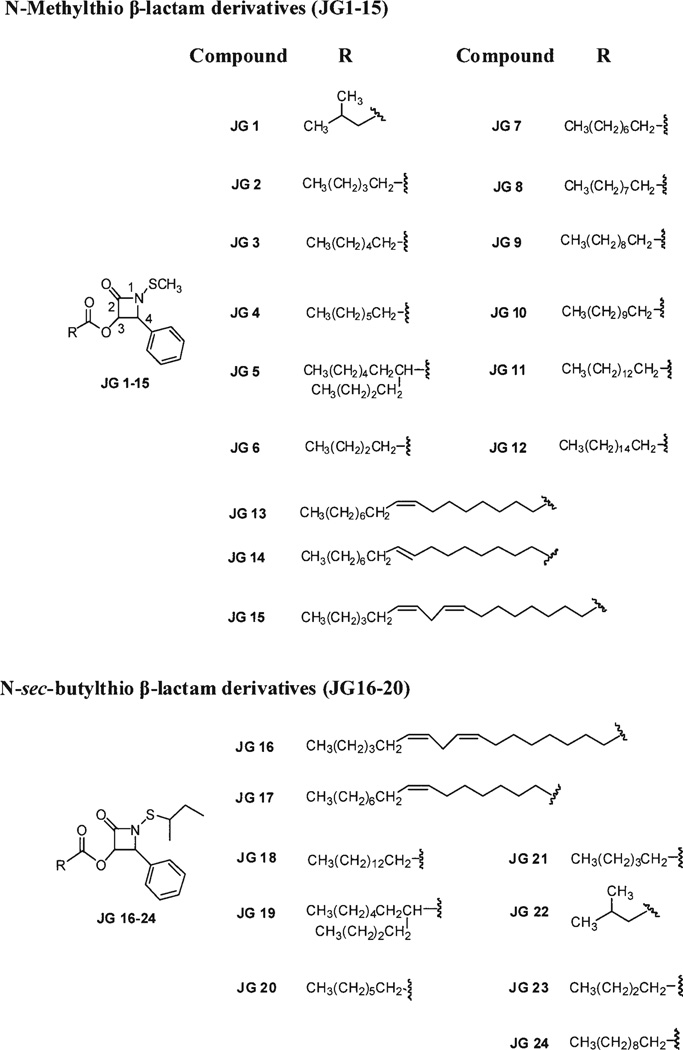
Figure 1.
Structures of 24 representative N-thiolated β-lactams with structural modifications at N1 and C3 of the lactam ring. Each β-lactam analog is designated as JG along with numerical representation.
Materials and methods
Materials
The β-lactam analogs were prepared using a procedure described previously (18). Hoechst 33258, cremophor, dimethylsulfoxide (DMSO) and trypan blue were purchased from Sigma-Aldrich (St. Louis, MO). Apoptag peroxidase in situ Apoptosis detection kit was from Chemicon International, Inc. (Temecula, CA). Fetal bovine serum was purchased from Aleken Biologicals (Texarkana, AR). A mixture of penicillin-streptomycin-L-glutamine, RPMI-1640 medium and sodium pyruvate were purchased from Invitrogen (Carlsbad, CA). Monoclonal antibodies to poly(ADP-ribose) polymerase were purchased from Biomol International LP (Plymouth Meeting, PA) and anti-mouse IgG-horseradish peroxidase was obtained from Santa Cruz Biotechnologies (Santa Cruz, CA).
Cell cultures and whole cell extract preparation
Human leukemic Jurkat T, Raji and HL-60 cells were cultured in RPMI-1640 medium, supplemented with 10% fetal bovine serum, 100 U/ml penicillin and 10 µg/ml streptomycin and 1% sodium pyruvate solution (100 mM). All cell lines were maintained in a humidified incubator at 37°C and 5% CO2. A whole-cell extract was prepared as described previously (19). Briefly, cells were harvested, washed with ice-cold PBS and homogenized in a lysis buffer (50 mM Tris-HCl, pH 7.5, 5 mM EDTA, 150 mM NaCl, 0.5% NP-40) for 25 min at 4°C. Afterwards, the lysates were centrifuged at 12,000 rpm for 15 min at 4°C and the supernatants collected as whole-cell extracts.
Western blot analysis
The cell extracts were separated by SDS-PAGE and transferred to a nitrocellulose membrane. Western blot analysis was performed using a specific antibody to PARP, followed by visualization using the enhanced chemiluminescence (ECL) reagent (Amersham Biosciences, Piscataway, NJ).
Trypan blue assay
The trypan blue dye exclusion assay was performed by mixing 50 µl of cell suspension with 20 µl of 0.4% trypan blue dye before injecting into a hemocytometer and counting. The number of cells that absorbed the dye and those that excluded the dye were counted, from which the percentage of nonviable cell number to total cell number was calculated.
Nuclear staining
A Zeiss Axiovert 25 microscope was used with fluorescence for nuclear morphology with Hoechst 33258 staining as described previously (20).
Human breast tumor xenograft experiments
Five-week-old female athymic nude mice were purchased from Taconic Research Animal Services (Hudson, NY) and housed under pathogen-free conditions according to Wayne State University animal care guidelines. The protocols of animal experiments were reviewed and approved by the Insitutional Laboratory Animal Care and Use Committee of Wayne State University. MDA-MB-231 cells were injected subcutaneously (s.c.) at one flank of the mice. The mice were then injected s.c. with either solvent (PBS:cremophor: ethanol: DMSO = 5: 2.7: 1.3: 1) as a control or 10 mg/kg with dose escalation to 20 mg/kg of either β-lactams JG-5 or JG-19 for 29 days. Tumor size was measured every other day using calipers. Tumor volume (V) was determined by the equation: V = (L × W2) ×0.5, where L is the length and W is the width of the tumor.
Terminal deoxyribonucleotidyl transferase-mediated dUTP nick end-labeling (TUNEL) assay
Tumor tissues were paraffin embedded and stained according to the manufacturer's instructions. Briefly, after deparaffinization and hydration, the tissue was incubated with working strength stop/wash buffer, conjugated with anti-digoxigenenin, and then stained with peroxidase substrate. Finally, the tissue was mounted under a glass cover slip in permount and viewed under a microscope.
H&E staining assay
Paraffin-embedded sample slides were deparaffinized and hydrated, and then stained with hematoxylin for 1 min. After rinsing, the slides were then stained with eosin for 1 min, followed by further rinsing, and cover-slips were mounted onto slides with permount.
Results
Library screen of N-thiolated β-lactams for cell death-inducing activity
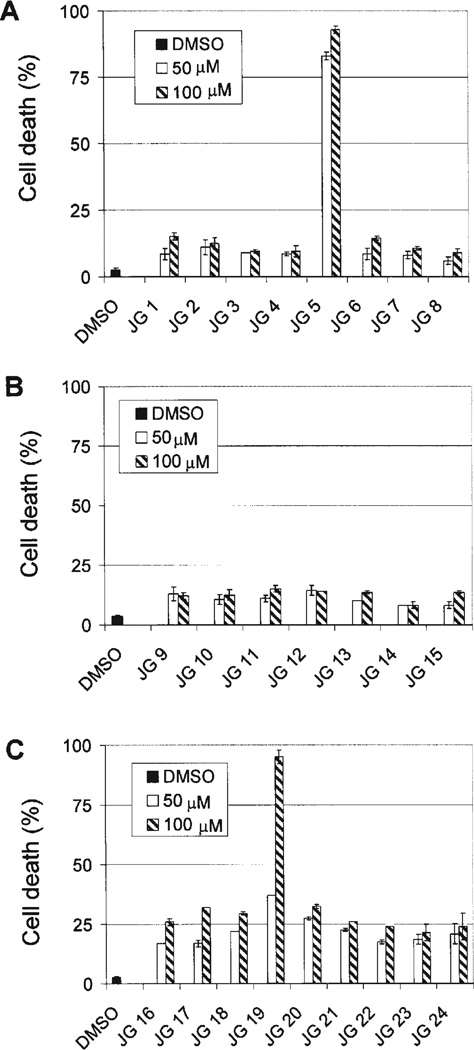
Our laboratories have previously shown that certain β-lactam analogs with structural modifications at N1 and C3 of the lactam ring possess apoptosis-inducing ability (16,17). A focused library of β-lactam analogs was synthesized having different alkyl chain lengths and branching properties on the C3 acyl side chain (Fig. 1), and then each was independently screened for cell death-inducing activity using a trypan blue exclusion assay (Fig. 2). We also examined a second family of N-thiolated β-lactams having a sec-butylthio moiety on the N1 center. The compound screening procedure was undertaken by treating leukemia cells with either 50 or 100 µM of indicated analog for 24 h, followed by measurement of non-viable cells (Fig. 2). Among the compounds tested, the branched long-alkyl chain derivative JG-5 was able to induce cell death by 83 and 93% at 50 and 100 µM, respectively (Fig. 2A). Additionally, the corresponding sec-butylthio analog JG-19 at these two concentrations was found to induce cell death at 37 and 95%, respectively (Fig. 2C). A common structure-activity relationship that exists between these two lactams JG-5 and JG-19 is the presence of a branched carbon chain moiety on C3 of the lactam ring. Additionally, the presence of a double bond within the carbon chain moiety at C3 of the lactam ring (JG-13 to JG-17) did not appear to significantly affect potency (Fig. 2B and C). Furthermore, lengthening of the carbon chain (without branching) was found to be inversely proportional to its cell killing activity, with increasing straight chain length leading to diminishing activity in this assay (Fig. 2).
Figure 2.
Library screen of β-lactams for more potent analogs. (A and B), Raji leukemic cells were treated with solvent (DMSO), 50 or 100 µM of each indicated compound for 24 h, followed by trypan blue exclusion assay. (C) HL-60 leukemic cells were treated with solvent (DMSO), 50 or 100 µM of each indicated analog for 24 h, followed by trypan blue exclusion assay. The numbers given are percentages of nonviable cells to total cells. Standard Deviations are shown with error bars from a mean of three different experiments.
Induction of apoptotic cell death by JG-5 and JG-19
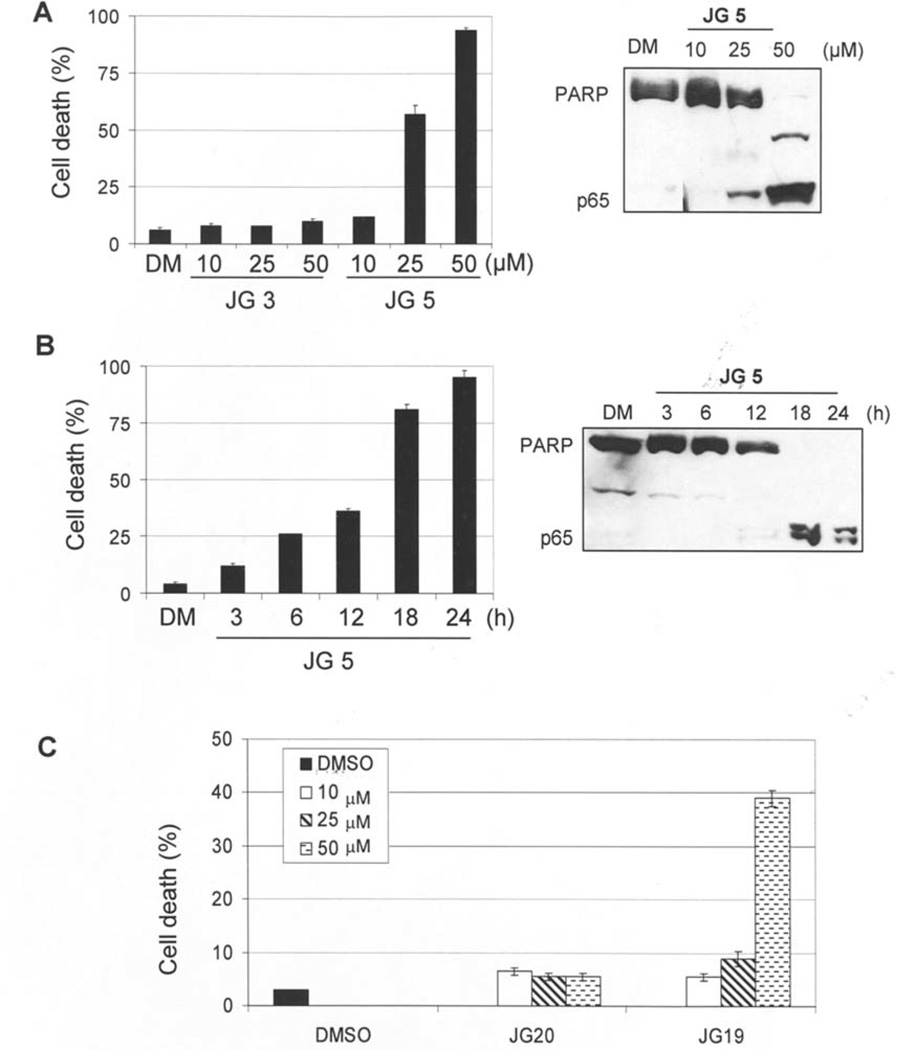
We then set out to determine if apoptosis is responsible for the cell-killing activity of the two most active compounds, JG-5 and JG-19, by measuring for apoptosis-specific PARP cleavage in a Western blot analysis (Fig. 3A and B). Jurkat T cells were first treated at different concentrations of JG-5 and compared to its smaller straight chain counterpart (JG-3) in a trypan blue assay, followed by detection of the cleaved PARP fragment (Fig. 3A). Our results showed that cells treated with JG-5 at 25 and 50 µM exhibited ~58 and ~95% cell death, respectively. In contrast, <10% cell death was induced by cells treated with JG-3 (Fig. 3A, left). Consistent with the presence of non-viable cells, low levels of PARP cleavage were visible in cells treated at 25 µM of JG-5. However, after treatment with 50 µM of JG-5, the full length PARP fragment was fully cleaved into p85 and p65 fragments (Fig. 3A, right). In contrast, no PARP cleavage was detectable in cells treated with JG-3 at the highest concentration tested (data not shown).
Figure 3.
Selective induction of apoptosis by branched lactam analogs (JG-5, JG-19) over their straight chain counterparts (JG-3, JG-20). (A) Jurkat T cells were treated at indicated concentrations for 24 h with either DMSO control, JG-3 or JG-5, followed by measurement of cell death and apoptosis-specific PARP cleavage by Western blot analysis. The intact PARP fragment (116 kDa) and PARP cleavage (p65) are shown. (B) Jurkat T cells were treated with JG-5 at 50 µM for the indicated hours, followed by measurement of cell death and apoptosis-specific PARP cleavage. (C) Jurkat T cells were treated with DMSO control, JG-20 or JG-19 for 24 h at indicated concentrations and cell death was measured. All results were obtained in three independent experiments.
In the kinetic experiment, Jurkat T cells were treated with 50 µM of JG-5 for various points (3–24 h), followed by measurement of nonviable cells and apoptosis-specific PARP cleavage (Fig. 3B). After 18 h and 24 h treatment, cell death was induced at 82 and 95%, compared to 12, 27, and 37% at 3, 6 and 12 h, respectively (Fig. 3B, left). In the same experiment, the p65/PARP cleavage fragment was detected at 18 and 24 h, compared to earlier time points where no detectable levels of p65/PARP were found (Fig. 3B, right). These results suggest that substitution at C3 of the lactam ring with a branched moiety confers enhanced anti-proliferative activity.
We then compared the potency of the branched acyl analog JG-19 and the straight chain variant JG-20 by measuring cell death in a trypan blue assay. Jurkat T cells were treated with 10–50 µM of JG-19 or JG-20 for 24 h and measured for non-viable cell population (Fig. 3C). We found that when cells were treated with 50 µM of JG-19, cell death was induced ~40%. In comparison, cells treated with JG-19 <50 µM were mostly viable similar to that obtained with JG-20-treated cells at all concentrations tested. In the same experiment, cells treated with JG-19 and JG-20 were measured for apoptosis-specific PARP cleavage (data not shown). PARP cleavage was detectable only in cell lysates treated with JG-19 at 50 µM compared to JG-20- and DMSO-treated cells. This suggests that introducing branched alkyl moiety on the sulfur side chain at the same time as having branching in the C3 acyl residue may partially mitigate the potency compared to JG-5 since our laboratory has previously shown that increasing the number of carbons from one to two on the N-alkylthio group attenuated cell killing activity (16,21).
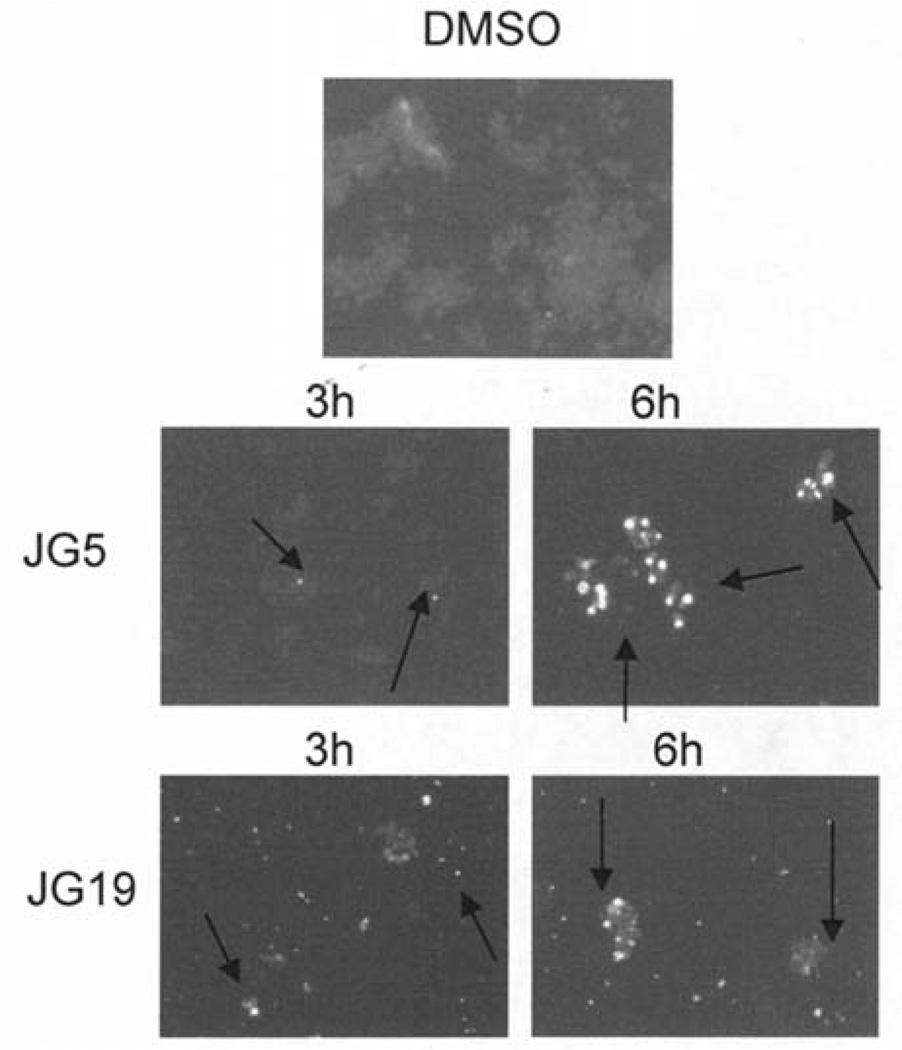
To further confirm that apoptosis is responsible for the cell killing of JG-5 and JG-19, we investigated the ability of the two branched lactams JG-5 and JG-19 to damage tumor cell DNA. Jurkat T cells were treated with 50 µM of either JG-5 or JG-19 for 3 or 6 h, followed by Hoechst staining and observation of nuclear morphology (Fig. 4). Cells treated with 50 µM of JG-5 for 6 h contain significant levels of bright, punctuate nuclei consistent with the onset of apoptosis (Fig. 4). In comparison, JG-19-treated cells contained lower levels of brightly stained apoptotic nuclei at 6 h (Fig. 4). Collectively, our results demonstrate tumor cell killing by branched acyloxy residues at C3 of these β-lactams, with enhanced activity conferred by JG-5.
Figure 4.
Apoptotic nuclear staining in cells treated with JG-5 and JG-19. To assay nuclear morphology, Raji cells treated with 50 µM of JG-5 or JG-19 for 3 or 6 h were fixed and stained with Hoechst 33258. The nuclear morphology is visualized by fluorescence microscopy (×100).
Cell death-inducing capability of JG-19 and JG-5 is associated with apoptosis induction in breast tumor xenografts
Our in vitro results show that JG-19 and JG-5 preferentially induce cell death associated with apoptosis in human leukemia cells. To investigate whether this effect can be emulated in vivo, we used mice bearing breast tumor xenografts. MDA-MB-231 cells were implanted s.c. into female nude mice and allowed to grow until the appearance of a palpable tumor (~80 mm3). The mice were then injected s.c. daily with solvent or 10 mg/kg with dose escalation to 20 mg/kg with lactam JG-19 or JG-5 for 29 days. At the end of the trial the mice were sacrificed and their tumor tissue was used for immunostaining assays. Measurement of tumor size showed that tumor growth was inhibited only ~18% with the mouse treated with JG-19 and ~49% in JG-5-treated mouse, compared to solvent control treatment.
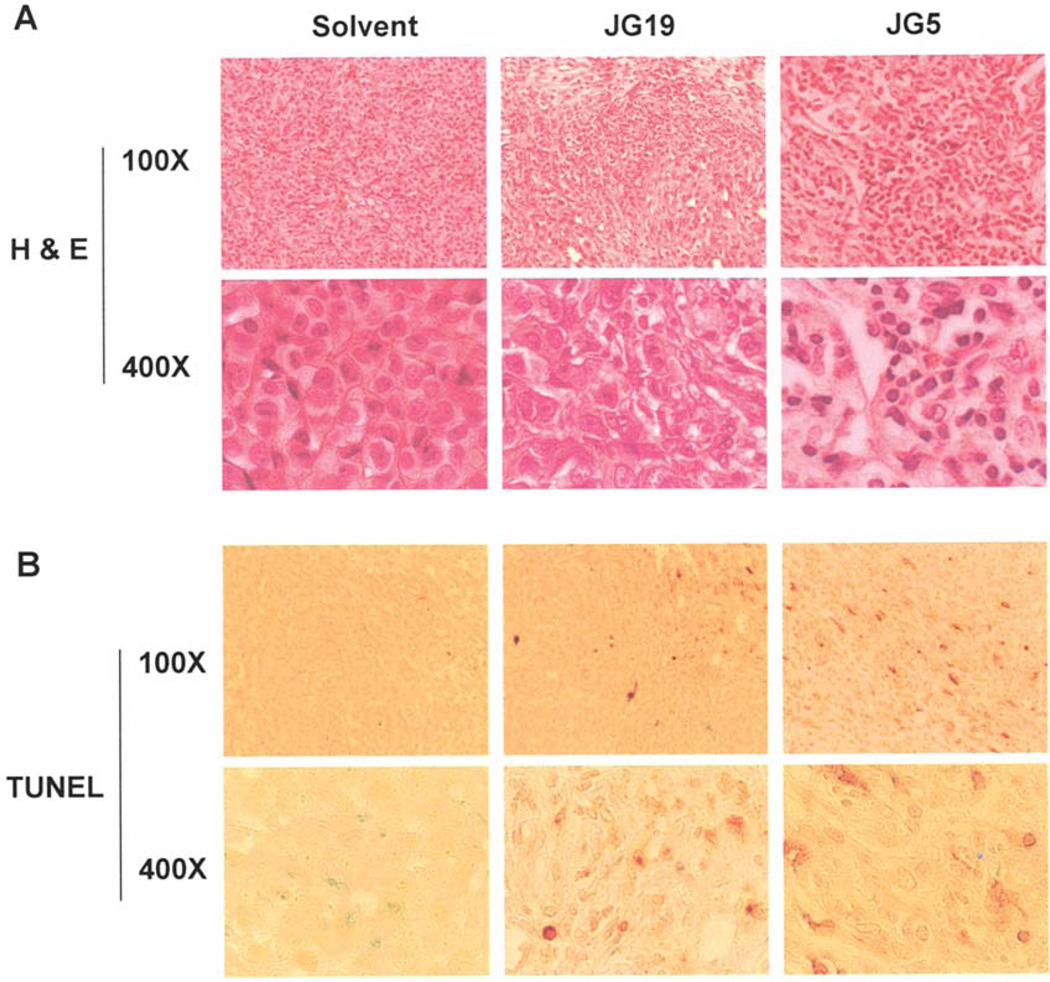
To determine if apoptosis was the predominant factor responsible for tumor growth inhibition, prepared tissue samples were used for H&E and TUNEL staining (Fig. 5). Induction of apoptosis was confirmed in tumors treated with JG-5 and JG-19 compared to control-treated tumors by measurable levels of condensed apoptotic nuclei detected in H&E staining (Fig. 5A) and the presence of TUNEL-positive cells (Fig. 5B). JG-19-treated tumor contained low levels of apoptotic markers whereas JG-5-treated tumors contained significantly higher levels of apoptotic markers (Fig. 5), consistent with the effect exhibited in vitro. No visible toxicity was observed in either treatment groups as indicated by a stable weight pattern. Collectively, these results clearly show that the branched acyloxy-substituted β-lactams (JG-19 and JG-5) can preferentially induce DNA damage and apoptosis in vivo.
Figure 5.
Hematoxylin and eosin (H&E) staining and TUNEL assays using mouse tumor samples. Tumors were collected after a 29-day treatment and the prepared tissue slides were used for immunostaining with H&E (A) and TUNEL staining (B) assays. Apoptotic-condensed nuclei and TUNEL-positive cells were found in tumor tissue from mice treated with JG-5 or JG-19. Magnification: ×100 and ×400 as indicated.
Discussion
Developing novel anti-cancer agents that preferentially induce apoptosis in tumor cells with an overall favorable toxicity profile is an ongoing endeavor in anti-cancer drug discovery. The established clinical effectiveness of β-lactams with limited toxicity in treating bacterial infections, and the unusual mode of action of N-thiolated β-lactam analogs, prompted our investigation into their effect towards tumor cells. N-thiolated β-lactams represent a novel class of anti-bacterial compounds that significantly inhibit the growth of methicillin-resistant Staphylococcus aureus (MRSA) and Bacillus anthracis (13,14,22). We have previously shown that certain N-thiolated β-lactams can induce DNA damage in a variety of cell lines, including prostate, breast, and head and neck leading to the induction of apoptosis through S-phase arrest, p38 activation, cytochrome c release and induction of caspase 3 (16,17). Furthermore, we have shown that some of these particular N-thiolated β-lactam modalities can selectively induce apoptosis in human leukemic Jurkat T cells, but not in non-transformed, immortalized human NK cells (15).
In this study, we screened a concentrated library of N-alkylthio β-lactam analogs to generate additional lead candidates for further preclinical development. Our laboratory has previously shown that substitution at each position of the lactam ring plays a unique role in conferring efficacy. The N-methylthio group is imperative for imparting biological activity and that eliminating this moiety or adding more carbon chains in the alkyl residue results in a substantial decrease of potency (21). The carbonyl at position 2 of the lactam ring is characterized as the backbone of the lactam molecule and therefore cannot be manipulated without losing its overall activity. We have previously reported on the effects of additions/manipulations at position 3 of the lactam ring (16). Our current study is a refined extension of the structure-activity relationship between various substituent groups at positions 1 and 3 of the lactam ring, and how this influences the overall efficacy of the molecule.
The results of our screen generated two potent analogs that are directly related to the nature of substituent groups at position N1 and C3 of the 4-membered lactam ring. JG-19 and JG-5 were selected from a group of 24 representative β-lactam analogs that possess a branched carbon chain moiety at position 3 of the lactam ring. Furthermore, JG-19 possesses a sec-butylthio group at the nitrogen position 1 of the lactam ring. Our results demonstrate that branched β-lactam analogs confer a selective advantage in inducing cell death. Additionally, this cell death-inducing ability is directly associated with apoptosis as indicated by apoptosis-specific PARP cleavage and the appearance of bright, punctuate apoptotic nuclei (Figs. 3 and 4). This effect was demonstrated in a time- and concentration-dependent manner. However, JG-19 demonstrated a much lower level of potency as indicated by the fact that cell death was only induced ~40% at 50 µM and generated only low levels of PARP cleavage (Fig. 3C and data not shown). This observation is consistent with previous work in our laboratory that suggests that increasing the number of carbons within the N-alkylthiol group decreases potency (17). This may also preclude tight binding to its cellular target within the apoptotic cascade requiring higher concentrations to reach a similar effect as JG-5.
An important aspect of our study is to investigate the apoptosis-inducing affect in vivo and whether this effect is associated with reduction of tumor burden in mice bearing breast tumor xenografts. Therefore, we tested the effects of JG-5 and JG-19 in mice bearing human MDA-MB-231 xenografts. Our data showed that mice treated with JG-5 reduced tumor growth by (~49%) and JG19 (~18%) with no visible levels of toxicity. Immunostaining by H&E and TUNEL (Fig. 5) confirmed that reduction of tumor burden is directly related to apoptosis.
While the molecular mechanism of N-alkylthio β-lactams are not fully characterized and still under investigation, our results and previous studies suggest that these unusual β-lactams possess selective apoptosis-inducing activity. Further investigation into their potential for use as anti-cancer drugs is warranted.
Acknowledgements
We thank Joan McCallum for her assistance. This study was supported by Karmanos Cancer Institute of Wayne State University (to Q.P. Dou), Department of Defense Breast Cancer Research Program Awards (W81XWH-04-1-0688 and DAMD17-03-1-0175 to Q.P. Dou), NCI Training Grant (T32-CA009531 to M. Frezza), and University of South Florida Graduate Multidisciplinary Scholarship (to J. Garay).
References
- 1.Danial NN, Korsmeyer SJ. Cell death: critical control points. Cell. 2004;116:205–219. doi: 10.1016/s0092-8674(04)00046-7. [DOI] [PubMed] [Google Scholar]
- 2.Fesik SW. Promoting apoptosis as a strategy for cancer drug discovery. Nat Rev Cancer. 2005;5:876–885. doi: 10.1038/nrc1736. [DOI] [PubMed] [Google Scholar]
- 3.Hengartner MO. The biochemistry of apoptosis. Nature. 2000;407:770–776. doi: 10.1038/35037710. [DOI] [PubMed] [Google Scholar]
- 4.Earnshaw WC. Nuclear changes in apoptosis. Curr Opin Cell Biol. 1995;7:337–343. doi: 10.1016/0955-0674(95)80088-3. [DOI] [PubMed] [Google Scholar]
- 5.Fulda S, Debatin KM. Extrinsic versus intrinsic apoptosis pathways in anticancer chemotherapy. Oncogene. 2006;25:4798–4811. doi: 10.1038/sj.onc.1209608. [DOI] [PubMed] [Google Scholar]
- 6.Kaufmann SH, Earnshaw WC. Induction of apoptosis by cancer chemotherapy. Exp Cell Res. 2000;256:42–49. doi: 10.1006/excr.2000.4838. [DOI] [PubMed] [Google Scholar]
- 7.Fulda S, Debatin KM. Targeting apoptosis pathways in cancer therapy. Curr Cancer Drug Targets. 2004;4:569–576. doi: 10.2174/1568009043332763. [DOI] [PubMed] [Google Scholar]
- 8.Makin G, Dive C. Apoptosis and cancer chemotherapy. Trends Cell Biol. 2001;11:S22–S26. doi: 10.1016/s0962-8924(01)02124-9. [DOI] [PubMed] [Google Scholar]
- 9.Nicholson DW. From bench to clinic with apoptosis-based therapeutic agents. Nature. 2000;407:810–816. doi: 10.1038/35037747. [DOI] [PubMed] [Google Scholar]
- 10.Nathwani D, Wood MJ. Penicillins. A current review of their clinical pharmacology and therapeutic use. Drugs. 1993;45:866–894. doi: 10.2165/00003495-199345060-00002. [DOI] [PubMed] [Google Scholar]
- 11.Abraham EP. The beta-lactam antibiotics. Sci Am. 1981;244:76–86. doi: 10.1038/scientificamerican0681-76. [DOI] [PubMed] [Google Scholar]
- 12.Kidwai M, Sapra P, Bhushan KR. Synthetic strategies and medicinal properties of beta-lactams. Curr Med Chem. 1999;6:195–215. [PubMed] [Google Scholar]
- 13.Turos E, Long TE, Heldreth B, et al. N-thiolated beta-lactams: a new family of anti-Bacillus agents. Bioorg Med Chem Lett. 2006;16:2084–2090. doi: 10.1016/j.bmcl.2006.01.070. [DOI] [PubMed] [Google Scholar]
- 14.Turos E, Long TE, Konaklieva MI, et al. N-thiolated beta-lactams: novel antibacterial agents for methicillin-resistant Staphylococcus aureus. Bioorg Med Chem Lett. 2002;12:2229–2231. doi: 10.1016/s0960-894x(02)00343-8. [DOI] [PubMed] [Google Scholar]
- 15.Kazi A, Hill R, Long TE, Kuhn DJ, Turos E, Dou QP. Novel N-thiolated beta-lactam antibiotics selectively induce apoptosis in human tumor and transformed, but not normal or non-transformed, cells. Biochem Pharmacol. 2004;67:365–374. doi: 10.1016/j.bcp.2003.09.017. [DOI] [PubMed] [Google Scholar]
- 16.Kuhn DJ, Wang Y, Minic V, et al. Structure-activity relationships of N-methylthiolated beta-lactam antibiotics with C3 substitutions and their selective induction of apoptosis in human cancer cells. Front Biosci. 2005;10:1183–1190. doi: 10.2741/1611. [DOI] [PubMed] [Google Scholar]
- 17.Smith DM, Kazi A, Smith L, et al. A novel beta-lactam antibiotic activates tumor cell apoptotic program by inducing DNA damage. Mol Pharmacol. 2002;61:1348–1358. doi: 10.1124/mol.61.6.1348. [DOI] [PubMed] [Google Scholar]
- 18.Ren XF, Konaklieva MI, Shi H, Dickey S, Lim DV, Gonzalez J, Turos E. Studies on nonconventionally fused bicyclic beta-lactams. J Org Chem. 1998;63:8898–8917. [Google Scholar]
- 19.Daniel KG, Gupta P, Harbach RH, Guida WC, Dou QP. Organic copper complexes as a new class of proteasome inhibitors and apoptosis inducers in human cancer cells. Biochem Pharmacol. 2004;67:1139–1151. doi: 10.1016/j.bcp.2003.10.031. [DOI] [PubMed] [Google Scholar]
- 20.An B, Goldfarb RH, Siman R, Dou QP. Novel dipeptidyl proteasome inhibitors overcome Bcl-2 protective function and selectively accumulate the cyclin-dependent kinase inhibitor p27 and induce apoptosis in transformed, but not normal, human fibroblasts. Cell Death Differ. 1998;5:1062–1075. doi: 10.1038/sj.cdd.4400436. [DOI] [PubMed] [Google Scholar]
- 21.Kuhn D, Coates C, Daniel K, et al. Beta-lactams and their potential use as novel anticancer chemotherapeutics drugs. Front Biosci. 2004;9:2605–2617. doi: 10.2741/1420. [DOI] [PubMed] [Google Scholar]
- 22.Revell KD, Heldreth B, Long TE, Jang S, Turos E. N-thiolated beta-lactams: Studies on the mode of action and identification of a primary cellular target in Staphylococcus aureus. Bioorg Med Chem. 2007;15:2453–2467. doi: 10.1016/j.bmc.2006.12.027. [DOI] [PMC free article] [PubMed] [Google Scholar]