Abstract
Purpose
To assess feasibility, complications, local tumor recurrences, overall survival (OS) and estimates of cost-effectiveness for multi-site cryoablation (MCA) of oligometastatic non-small cell lung cancer (mNSCLC).
Materials and Methods
49 CT and/or US-guided percutaneous MCA procedures were performed on 60 tumors in 31 oligo-mNSCLC patients. Average patient age was 65 years, including 19 females and 12 males. Tumor location was grouped according to common metastatic sites. Median OS was determined using the Kaplan-Meier method and defined life years gained (LYG). Estimates of MCA costs per LYG were compared with established values for systemic therapies.
Results
Total number of tumors and cryoablation procedures for each anatomical site are as follows: 20, 18 – lung; 9, 7 – liver; 12, 11 - superficial; 7, 7 – adrenal; 2, 2 – para-aortic/isolated; and 10, 7 – bone. A mean 1.6 procedures per patient were performed with a median clinical follow-up of 11 months. Major complication and local recurrence rates were 8% (4/49) and 8% (5/60), respectively. Median OS for MCA was 1.33 years with an estimated 1-year survival of ~53%. MCA appeared cost-effective even when added to the cost of BSC or systemic regimens, with an adjunctive cost-effectiveness ratio (ACER) of $49,008 – $87,074.
Conclusions
Multi-site cryoablation had very low morbidity and local tumor recurrence rates for all anatomic sites, and possibly increased OS. Even as an adjunct to systemic therapies, MCA appeared cost-effective for palliation of oligo-mNSCLC.
Introduction
Lung cancer is the leading cause of cancer related deaths, with an estimated 222,520 new diagnoses and 157,300 deaths in the United States in 2010. Non-small cell lung cancer (NSCLC) is the most prevalent form accounting for approximately 85% of all lung cancers (1). Approximately 7% (15,576) of NSCLC patients will initially present with limited or oligometastatic disease (2–3). Survival for NSCLC patients varies considerably depending on stage, with a 5-year survival of 15.8% observed in all cases and a 5-year survival of 3.5% observed in metastatic patients (4).
The primary treatment for advanced ]NSCLC patients is platinum-based chemotherapy, but is mainly for palliation since survival generally only increased to 6 months, versus 4.5 months for best-supportive care (BSC) (5–6). Targeted treatments such as bevacizumab further extended survival up to 14 months but is only safe in non-squamous cell carcinoma patients due to bleeding risks (7–9). Radiation therapy is effective in palliation of NSCLC symptoms but offers limited survival benefits of under 10% at two years for both high and low dose treatment (10). Regardless of the specific treatment, nearly all NSCLC patients who receive systemic therapy will eventually relapse (5, 11).
Minimally invasive ablation techniques such as radiofrequency ablation (RFA) and cryoablation offer unique benefits in the management of symptomatic and/or isolated metastases (12–14). RFA may be more limited in its ability to treat metastases in diverse soft tissue locations, such as near the skin or in close proximity to crucial structures (15). The visible treatment zone of cryoablation, lower pain, and better healing allowed us to apply our established cryoablation techniques (16–21) to many anatomic sites for local control of limited, or oligo-mNSCLC.
The purpose of this study was to assess the potential role of multi-site cryoablation (MCA) of oligo-mNSCLC by evaluating complications, local recurrences, survival, and projected procedure costs in relation to systemic treatments. Estimates of MCA cost-effectiveness we compared to best supportive care (BSC) and emerging chemo/targeted therapy regimens (22–24) in order to place an economic perspective upon our outcomes for this select group of patients.
Methods
Patients
Consecutive patients with limited or oligo-mNSCLC read and signed an authorization form issued under the Health and Insurance Portability and Accountability Act of 1996 (HIPAA). All patients also signed a separate consent form detailing the procedure, as well as an investigational review board approved consent form for prospective collection of procedure, imaging, and clinical parameters. Average patient age was 65 years (range 45–90 years) at time of first procedure. The six procedural locations included lung, liver and 4 soft tissue sites: adrenal, para-aortic/isolated, superficial, and bone (Table 1).
Table 1. Patient, Procedure and Tumor Characteristics.
The 6 procedural locations included lung, liver and 4 soft tissue sites: adrenal, para-aortic/isolated, superficial and bone. Lung tumor locations consisted of metastatic lesions in lung parenchyma and/or chest wall, but did not include mediastinal or hilar adenopathy. Superficial tumor locations consisted of predominantly subcutaneous, muscular and/or lymph node metastases within the extremities or torso wall. Tumors in bone locations were limited metastatic deposits in non-weight bearing locations with the epicenter in osseous structures.
| Location | Lung | Liver | Soft Tissue |
Total* | ||||
|---|---|---|---|---|---|---|---|---|
| Superficial | Adrenal |
Para-aortic or isolated |
Bone | Subtotal* | ||||
| # of Patients | 13 | 6 | 4 | 7 | 2 | 6 | 15 | 31 |
| # of Procedures | 18 | 7 | 11 | 7 | 2 | 7 | 25 | 49 |
| # of Tumors | 20 | 9 | 12 | 7 | 2 | 10 | 31 | 60 |
| Average Tumor Diameter (cm3) | 2.6 | 2.7 | 2.8 | 4.3 | 2.2 | 4.3 | 3.6 | 3.1 |
| Average Ablation Diameter (cm3) | 4.4 | 5.2 | 5.1 | 5.9 | 4.5 | 6.5 | 5.7 | 5.2 |
Totals do not equal the summation because soft tissue is broken down into 4 categories and overlap in the total. Retreatment of a single tumor, as well as a single procedure involving multiple locations also overlaps totals.
Inclusion criteria for cryoablation consisted of a localized mass <7 cm which was either biopsy proven, deemed suspicious from a CT showing an enhancing or growing mass, or found positive via PET scan. Patients should not have more than 5 cancerous foci in an organ site to avoid compromising safety in patients with advanced disease, as well as only conducting MCA if it is believed all metastases present at the time of first procedure can be ablated over the course of one or multiple procedures, and were generally referred by oncologists for local control of oligo-mNSCLC. Tumors in different locations were treated in single or multiple staged cryoablation procedures according to projected feasibility and/or safety. MCA also referred to additional foci developing over time which were targeted for local control in subsequent procedures. All cases were reviewed and performed by a single radiologist with 20 years of interventional and cross-sectional imaging experience (PJL).
The patients included in this study were retrospectively confirmed as mNSCLC through thorough review of their patient charts, pathology reports, imaging findings, and correlation with PET positive lesions. Patients with pulmonary lesions were only deemed positive for metastatic disease if other discrete metastases were already present since a metastatic focus is then much more likely than a second primary tumor. Patients who were proven to have locally recurrent tumors after chemo/radiation therapy, surgical resection, or prior ablation without biopsy-proven metastatic lung cancer were excluded from this study. Also, patients with biopsy proven lesions suspicious for a second primary or a metastatic lesion from an extrathoracic primary were excluded from this study. Patient charts were reviewed by a pulmonary oncologist with greater than 20 years of experience (SG). Patients who received BSC or any chemo-targeted therapy regimen before or after MCA were also noted. For comparison in our cost evaluations, these regimens included erlotinib (tarceva), cisplatin with vinorelbine, cisplatin with gemcitabine, paclitaxel with carboplatin, and bevacizumab with paclitaxel and carboplatin. Table 2 displays the breakdown of administration of these systemic regimens in our patient group.
Table 2. Breakdown of Patients Receiving Systemic Regimens Before/After MCA.
The following percentages of patients were exposed to treatment regimens before, or after MCA, respectively: 10% (3/31): 6% (2/31) erlotinib (tarceva); 0% (0/31): 0% (0/31) cisplatin with vinorelbine; 3% (1/31): 0% (0/31) cisplatin with gemcitabine; 19% (6/31): 3% (1/31) paclitaxel with carboplatin; 6% (2/31): 0% (0/31) bevacizumab with paclitaxel and carboplatin, and 58% (18/31): 26% (8/31) other. Therefore, 84% (26/31) of our patients had some form of chemo-targeted therapy before MCA and 35% (11/31) received some regimen after MCA, with a total of 84% (26/31) of patients having received chemo-targeted therapy at some point. Therefore 16% of patients did not receive any systemic therapy in conjunction with cryoablation.
| ER | CS + VN | CS + GM | PT + CB | PT + CB + BV |
Other | Total | Total (%) | |
|---|---|---|---|---|---|---|---|---|
| Before MCA | 3 | 0 | 1 | 6 | 2 | 18 | 26* | 84% |
| After MCA | 2 | 0 | 0 | 1 | 0 | 8 | 11 | 35% |
| Total | 5 | 0 | 1 | 7 | 2 | 26 | 26** | 84% |
Multiple patients received more than one systemic regimen which results in overlapping data. The actual number of patients receiving chemo-targeted therapy before MCA was 26.
Some patients received chemo-targeted therapy before and after MCA, resulting in overlapping data. Actual number of patients receiving systemic regimens at some point was 26.
Cryoablation Procedure
The primary technique principal for cryoablation procedures was to achieve sufficient probe distribution [e.g., ~1 cryoprobe for each cm tumor diameter] to reach cytotoxic temperatures less than −20°C covering all tumor margins. Probe type (i.e., 1.7 or 2.4 mm outer diameter) and number were recorded for each ablation site. Cryoablation planning techniques/procedural details and associated hydro-dissection protection measures for renal, pulmonary, soft tissue, and breast tumors have been previously described (16–21).
Imaging and Follow-up
Real-time ultrasound (US) (Logiq 700; GE Medical Systems, Milwaukee, WI) was used to place and monitor cryoprobes during procedures solely in superficial locations which consisted of predominantly subcutaneous, muscular and or lymph node metastases within the extremities or torso wall. Computed tomography (CT) was used as the primary imaging modality for planning, procedure guidance and treatment follow-up in the remaining procedural sites. MR imaging was used as needed for improved tissue:tumor discrimination or iodine allergies. Tumors and ablation zones were measured in three dimensions, noted on axial images in their greatest transverse and anteroposterior extent, with sagittal and/or coronal reconstructions used to obtain craniocaudal measurements. In follow-up, enhanced CT or MRI images were obtained at 1, 3, 6, 12, 18 and 24 months and yearly thereafter as available.
Complications
All treatment-related complications were categorized in accordance with the Common Terminology Criteria for Adverse Events Version 3.0 (CTCAE v3) of the National Cancer Institute, similar to prior cryoablation series (17–19). Complications were not linked to cost estimates. A formal decision analysis model was not yet considered appropriate for initial cost-effectiveness estimates.
Recurrences
The therapeutic goal of cryoablation is to achieve complete ablation of a tumor focus with minimal damage to surrounding soft tissues. However tumor recurrence may occur at the site of cryoablation. Local recurrences were separated into procedural and satellite etiology and do not address additional metastatic disease since patients were stage 4 and treated for palliation. A procedure-related recurrence was defined as any recurrence within the ablation zone resulting from an inadequate, sub-lethal isotherm likely along the tumor rim (positive margins). Satellite lesions were located less than 1 cm beyond the ablation zone likely resulting from adjacent microscopic foci of the tumor.
Survival
Overall survival was analyzed using the Kaplan-Meier (K–M) estimator in the Lifetest procedure in SAS 9.2 software (SAS Institute, Inc., Cary, NC). Progression-free survival was deemed not appropriate for evaluation of a local treatment in patients treated only for palliation of symptoms. Thus, additional sites of disease progression were not assessed after ablation. OS was measured from the time of the first MCA procedure until death or until the most recent follow-up for vital status determination. Due to modest sample sizes (or numbers of events), OS statistics (e.g., median, 1-year rate, etc.) were estimated more conservatively using linear interpolation between successive event times on the K–M curve (25). All point estimates of OS statistics were accompanied by a 95% confidence interval (CI). OS for MCA was compared to OS for BSC and five established mNSCLC regimens: erlotinib (tarceva); cisplatin with vinorelbine; cisplatin with gemcitabine; paclitaxel with carboplatin; and paclitaxel with carboplatin and bevacizumab (22–24).
Cost
We explored inflated cost estimates for MCA to gain insight whether the palliative use of MCA had reasonable potential for future more detailed cost-effectiveness analyses. Our cost estimates also contain billing charges, rather than estimates of direct and/or indirect costs (26). These cost estimates served as a potential economic counterbalance to any survival benefit noted for MCA, especially since ablation may be perceived as only adding costs to a palliative disease state.
A total cost of $11,000 per cryoablation procedure represents a high-end estimate from mean professional fees ($2,000), disposable equipment fees ($4,000 for 3 cryoprobes) and hospital fees ($5,000). Mean cost of more frequent follow-up imaging examinations of $42,000 encompassed 6 follow-up CT imaging sessions at $7,000/CT (e.g., 1, 3, 6, 12, 18 and 24 months and yearly thereafter). Each CT session reflected our institution’s 2010 Medicare technical component guidelines of $2,171, $2,396 and $1,390 for chest, abdomen and pelvic CT, respectively, and professional fees of approximately $350/scan. No significant cost difference was assumed for MR based on our 2010 Medicare guideline of $2,171 for each MR exam per anatomic site. The mean number of procedures per patient was used to determine the cost per patient. The overlapping schedule in follow-up imaging after a second ablation did not justify counting follow-up imaging charges more than once.
Additionally, patients in this study may have had chemo-targeted treatments at some point. Costs of MCA were thus also considered in an adjunctive role and added to each therapy comparison, then divided by the overall LYG for MCA in this study. We termed this approach an adjunctive cost-effectiveness ratio (ACER) to more accurately estimate scenarios encountered by our patients. ACERs below $100,000 per LYG were considered cost-effective (27).
Results
Patients, Procedure, and Follow-Up
A total of 31 patients underwent 49 procedures on 60 tumors (Table 1). The mean number of procedures per patient was 1.6 (49/31). The cryoablation zone was well defined by CT as a hypodense ice ball (Figure 1, 2) with an average ablation diameter of 5.2 cm, generated by mean probe number of 3.4 for a mean tumor diameter of 3.1 cm. Of our patients, 84% (26/31) and 35% (11/31) received some form of chemo-targeted therapy before or after MCA, respectively, with a total of 84% (26/31) receiving a systemic regimen at some point. Table 2 shows the breakdown of these regimens in our patient group.
Figure 1.
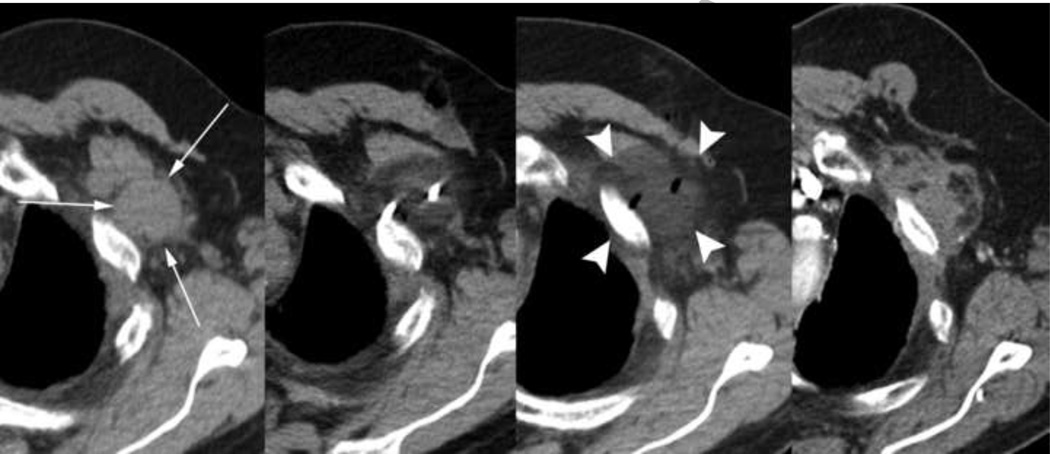
68-year-old male with metastatic adenocarcinoma who received localized radiation treatment to the chest and had discontinued tarceva secondary to diffuse skin eruptions presented with a left chest wall metastatic lesion abutting the 2nd rib near the site of prior thoracotomy for a left lung mass one year prior. Images (from left to right) show the pre-operative, intra-procedural, immediate post-ablation, and two year follow-up enhanced axial CT appearance of the mass. Initial measurements were 5.1 × 3.2 × 4.0 cm which resorbed into a diffusely hypovascular mass measuring 3.8 × 2.2 × 2.0 cm approximately 22 months later. A well-demarcated hypodense ablation zone (arrowheads) is shown in the immediate post-ablational axial CT.
Figure 2.
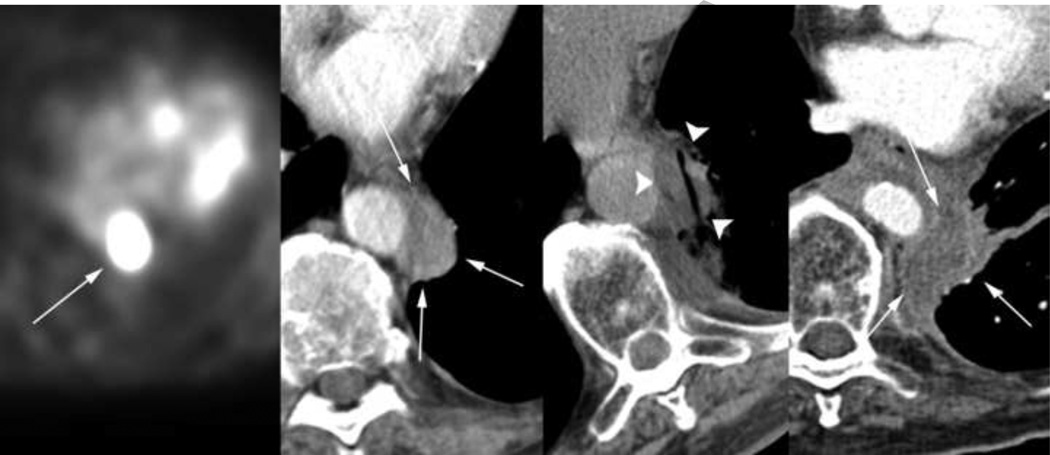
The same 68-year-old patient was found to have an FDG PET-positive left para-aortic node (first image) 4 months after the ablation procedure described in Figure 1. This enhancing lesion measuring 1.8 × 3.5 × 4.2 cm (second image) underwent cryoablation (third image), resulting in a resorbed hypodense non-enhancing soft tissue site on follow-up axial CT (fourth image). As demonstrated in the third image, well-demarcated hypodense margins of the iceball allow safe ablation adjacent to crucial structures such as the aorta.
Complications
Overall, cryoablation procedures on this patient cohort resulted in an 8% (4/49) complication rate of grade 3 or worse. A detailed breakdown of complication grade and location is shown in Table 3. One patient undergoing an ablation of a metastasis on the chest wall (Figure 3) resulted in a grade 4 pericardial tamponade. A pericardiocentesis was performed to remove 300 cc of clear fluid from the pericardium after which blood pressure increased from 130 to 180 and pulse decreased from 130 to 110. Another patient was classified as grade 5 because his death occurred within the one month window after the procedure; however, it was deemed unrelated.
Table 3. Procedure Complications.
Complication rates per procedure broken down into their respective anatomical locations
| Location | # of Procedures |
Grades 1 and 2 |
Grade 3 |
Grade 4 |
Grade 5 |
# of Complications ≥ Grade 3 |
|
|---|---|---|---|---|---|---|---|
| Lung | 18 | 6 | 1 | 1 | 2 | ||
| Liver | 7 | 2 | |||||
| Soft Tissue | Superficial | 11 | 1 | ||||
| Adrenal | 7 | 1 | |||||
| Para-aortic or isolated | 2 | ||||||
| Bone | 7 | 1 | 1 | 2 | |||
| Subtotal | 25* | 2 | |||||
| Total | 49** | 10 | 2 | 1 | 1 | 4 | |
| Total (%) | 20% | 4% | 2% | 2% | 8% | ||
Two patients had a procedure involving two soft tissue locations. Actual procedure count is 25 instead of 27
One patient had one procedure in both soft tissue and lung locations. Actual procedure count is 49 instead of 50.
Figure 3.
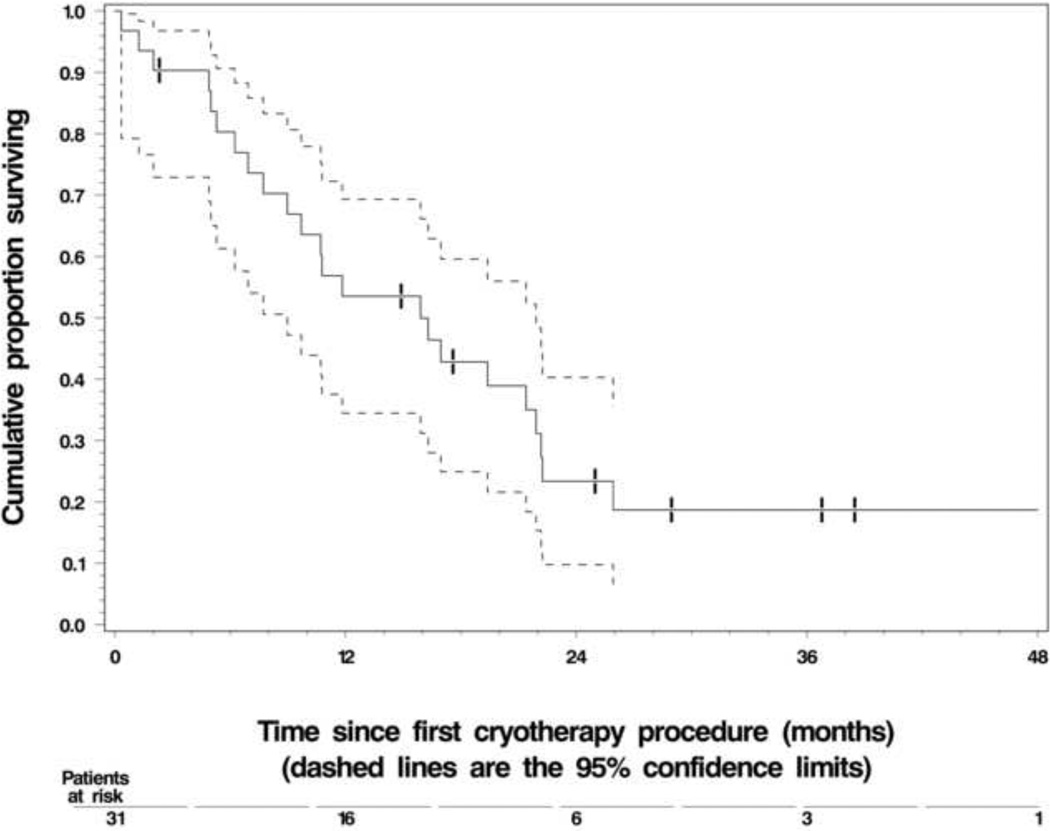
The Kaplan-Meier estimate of overall survival (OS) in the 31 study-eligible patients. The dashed lines represent the 95% confidence interval (CI) about each successive estimate of the survival rate. The median OS was 15.9 months (1.33 years) (95% CI, 8.9 – 21.9 months). The 1-year OS rate was 53% (95% CI, 35 – 71 %).
Recurrences
The mean follow up time for all patients was 11 months (range 0–60 months), and Table 4 shows the anatomic breakdown for local recurrences. Procedures on 60 tumors resulted in a 2% (1/60) procedural recurrence rate and 7% (4/60) satellite recurrence rate, for an overall local recurrence rate of 8% (5/60). Average time to recurrence was 4 months.
Table 4. Local Tumor Recurrence.
Anatomical location of local recurrences which occurred during the 60 procedures in this study. Of the five total recurrences, three (1 lung, 2 liver) satellite recurrences were noted to have occurred within the presence of major vasculature. While it is possible these recurrences may be attributed to adjacent microscopic satellite foci, it is also possible the vessels created a “heat-sink” affect resulting in incomplete ablation of all tumor margins.
| Location | # of Tumors |
Average Tumor Diameter (cm) |
Procedural (Type 1) |
Satellite (Type 2) |
Total Local Recurrences |
Mean Time to Recurrence (months) |
|
|---|---|---|---|---|---|---|---|
| Lung | 20 | 2.6 | 1 | 1 | 2 | 4 | |
| Liver | 9 | 2.7 | 2 | 2 | |||
| Soft Tissue | Superficial | 12 | 2.8 | 1 | 1 | 10 | |
| Adrenal | 7 | 4.3 | |||||
| Para-aortic or isolated | 2 | 2.2 | |||||
| Bone | 10 | 4.3 | 2 | ||||
| Subtotal | 31 | 3.6 | |||||
| Total | 60 | 3.1 | 1 | 4 | 5 | ||
| Total (%) | 1.7% | 6.7% | 8.3% | ||||
Survival
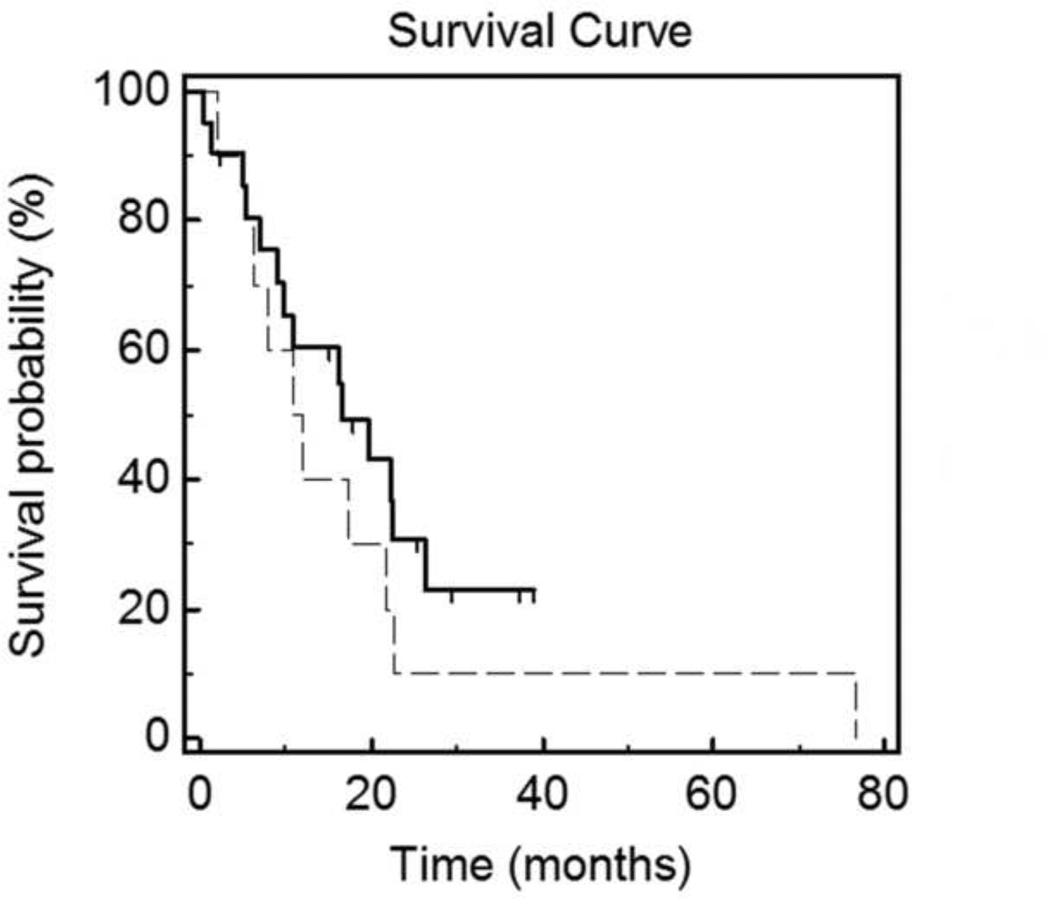
The calculated OS is shown in Figure 3. Of the original 31 patients, 27 have expired. The mean observed OS was 15.9 months or 1.33 years. Projected one year survival rate was ~53% for these patients. Figure 4 displays the estimated OS of patients who received only best supportive care following their first MCA procedure (median survival = 16.5 months) versus patients who received systemic therapy before and after MCA (median survival = 12 months).
Figure 4.
The Kaplan-Meier estimate of overall survival for patients who received chemo-targeted therapy regimens before and after MCA (dashed-line) versus patients who received no systemic therapy following MCA (solid-line). The median OS was 12 months for patients who were administered systemic therapy before/after MCA, and the median OS was 16.5 months for patients who received only best supportive care after MCA.
Cost
In all cases, “upper bound” cost estimates produced total cost of each cryoablation procedure and frequent imaging follow-up of $53,000 ($11,000/procedure plus $42,000 total for imaging follow-up). Multiple metastatic lesions were treated in an average of 1.6 procedures per patient, making the estimated upper cost per patient of $59,600 (i.e., $11,000*1.6 + $42,000).
Table 5 demonstrates our adjunctive cost effectiveness (ACER) evaluations for MCA based on comparisons with established values for five mNSCLC therapies: BSC; erlotinib; cisplatin with vinorelbine; cisplatin with gemcitabine; paclitaxel with carboplatin; and paclitaxel with carboplatin and bevacizumab (22–24). Our MCA estimate of cost per total LYG of $44,812 (i.e., $59,600 / 1.33) appears encouraging for future detailed analysis, especially since the ACER for MCA was cost-effective versus all chemo-targeted therapy protocols, with the average being $60,610 per LYG.
Table 5. Preliminary Cost-Effectiveness Estimates.
Cost-effectiveness estimates for BSC and five established therapies (22–24) for mNSCLC are noted in conjunction with upper-bound estimates of cost for MCA. Our proposed adjunctive cost-effectiveness ratio, or ACER, was used to calculate the estimated cost of MCA when paired with systemic regimens
| BSC | ER | CS + VN | CS + GM | PT + CB | PT + CB + BV |
MCA | |
|---|---|---|---|---|---|---|---|
| LYG | 0.44 | 0.56 | 0.79 | 0.82 | 0.83 | 1.03 | 1.33 |
| Total Cost ($)** | $5,581 | $16,487 | $15,564 | $13,517 | $18,709 | $56,209 | $59,600* |
| $/LYG | $12,684 | $29,441 | $19,701 | $16,484 | $22,541 | $54,572 | $44,812 |
| ACER ($/LYG)*** | $49,008 | $57,208 | $56,514 | $54,975 | $58,879 | $87,074 | AVG: $60,610 |
assumes 1.6 cryoablation procedures per patient and more image intensive follow-up
a conversion factor of 1.67 from pounds to dollars was used to allow easier comparison and conforms to the difference between established definitions of cost efficacy of $100,000 (22, 27).
ACER = adjunctive role for MCA = assumes costs are additive and divided by a total LYG of 1.33 for MCA
MCA = Multi-site cryoablation
LYG = Life years gained
ACER = Adjunctive cost effectiveness ratio
BSC = Best supportive care
ER = Erlotinib (Tarceva)
CS = Cisplatin
VN = Vinorelbine
GM = Gemcitabine
PT = Paclitaxel
CB = Carboplatin
BV = Bevacizumab
Discussion
This study suggests feasibility, safety and potential cost-effectiveness of multi-site cryoablation as an adjunct to the palliative care of oligo-mNSCLC patients. We first summarize our findings and then address specific implications. The estimated 1-year survival observed in our patient population of ~53% and a median survival of 15.9 months suggests extended survival over known systemic options; this is also an increase compared to the observed survival of patients receiving only best supportive care, which typically cannot provide median survivals beyond 6 months. Low rates of local recurrences and complications in our patient group suggest feasibility and safety, and did not appear dependent on tumor location. Overall, 84% of our patients received systemic treatment at some point, with many failing multiple regimens. Of our patients, 65% received no chemo-targeted therapy after MCA, indicating MCA alone is capable of achieving at least local control of oligo-mNSCLC
Although a relatively low percent of cases present with oligo-mNSCLC, these patients still represent nearly 20,000 cases, not including local failures after chemotherapy (28). The ability to provide local control for persistent disease foci is important when addressing treatment options, particularly when metastases can present in multiple locations. A unique aspect of cryoablation over heat-based (i.e., RFA or microwave) ablations is its flexibility for both pulmonary and soft tissue locations, which are commonly observed in mNSCLC; for instance, hepatic, adrenal, bone, and abdominal lymph node metastases are seen in up to 29–40% of mNSCLC patients (29), and more than 58% (18/31) of our patients, or 52% (31/60) of tumor’s were treated for soft tissue metastases. For centrally located pulmonary metastases, cryoprobes can be placed closely to mediastinal and hilar vessels under CT guidance to negate the thermal exchange occurring between vasculature and the ablation zone without fear of damaging vessel or bronchial architecture (16, 30)(Figure 2). This ability to safely counteract heat-sink effects likely led to our observed local recurrence rate of 8%, which compare well to RF and surgery (12–14).
Cryoablation effectively treated even larger tumors that are difficult to manage. In our study, 60% (12/20) of lung lesions, 56% (5/9) liver lesions and 68% (21/31) of soft tissue lesions were ≥ 3cm, or 63% of overall. In comparison to RFA for pulmonary masses, only 23 – 30% of tumors ≥ 3cm were fully ablated (12, 14). The mean survival for patients with incomplete necrosis from RFA was 8.7 months vs. 19.7 for complete (12, 14).
Although chemotherapy is the gold standard in the palliative care of mNSCLC, local tumor control plays a major role for quality of life in long-term treatment. A study on over 700 patients who had received two or more prior chemotherapy regimens for advanced NSCLC determined that survival and local control effectiveness diminished greatly with each subsequent regimen and resulted in a considerable increase in chemical toxicity (31). Furthermore, most patients undergoing second-line therapy will not be treated further due to the increased burden of morbidities and insignificant survival gains (3). In this study we assessed the complication rates of cryoablation procedures in order to elucidate any potential beneficial or harmful aspects of our technique on a patient’s quality of life. In 49 total procedures, 8% (4/49) incurred grade 3 or higher complications requiring surgical intervention, increased hospital stay for observation, or death. The grade 5 death occurred within the one month window after MCA but was considered unrelated to the procedure. Therefore, over 90% of procedures had only transient (i.e., 2–3 days) impact on quality of life after MCA. The potential for reduction of chemo-targeted therapy toxicities for oligo-mNSCLC thus appears much greater than the risks of procedural complications
Cost-effectiveness estimates for this study were validated by a health economist with over 30 years of experience (ACG) (27), and were conducted to evaluate the economic impact of MCA in an adjunctive role by considering the added cost for palliation. We acknowledge that thorough cost-effectiveness estimates should include utility estimates for quality adjusted life years (QALY), as well as sensitivity analyses for both probability and cost assumptions within the framework of a Markov, or Monte Carlo decision model (22). Such in depth analyses are beyond the scope of this study which was focused on the feasibility, safety and OS assessments of MCA for palliation in relation to potential cost-effectiveness.
Weaknesses in this study relate to the relatively small patient population compared to large multicenter drug trials and associated potential selection bias. Our study sample size was limited to oligo-mNSCLC patients in order to compare survival outcomes but precluded sufficient analyses of procedural details for pulmonary and/or soft tissue cryoablation. Detailed assessments of progression-free survival were also beyond the scope of this study for local control. As noted, most of our patients had some form of initial chemotherapy or targeted therapy which likely also improved OS duration. Also, while the definition of oligo-mNSCLC varies across medical literature, such lesions are generally considered less biologically aggressive (29). Patients with oligo-mNSCLC may have survival potential greater than traditional stage IV patients. Therefore, any survival gain in our MCA patients may have been simply achieved by selection rather than any MCA effect. Similarly, detailed assessments of morbidities were not feasible for the 84% of patients who received some form of chemo-targeted therapy. It was interesting to find the median survival in patients was longer in the patient group which only received best-supportive care following MCA, however this may not be an accurate assessment. Given that our study involved a relatively small patient pool, the improved survival in this group may be due to a less extent of disease aggressiveness/severity. Morbidity associated with chemo-targeted regimens may also be a significant factor, however we do not feel our limited data is able to conclusively make such claims, but rather introduce a possible benefit of cryoablation in reducing chemotoxicity. Nevertheless, the future assessment of potential reductions of chemotoxicity by use of MCA, or other ablation modalities, for oligo-mNSCLC appears promising. Further work is needed to convert LYG to QALY for this adjunctive role of MCA, as well as in-depth procedural and peri-procedural true cost assessments.
Our cost analyses were also limited. A more comprehensive “social” cost-effectiveness analysis would require enumeration of additional costs on the patient’s end. These would include travel cost to and from the treatment facility, foregone wages from lost work-days, and incremental costs (if any) incurred by family members in the provision of treatment. Inclusion of these costs would increase the total cost estimates, yet would likely not contribute to our already upper bound cost estimates. However, the social and economic impacts of MCA’s very low complication and tumor recurrence rates were also not considered for this study, but will likely favor conversion of LYG to QALY, especially in relation to chemotoxicities.
In summary, percutaneous cryoablation of oligo-mNSCLC appears well-tolerated with minimal morbidity and low local recurrence rates and may extend OS beyond current systemic treatments alone. Future potential for reduction of chemotoxicities by use of MCA for oligo-mNSCLC appears promising.
Acknowledgements
The authors would like to thank Barbara Adam, NP, for her tireless hours assisting in the clinical aspects of this study, as well as for her assistance in data collection.
This study was partially supported by NIH Cancer Support Grant CA-22453.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Cancer Facts and Figures 2010. Atlanta, GA: American Cancer Society; 2010. [Accessed on July 6, 2011]. Available at: http://www.cancer.org/acs/groups/content/@nho/documents/document/acspc-024113.pdf. [Google Scholar]
- 2.Luketich JD, Martini N, Ginsberg RJ, Rigberg D, Burt ME. Successful treatment of solitary extracranial metastases from non-small cell lung cancer. Ann Thorac Surg. 1995;60:1609–1611. doi: 10.1016/0003-4975(95)00760-1. [DOI] [PubMed] [Google Scholar]
- 3.Albain KS, Crowley JJ, LeBlanc M, Livingston RB. Survival determinants in extensive-stage non-small-cell lung cancer: the Southwest Oncology Group experience. J Clin Oncol. 1991;9:1618–1626. doi: 10.1200/JCO.1991.9.9.1618. [DOI] [PubMed] [Google Scholar]
- 4.Howlader N, Noone AM, Krapcho M, et al. SEER Cancer Statistics Review. Bethesda, MD: National Cancer Institute; 1975–2008. http://seer.cancer.gov/csr/1975_2008/, based on November 2010 SEER data submission, posted to the SEER web site, 2011. [Google Scholar]
- 5.Froudarakis ME, Briasoulis E. Advanced non-small cell lung cancer: on relapse rechallenge the tumor, not the patient. BMC Research Notes. 2010;3:195. doi: 10.1186/1756-0500-3-195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Non-Small Cell Lung Cancer Collaborative Group. Chemotherapy and supportive care versus supportive care alone for advanced non-small cell lung cancer. Cochrane Database of Systematic Reviews. 2010 May 12;5:CD007309. doi: 10.1002/14651858.CD007309.pub2. PubMed PMID: 20464750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Blumenschein GR, Kabbinavar F, Menon H, et al. A phase II, multicenter, open-label randomized study of motesanib or bevacizumab in combination with paclitaxel and carboplatin for advanced nonsquamous non-small-cell lung cancer. Ann Oncol. 2011 Feb 14; doi: 10.1093/annonc/mdq731. PubMed PMID: 21321086. [DOI] [PubMed] [Google Scholar]
- 8.Cagle PT, Allen TC, Dacic S, et al. Revolution in lung cancer; New challenges for the surgical pathologist. Arch Pathol Lab Med. 2011;135:110–116. doi: 10.5858/2010-0567-RA.1. [DOI] [PubMed] [Google Scholar]
- 9.Hang XF, Xu WS, Wang JX, et al. Risk of high-grade bleeding in patients with cancer treated with bevacizumab: a meta-analysis of randomized control trials. Eur J Clin Pharmacol. 2011;67:613–623. doi: 10.1007/s00228-010-0988-x. [DOI] [PubMed] [Google Scholar]
- 10.Fairchild A, Harris K, Barnes E, et al. Palliative thoracic radiotherapy for lung cancer: A systematic review. J Clin Oncol. 2008;26:4001–4011. doi: 10.1200/JCO.2007.15.3312. [DOI] [PubMed] [Google Scholar]
- 11.Gridelli C, Ardizzoni A, Ciardiello F, et al. Second-line treatment of advanced non-small cell lung cancer. J Thorac Oncol. 2008;3:430–440. doi: 10.1097/JTO.0b013e318168c815. [DOI] [PubMed] [Google Scholar]
- 12.Thanos L, Mylona S, Ptohis N, et al. Percutaneous radiofrequency thermal ablation in the management of lung tumors: presentation of clinical experience on a series of 35 patients. Diagn Interv Radiol. 2009;15:290–296. doi: 10.4261/1305-3825.DIR.1828-08.2. [DOI] [PubMed] [Google Scholar]
- 13.Yan TD, King J, Sjarif A, Glenn D, et al. Treatment failure after percutaneous radiofrequency ablation for nonsurgical candidates with pulmonary metastases from colorectal carcinoma. Ann Surg Oncol. 2007;14:1718–1726. doi: 10.1245/s10434-006-9271-x. [DOI] [PubMed] [Google Scholar]
- 14.Lee JM, Jin GY, Goldberg SN, et al. Percutaneous radiofrequency ablation for inoperable non-small cell lung cancer and metastases: Preliminary report. Radiology. 2004;230:125–134. doi: 10.1148/radiol.2301020934. [DOI] [PubMed] [Google Scholar]
- 15.Arellano RS, Flanders VL, Lee SI, Mueller PR, Gervais DA. Imaging-guided percutaneous radiofrequency ablation of retroperitoneal metastatic disease in patients with gynecologic malignancies: clinical experience with eight patients. AJR Am J Roentgenol. 2010;194:1635–1638. doi: 10.2214/AJR.09.3561. [DOI] [PubMed] [Google Scholar]
- 16.Wang H, Littrup PJ, Duan Y, Zhang Y, Feng H, Nie Z. Thoracic masses treated with percutaneous cryotherapy: initial experience with more than 200 procedures. Radiology. 2005;235:289–298. doi: 10.1148/radiol.2351030747. [DOI] [PubMed] [Google Scholar]
- 17.Littrup PJ, Ahmed A, Aoun HD, et al. CT-guided percutaneous cryotherapy of renal masses. J Vasc Interv Radiol. 2007;18:383–392. doi: 10.1016/j.jvir.2006.12.007. [DOI] [PubMed] [Google Scholar]
- 18.Solomon LA, Munkarah AR, Vorugu VR, et al. Image-guided percutaneous cryotherapy for the management of gynecologic cancer metastases. Gynecol Oncol. 2008;111:202–207. doi: 10.1016/j.ygyno.2008.08.003. [DOI] [PubMed] [Google Scholar]
- 19.Littrup PJ, Jallad B, Chandiwala-Mody P, D’Agostini M, Adam BA, Bouwman D. Cryotherapy for breast cancer: a feasibility study without excision. J Vasc Interv Radiol. 2009;20:1329–1341. doi: 10.1016/j.jvir.2009.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Littrup PJ, Jallad B, Vorugu V, et al. Lethal isotherms of cryoablation in a phantom study: effects of heat load, probe size, and number. J Vasc Interv Radiol. 2009;20:1343–1351. doi: 10.1016/j.jvir.2009.05.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kam AW, Littrup PJ, Walther MM, Hvizda J, Wood BJ. Thermal protection during percutaneous thermal ablation of renal cell carcimona. J Vasc Interv Radiol. 2004;15:753–758. doi: 10.1097/01.rvi.0000133535.16753.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Clegg A, Scott DA, Sidhu M, Hewitson P, Waugh N. A rapid and systematic review of the clinical effectiveness and cost-effectiveness of paclitaxel, docetaxel, gemcitabine and vinorelbine in non-small-cell lung cancer. Health Technology Assessment. 2001;5:1–195. doi: 10.3310/hta5320. [DOI] [PubMed] [Google Scholar]
- 23.Bradbury PA, Dongsheng T, Seymour L, et al. Economic analysis: Randomized placebo-controlled clinical trial of erlotinib in advanced non-small cell lung cancer. J Natl Cancer Inst. 2010;102:298–306. doi: 10.1093/jnci/djp518. [DOI] [PubMed] [Google Scholar]
- 24.Novello S, Kielhorn A, Stynes G, et al. Cost-minimisation analysis comparing gemcitabine/cisplatin, paclitaxel/carboplatin, and vinorelbine/cisplatin in the treatment of advanced non-small cell lung cancer in Italy. Lung Cancer. 2005;48:397–387. doi: 10.1016/j.lungcan.2004.11.014. [DOI] [PubMed] [Google Scholar]
- 25.Lee E, Wang JW. Statistical Methods for Survival Data Analysis. 3 Ed. Hoboken, NJ: Wiley & Sons, Inc.; 2003. pp. 76–91. [Google Scholar]
- 26.Porter GA, Cantor SB, Walsh GL, et al. Cost-effectiveness of pulmonary resection and systemic chemotherapy in the management of metastatic soft tissue sarcoma: a combined analysis from the University of Texas M. D. Anderson and Memorial Sloan-Kettering Cancer Centers. J Thorac Cardiovasc Surg. 2004;127:1366–1372. doi: 10.1016/j.jtcvs.2003.11.016. [DOI] [PubMed] [Google Scholar]
- 27.Folland S, Goodman AC, Stano M. Cost benefit analysis and other tools of economic evaluation.in, The Economics of Health and Health Care. 6th ed. Upper Saddle River: Prentice Hall; 2010. pp. 74–96. [Google Scholar]
- 28.Pfannschmidt J, Dienemann H. Surgical treatment of oligometastatic non-small cell lung cancer. Lung Cancer. 2010;69:251–258. doi: 10.1016/j.lungcan.2010.05.003. [DOI] [PubMed] [Google Scholar]
- 29.Quint LE, Tummala S, Brisson LJ, et al. Distribution of Distant Metastases from Newly Diagnosed Non-Small Cell Lung Cancer. The Society of Thoracic Surgeons. 1996;62:246–250. doi: 10.1016/0003-4975(96)00220-2. [DOI] [PubMed] [Google Scholar]
- 30.Tath S, Acar M, Tuncah K, Morrison PL, Silverman S. Percutaneous cryoablation techniques and clinical applications. Diagn Interv Radiol. 2010;16:90–95. doi: 10.4261/1305-3825.DIR.1922-08.0. [DOI] [PubMed] [Google Scholar]
- 31.Massarelli E, Andre F, Liu DD, et al. A retrospective analysis of the outcome of patients who have received two prior chemotherapy regimens including platinum and docetaxel for recurrent non-small-cell lung cancer. Lung Cancer. 2003;39:55–61. doi: 10.1016/s0169-5002(02)00308-2. [DOI] [PubMed] [Google Scholar]