Abstract
There are currently no comparison measurements of stress-induced changes in vascular function during acute mental stress tests to measurements made by BIOPAC MP150 systems technology, a standard polygraph device used to detect deception during polygraph examinations in military or law enforcement applications. Vascular responses to reactive hyperaemia and acute mental stress in 25 healthy subjects were measured by both peripheral arterial tonometry (EndoPAT) and a blood pressure cuff attached to a pressure transducer (BIOPAC) and compared. Reactive hyperaemia was performed at baseline and following three acute mental stress tests. There was no difference in vascular reactivity at baseline and following acute mental stress, as measured by EndoPAT or BIOPAC systems (p > 0·05). Mental stress ratios measured by EndoPAT were significantly different than those measured by BIOPAC (p < 0·01). These data suggest that EndoPAT measurements of vascular responses to acute mental stress may be more specific and sensitive than measurements using the BIOPAC system.
Keywords: deception detection, endothelial function, mental stress, peripheral arterial tonometry, plethysmography, polygraph, reactive hyperaemia, vascular response
INTRODUCTION
Since the early 1900s, the detection of changes in physiological and cardiovascular parameters has been used as an indicator for the individuals’ ability to cope in response to questioning or interrogation mediated by mental stress testing. There are several techniques that are currently used and several others that are being developed to aid in the detection of deception,1-5 of which the standard polygraph examination is the most widely used and reliable (80–90% reliability6) Although the polygraph system has become the most common method used to detect deception, it is not widely accepted and has several weaknesses.2,5,7,8
Because deception detection with the polygraph system is based almost entirely on the measurement of the sympathetic nervous system response, one of the main drawbacks of the current polygraph system used by the Defence Academy for Credibility Assessment (the BIOPAC MP150 system), as well as field model polygraphs, is the inability to control for fluctuation of the examinee’s emotional state or response to mental stress that are also under the control of the sympathetic nervous system.2,5,7,8 Moreover, the current polygraph technology may be affected by the examinee’s health, integrity of the examinee’s vascular system and the presence of cardiovascular disease or cardiovascular risk factors, such as high blood pressure, smoking and diabetes mellitus. Finally, the standard polygraph blood pressure cuff is very uncomfortable.5,8 Therefore, the ideal polygraph system should be independent of the individuals’ vascular system integrity and status, as well as independent of traditional and new vascular and heart disease risk factors.
Peripheral arterial tonometry (PAT) technology can be used to assess both vascular health, as determined by endothelial function, and the physiological response to mental stress simultaneously in the same individuals and may provide important information on the relationship between the vascular status of the individuals and their response to interrogation associated with mental stress. This methodology is independent of the subject’s knowledge or conscious control of the signals and is designed to eliminate environmental interference. Under conditions of mental stress, there are certain changes in physiology (such as endothelial function) that may be used to assess and quantify the individual’s response. Thus, the aim of the study was to test the hypotheses that there are significant differences in the measurement of stress-induced changes in vascular function during acute mental stress tests as measured by EndoPAT 2000 and BIOPAC MP150 systems technology, the system used by the Defence Academy for Credibility Assessment for polygraph testing.
METHODS
All study protocols were reviewed and approved by the Institutional Review Board, Mayo Clinic and Foundation. Written informed consent was obtained from each participant and all studies were conducted under in accordance with the Declaration of Helsinki. Subjects between the ages of 18 and 60 years old, without any history of ischaemic or coronary artery disease, hypertension, diabetes mellitus or smoking, were recruited by advertisements for this study. Subjects (19 F, 6 M) were asked to fast for 4 h prior to the study, and abstain from strenuous exercise and products containing coffee or tobacco the day of the examination.
General experimental protocol
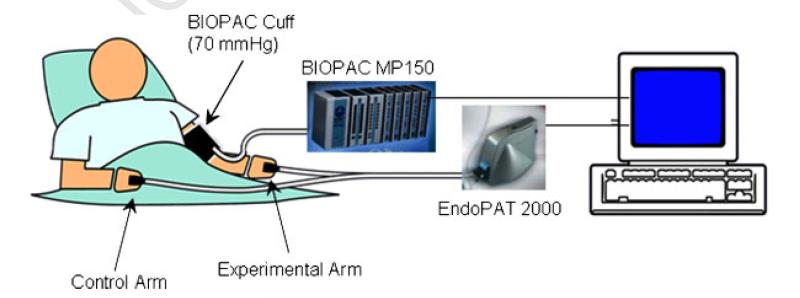
All studies were performed in a designated quiet, temperature controlled and uniformly lit room. Subjects were seated comfortably in a padded chair with armrests, with their feet flat on the floor. The goal was to measure blood pressure from the BIOPAC blood pressure cuff and peripheral arterial tonometry from the EndoPAT cuffs simultaneously during the entire protocol. Therefore, the BIOPAC blood pressure cuff, attached to a pressure transducer and the acquisition system, was placed on one arm. The finger cuffs of the EndoPAT 2000 device were attached to the middle finger of each hand (Figure 1). Fingers were separated by soft foam cushions to prevent them from touching the pressure sensitive finger cuff.
Figure 1.
The experimental set-up is shown. The BIOPAC cuff is attached to the experimental arm of the subject, which is connected to the BIOPAC MP150 system for pressure measurement and the computer. The EndoPAT finger cuffs are attached to the middle fingers of each hand, which are connected to the EndoPAT 2000 machine and the computer.
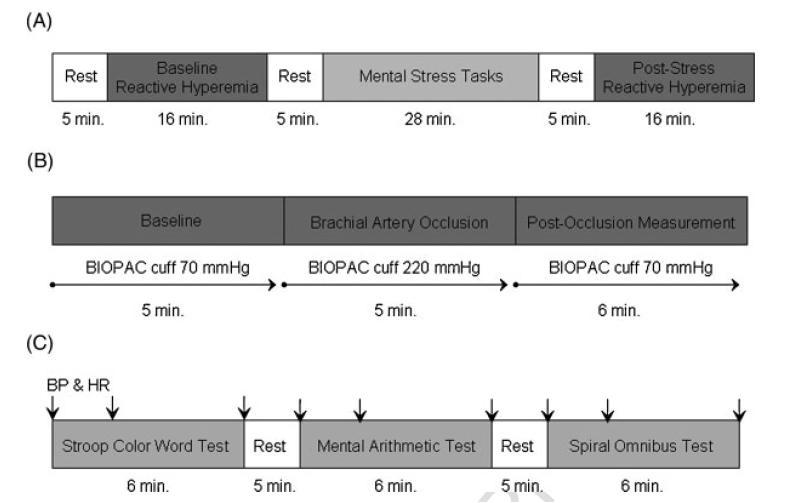
The experimental timeline is shown in Figure 2. Subjects were instructed to relax for 5 min before baseline systolic and diastolic blood pressure and heart rate were obtained via a digital automated blood pressure cuff (Omron Healthcare, Inc., Vernon Hills, Illinois, USA). The BIOPAC blood pressure cuff was then inflated to 70 mmHg and blood pressure measurements were started. The EndoPAT finger cuffs were inflated at the same time as the BIOPAC blood pressure cuff and continuous signal recording was started. Following baseline measurements, the first reactive hyperaemia protocol was performed. This consisted of 5 min of arterial occlusion by increasing the inflation of the BIOPAC cuff from 70 mmHg to 220 mmHg followed by a 6-min post-occlusion measurement of hyperaemia, with the BIOPAC cuff released from 220 mmHg to70 mmHg, as described in a later section. Subjects were allowed to relax for 5 min before the start of the sequence of mental stress tasks (Figure 2).
Figure 2.
(A) The experimental timeline for the entire study protocol is shown. Subjects rested for 5 min and then underwent baseline reactive hyperaemia testing for 16 min. Following another 5-min rest, subjects underwent three mental stress tasks, another 5 min of rest, and the post-stress reactive hyperaemia test. (B) The experimental timeline for the reactive hyperaemia test is shown. Baseline measurements are taken for 5 min with both the BIOPAC arm cuff and the EndoPAT finger cuffs inflated to 70 mmHg. The BIOPAC cuff is then inflated from 70 mmHg to 220 mmHg to occlude the experimental arm for 5 min. The BIOPAC cuff is then released back down from 220 mmHg to 70 mmHg to allow for 6 min of post-occlusion measurements with both the BIOPAC and EndoPAT cuffs. (C) The experimental timeline for the mental stress testing is shown. Each of the three mental stress tasks (Stroop colour word test, mental arithmetic test, and spiral omnibus test) is performed for 6 min with 5-min rest periods between each task. Blood pressure (BP) and heart rate (HR) measurements taken by the digital automated blood pressure cuff are indicated by the arrows and are taken at the beginning, 2 min into, and at the end of each mental stress task.
Systolic and diastolic blood pressure and heart rate measurements were taken by the digital automated blood pressure cuff 30s before, 2 min into and at the end of each 6-min mental stress task, which are described in a later section. Subjects had 5-min baseline rest periods between each mental stress task, during which they were able to relax (Figure 2). At the end of the mental stress tasks, the reactive hyperaemia protocol was repeated. Double product was calculated as systolic blood pressure multiplied by heart rate.
BIOPAC system measurements
Arterial blood pressure in the control arm was measured using the oscillometric or auscultatory (Korotkoff sounds) plethysmographic technique with a TSD120 pressure transducer connected the computerized MP150-BIOPAC data acquisition system via to a DA100C amplifier (BIOPAC Systems, Goleta, CA, USA).9-11 The cuff was inflated to and held at 70 mmHg pressure to prevent venous pooling, thus avoiding veno-arteriolar reflex vasoconstriction. Pulsatile volume changes of the brachial artery induce a pressure alteration in the cuff, which is sensed by the pressure transducer, and then transmitted, amplified and recorded by the BIOPAC acquisition system. After analogue-to-digital conversion using a peak and rate detector (AcqKnowledge 3.0 software), values of mean arterial blood pressure were determined.
Peripheral arterial tonometry
PAT signals were obtained using the EndoPat 2000 device (Itamar Medical Inc. Ltd, Caesarea, Israel), which has been validated and used previously to assess peripheral arterial tone in other populations.12-17 Specially designed finger probes were placed on the middle finger of each subject’s hand. These probes comprised of a system of inflatable latex air cuffs connected by pneumatic tubes to an inflating device controlled through a computer algorithm. A constant counter pressure (pre-determined by baseline diastolic blood pressure) was applied through the air cushions. This prevented venous pooling thus avoiding veno-arteriolar reflex vasoconstriction. There was no occlusion of arterial blood flow.
Pulsatile volume changes of the distal digit induced pressure alterations in the finger cuff, which were sensed by pressure transducers and transmitted to and recorded by the EndoPat 2000 device. A decrease in the arterial blood volume in the distal finger tip caused a decrease in pulsatile arterial column changes, reflected as a decrease in the measured PAT signal, and vice versa. Blood pressure and heart rate were measured using an automated blood pressure monitor (Omron Healthcare Inc. model # HEM-907XL).
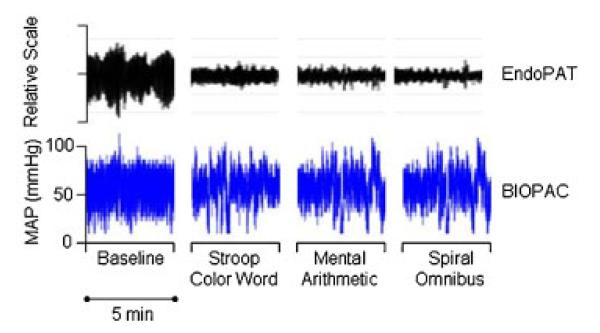
The PAT response to mental stress in patients with cardiovascular disease has been reported previously.17,18 There is a characteristic PAT signal response to mental stress, with diminution of the signal amplitude during stress (Figure 3). A ratio of the stress PAT amplitude to baseline PAT amplitude (PAT stress score) of 0.8 or less is considered a positive response to mental stress and considered a marker for future stress-related cardiovascular events.14
Figure 3.
An example tracing from one subject during one mental stress task is shown. The panel on the top shows the EndoPAT pulsatile volume tracing (relative scale) and the panel on the bottom shows the BIOPAC arterial blood pressure tracing for a 5-min baseline period, and 5-min segments of the Stroop colour word test, the mental arithmetic test and the spiral omnibus test.
Because the EndoPAT records the amplitude of the pulsatile volume changes of the distal digit, and the output is ratios of signal amplitude, we attempted to compare the EndoPAT measurements to the BIOPAC measurements by simultaneously calculating the EndoPAT ratios and the ratios of the blood pressure signal amplitude from the BIOPAC blood pressure cuff. Therefore, mental stress arterial blood pressure measurements from the blood pressure cuff were taken at the same times that PAT signals were measured and similarly compared (a ratio of arterial blood pressure signal amplitude during stress to arterial blood pressure signal amplitude at baseline; BIOPAC stress score; Figure 3). The PAT stress score was compared to the BIOPAC stress score.
Reactive hyperaemia protocol
Vascular reactivity was measured via a reactive hyperaemia, as previously described.12,13,17,19,20 A reactive hyperaemia protocol consists of a 5-min baseline measurement, after which the BIOPAC blood pressure cuff on the test arm was inflated to 60 mmHg above baseline systolic blood pressure, or at least 220 mmHg for 5 min. Occlusion of pulsatile arterial flow was confirmed by the reduction of the EndoPAT and BIOPAC tracings to zero. After 5 min, the cuff was deflated to 70 mmHg to restore arterial blood flow and the EndoPAT and BIOPAC tracings were recorded for a further 6 min. The ratio of the EndoPAT signal after cuff release compared to baseline was calculated through a computer algorithm automatically normalizing for baseline signal, and indexed to the contra lateral arm. The calculated ratio reflects the reactive hyperaemia–PAT index.
BIOPAC reactive hyperaemia pressure measurements from the blood pressure cuff were taken at the same times that PAT signals were measured and similarly compared (a ratio of arterial blood pressure signal amplitude during reactive hyperaemia to arterial blood pressure signal amplitude at baseline; BIOPAC reactive hyperaemia index). The PAT reactive hyperaemia index was compared to the BIOPAC hyperaemia index.
Mental stress protocol
The mental stress testing protocol was administered under the supervision of a licensed clinical psychologist. Following the first reactive hyperaemia trial and a subsequent rest period, the three different mental stress tasks of 6-min duration were performed. Each test was designed to address a different mental skill set. This was designed specifically to prevent mental fatigue and keep subjects motivated. All tasks had intra-test levels of varying difficulty and were externally paced, with the goal of stressing subjects as much as tolerated as judged by the psychologist administering the tests. It is known that external task pacing accentuates the induced mental stress.21 The aim was to keep subjects engaged and focused.
The tasks were performed in random order. They consisted of a number–letter recall challenge of increasing length and complexity testing short-term memory (spiral omnibus), number subtraction of increasing difficulty (1 digit from 2 digits up to 3 digits from 3 digits) testing math skills, and a computerized version of the Stroop word–colour conflict.18,22 The Stroop word–colour conflict test consisted of three coloured words (red, blue and green) displayed in random order on a computer screen. Each word could appear in either its own colour (visually concordant), or in one of the other 2 colours (visually discordant). The subject’s task was to enunciate the colour of the word, not the actual word itself. A rectangular box surrounded the stimulus colour–word, and this task was also externally paced, with the subject having to keep pace with the advancing box.
Statistical analysis
Results are expressed as mathematical mean±standard deviation. Differences between groups were analysed using unpaired student’s t-test or analysis of variance ANOVA, when comparing multiple groups. Statistical significance was accepted at p < 0·05.
RESULTS
Subject characteristics
Baseline subject characteristics are shown in Table 1. There were significantly more female subjects (n=19) than male subjects (n=6). There were no significant differences in age, cardiovascular risk factors, resting blood pressure, or heart rates between female and male subjects (p=0·05). Male subjects had significantly higher height and weight as compared to female subjects (p=0·003 and p=0·03, respectively). Mean age (years) for female subjects (n=19), male subjects (n=6) and all subjects combined were 27±7, 29±8 and 28±8, respectively.
Table 1.
Baseline characteristics of the subject population
| Female subjects (n=19) |
Male subjects (n=6) |
All subjects (n=25) |
|
|---|---|---|---|
| Age (years) | 27±7 | 29±8 | 28±8 |
| Height (cm) | 166·1±6·2 | 178·1±6·3* | 168·9±8·0 |
| Weight (kg) | 64·8±10·6 | 91·5±22·5* | 70·9±17·9 |
| BMI (kgm −2) | 23·4±3·2 | 28·5±5·3 | 24·6±4·3 |
| Systolic BP (mmHg) | 107±9 | 117±11 | 109±10 |
| Diastolic BP (mmHg) | 68±10 | 72±12 | 69±10 |
| Mean BP (mmHg) | 81±9 | 87±11 | 82±10 |
| Heart rate (beats min−1) | 72±12 | 71±14 | 72±12 |
| Baseline | 7702±1376 | 8370±2036 | 7856±1533 |
| double product |
Values are mean±standard deviation; BP denotes blood pressure; BP and heart rate measurements made by digital automated BP cuff; double product calculated as systolic BP multiplied by heart rate.
p < 0·05 for female versus male subjects.
Reactive hyperaemia
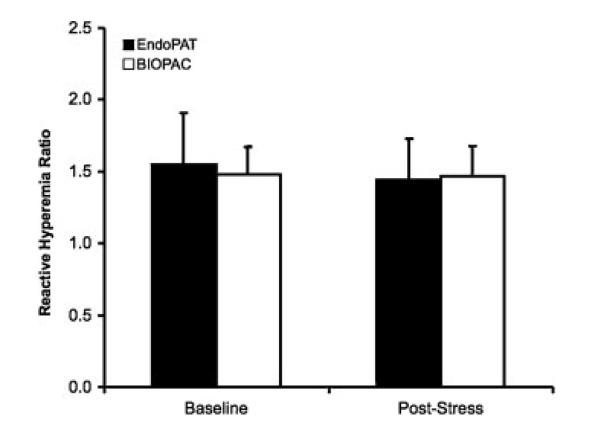
There was no significant difference in reactive hyperaemia to baseline ratios measured by either the EndoPAT pulsatile volume signal amplitude or BIOPAC arterial blood pressure signal amplitude at baseline or following mental stress, as shown in Figure 4 (p=0·38 and p=0·61, respectively). EndoPAT pulsatile volume and BIOPAC arterial BP reactive hyperaemia ratios were 1·55±0·36 and 1·48±0·19, respectively, at baseline and 1·44±0·29 and 1·47±0·21, respectively, following mental stress (Figure 4). There were no differences in reactive hyperaemia ratios between male and female subjects (p=0·75).
Figure 4.
Reactive hyperaemia ratios (ratio of signal amplitude after releasing BIOPAC arterial blood pressure cuff during reactive hyperaemia to signal amplitude at baseline) for EndoPAT (pulsatile volume signal amplitude) and BIOPAC (arterial blood pressure signal amplitude) at baseline and following acute mental stress testing.
Mental stress
Systolic blood pressure, diastolic blood pressure, mean blood pressure and heart rate, as measured by the automated digital automated blood pressure cuff, and the calculated double product values during acute mental stress testing are shown in Table 2. There were no significant increases in any systolic or diastolic blood pressure, heart rate or double product values during any mental stress test (p > 0·05). There were no differences between male and female subjects (p > 0·05).
Table 2.
Blood pressure, heart rate, and double product values during mental stress
| Female subjects (n=19) |
Male subjects (n=6) |
All subjects (n=25) |
|
|---|---|---|---|
| Stroop Colour Word Test | |||
| Systolic BP (mmHg) | 115±14 | 120±7 | 116±12 |
| Diastolic BP (mmHg) | 77±11 | 76±9 | 77±10 |
| Mean BP (mmHg) | 90±12 | 91±6 | 90±10 |
| Heart rate (beats min−1) | 78±11 | 73±14 | 77±12 |
| Double product | 9063±1894 | 8758±1685 | 8993±1820 |
| Mental Arithmetic Test | |||
| Systolic BP (mmHg) | 113±14 | 122±11 | 115±14 |
| Diastolic BP (mmHg) | 76±14 | 78±10 | 77±13 |
| Mean BP (mmHg) | 88±13 | 93±9 | 89±13 |
| Heart rate (beats min−1) | 78±12 | 74±13 | 77±12 |
| Double product | 8873±2013 | 9145±2337 | 8936±2046 |
| Spiral Omnibus Test | |||
| Systolic BP (mmHg) | 112±13 | 124±13 | 115±13 |
| Diastolic BP (mmHg) | 76±12 | 77±9 | 76±11 |
| Mean BP (mmHg) | 88±12 | 93±8 | 89±11 |
| Heart rate (beats min−1) | 75±10 | 79±13 | 76±10 |
| Double product | 8423±1783 | 9882±2532 | 8760±2023 |
Values are mean±standard deviation; BP denotes blood pressure; BP and heart rate measurements made by digital automated BP cuff; double product calculated as systolic BP multiplied by heart rate; p > 0·05 for all values in female versus male subjects.
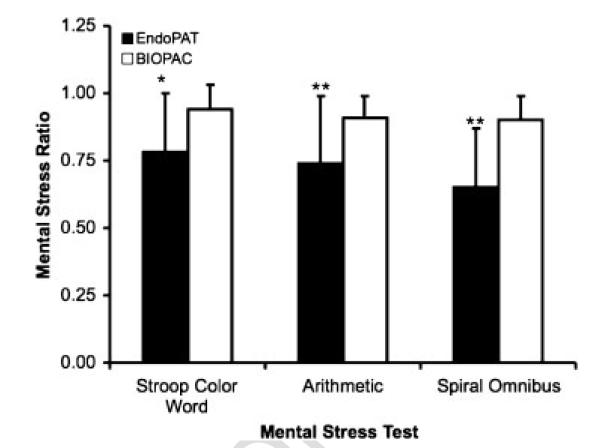
The ratio of pulsatile volume signal amplitude during mental stress to baseline pulsatile volume signal amplitude during each mental stress task as measured by EndoPAT (PAT mental stress ratio) were significantly lower than the ratio of BIOPAC arterial blood pressure signal amplitude during mental stress to baseline arterial blood pressure signal amplitude during each mental stress task (p=0·017 for Stroop colour word test, p=0·0001 for mental arithmetic and p=0·0001 spiral omnibus tests; Figure 5). Mental stress ratios for the Stroop colour word test, mental arithmetic and spiral omnibus memory test were 0·78±0·22, 0·74±0·25 and 0·65±0·22, respectively, for EndoPAT and 0·94±0·08, 0·91±0·208 and 0·90±0·09, respectively, for BIOPAC. There was a significant increase in blood pressure during each mental stress task as compared to baseline, as measured by the BIOPAC cuff (p <0·00001). There also was a significant difference between acute mental stress tasks measured by EndoPAT (mental stress ratio during each consecutive mental stress task was lower than that measured during the previous task; p < 0·05), but were no differences between stress tests as measured by BIOPAC system (p > 0·05). There were no differences in mental stress ratios between male and female subjects (p > 0·05).
Figure 5.
Mental stress ratios (ratio of signal amplitude during mental stress to signal amplitude at baseline) for EndoPAT (pulsatile volume signal amplitude) and BIOPAC (arterial blood pressure signal amplitude) at baseline and following acute mental stress testing. *p < 0·01 for EndoPAT versus BIOPAC mental stress ratios. **p < 0·0001 for EndoPAT versus BIOPAC mental stress ratios.
DISCUSSION
We demonstrated that there is a significant difference in the measurement of stress-induced changes in vascular function during acute mental stress tests as measured by the EndoPAT system compared to the BIOPAC MP150 system, used by the Defence Academy for Credibility Assessment for polygraph testing. There were no differences in vascular signals during mental stress measured by the BIOPAC system.
These data may suggest that EndoPAT may be an extremely useful adjuvant tool in the detection of mental stress, as in the detection of deception in military or law enforcement applications. Whereas the BIOPAC system did measure a decrement in signal measurement during mental stress, as compared to baseline, the differences were not significant. EndoPAT, on the other hand, measured significant decrement in signals measured during stress as compared to baseline. Furthermore, PAT stress ratios were significantly smaller in each successive acute mental stress test as measured by EndoPAT (PAT mental stress ratios from highest to lowest were Stroop colour word test, arithmetic test and spiral omnibus test). Also, it is likely that part of the ability of EndoPAT to better measure effects of mental stress on vascular function in normal, healthy subjects, is based on the ability of the EndoPAT to measure downstream vascular effects (in the microcirculation) where most of the vascular changes take place. On the other hand, the BIOPAC blood pressure cuff measures changes in a feed (brachial) artery. These feed arteries do not vasodilate or react as much as smaller, downstream vessels, and, therefore, may not be comparable to downstream (microvascular) effects. These findings suggest that further investigation in this area, especially using methods more common in lie detection, such as mock crimes, is warranted and may support the idea of utilizing EndoPAT as an essential tool in detection of deception.
The current format of polygraph examination by the Defence Academy for Credibility Assessment utilizes an inflated BIOPAC arterial blood pressure cuff for recording cardiovascular physiological activities (CPA). This has proven to be uncomfortable for the examinee, and necessitates shorter test formats, with periods of rest in between.2,5,7,8 Additionally, some individuals are highly sensitive to the discomfort brought about by the cuff. The design of the PAT device itself allows for longer periods of use. It can be left on for extended periods of time without discomfort to the examinee, and allows for the development of lengthier test formats that could potentially increase the sensitivity and specificity of polygraph testing.
Under similar conditions of high mental stress, individuals manifest different behavioural responses. Some appear outwardly unaffected by high stress situations (‘cool’ under pressure), whilst others are overwhelmed.2,5,7,8 It remains unclear whether this dichotomy in behavioural response to mental stress is due to a fundamental difference in physiology (specifically in vascular wall physiology and biology) or a behavioural adaptation. For example, some people are more adept at controlling their outward manifestations of stress than others, but still have the same physiologic response to the stimulus of mental stress. The lack of information regarding the possible link between the response to mental stress and vascular function may be in part secondary to the lack of innovative technology used in polygraph testing, such as the EndoPAT technology, that will allow assessment of these two physiological responses in the same individuals in a non-invasive manner.
Study limitations
Although the results are suggestive of the increased ability of EndoPAT to measure the effect of mental stress on cardiovascular responses, as compared to BIOPAC, further studies with larger and more diverse populations may be warranted. Although there has been reported sex-based differences in vascular responses to mental stress,17,23,24 because this study directly compared measurements taken by the EndoPAT and BIOPAC systems in the same individual, any sex-related effects were likely minimized.
Because both modalities also use similar technology (occlusion of venous flow) to make different measurements, direct comparison of EndoPAT and BIOPAC systems in the same study subjects is less than ideal, due to the need to use the same experimental arm for both modalities. For example, the BIOPAC arterial blood pressure cuff decreased the EndoPAT signal amplitude (by preventing venous return) measured in the experimental arm, but not the control arm of each subject. This may have affected the reactive hyperaemia EndoPAT scores, also, as these scores are lower than scores from previous studies.17 This may suggest that the measurement of EndoPAT on the same arm with the inflated BIOPAC cuff may decrease the absolute EndoPAT score. Furthermore, subjects complained of significant discomfort from duration of inflation of BIOPAC cuff, which may have elicited pain-mediated vascular changes erroneous of the mental stressors. This may lead to questionable efficacy of measuring a ratio of BIOPAC cuff signals using these specific protocols.
We used acute mental stress tests and the response of the peripheral circulation in the laboratory to mimic stress that subjects may encounter in their daily lives.14,22,25-28These acute mental stress tests may not be representative of the stress an examinee may face during a polygraph exam. Although no specific physiological change has been associated, these studies may provide insight into similar collective physiological responses demonstrated during polygraph examination.
CONCLUSIONS
We conclude that EndoPAT is effective in measuring significant differences in peripheral vascular responses to mental stress in multiple populations. These studies indicate a significant ability of EndoPAT to measure acute changes in vascular function associated with mental stress, and possibly deception. It may be speculated that EndoPAT may be an effective adjuvant tool in detecting deception, but further studies are required to evaluate the ability of EndoPAT to measure vascular changes during a protocol more specific to current deception testing, such as following mock crime.
ACKNOWLEDGEMENTS
This work was supported by funding from the Department of Defence (grant #W74V8H-05-1-0001) and Mayo Foundation.
Footnotes
CONFLICT OF INTEREST
None known.
REFERENCES
- 1.Bell BG, Kircher JC, Bernhardt PC. New measures improve the accuracy of the directed-lie test when detecting deception using a mock crime. Physiol Behav. 2008;94:331–340. doi: 10.1016/j.physbeh.2008.01.022. [DOI] [PubMed] [Google Scholar]
- 2.Lykken DT. Psychology and the lie detector industry. Am Psychol. 1974;29:725–739. doi: 10.1037/h0037441. [DOI] [PubMed] [Google Scholar]
- 3.Mohamed FB, Faro SH, Gordon NJ, Platek SM, Ahmad H, Williams JM. Brain mapping of deception and truth telling about an ecologically valid situation: functional MR imaging and polygraph investigation–initial experience. Radiology. 2006;238:679–688. doi: 10.1148/radiol.2382050237. [DOI] [PubMed] [Google Scholar]
- 4.Pavlidis I, Eberhardt NL, Levine JA. Seeing through the face of deception. Nature. 2002;415:35. doi: 10.1038/415035a. [DOI] [PubMed] [Google Scholar]
- 5.Steinbrook R. The polygraph test–a flawed diagnostic method. N Engl J Med. 1992;327:122–123. doi: 10.1056/NEJM199207093270212. [DOI] [PubMed] [Google Scholar]
- 6.Nardini W. The polygraph technique: an overview. J Police Sci Admin. 1987;15:239–249. [Google Scholar]
- 7.Ford EB. Lie detection: historical, neuropsychiatric and legal dimensions. Int J Law Psychiatry. 2006;29:159–177. doi: 10.1016/j.ijlp.2005.07.001. [DOI] [PubMed] [Google Scholar]
- 8.U.S. Congress . O.o.T.A. Scientific Validity of Polygraph Testing: A Research Review and Evaluation - A Technical Memorandum. DIANE Publishing; Washington, D.C.: 1983. [Google Scholar]
- 9.Arriaga P, Esteves F, Carneiro P, Monteiro MB. Are the effects of unreal violent video games pronounced when playing with a virtual reality system? Aggress Behav. 2008;34:521–538. doi: 10.1002/ab.20272. [DOI] [PubMed] [Google Scholar]
- 10.Carmel S, Macy A. Physiological signal processing laboratory for biomedical engineering education. Conf Proc IEEE Eng Med Biol Soc; 2005. pp. 859–862. [DOI] [PubMed] [Google Scholar]
- 11.Wang YT, Chen S, Limroongreungrat W, Change LS. Contributions of selected fundamental factors to wheelchair basketball performance. Med Sci Sports Exerc. 2005;37:130–137. doi: 10.1249/01.mss.0000150076.36706.b2. [DOI] [PubMed] [Google Scholar]
- 12.Bonetti PO, Barsness GW, Keelan PC, et al. Enhanced external counterpulsation improves endothelial function in patients with symptomatic coronary artery disease. J Am Coll Cardiol. 2003a;41:1761–1768. doi: 10.1016/s0735-1097(03)00329-2. [DOI] [PubMed] [Google Scholar]
- 13.Bonetti PO, Lerman LO, Lerman A. Endothelial dysfunction: a marker of atherosclerotic risk. Arterioscler Thromb Vasc Biol. 2003b;23:168–175. doi: 10.1161/01.atv.0000051384.43104.fc. [DOI] [PubMed] [Google Scholar]
- 14.Goor DA, Sheffy J, Schnall RP, et al. Peripheral arterial tonometry: a diagnostic method for detection of myocardial ischemia induced during mental stress tests: a pilot study. Clin Cardiol. 2004;27:137–141. doi: 10.1002/clc.4960270307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Halligan SC, Murtagh B, Lennon RJ, et al. Effect of long-term hormone replacement therapy on coronary endothelial function in postmenopausal women. Mayo Clin Proc. 2004;79:1514–1520. doi: 10.4065/79.12.1514. [DOI] [PubMed] [Google Scholar]
- 16.Lavie P, Shlitner A, Sheffy J, Schnall RP. Peripheral arterial tonometry: a novel and sensitive non-invasive monitor of brief arousals during sleep. Isr Med Assoc J. 2000;2:246–247. [PubMed] [Google Scholar]
- 17.Martin EA, Tan SL, Macbride LR, Lavi S, Lerman LO, Lerman A. Sex differences in vascular and endothelial responses to acute mental stress. Clin Auton Res. 2008;18:339–345. doi: 10.1007/s10286-008-0497-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sheps DS, McMahon RP, Becker L, et al. Mental stress-induced ischemia and all-cause mortality in patients with coronary artery disease: results from the Psychophysiological Investigations of Myocardial Ischemia study. Circulation. 2002;105:1780–1784. doi: 10.1161/01.cir.0000014491.90666.06. [DOI] [PubMed] [Google Scholar]
- 19.Bonetti PO, Pumper GM, Higano ST, Holmes DR, Jr, Kuvin JT, Lerman A. Noninvasive identification of patients with early coronary atherosclerosis by assessment of digital reactive hyperemia. J Am Coll Cardiol. 2004;44:2137–2141. doi: 10.1016/j.jacc.2004.08.062. [DOI] [PubMed] [Google Scholar]
- 20.Martin EA, Prasad A, Rihal CS, Lerman LO, Lerman A. Endothelial function and vascular response to mental stress are impaired in patients with apical ballooning syndrome. J Am Coll Cardiol. 2010;56:1840–1846. doi: 10.1016/j.jacc.2010.03.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Renaud P, Blondin JP. The stress of Stroop performance: physiological and emotional responses to color-word interference, task pacing, and pacing speed. Int J Psychophysiol. 1997;27:87–97. doi: 10.1016/s0167-8760(97)00049-4. [DOI] [PubMed] [Google Scholar]
- 22.Goldberg AD, Becker LC, Bonsall R, et al. Ischemic, hemodynamic, and neurohormonal responses to mental and exercise stress. Experience from the Psychophysiological Investigations of Myocardial Ischemia Study (PIMI) Circulation. 1996;94:2402–2409. doi: 10.1161/01.cir.94.10.2402. [DOI] [PubMed] [Google Scholar]
- 23.Becker LC, Pepine CJ, Bonsall R, et al. Left ventricular, peripheral vascular, and neurohumoral responses to mental stress in normal middle-aged men and women. Reference Group for the Psychophysiological Investigations of Myocardial Ischemia (PIMI) Study. Circulation. 1996;94:2768–2777. doi: 10.1161/01.cir.94.11.2768. [DOI] [PubMed] [Google Scholar]
- 24.Hassan M, Li Q, Brumback B, et al. Comparison of peripheral arterial response to mental stress in men versus women with coronary artery disease. Am J Cardiol. 2008 doi: 10.1016/j.amjcard.2008.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Blumenthal JA, Jiang W, Waugh RA, et al. Mental stress-induced ischemia in the laboratory and ambulatory ischemia during daily life. Association and hemodynamic features. Circulation. 1995;92:2102–2108. doi: 10.1161/01.cir.92.8.2102. [DOI] [PubMed] [Google Scholar]
- 26.Krantz DS, Santiago HT, Kop WJ, Bairey Merz CN, Rozanski A, Gottdiener JS. Prognostic value of mental stress testing in coronary artery disease. Am J Cardiol. 1999;84:1292–1297. doi: 10.1016/s0002-9149(99)00560-3. [DOI] [PubMed] [Google Scholar]
- 27.Rozanski A, Blumenthal JA, Kaplan J. Impact of psychological factors on the pathogenesis of cardiovascular disease and implications for therapy. Circulation. 1999;99:2192–2217. doi: 10.1161/01.cir.99.16.2192. [DOI] [PubMed] [Google Scholar]
- 28.Yeung AC, Vekshtein VI, Krantz DS, et al. The effect of atherosclerosis on the vasomotor response of coronary arteries to mental stress. N Engl J Med. 1991;325:1551–1556. doi: 10.1056/NEJM199111283252205. [DOI] [PubMed] [Google Scholar]