Abstract
Importance
Although leukocyte telomere length is associated with mortality and many chronic diseases thought to be manifestations of age-related functional decline, it is not known whether it relates to acute disease in younger healthy populations.
Objective
To determine whether shorter telomeres in leukocytes, especially CD8CD28− T cells, are associated with decreased resistance to upper respiratory infection and clinical illness in young to midlife adults.
Design, Setting, and Participants
Between 2008 and 2011, telomere length was assessed in peripheral blood mononuclear cells (PBMCs) and T-cell subsets (CD4, CD8CD28+, CD8CD28−) from 152 healthy 18- to 55-year-old residents of Pittsburgh, Pennsylvania. Participants were subsequently quarantined (single rooms), administered nasal drops containing a common cold virus (rhinovirus 39), and monitored for 5 days for development of infection and clinical illness.
Main Outcome Measures
Infection (virus shedding or 4-fold increase in virus-specific antibody titer) and clinical illness (verified infection plus objective signs of illness).
Results
Rates of infections and clinical illness were 69% (n = 105) and 22% (n = 33), respectively. Shorter telomeres were associated with greater odds of infection, independent of prechallenge virus-specific antibody, demographics, contraceptive use, season, and body mass index (PBMC odds ratio [OR] per 1-SD decrease in telomere length, 1.71 [95% CI, 1.08–2.72]; n = 128 [shortest tertile 77% infected; middle, 66%; longest, 57%]; CD4: OR, 1.76 [95% CI, 1.15–2.70]; n = 146 [shortest tertile 80% infected; middle, 71%; longest, 54%]; CD8CD28+: OR, 1.93 [95% CI, 1.21–3.09], n = 132 [shortest tertile 84% infected; middle, 64%; longest, 58%]; CD8CD28-: OR, 2.02 [95% CI, 1.29–3.16]; n = 144 [shortest tertile 77% infected; middle, 75%; longest, 50%]). CD8CD28− was the only cell population in which shorter telomeres were associated with greater risk of clinical illness (OR, 1.69 [95% CI, 1.01–2.84]; n = 144 [shortest tertile, 26%; middle, 22%; longest, 13%]). The association between CD8CD28− telomere length and infection increased with age (CD8CD28− telomere length-X-age interaction, b = 0.09 [95% CI, 0.02–0.16], P = .01, n = 144).
Conclusion and Relevance
In this preliminary study among a cohort of healthy 18- to 55-year-olds, shorter CD8CD28− T-cell telomere length was associated with increased risk for experimentally induced acute upper respiratory infection and clinical illness.
Telomeres, the DNA-protein complexes at the end regions of chromosomes, decrease in length with every cell division.1 In primary blood cells, telomeres are partly reconstructed by the activity of telomerase, a specialized intracellular enzyme that adds subunit repeats to telomeres.2 Despite the activity of telomerase, telomeres continue to shorten with repeated cell divisions, leading to disrupted cell function and eventual cell senescence.3,4
Telomere shortening in leukocytes has implications for immunocompetence1–4 and is associated with increased synthesis of proinflammatory cytokines and poorer antibody response to vaccines.5–7 Shorter leukocyte telomere length also is associated with aging-related morbidity and mortality from conditions with immune system involvement, including infectious diseases,8,9 cancer,10 and cardiovascular disease.11
The rate of progression to senescence differs among lymphocyte subsets, with an advanced rate of telomere shortening in the cytolytic CD8 T cells.12 This is especially important for cancer and virally induced infectious diseases, because rapid loss of telomere length in cytolytic CD8 T cells causes cell senescence marked by loss of expression of CD28,13 a costimulatory molecule important for antiviral function.
In this study we assessed whether telomere length in leukocytes is associated with host resistance to experimentally induced viral upper respiratory infection in young to midlife adults. Our expectation was that shorter leukocyte telomere length, especially in CD8CD28− cells, would be associated with an increased risk for infection and clinical illness.
METHODS
Participants
Participants were 152 healthy residents of the greater Pittsburgh, Pennsylvania area aged 18 through 55 years and recruited by newspaper advertisements to participate in a study of the causes of the common cold. Each received $1000 for participating in the study. The study received approval from the Carnegie Mellon University and University of Pittsburgh human participants review boards, and all participants provided signed informed consent.
Design
Healthy participants who had their blood drawn for telomere assessment were subsequently quarantined, administered nasal drops containing a rhinovirus that causes the common cold (rhinovirus type 39 [RV39]), and monitored for 5 days for development of infection and clinical illness.
Data were collected between 2008 and 2011. Volunteers were screened 6 to 8 weeks before viral administration and enrolled only if they reported no acute or chronic illnesses; were in good health as assessed by a complete physical examination that included examination of the ear, nose, and throat, complete blood and urine panels, and human immunodeficiency virus testing; did not take prescription medications, with the exception of birth control; and had specific neutralizing serum antibody titers to the experimental rhinovirus of 4 or less. Participants were later excluded from the study if they reported to quarantine with symptoms or signs of illness, had rhinovirus isolated in their nasal lavage fluid on that day, tested positive for pregnancy on that day, or had a nonchallenge strain of rhinovirus isolated during cloister.
During the period before viral challenge, data on 7 control variables (covariates) were collected to exclude potential alternative explanations for associations between telomere length and infections or colds: prechallenge viral-specific antibody titer (day before challenge), body mass index (BMI) (calculated as weight in kilograms divided by height in meters squared), sex/birth control use (men, women taking contraceptives, women not taking contraceptives), season of exposure (spring, summer, winter), number of days before viral challenge that blood for telomere length assay was drawn, and self-reported age (years) and race (selected from white, black, Native American, Asian/Pacific Islander, Hispanic/Latino, or other). Because of small numbers of participants endorsing nonblack minority racial categories, race was recoded into a dichotomous variable (white = 0, other = 1) for analyses.
Blood for assessment of telomere length was collected, processed by cell separation, and stored for later assay. Participants were then quarantined in a local hotel for 6 days. After the first 24 hours of quarantine (quarantine day 0), participants received nasal drops containing 100 tissue culture infectious doses of RV39. Daily throughout quarantine, nasal lavage samples were collected for isolation of the challenge virus and participants were evaluated for objective signs of illness. Following approximately 28 days after exposure to the virus, blood samples were taken for assay of convalescent serum-specific antibody titer to the challenge virus. Investigators conducting the telomere length assays, which were performed in batch after all trials were concluded, were blinded to all participant data.
Procedures of the Viral Challenge Trial
Infection
Infection was defined as isolation of the challenge virus in nasal secretions (cultured using standard procedures14) on any of the postexposure quarantine days or as a 4-fold or greater increase in specific antibody titer to the challenge virus (microtiter neutralization assay15) from before to 28 days after exposure.
Clinical Illness
Individuals were considered to have a cold on meeting the criteria for both infection and objective signs of illness. Objective signs of illness were assessed using 2 daily measures: nasal mucociliary clearance time and mucus weight. Nasal mucociliary clearance time—an indicator of nasal congestion--was measured as the time (seconds) needed for a flavored dye administered to the inferior turbinates to arrive at the nasopharynx.16 Mucus weight (grams)—a marker of nasal secretion production--was measured by weighing sealed plastic bags containing the participants’ used paper tissues and then subtracting the weights of unused tissues and empty plastic bags.16 These measures were calculated on a daily basis with all daily measures adjusted for baseline by subtracting baseline values (mode values, 0 seconds and 0 g) from the daily values. Negative adjusted values were given a score of 0. The criterion for illness was a total (sum of 5 days) adjusted weight of mucus of 10 g or greater or an average adjusted time of nasal mucociliary clearance of 420 seconds (7 minutes) or longer.17
Telomere Length
Because we could not conduct timely cell separations and preservation for the entire sample in a single day, whole blood for telomere length assay was collected from a random one-third of participants on each of 3 days: during the baseline screening visit (6–8 weeks prequarantine), 3 to 5 days prequarantine, or on the baseline day of quarantine. Blood samples were collected by standard venipuncture into 3 green-top (heparinized) collection tubes. Lymphocyte subsets from each blood sample were labeled with fluorochrome conjugated mouse anti-human monoclonal antibodies (BD Bioscience Pharmingen) in RosetteSep cocktails, isolated immediately using an automated immunomagnetic cell separator (RoboSep, StemCell Technologies), and then stored at −80°C.
Total DNA was purified from each cell subset, including peripheral blood mononuclear cells (PBMCs) and CD4, CD8CD28+, and CD8CD28− cells (DNeasy Blood & Tissue Kit, Qiagen). Duplicate DNA samples (20 ng) from each cell subpopulation were amplified using primers of telomere (forward, 5’-CGGTTT[GTTTGG]5GTT-3’; reverse, 5’-GGCTTG[CCTTAC]5CCT-3’) and a single copy gene (36B4; forward, 5’-CAGCAAGTGGGAAGGTGTAATCC-3’; reverse, 5’-CCCATTCTATCATCAACGGGTACAA-3’), separately, on the same plate in 1 x SYBR Green master mix (Applied Biosystems) using a real-time quantitative polymerase chain reaction assay (qRT-PCR) (7300 Fast Real Time PCR system, Applied Biosystems) as per a published protocol.18 Applied Biosystems SDS software was used to generate standard curves and to determine the dilution factors of standards corresponding to the telomere (T) and single-copy gene (S) amounts in each sample. From these data, a T:S ratio was computed, providing an index of average telomere length. Coefficients of variation were 12% and 13% for T and S, respectively. In a subsample of 26 participants, qRT-PCR-derived T:S ratio was correlated (r = .64, P < .001 with telomere length as determined by terminal restriction fragment Southern blot analysis.
Although the blood volumes used for telomere assays were the same across cell types, samples differed randomly in cell number and thus in DNA concentration. This, in combination with random cell loss during separation and DNA purification, resulted in some samples containing insufficient DNA for telomere assay.
Statistical Analyses
Pearson correlations (r and P values) were used to assess associations between continuous variables, analysis of variance (F and P values) to test differences in telomere length among categorical control variables, and paired (within person) t-tests (t and P values) to compare telomere length differences between cell types. We used multiple logistic regression19 to predict the binary outcomes, incidence of infection, and clinical illness. All regression equations included the 7 control variables (covariates) described in the design section. Telomere length (T:S ratio) was normally distributed in all cell populations and for analysis was standardized (Z score) and treated as a continuous variable. Shorter telomere length could be related to risk for clinical colds through an association with either increased risk of infection among all participants, increased risk of developing clinical illness among participants who became infected, or both. We tested the second hypothesis by conducting a multiple logistic regression (including all standard covariates) with clinical illness as the outcome and using data only from the subset of participants who became infected with the challenge virus. Interaction terms were entered in separate analyses including the covariates and relevant main effects.
For all main effects of the logistic regressions we report odds ratios (ORs) and 95% CIs. To facilitate interpretation, we present inverse ORs, which indicate the change in the odds of developing an infection or cold with each 1-SD decrease in standardized telomere length. Interactions are reported as unstandardized logistic regression coefficients (b), with 95% CIs and P levels. Likelihood ratio tests are used to compare models. As a result of the high prevalence of infection and colds, the ORs presented herein should not be interpreted as risk ratios. Consequently, we also transformed ORs to relative risks for significant associations20 using the longest quartile of telomere length as the unexposed value.21
Power for this study was estimated at 80% based on an expected OR of 2.0 for each 1-SD decrease in telomere length, 2-tailed tests with P < .05, and an N of 125 or greater. All statistics were performed using IBM SPSS Statistics version 20 (SPSS Inc.). All tests were 2-tailed.
RESULTS
Sample Characteristics
Table 1 presents descriptive information on sample demographic characteristics, other covariates, and telomere length.
Table 1.
Participant Characteristics (N = 152)
| Characteristic | No. (%)a |
|---|---|
| Age, mean (SD), y | 29.92 (10.71) |
| Women | 63 (41) |
| Race/ethnicityb | |
| White | 109 (72) |
| Black | 34 (22) |
| Other | 9 (6) |
| Education | |
| High school or less | 37 (24) |
| <2 y college, no degree | 43 (28) |
| 2 y college + associate’s degree | 31 (20) |
| Bachelor’s degree or higher | 41 (27) |
| Body mass index, mean (SD)c | 26.44 (5.68) |
| Days from blood draw to viral challenge, median (IQR) | 4 (3–50) |
| Prechallenge viral-specific antibody titer < 4 | 123 (81) |
| Season of exposure | |
| Winter | 39 (26) |
| Spring | 50 (33) |
| Summer | 63 (41) |
| Lymphocyte-relative T:S ratio, mean (SD), raw data | |
| PBMC (n = 128) | 0.81 (0.22) |
| CD4 (n = 146) | 0.53 (0.24) |
| CD8CD28+ (n = 132) | 0.55 (0.20) |
| CD8CD28− (n = 144) | 0.58 (0.27) |
Abbreviations: IQR, interquartile range; PBMC, peripheral blood mononuclear cell; T:S, ratio of telomere to single-copy gene amounts.
Except where otherwise noted. Ns differ between cell types owing to insufficient DNA content in some blood samples. Analyses involving each cell population were conducted with data only from those participants with complete telomere length data for that cell type.
”White” category includes white Hispanic. “Other” category includes Asian/Pacific Islander (3%); Native American (<1%); mixed race (3%).
Calculated as weight in kilograms divided by height in meters squared.
Correlations Between Telomere Lengths in Different Cell Populations
Table 2 presents telomere length correlations among the different cell types. Mean PBMC telomere length was longer than telomere length in all 3 T-cell subsets (PBMC telomere length vs CD4 telomere length, t124 = 12.57, P = .001; PBMC telomere length vs CD8CD28+ telomere length, t110 = 11.67, P = .001; PBMC telomere length vs CD8CD28− telomere length, t122 = 9.51, P = .001).
Table 2.
Intercorrelations Among Telomere Lengths in the 4 Leukocyte Populationsa
| PBMC T:S ratio | CD4 T:S ratio | CD8CD28+ T:S ratio | |
|---|---|---|---|
| CD4 T:S ratio | |||
| Pearson r | 0.46 | NA | |
| P value | .001 | ||
| No.b | 125 | ||
| CD8CD28+ T:S ratio | |||
| Pearson r | 0.39 | 0.36 | NA |
| P value | .001 | .001 | |
| No.b | 111 | 127 | |
| CD8CD28− T:S ratio | |||
| Pearson r | 0.50 | 0.37 | 0.42 |
| P value | .001 | .001 | .001 |
| No.b | 123 | 138 | 131 |
Abbreviations: NA, not applicable; PBMC, peripheral blood mononuclear cell.
Ratio of telomere to single-copy gene amounts (T:S ratio) used as an index of average telomere length.
Ns differ between cell types owing to insufficient DNA content in some blood samples.
Associations of Control Variables With Telomere Length, Infection, and Colds
Older age was correlated with shorter telomere length in PBMCs (n = 128, r = −0.17, P = .05), CD8CD28+ cells (n = 132, r = −0.21, P = .02), and CD8CD28− cells (n = 144, r = −0.19, P = .02), and higher BMI was correlated with shorter telomere length in PBMCs (n = 128, r = −0.19, P = .03) and CD8CD28− cells (n = 144, r = −0.16, P = .05). PBMC telomere length also differed by season (F2,125 = 3.55, P = .03, n = 128), with participants monitored during the winter having shorter telomeres than those monitored during the summer. No other relations of telomere length with covariates were significant. In separate logistic regressions with the 7 control variables as predictors, only antibody level less than 4 (OR, 2.55 [95% CI, 1.11–5.84]; n = 152) was associated with infection, and only older age (OR, 1.05 [95% CI, 1.01–1.08]; n = 152) was associated with colds.
Telomere Length and Infection
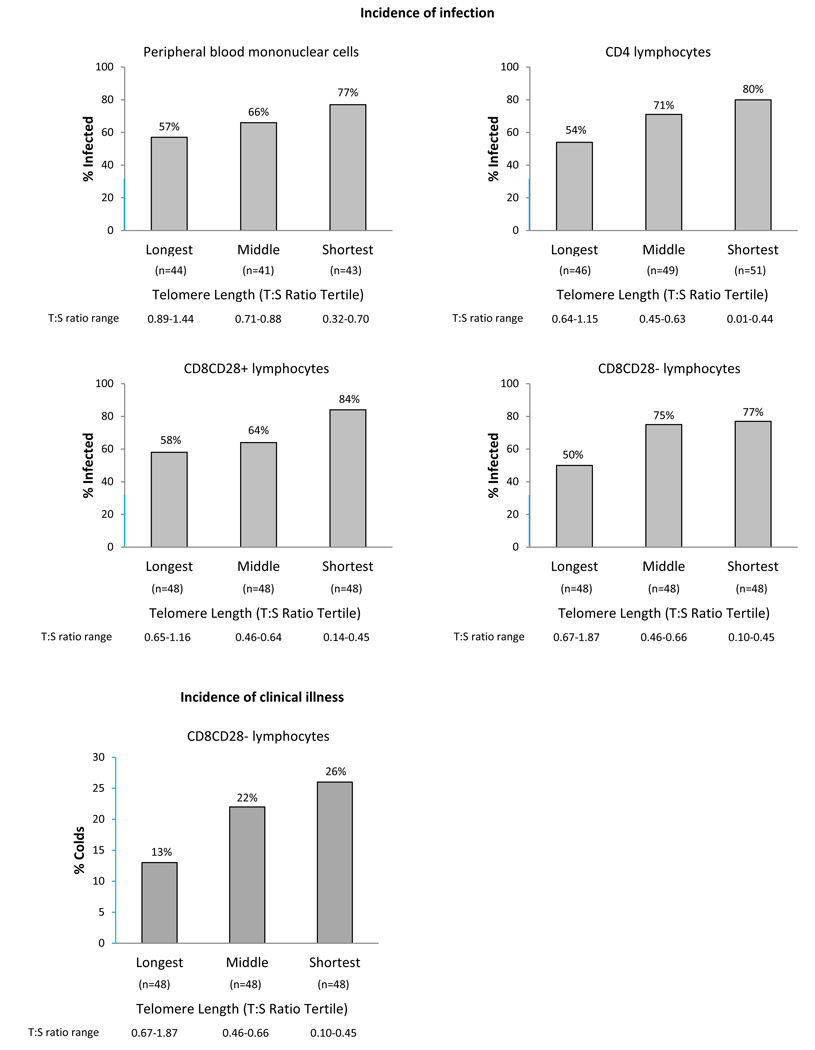
Sixty-nine percent of participants (n = 105) were infected. Results of the separate multiple logistic regressions for each cell population are summarized in Table 3. To facilitate the interpretation of these relations, Figure 1 presents infection rates by tertile of telomere length. In all 4 cell populations, the shorter the telomere length, the greater the risk of infection.
Table 3.
Multivariable Associations of Lymphocyte Telomere Length With Viral Upper Respiratory Infection and Clinical Illness (Colds)a
| PBMC T:S Ratiob | CD4 T:S Ratiob | CD8CD28+ T:S Ratiob | CD8CD28− T:S Ratiob | ||
|---|---|---|---|---|---|
| (n = 128) | (n = 146) | (n = 132) | (n = 144) | ||
| Infection | OR (95% CI) | 1.71 (1.08 – 2.72) | 1.76 (1.15 – 2.70) | 1.93 (1.21 – 3.09) | 2.02 (1.29 – 3.16) |
| RR (95% CI) | 1.22 (1.03 – 1.38) | 1.27 (1.07 – 1.44) | 1.26 (1.08 – 1.40) | 1.38 (1.14 – 1.59) | |
| R2c | 0.26 | 0.19 | 0.23 | 0.23 | |
| ΔR2 | 0.05 | 0.06 | 0.08 | 0.09 | |
| LRTd | X2=5.35, df=1, P=.02 | X2=7.24, df=1, P=.007 | X2=8.47, df=1, P=.004 | X2=11.07, df=1, P=.001 | |
|
Clinical Illness |
OR (95% CI) | 1.38 (0.85 – 2.23) | 1.14 (0.74 – 1.76) | 1.36 (0.83 – 2.23) | 1.69 (1.01 – 2.84) |
| RR (95% CI) | 1.26 (0.88 – 1.71) | 1.11 (0.78 – 1.55) | 1.29 (0.85 – 1.88) | 1.57 (1.01 – 2.35) | |
| R2c | 0.19 | 0.16 | 0.20 | 0.21 | |
| ΔR2c | 0.02 | 0 | 0.02 | 0.04 | |
| LRTd | X2=1.77, df=1, P=.18 | X2=0.35, df=1, P=.55 | X2=1.47, df=1, P=.23 | X2=4.37, df=1, P=.04 |
Abbreviations: OR, odds ratio; PBMC, peripheral blood mononuclear cell; RR, relative risk; T:S, ratio of telomere to single-copy gene amounts; LRT, likelihood ratio test.
Ratio of telomere to single-copy gene amounts (T:S ratio) used as an index of average telomere length. All logistic regression models were adjusted for prechallenge viral-specific antibody titer, age, body mass index, race, sex/contraceptive use (men, women taking contraceptives, women not taking contraceptives), season of exposure, and days between blood draw for telomere assay and viral challenge. Each association was generated from an equation including telomere length in only the 1 cell type. ORs are standardized to indicate change in odds of infection or clinical illness with each 1-SD decrease in telomere length. ORs were converted to RRs using the formula of Zhang & Yu20 and approximating the incidence of the outcome in the unexposed group to be equivalent to the incidence among the longest quartile of telomere length for each relevant cell type.
Ns differ between cell types owing to insufficient DNA content in some blood samples. Analyses involving each cell population were conducted with data only from those participants with complete telomere length data for that cell type.
R2 = Nagelkerke pseudo-R2 for fully adjusted model (ie, telomere length + covariates); ΔR2 = absolute change in Nagelkerke pseudo-R2 after addition of telomere length to model including covariates only.
Likelihood ratio test comparing the fully adjusted model including all covariates and telomere length with the model including covariates only.
Figure 1.
Incidence of infection and colds by tertile of leukocyte telomere length. Ratio of telomere to single-copy gene amounts (T:S ratio) used as an index of average telomere length. Total Ns differ between cell types owing to insufficient DNA content in some blood samples. Data for each cell population are presented based on data from only those participants with complete telomere length data for that cell type. Y-axis segments in blue indicate range y = 0 to y = 30.
In analyses of infection rates limited to the 126 participants with telomere length data for all 3 T-cell subsets, associations were equivalent to those found with the maximum sample sizes (CD4: OR, 1.80 [95% CI, 1.14–2.86]; CD8CD28+: OR, 1.88 [95% CI, 1.17–3.00]; CD8CD28−: OR, 2.07 [95% CI, 1.26–3.40]). When telomere length from all 3 subsets were simultaneously examined in the same regression (PBMC telomere length was not included in this analysis because that mixed population includes the other 3 cell types), we found that CD8CD28− telomere length was the only one of the 3 measures to enter the equation. That is, CD8CD28− telomere length had the strongest association with infection, and once it was included in the model, telomere length in the remaining cell types did not improve the prediction.
Telomere Length and Clinical Illness (Colds)
Twenty-two percent of the entire sample (n = 33) developed a clinical illness (common cold). As shown in Table 3 and Figure 1, only telomere length in the CD8CD28− subset was associated with risk for clinical illness, with shorter telomeres being associated with greater risk.
Pathways Linking CD8CD28− Telomere Length to Clinical Illness
In the analysis testing whether telomere length is associated with the development of signs of illness among infected individuals, limited to the participants who were infected (n = 97), there was no association between telomere length and colds (OR, 1.11 [95% CI, 0.59–2.07]).
Telomere Length, Infection, and Clinical IIlness, by Age
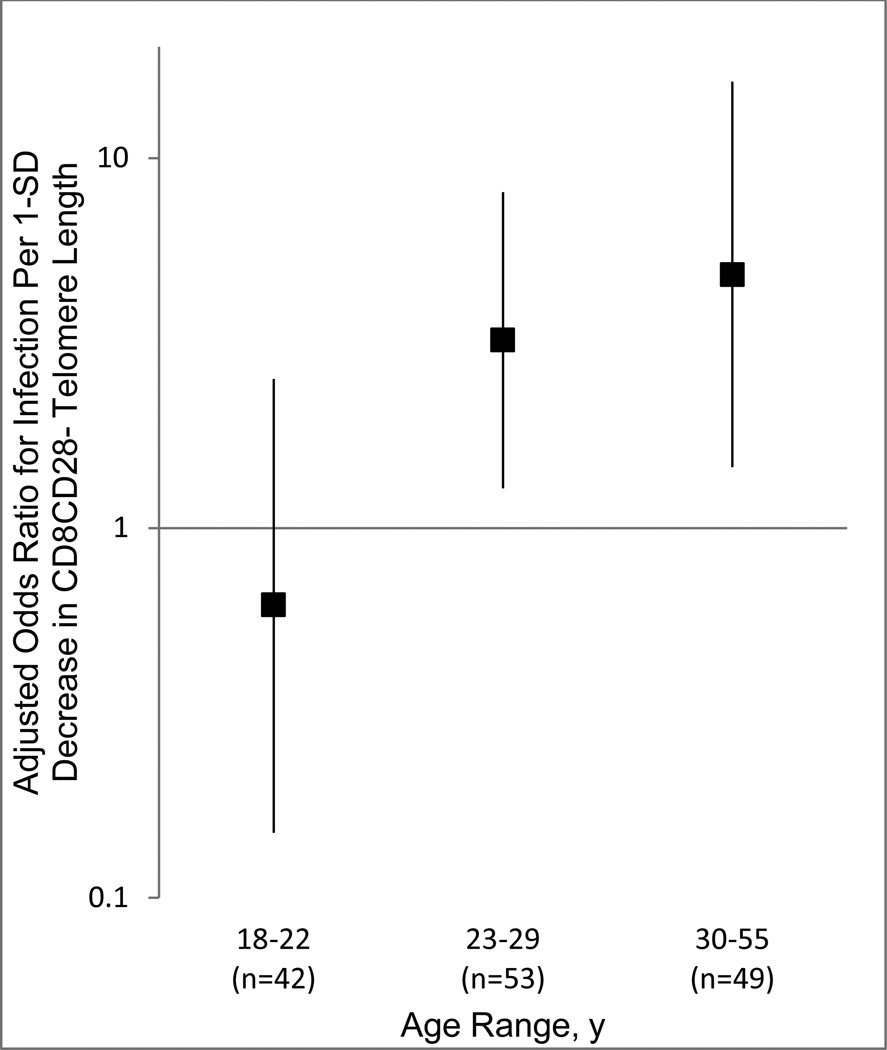
We tested whether the magnitude of the association of CD8CD28− telomere length with infection and clinical illness varied across age. The magnitude of the association between telomere length and infection increased with increasing age (interaction, b = 0.09 [95% CI, 0.02–0.16], P = .01, n = 144; X2 = 9.12 by likelihood ratio test, P = .003). To facilitate the interpretation of the interaction, ORs for separate analyses of each age tertile are presented in Figure 2. For colds, the form of the interaction was similar to that of infection but did not reach statistical significance, and addition of the interaction term did not significantly improve the model fit (b = 0.05 [95% CI, −0.001–0.10], P = .09; X2 = 3.16 by likelihood ratio test, P = .08).
Figure 2.
Association of CD8CD28− Telomere Length and Infection With Increasing Age. Ratio of telomere to single-copy gene amounts (T:S ratio) used as an index of average telomere length. Age is presented in tertiles in the figure to facilitate interpretation. Error bars indicate 95% CIs. Odds ratios (ORs) can be interpreted as the increase in the odds of infection with each 1-SD decrease in telomere length. Each age group was analyzed in a separate logistic regression analysis that included the following control variables: prechallenge viral-specific antibody titer, body mass index, age, race, sex/contraceptive use (men, women taking contraceptives, women not taking contraceptives), season of exposure, and days between blood draw for telomere assay and viral challenge. Conversion of ORs to relative risks (RRs) using the formula of Zhang & Yu20 and setting the infection rate for the unexposed group equal to that of the quartile of longest CD8CD28− telomere length (ie, 50%) yields RR, 0.75 (95% CI, 0.25–1.49) for 18- to 22-year-olds; RR, 1.65 (95% CI, 1.13–1.91) for 23- to 29-year-olds; and RR, 1.76 (95% CI, 1.21–2.04) for 30- to 55-year-olds.
COMMENT
Leukocyte telomere length has emerged as a predictor of earlier onset of aging-related morbidity and mortality in older adults.8–11 Telomere length traditionally has been thought important in age-related functional decline and development of chronic disease. No studies have examined whether shorter telomere length might also be related to acute functional consequences in younger, healthy populations. In this study, we used a prospective viral-challenge methodology to test whether leukocyte telomere length is associated with host resistance to standardized exposure to a virus that causes the common cold in humans (RV39). Further, because CD8CD28− T cells appear to play an important role in immune response to immunization and are particularly prone to early telomere shortening, we compared telomere length in these cells with length in several other peripheral blood cell populations.
We found that shorter telomere lengths in all 4 cell types were associated with greater odds of infection following experimental exposure to RV39. However, CD8CD28− telomere length had the largest association with infection. Moreover, after CD8CD28− telomere length was entered into the logistic equation, adding the telomere length for other cell types did not improve the prediction. Further, only CD8CD28− telomere length was associated with clinical illness.
When examining these relations by age, we found that the association between CD8C28− telomere length and infection increased with increasing age. The increasing importance of leukocyte telomere length with advancing age may be attributable to cells with very short telomeres becoming more prevalent as individuals get older (eg, 31% of 30- to 55-year-olds vs 17% of 18- to 22-year-olds had CD8CD28− telomere lengths in the bottom 20% of the distribution); to a younger immune system more effectively compensating for the level of CD8 cell senescence; or to CD8 cell senescence contributing to immune impairment, especially impairment occurring within the context of other age-related biological changes.
This is a preliminary study with a small volunteer sample and modest effect sizes. However, because these analyses were prospective, we can eliminate reverse causation (developing an infection or illness in this study did not cause shortening of telomere length) as an alternative explanation. Use of multiple control variables (age, sex/birth control status, race, prechallenge antibody, season, BMI, day of blood draw to assess telomere length) eliminated many potential spurious explanations. Even so, the possibility remains that alternative unspecified third variables, eg, a common genetic contributor to both telomere length and susceptibility to infection, could account for our results. Also, the generalizability of the results may be limited if study volunteers differ significantly from the wider population.
There are no published functional data (eg, differences in virus-stimulated proliferation or cytokine production) comparing CD8CD28+ and CD8CD28− cells as they pertain specifically to RV infection. Consequently, the explanations for our findings are speculative and based on a more general understanding of the function of these cells. Because CD8 lymphocytes are important for eliminating virus-infected cells, a decreased ability to replicate would likely contribute to an increased susceptibility to viral infection. CD8CD28− cells with short telomeres are near or have already reached replicative senescence22 and have poor antigen-induced proliferation.23 Thus, the number of effector cells available to respond to the virus may be reduced in persons with a large number of senescent or near-senescent CD8 cells.
There is a close relationship between the CD28 molecule and the telomere/telomerase aging system.18,24 Without CD28, cells can no longer upregulate telomerase during activation, which is essential for proliferation, cytokine/chemokine production, and antiviral activity.22 Maintaining CD28 (through gene transduction) slows immunosenescence by increasing telomerase activity and cell proliferation and by reducing proinflammatory cytokine expression in vitro.25 Given these in vitro findings, it is reasonable to expect that in vivo loss of CD28 from CD8 cells should impair the host’s ability to fight infection.
Previous research has shown telomere length to be shorter in CD8CD28− relative to CD8CD28+ cells, with that research being restricted to samples of patients who are older26 or human immunodeficiency virus-positive27 and to 1 small study (n = 10) of 29- to 59-year-olds.28 In contrast, we found no difference in telomere length between CD8CD28− and CD8CD28+ cells in our healthy younger sample (mean age, 29.9 years). Nevertheless, CD8CD28− cells did show a wide range of telomere lengths.
In sum, this study found an association between leukocyte telomere length and resistance to a common virus infection in healthy young and midlife adults. These findings are consistent with the wide variance in leukocyte telomere length found in young adults.18 The presence of short telomeres among young people could result from several factors including genetics,29–30 younger paternal age at conception,26 poor health behaviors, and oxidative31 and chronic psychological stress.32,33 CD8 cell senescence has also been attributed to the presence of latent viral infections34,35 and proinflammatory environments.36
Implicit in the relationship between telomere length and colds in this study is that telomere length is relatively stable over the course of at least 1 to 2 months. Published data from a small study of 30- to 50-year-olds found that telomere length in leukocytes is quite stable over the course of 7 months (r = 0.93),37 whereas a 10-year follow-up of a large sample of older adults (>53 years) found a still substantial but smaller association (r = 0.65).38 Evidence of long-term stability is also suggested by correlations of telomere length with stable individual characteristics including genetic markers,29,30 education,39 and stable psychological dispositions.40,41 A provocative possibility is that telomere length is a very stable marker of disease susceptibility, with associations between telomere length and clinical outcomes beginning to emerge in early adulthood.
In this study of healthy young and midlife adults, shorter CD8CD28− cell telomere length was associated with upper respiratory tract infection and clinical illness following experimental exposure to rhinovirus. Because these data are preliminary, their clinical implications are unknown.
Acknowledgments
Study concept and design: Cohen, Casselbrant, Li-Korotky, Epel, Doyle.
Acquisition of data: Cohen, Turner, Casselbrant, Li-Korotky, Doyle.
Analysis and interpretation of data: Cohen, Janicki-Deverts, Epel, Doyle.
Drafting of the manuscript: Cohen, Janicki-Deverts, Casselbrant, Epel, Doyle.
Critical revision of the manuscript for important intellectual content: Janicki-Deverts, Turner, Li-Korotky, Doyle.
Statistical analysis: Cohen, Janicki-Deverts.
Obtained funding: Cohen, Casselbrant, Doyle.
Administrative, technical, or material support: Turner, Li-Korotky.
Study supervision: Cohen, Li-Korotky, Doyle.
Funding/Support: This research was supported by the National Center for Complementary and Alternative Medicine (AT006694 and RC1 AT005799) and the National Institute of Allergy and Infectious Diseases (AI066367), and through supplemental support from the John D. and Catherine T. MacArthur Foundation’s Research Network on Social Economic Status and Health, the Eberly Foundation, the Hamburg Fellowship, and National Institutes of Health funding to the University of Pittsburgh Clinical and Translational Science Institute (UL1 RR024153, and UL1TR000005).
Role of the Sponsor: None of the funders had any role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; or the preparation, review, or approval of the manuscript.
Footnotes
Author Contributions: Dr. Cohen had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Conflict of Interest Disclosures: All authors have completed and submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Dr. Turner reported serving as a consultant for, and receiving grants/grants pending from, Janssen Research and Development. Dr. Epel reported that she is a cofounder of, and holds stock or stock options in, Telome Health Inc., a telomere measurement company. No other authors reported disclosures.
Additional Contributions: We are indebted to Ellen Conser, MA (Carnegie Mellon University [CMU]), James Seroky, MA, Julie Banks, BS (both Pittsburgh Children’s Hospital), and Wesley Barnhart, BS (formerly CMU) for their assistance in data collection and to Cathy (Chia Chow) Lo, MS, and Allison Cullen Doyle, MS (both Pittsburgh Children’s Hospital), for their assistance in conducting assays; we also thank Judith A. Woodfolk, MBChB, PhD (University of Virginia Health Sciences Center), for her comments on an early draft. All but Dr. Woodfolk received payment for their participation.
Contributor Information
Sheldon Cohen, Email: scohen@cmu.edu.
Denise Janicki-Deverts, Email: djanicki@andrew.cmu.edu.
Ronald B. Turner, Email: rbt2n@virginia.edu.
Margaretha L. Casselbrant, Email: margaretha.casselbrant@chp.edu.
Ha-Sheng Li-Korotky, Email: ha-sheng@pnwaudiology.com.
Elissa S. Epel, Email: eepel@lppi.ucsf.edu.
William J. Doyle, Email: docdoyle666@hotmail.com.
References
- 1.Harley CB, Futcher AB, Greider CW. Telomeres shorten during ageing of human fibroblasts. Nature. 1990;345(6274):458–460. doi: 10.1038/345458a0. [DOI] [PubMed] [Google Scholar]
- 2.Kipling D, Wynford-Thomas D, Jones CJ, et al. Telomere-dependent senescence. Nat Biotechnol. 1999;17(4):313–314. doi: 10.1038/7827. [DOI] [PubMed] [Google Scholar]
- 3.Effros RB, pawelec G. Replicative senescence of T cells: does the Hayflick Limit lead to immune exhaustion? Immunol Today. 1997;18(9):450–454. doi: 10.1016/s0167-5699(97)01079-7. [DOI] [PubMed] [Google Scholar]
- 4.Effros RB. Ageing and the immune system. Novartis Found Symp. 2001;235:130–139. doi: 10.1002/0470868694.ch12. discussion 139–145, 146–149. [DOI] [PubMed] [Google Scholar]
- 5.Effros RB, Dagarag M, Spaulding C, Man J. The role of CD8 T-cell replicative senescence in human aging. Immunol Rev. 2005;205:147–157. doi: 10.1111/j.0105-2896.2005.00259.x. [DOI] [PubMed] [Google Scholar]
- 6.Saurwein-Teissl M, Lung TL, Marx F, et al. Lack of antibody production following immunization in old age: association with CD8(+)CD28(−) T cell clonal expansions and an imbalance in the production of Th1 and Th2 cytokines. J Immunol. 2002;168(11):5893–5899. doi: 10.4049/jimmunol.168.11.5893. [DOI] [PubMed] [Google Scholar]
- 7.Goronzy JJ, Fulbright JW, Crowson CS, Poland GA, O’Fallon WM, Weyand CM. Value of immunological markers in predicting responsiveness to influenza vaccination in elderly individuals. J Virol. 2001;75(24):12182–12187. doi: 10.1128/JVI.75.24.12182-12187.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cawthon RM, Smith KR, O’Brien E, Sivatchenko A, Kerber RA. Association between telomere length in blood and mortality in people aged 60 years or older. Lancet. 2003;361(9355):393–395. doi: 10.1016/S0140-6736(03)12384-7. [DOI] [PubMed] [Google Scholar]
- 9.Fitzpatrick AL, Kronmal RA, Kimura M, et al. Leukocyte telomere length and mortality in the Cardiovascular Health Study. J Gerontol A Biol Sci Med Sci. 2011;66(4):421–429. doi: 10.1093/gerona/glq224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Willeit P, Willeit J, Mayr A, et al. Telomere length and risk of incident cancer and cancer mortality. JAMA. 2010;304(1):69–75. doi: 10.1001/jama.2010.897. [DOI] [PubMed] [Google Scholar]
- 11.Weischer M, Bojesen SE, Cawthon RM, Freiberg JJ. Tybjærg-Hansen A, Nordestgaard BG. Short telomere length, myocardial infarction, ischemic heart disease, and early death. Arterioscler Thromb Vasc Biol. 2012;32(3):822–829. doi: 10.1161/ATVBAHA.111.237271. [DOI] [PubMed] [Google Scholar]
- 12.Schmid I, Dagarag MD, Hausner MA, et al. Simultaneous flow cytometric analysis of two cell surface markers, telomere length, and DNA content. Cytometry. 2002;49(3):96–105. doi: 10.1002/cyto.10163. [DOI] [PubMed] [Google Scholar]
- 13.Valenzuela HF, Effros RB. Divergent telomerase and CD28 expression patterns in human CD4 and CD8 T cells following repeated encounters with the same antigenic stimulus. Clin Immunol. 2002;105(2):117–125. doi: 10.1006/clim.2002.5271. [DOI] [PubMed] [Google Scholar]
- 14.Gwaltney JM, Colonno RJ, Hamparian VV, Turner RB. Rhinovirus. In: Schmidt NJ, Emmons RW, editors. Diagnostic Procedures for Viral, Rickettsial and Chlamydial Infections. Washington, DC: American Public Health Association; 1989. pp. 579–614. [Google Scholar]
- 15.Al Nakib W, Tyrrell DAJ. Picornviridae: Rhinoviruses—common cold viruses. In: Lennett EH, Halnen P, Murphy FA, editors. Laboratory Diagnosis of Infectious Diseases: Principles and Practice. Vol. 2. New York, NY: Springer-Verlag; 1988. pp. 723–742. [Google Scholar]
- 16.Doyle WJ, McBride TP, Swarts JD, Hayden FG, Gwaltney JM. The response of the nasal airway, middle ear and Eustachian tube to provocative rhinovirus challenge. Am J Rhinol. 1988;2:149–154. doi: 10.1097/00006454-198803000-00033. [DOI] [PubMed] [Google Scholar]
- 17.Cohen S, Doyle WJ, Skoner DP, Rabin BS, Gwaltney JM. Social ties and susceptibility to the common cold. JAMA. 1997;277(24):1940–1944. [PubMed] [Google Scholar]
- 18.O’Callaghan NJ, Dhillon VS, Thomas P, Fenech M. A quantitative real-time PCR method for absolute telomere length. BioTechniques. 2008;44(6):807–809. doi: 10.2144/000112761. [DOI] [PubMed] [Google Scholar]
- 19.Hosmer DW, Jr., Lemeshow S. Applied Logistic Regression. New York, NY: John Wiley & Sons; 1989. [Google Scholar]
- 20.Zhang J, Yu KF. What’s the relative risk? A method of correcting the odds ratio in cohort studies of common outcomes. JAMA. 1998;280(19):1690–1691. doi: 10.1001/jama.280.19.1690. [DOI] [PubMed] [Google Scholar]
- 21.Alexander GC, Casalino LP, Meltzer DO. Patient-physician communication about out-of-pocket costs. JAMA. 2003;290(7):953–958. doi: 10.1001/jama.290.7.953. [DOI] [PubMed] [Google Scholar]
- 22.Dock JN, Effros RB. Role of CD8 T cell replicative senescence in human aging and in HIV-mediated immunosenescence. Aging Dis. 2011;2(5):382–397. [PMC free article] [PubMed] [Google Scholar]
- 23.Azuma M, Phillips JH, Lanier LL. CD28− T lymphocytes: antigenic and functional properties. J Immunol. 1993;150(4):1147–1159. [PubMed] [Google Scholar]
- 24.Butler MG, Tilburt J, DeVries A, et al. Comparison of chromosome telomere integrity in multiple tissues from subjects at different ages. Cancer Genet Cytogenet. 1998;105(2):138–144. doi: 10.1016/s0165-4608(98)00029-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Parish ST, Wu JE, Effros RB. Sustained CD28+expression delays multiple features of replicative senescence in human CD8 T lymphocytes. J Clin Immunol. 2010;30(6):798–805. doi: 10.1007/s10875-010-9449-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lin J, Epel E, Cheon J, et al. Analyses and comparisons of telomerase activity and telomere length in human T and B cells: Insights for epidemiology of telomere maintenance. J Immunol Methods. 2010;352:71–80. doi: 10.1016/j.jim.2009.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Effros RB, Allsopp R, Chiu CP, et al. Shortened telomeres in the expanded CD28-CD8+ cell subset in HIV disease implicate replicative senescence in HIV pathogenesis. AIDS. 1996;10(8):F17–F22. doi: 10.1097/00002030-199607000-00001. [DOI] [PubMed] [Google Scholar]
- 28.Monteiro J, Batliwalla F, Ostrer H, Gregersen PK. Shortened telomeres in clonally expanded CD28-CD8+ T cells imply a replicative history that is distinct from their CD28+CD8+ counterparts. J Immunol. 1996;156:3587–3590. [PubMed] [Google Scholar]
- 29.Unryn BM, Cook LS, Riabowol KT. Paternal age is positively linked to telomere length of children. Aging Cell. 2005;4(2):97–101. doi: 10.1111/j.1474-9728.2005.00144.x. [DOI] [PubMed] [Google Scholar]
- 30.Soerensen M, Thinggaard M, Nygaard M, et al. Genetic variation in TERT and TERC and human leukocyte telomere length and longevity: a cross-sectional and longitudinal analysis. Aging Cell. 2012;11(2):223–227. doi: 10.1111/j.1474-9726.2011.00775.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.von Zglinicki T. Oxidative stress shortens telomeres. Trends Biochem Sci. 2002;27(7):339–344. doi: 10.1016/s0968-0004(02)02110-2. [DOI] [PubMed] [Google Scholar]
- 32.Damjanovic AK, Yang Y, Glaser R, et al. Accelerated telomere erosion is associated with a declining immune function of caregivers of Alzheimer’s disease. J Immunol. 2007;179(6):4249–4254. doi: 10.4049/jimmunol.179.6.4249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Epel E, Blackburn EH, Lin J, et al. Accelerated telomere shortening in response to life stress. Proc Natl Acad Sci U S A. 2004;101(49):17312–17315. doi: 10.1073/pnas.0407162101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bestilny LJ, Gill MJ, Mody CH, Riabowol KT. Accelerated replicative senescence of the peripheral immune system induced by HIV infection. AIDS. 2000;14(7):771–780. doi: 10.1097/00002030-200005050-00002. [DOI] [PubMed] [Google Scholar]
- 35.Palmer LD, Weng N, Levine BL, June CH, Lane HC, Hodes RJ. Telomere length, telomerase activity, and replicative potential in HIV infection: analysis of CD4+ and CD8+T cells from HIV-discordant monozygotic twins. J Exp Med. 1997;185(7):1381–1386. doi: 10.1084/jem.185.7.1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Weng NP, Akbar AN, Gorozny J. CD28(−) T cells: their role in the age-associated decline of immune function. Trends Immunol. 2009;30(7):306–312. doi: 10.1016/j.it.2009.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chen W, Kimura M, Kim S, et al. Longitudinal versus cross-sectional evaluations of leukocyte telomere length dynamics: Age-dependent telomere shortening is the rule. J Gerontol A Biol Sci Med Sci. 2011;66A(3):312–319. doi: 10.1093/gerona/glq223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ehrlenbach S, Willeit P, Kiechl S, et al. Influences on the reduction of relative telomere length over 10 years in the population-based Bruneck Study: Introduction of a well-controlled high-throughput assay. Int J Epidemiol. 2009;38(6):1725–1734. doi: 10.1093/ije/dyp273. [DOI] [PubMed] [Google Scholar]
- 39.Steptoe A, Hamer M, Butcher L, et al. Educational attainment but not measures of current socioeconomic circumstances are associated with leukocyte telomere length in healthy older men and women. Brain Behav Immun. 2011;25(7):1292–1298. doi: 10.1016/j.bbi.2011.04.010. [DOI] [PubMed] [Google Scholar]
- 40.Brydon L, Lin J, Butcher L, et al. Hostility and cellular aging in men from the Whitehall II cohort. Biol Psychiatry. 2012;71(9):767–773. doi: 10.1016/j.biopsych.2011.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.O'Donovan A, Lin J, Dhabhar FS, et al. Pessimism correlates with leukocyte telomere shortness and elevated interleukin-6 in post-menopausal women. Brain Behav Immun. 2009;23(4):446–449. doi: 10.1016/j.bbi.2008.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]