Abstract
Copper is an essential element for multiple biological processes. Its concentration is elevated to a very high level in cancer tissues for promoting cancer development through processes such as angiogenesis. Organic chelators of copper can passively reduce cellular copper and serve the role as inhibitors of angiogenesis. However, they can also actively attack cellular targets such as proteasome, which plays a critical role in cancer development and survival. The discovery of such molecules initially relied on a step by step synthesis followed by biological assays. Today high-throughput chemistry and high-throughput screening have significantly expedited the copper-binding molecules discovery to turn “cancer-promoting” copper into anti-cancer agents.
Keywords: Copper, oxidative stress, proteasome inhibitor, ROS, angiogenesis
1. INTRODUCTION
Copper is an essential trace element that can affect many biological pathways in the human body [1, 2]. Copper can activate some critical enzymes such as Cu-Zn superoxide dismutase, tyrosinase, ceruloplasmin and cytochrome oxidase, which are important in fundamental biological pathways [3].
In healthy human adults, the necessary copper daily dietary intake is 1–2 mg [4]. Copper can be absorbed from the stomach and intestine through different protein transporters. Then, copper associated with albumin and transcuprein is deposited in the liver [4]. Cu2+ is reduced to Cu+ and then carried into cells by various transmembrane transporters such as Ctr1 and Ctr3. In the blood, the major copper carrying protein is ceruloplasmin [5] and the rest of copper is transported by albumin and histidine [6].
Upon entering cells, copper ions bind to different sites such as metallothionein, cytochrome oxidase, superoxide dismutase and copper chaperones to avoid its oxidative damage to proteins, DNA and cellular membranes. If the balance of copper in cells is impaired, diseases will occur. Homeostatic imbalances of copper can be caused by the uptake of excessive amounts of copper or some genetic defects [7]. Wilson’s disease is an autosomal recessive disorder resulting from dysfunction of a copper binding ATPase, ATP7B [8, 9]. The accumulation of copper in the liver can cause liver damage [8, 9]. X-linked recessive mutation in copper transporting ATPase ATP7A will cause the Menkes disease [10, 11].
Over the past two decades, evidence has shown that copper plays an important role in inflammation and the growth of tumors [12–14]. The copper concentration in serum and tumor tissue is significantly higher than that of healthy subjects [15–17]. Evidence has showed that tumour cells can readily take up copper from ceruloplasmin fractions of the plasma [18]. In tumour tissues, the concentration of ceruloplasmin and other copper-binding components, such as transcuprein, appear to be increased [19]. From a clinical perspective, disease stage has been validated by measuring ceruloplasmin oxidase activity [20].
2. COPPER AND ANGIOGENESIS
Blood vessels function to supply nutrients and oxygen and are fundamental to the survival and development of tumors. The growth of tumors relies on the formation of new blood vessels [21–24]. There are two different ways to form new blood vessels: one is the development of existing vessels and the other is to develop new vessels from endothelial cell precursors angioblasts [25, 26]. There is overwhelming evidence that copper is an important angiogenic factor in tumors [27]. Results on a comparative study of trace elements in normal and tumour tissues [1] showed that the average copper concentration of cancerous tissues is significantly different from the average of normal tissue. Evidence shows that copper can stimulate the proliferation and migration of endothelial cells [28]. Vascular endothelial growth factor (VEGF) [12] is another critical factor in angiogenesis [29]. The high level of VEGF reduced the effect of endocrine therapy and radiotherapy of breast cancer [30, 31]. Some effective treatment methods targeting VEGF have been developed [32–35].
Upon the activation of endothelial cells, the subsequent steps, such as proteolytic enzymes, facilitate, endothelial cell migration, and new basal membrane formation [36] can be targeted in anti-angiogenesis treatment [37–39]. By using rabbit cornea angiogenesis model [40], researchers have shown that copper can stimulate new blood vessel formation. Implanting tumor cells into the brains of animals and subsequent treatment with the anticopper drug penicillamine resulted in the inhibition of blood vessel formation [41]. Studies showed that the anticopper drug can decrease some proangiogenic mediators, such as VEGF, fibroblast growth factor (FGF), interleukin (IL)-1α and IL-8 [42]. Angiogenin, a potent blood vessels formation inducer, binds to endothelial cell receptors with high affinity. The binding between angiogenin and endothelial cells can increase 4.3-fold in the presence of Cu2+ [43]. The anti-copper drug tetrathiomolybdate (TM) can significant inhibit bleomycin induced tumor necrosis factor-α and transforming growth factor-β [44]. NFκB is an important regulator of cytokines and other cellular mediators. In recent years, studies have suggested that NFκB might be an important therapeutic target for cancer treatment. Studies relating to the effect of divalent copper on hydrogen peroxide-induced NFκB activation and dissociation of NF κB-IκB complex showed that Cu2+ can interrupt key steps of this signalling pathway which includes the phosphorylation of IκB [45]. It was also found that the degree of Cu-oxidized LDL (low density lipoproteins) was proportional to the extent of NFκB activation. Additionally, NFκB activity is known to be involeved in the activation of fibroblasts, smooth muscle cells and endothelial cells [46].
3. COPPER REDUCTION AS AN ANTI-CANCER STRATEGY
Anti-copper agents such as zinc, TM, trientine, D-penicillamine [9, 47, 48] have been used to treat the copper-related disease, such as Wilson’s disease. Using one or a combination of these drugs reversed copper accumulation. Copper is an essential trace element and is a key component of many important enzymes. Therefore, a precise balance of cellular copper is required to maintain normal homeostatic function. Reducing copper levels beyond a certain threshold can result in copper deficiency toxicity and has been found to be associated with anemia. The earliest toxicity of copper deficiency is anemia. The following manifestations of this toxicity would include peripheral neuropathy, hair loss, and delayed wound healing. The aim of copper depletion therapy is to decrease copper to an adequate level where the signaling pathways of cytokines can be reduced but copper deficiency toxicity does not occur. For different types of cancers, the mechanisms required for angiogenesis at the primary and metastatic sites are different. These processes depend on the imbalance between angiogenesis activators and inhibitors. Therefore, the development of an antiangiogenic therapy that would affect multiple activators of angiogenesis is desirable [49, 50]. Compounds that reduce copper levels seem to meet this requirement because copper is a required cofactor of several key angiogenic factors [51].
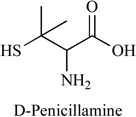
In 1956, the first successful oral anticopper drug D-penicillamine was developed, Table 1. Combined with low copper diet, the antitumor and antiangiogenic activities of penicillamine, and the feasibility of nutritional and metabolic intervention for patients with brain tumors have been demonstrated [41, 52]. Toxicity was consistent with predominantly hematological effects and copper deficiency. These studies showed that penicillamine could inhibit the human endothelial cell proliferation and neovascularization [53]. A phase II clinical study was carried out to investigate the angiosuppressive activity of D-penicillamine in the treatment of glioblastoma [54]. The oral dose of D-penicillamine was escalated from 250 mg/day to 2 g/day in five weeks with low copper diet (0.5 mg/day) and radiation therapy. The results showed that the copper level was reduced and the antioxidative activity of Cu/Zn-Superoxide Dismutase (SOD) was probably also decreased. But there are some side effects for long-term administration of D-penicillamine, such as fever, rash, neurological symptoms and some autoagressive diseases [54]. The pharmacokinetics of this drug after iv administration showed that the plasma elimination half-life (T 1/2 beta) was 62.7 +/− 5.3 min, plasma clearance (CI) 560.7 +/− 42.8 ml/min, volume of distribution (Vd), 57.0 +/− 9.3 l, and % D-penicillamine excreted within 24 h was 42.1% +/−6.2%. Following oral administration, T 1/2 beta was 60.7 +/−8.2 min; % D-penicillamine excreted within 24 h was 21.2 +/− 2.3%; and fraction of absorption was 41.2 +/− 5.5%. Urinary copper excretion paralleled urine and plasma D-penicillamine concentrations.
Table 1.
Copper-Chelating Compounds with Anti-Cancer Activity
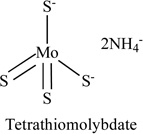
TM, a complex of molybdenum and sulphur, is a fast acting chelator with copper, Table 1. The ammonium salt of TM can be absorbed readily [55, 56]. TM affects angiogenesis by inhibiting NFκB, VEGF, IL-8, and IL-1α [42]. A phase I/II clinical trial with TM therapy in a variety of advanced and metastatic cancers was initiated at the University of Michigan in the late 1990s. Studies showed that oral dose of 120 mg of TM per day was the most effective dose to bring copper concentrations to the target level without causing side effects. Long term administration of TM was associated with toxicity that was limited to easily reversible anemia, and sometimes with leucopoenia [57]. Research also suggested that combination of cytotoxic chemotherapy (e.g. with doxorubicin) or radiotherapy with TM resulted in additive effects [58, 59]. TM was also found to have antiangiogenic effects in malignant pleural mesothelioma patients [60]. TM (1.7 mg kg-1) was administered intravenously to sheep, blood samples were collected at intervals and different pharmacokinetic parameters for TM were determined. Serum molybdenum concentration data were best described by a 2-compartmental open model with first order rate constants. An elimination half-life (T1/2 beta) of 396.8 min, steady state volume of distribution (Vdss) of 0.8 e kg-1 and total body clearance (C1B) of 1.53 ml kg-1 min-1 were observed using non-linear compartmental analysis. A significant (p < or = 0.05) increase in molybdenum excretion in the faeces occurred at 24 and 48 h following TM administration [61].
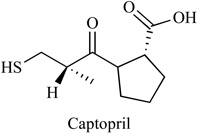
Hypertension drug Captopril was an inhibitor of the angiotensin converting enzyme (ACE), Table 1. Copper (II) can react with its thiol group to form Cu (I)-thiolate complexes. Captopril antagonizes the angiogenic process via several distinct pathways through copper chelation, metalloprotease inhibition, and conversion of plasminogen to angiostatin [62]. Common side effects of captopril include anaemia, low blood pressure, unexplained rash, itching, or cough. Other less common side effects (<2% of patients) may include diarrhea, constipation, stomach pain, and headache. The kinetics of captopril plasma levels and of the druginduced plasma converting enzyme activity (PCEA), plasma renin activity (PRA) and diastolic blood pressure (DBP) modifications were studied over 24 h after oral administration of captopril, 1 mg/kg, to ten hypertensive patients. Free unchanged captopril pharmacokinetic parameters were: t1/2, alpha: 0.45 +/− 0.06 h; tmax: 0.98 +/− 0.13 h; Cmax: 1.31 +/−0.20 mg l-1; t1/2,z: 0.66 +/− 0.13 h; V: 0.614 +/− 0.104 1 kg-1 and CLtot: 0.690 +/− 0.082 l h-1 kg-1. At 6 h captopril was no longer detectable in plasma. The onset of PCEA inhibition and of DBP decrease closely followed the rise of captopril's plasma levels, while that of PRA increase was delayed. In contrast, while captopril rapidly disappeared from plasma, its biological and antihypertensive effects were long-lasting [63].
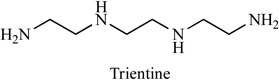
Trientine (sold as Syprine) can be used to treat patients with Wilson’s disease, Table 1. It is administered as an alternative drug for patients with penicillamine intolerance due to severe side effects, such as induction of auto-immune diseases and bone marrow suppression. The antiangiogenic effect of trientine occur through suppression of interleukin 8 production [64]. Researchers examined the effects of trientine on tumor development in a murine xenograft model. Oral administration of trientine (with food or water) between 1000 mg and 2000 mg per day can significant inhibit tumour growth in mice inoculated subcutaneously with hepatocellular carcinoma cells (HCC). In addition, treatment with trientine suppressed tumor blood vessel formation, increased the apoptotic index and inhibited endothelial cell proliferation and migration [65, 66]. Side effects include severe allergic reactions (rash, hives, difficulty breathing, tightness in the chest), fever, swollen, or blistered skin. A high concentration of metabolites appeared in blood samples of patients and rats in the early stage after administration of trientine. Furthermore, large amount of trientine metabolites were excreted into the urine of patients. These results suggest that trientine is remarkably subjected to a first-pass effect. The drug concentration area under the curve (AUC) of the unchanged form and the metabolites of trientine in patients was not dependent on the administered dosage. It seems that the absorption process is an important factor for the disposition behavior of trientine. The uptake characteristics of trientine by rat intestinal brush-border membrane vesicles were similar to the physiological polyamines, spermine and spermidine. The uptake mechanism of trientine in rat small intestinal brush-border membrane vesicles was almost identical to that of spermine and spermidine, and that the physiological polyamines seem to have the ability to inhibit the absorption of trientine from the gastrointestinal tract [67].
5-Chloro-7-iodo-quinolin-8-ol (Clioquinol) is a halogenated 8-hydroxyquinoline that was used in the 1950s–1970s as an oral anti-parasitic agent for the treatment and prevention of intestinal amebiasis, Table 1. A daily dosage of up to 750 mg for a period of two weeks is considered to present no risk of neurotoxicity or other side effects. In the 1970s, oral Clioquinol was withdrawn from the market due to reports of neurotoxicity in Japanese patients. Severe allergic reactions (rash; hives; itching; difficulty breathing; tightness in the chest; swelling of the mouth, face, lips, or tongue); acne-like rash; burning, cracking, irritation, or peeling not present before you began using Clioquinol/Hydrocortisone Cream; excessive hair growth; inflamed hair follicles; inflammation around the mouth; muscle weakness; thinning, softening, or discoloration of the skin; unusual weight gain, especially in the face. Pharmacokinetics of clioquinol in hamster plasma, spleen and brain after single and repeated oral or intraperitoneal administration (50 mg/kg) led to peak plasma clioquinol concentrations after 15 min (Tmax), followed by a decay with an apparent half-life of 2.20 +/− 1.1 h. After oral administration, Tmax was reached after 30 min and was followed by a similar process of decay. The Cmax and AUC values in spleen after a single administration were about 65% (i.p.) and 25% (p.o.) of those observed in blood; those in liver were 35% (p.o.) of those observed in blood and those in brain were 20% (i.p.) and 10% (p.o.) of those observed in plasma. After repeated oral doses, the plasma, brain and spleen concentrations were similar to those observed at the same times after a single dose [68]. Recently, Clioquinol has reemerged for the treatment of non-infectious indications such as malignancy. Clioquinol has anticancer properties, as shown by both in vitro and in vivo experiments, and results of these experiments proved that clioquinol induces apoptosis and PARP cleavage in Raji cells [69].
4. INDUCTION OF OXIDATIVE STRESS AS AN ANTI-CANCER STRATEGY
The human body maintains a fine balance between oxidant and antioxidant species [70, 71]. If the oxidant-antioxidant balance is disrupted, diseases such as atherosclerosis, cancer, and Alzheimer’s disease [72, 73] may occur.
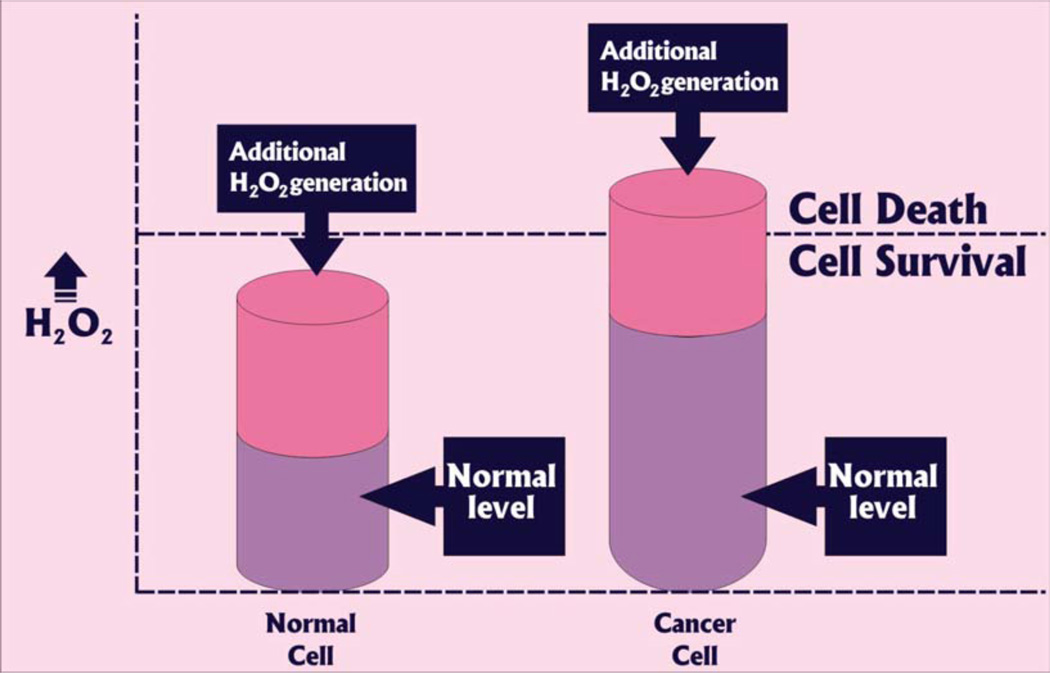
Reactive oxygen species (ROS) that are oxygen containing oxidizing agents are normally divided into two groups: free radicals (superoxide radicals O2·−) and non-radicals (hydrogen peroxide H2O2). Free radicals that have one or more unpaired electrons are highly reactive chemical agents. The activity of non-radicals is lower than free radicals, but the non-radicals can be converted to radical agents by reacting with other radicals. One of the hallmarks of cancer cells is the increased levels of ROS compared to normal cells [74]. The increased ROS levels in cancer cells may be attributed to mitochondrial malfunction, an increase in metabolic activity, and other factors [75, 76], Fig (1).
Fig. 1.
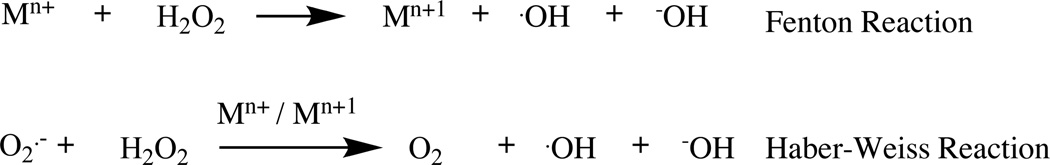
Fenton reaction and Haber-Weiss reaction.
The transition metals such as iron (II) and copper (I) oxidized by H2O2 can generate the hydroxyl radical (Fenton Reaction). The Haber-Weiss reactions can also lead to generation of hydroxyl radical, Fig. (1).
It is suggested that inhibition of the antioxidant enzymes and induction of apoptosis and necrosis in cancer cells caused by the ROS production can be a potential treatment for cancer [77–80], Fig. (2).
Fig. 2.
Mechanism of ROS-based anti-cancer therapies. Reprinted with permission from Cancer Lett. 2007, 252, 1-8. Copyright© 2006, Elsevier Ireland Ltd.
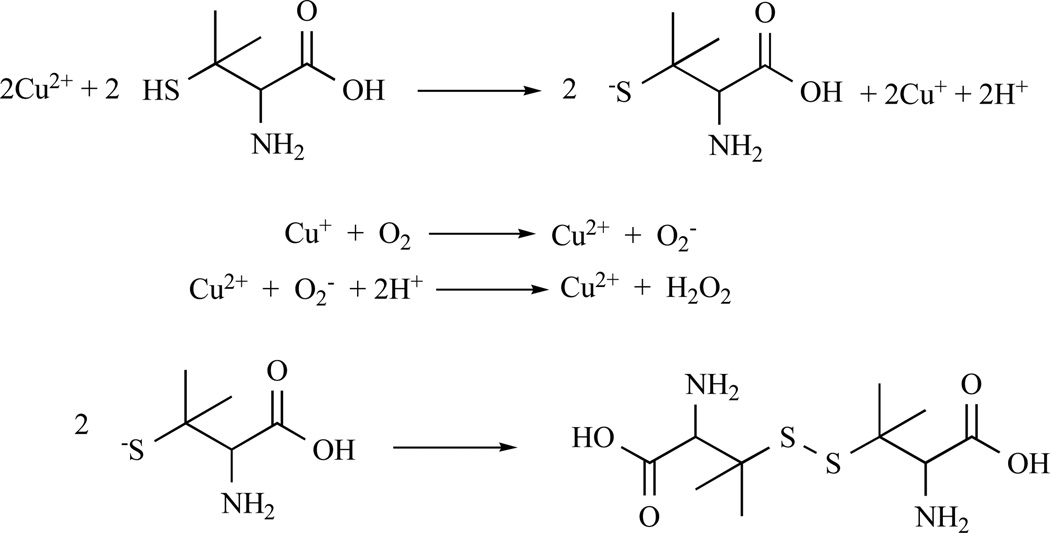
As discussed above, penicillamine is an effective copper chelator that has been successful in removing copper from tumor tissues. In the presence of copper, addition of penicillamine in endothelial cells and lymphocytes have been reported to lead to cell death by generation of ROS [53, 81, 82], Fig. (3).
Fig. 3.
The mechanism of copper catalyzed penicillamine oxidation.
The novel chemotherapeutic agent Casiopeina III-ia [(1,1’-dimethyl-2,2’-bipiridine)(acetylacetonate) Copper (II) nitrate], Table 2, was evaluated for its anti-tumor effect in vitro and in vivo [83]. It strongly inhibited colon adenocarcinoma HCT-115 cells and increased levels of BAX and inter-nucleosomal DNA fragmentation. Studies also showed that it was capable of inducing ROS [84], binding DNA, and blocking oxidative phosphorylation.
Table 2.
Anti-Cancer Copper Complexes Acting Through ROS Generation
| Compound | Cell Line | Oxidative Stress | Ref. |
|---|---|---|---|
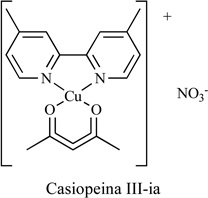 |
HCT-15 | Yes | [83] |
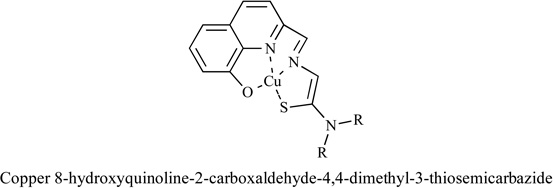 |
SK-N-DZ neuroblastoma cells | Yes | [92] |
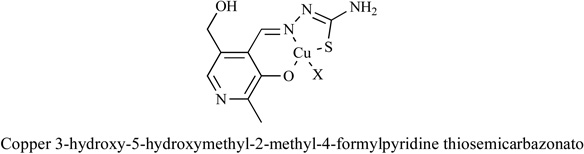 |
Ehrlich ascites tumor cell | Yes | [91] |
 |
Friend Erithroleukemia Cell; Melanoma B16F10 cell | Yes | [88] |
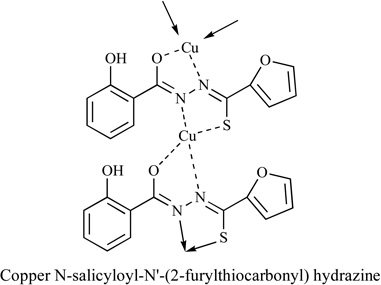 |
Solid Fibrosarcoma; As-cites Dalton’s Lymphoma | Yes | [87] |
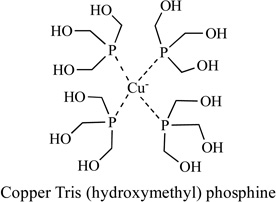 |
Human Ovarian Adenocarcinoma Cell; Human Cervix Squamous Carcinoma Cells; Human Colon Adenocarcinoma Cells | Yes | [93] |
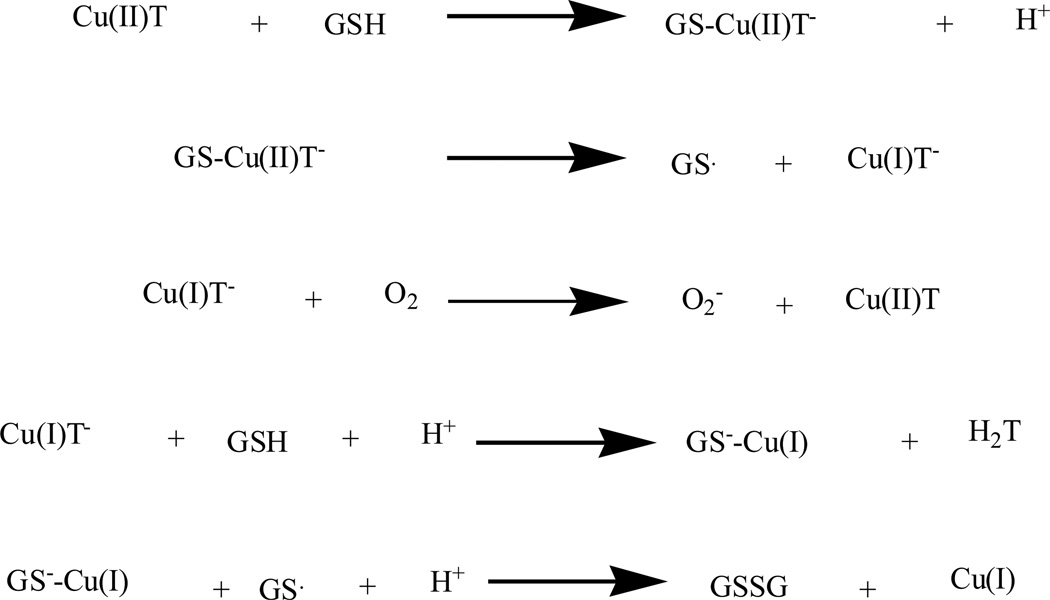
Metal complexes of thiosemicarbazone and their analogues were investigated [85–88]. It was found that the anti-tumor activities of thiosemicarbazones were correlated with the parent ketone or aldehyde group and their ability of metal chelation. Thiosemicarbazones and their analogues inhibited cancer cell proliferation by various mechanisms [89–91], including induction of oxidative stress [91]. The mechanism of ROS generation by metal thiosemicarbazone complexes can be explained by their interaction with GSH, Fig. (4).
Fig. 4.
ROS generation mechanism of metal thiosemicarbazone complexes.
5. COPPER COMPLEXES AS INHIBITORS OF THE PROTEASOME
5.1. Proteasome and Proteasome Inhibitors
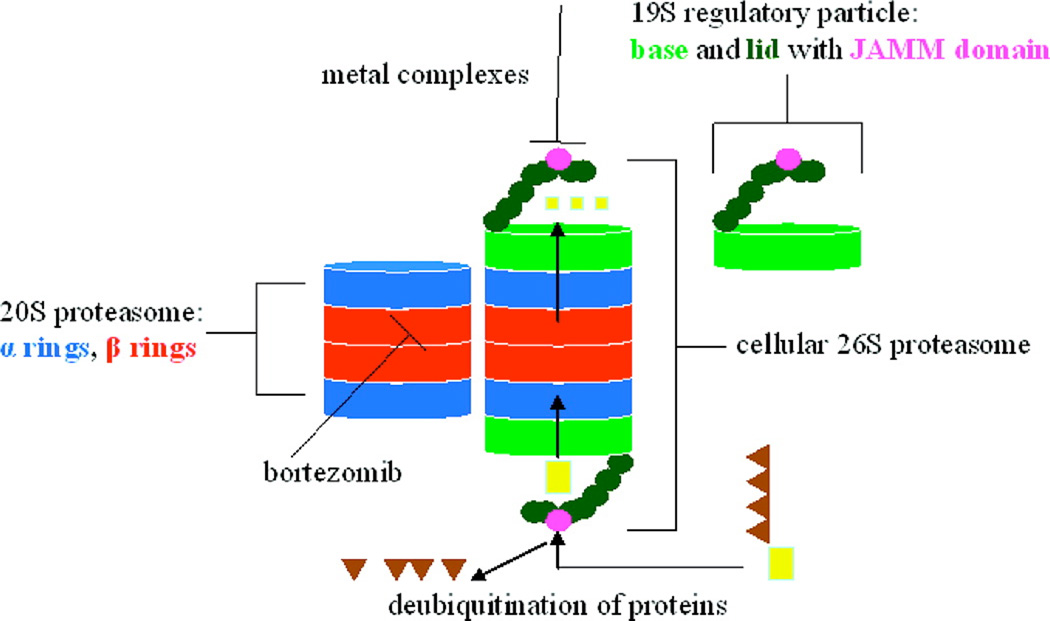
The proteasome is a large protein complex that is responsible for the timely degraradation of proteins and maintaining normal cellular homeostasis. Proteasomes can affect myriad biological processes such as protein quality control, signal transduction, antigen processing, cell cycle control, and apoptosis. The 26S proteasome complex in eukaryotes is a multifunctional molecule. It is composed of the 20S proteasome, which is the cylinder-shaped component and the 19S proteasome complex which is the regulatory component capped at each end of 26S proteasome [94, 95]. The 19S proteasomal complex recongnizes the ubiquitinated proteins, which are then translocated into the the lumen of 20S proteasome for degradation by proteolytically active sites [96, 97].
The de-ubiquitinating activity of 19S particle depends on a metal-loisopeptidase. This motif in 19S particle so-called JAB1/MPN/Mov34 domain or JAMM domain has been suggested to be a possible target for anticancer drugs [98], Fig. (5). We have reported that the DTC-Cu complex had different inhibitory effects in purified 20S proteasome (without 19S particles containing JAMM domain) and cellular 26S proteasome (with 19S particles containing JAMM domain). We found that Cu(PDTC)2 could inhibit CT-like activity of the cellular 26S proteasome but not purified 20S, suggesting that the effect of DTC-Cu complexes required interactions with both CT-like catalytic site located on the 20S catalytic core and de-ubiquitinating site located on the 19S particle containing JAMM domain within the 26S proteasome. In this report, we also synthesized and characterized another two complexes of metals (Zn and Ni) with DTC. We found that DTC-Ni was quite inactive to cancer cells in the experiment. But the DTC-Zn complex showed almost the same activity of DTC-Cu.
Fig. 5.
The suggested mechanism for metal-DTC complex to inhibit 26S proteasome. Reprinted with permission from J. Med. Chem. 2008, 51, 6256. Copyright© 2008, American Chemical Society.
Proteasome inhibitors can affect various biological processes through inhibiting the ubiquitin-proteasomal degradation pathway [99–102]. It has been shown that cancer cells are more sensitive to proteasome inhibition than normal cells, highlighting a favorable therapeutic strategy in cancer therapy [103, 104]. Velcade is the first FDA approved myeloma treatment drug known as proteasome inhibitor. It can inhibit the proteasome activity and disrupts the processes which relate to the growth and transformation of tumor cells. We reported a novel dipeptidyl proteasome inhibitor that can rapidly induce apoptosis in human Jukat T cells overexpression Bcl-2. This compound can selectively accumulate the cyclin-dependent kinase inhibitors p27 and p21, and induce apoptosis in cancer cells, but not the normal human cells [103].
Previously, we reported that certain organo-metallic compounds, such as 8-hydroxyquinoline and pyrrolidine dithiocarbamate, could react with Cu and form complexes that exhibit strong proteasome-inhibitory and apoptosis-inducing abilities in cultured human cancer cells. However, these compounds alone or its metal salt alone did not show the same effects in cultured cells. Clioquinol (CQ) is a lipophilic compound of the quinoline class that is capable of forming stable complexes with Cu(II) ions. Indeed, when we mixed a solution of CQ with a solution of CuCl2 at 1:1 molar ratio, a dramatic color change was observed, indicating that a chemical reaction had occurred [105]. To further verify that the color change of the CQ-Cu mixture indicates a formation of a stable CQ-Cu complex, we analyzed a series of samples including CQ, CuCl2 and a mixture of CQ and CuCl2 by means of X-ray absorption near-edge spectroscopy (XANES) and extended X-ray absorption fine structure spectroscopy (EXAFS). The results showed that the CQ-Cu mixture had a different Cu oxidation state compared with those of CuCl2 and Cu metal, confirming that a chemical reaction occurred between CQ and Cu [105]. We further tested the biological effects of CQ-Cu complex in human prostate cancer LNCaP (androgen-dependent) and C4-2B (androgen-independent) cells. The results showed that inhibition of proteasome activity, reduction of androgen receptor (AR) protein expression, suppression of cell proliferation, and induction of apoptotic cell death were observed in both prostate cancer cell lines treated with CQ-Cu complex, but not with CQ alone. Furthermore, animal studies of mice bearing human C4-2B xenografts showed that treatment with CQ (10 mg/kg/day) for 30 days resulted in significant inhibition of tumor growth (66%) compared to the control, associated with proteasome inhibition, induction of apoptosis, suppression of AR expression, and inhibition of angiogenesis in CQ-treated tumor tissues.
For a long time, it was speculated or inferred by indirect evidence that anti-cancer activities of 8-OHQ and CQ require their chelation with copper. This was experimentally proven by synthesizing 8-OHQ and CQ analogues with nearly intact structures, but missing only the 8-hydroxyl group. These compounds were found to lose their copper-binding ability, and ultimately their anti-cancer effects [106].
We further tested disulfiram (DSF) in cultured human breast cancer cells and used the Cu chelator TM as a control. Interestingly we observed inhibition of proteasome activity and induction of apoptotic cell death only after the treatment of DSF-Cu complex, but not DSF alone, TM alone or TM-Cu complex. Based on these findings, we hypothesized that after entering into tumor cells DSF could react with endogenous Cu and form a complex that displays similar biological activity as that of the DSF-Cu in situ mixture. Similar to DSF-Cu complex, DSF alone, but not TM, was able to inhibit the proteasome activity and induce apoptotic cell death in Cu-enriched MDA-MB-231 cells. In order to verify the finding of DSF in cultured cells, we treated the mice bearing human breast MDA-MB-231 xenografts with DSF (50 mg/kg/day) and found that after 29 days DSF indeed significantly inhibited the tumor growth of mice by 74%. The inhibition of proteasome activity and induction of apoptosis were observed in the tumor tissues treated with DSF [107]. Our findings demonstrated that DSF was a potent proteasome inhibitor and apoptosis inducer in vitro and in vivo only when Cu was present. We also found that DSF-Cu complex selectively inhibited proteasome activity and induced apoptosis in cancer but not normal cells and although both of DSF and TM could chelate Cu, DSF had advantage over TM because DSF-Cu, but not TM-Cu complex had proteasome inhibitory and anti-tumor activities.
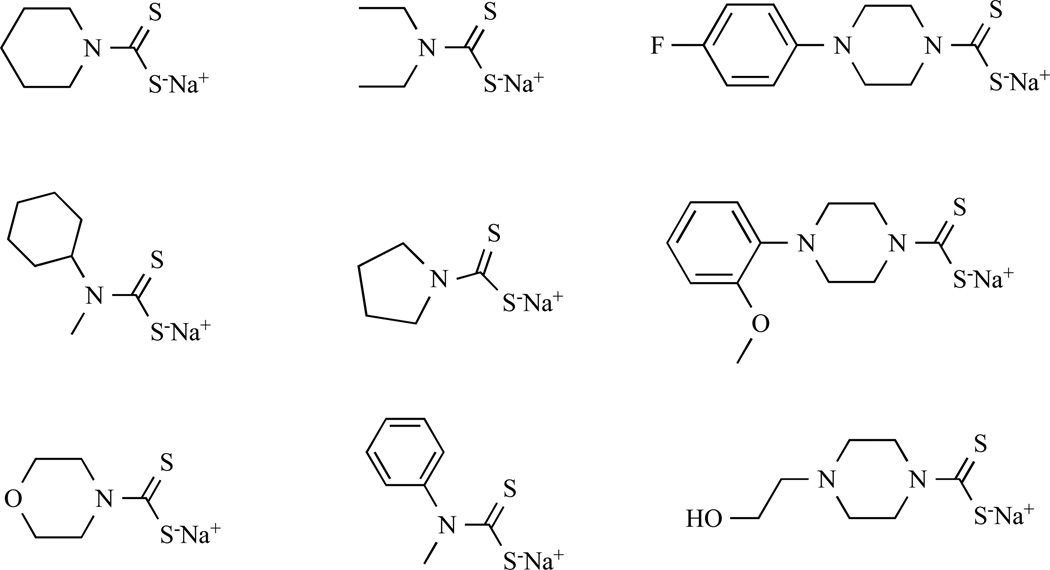
Dithiocarbamates (DTCs) are metal chelating and antioxidant compounds, Table 3. These compounds are usually used in treating fungal diseases and bacteria. More recently, researchers have found that various DTC compounds can induce metal uptake, especially copper and induce apotosis [108–110]. For example, Pyrrolidine dithiocarbamate (PDTC) is a synthetic antioxidant dithiocarbamate that has the potential to inhibit NFκB activation [111].
Table 3.
Anti-Cancer Copper Complexes as Proteasome Inhibitors
| Compounds | Target | Cell line | IC50/µM | Ref. |
|---|---|---|---|---|
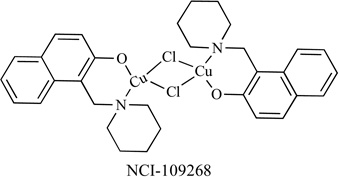 |
Proteasome Inhibitor | Jurkat T HL-60 PC-3, YT |
6 <6 <6 |
[114] |
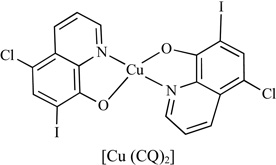 |
Proteasome Inhibitor | MCF10DCIS.com MDA-MB-231 LNCaP and C4-2B |
- | [115] |
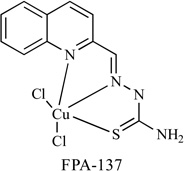 |
Proteasome Inhibitor | PC-3 LNCaP |
4 3.2 |
[116] |
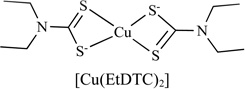 |
Proteasome Inhibitor | MDA-MB-231 | - | [98] |
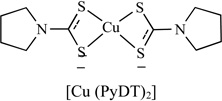 |
Proteasome Inhibitor | MDA-MB-231 | 1 | [117] |
 |
DNA | HepG 2 HeLa A549 Eca-109 |
10 6 8 3.5 |
[118] |
 |
DNA | HL-60 BGC-823 MDA-MB-435 |
- | [119] |
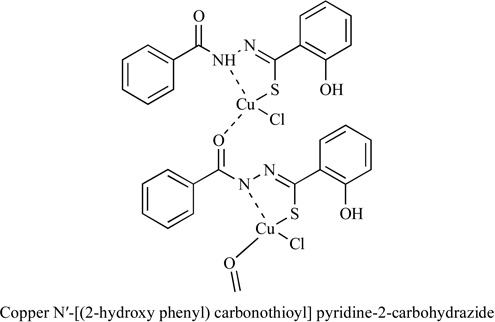 |
NMT | HT29 | 12.2 | [120] |
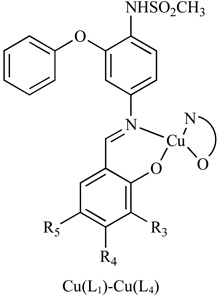 |
VEGF and COX | BxPC-3 MiaPaCa |
3–26 5–9 |
[121] |
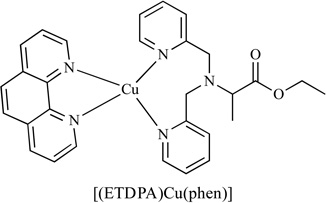
Copper (II) complexes of ethyl 2-[bis(2-pyridylmethyl) amino]propionate ligand (ETDPA) have been reported to have anti-cancer activity [107], Table 3. The DNA-binding properties of stable complexes [(ETDPA)CuCl2] and [(ETDPA)Cu(phen)](ClO4)2 were investigated. Results showed that the binding mode of these two copper complexes was different. The biological assay showed that the anti-cancer activity of [(ETDPA)Cu(phen)](ClO4)2 is more active than [(ETDPA)CuCl2].
5.2. High-Throughput Chemistry to Discover Proteasome Inhibitors
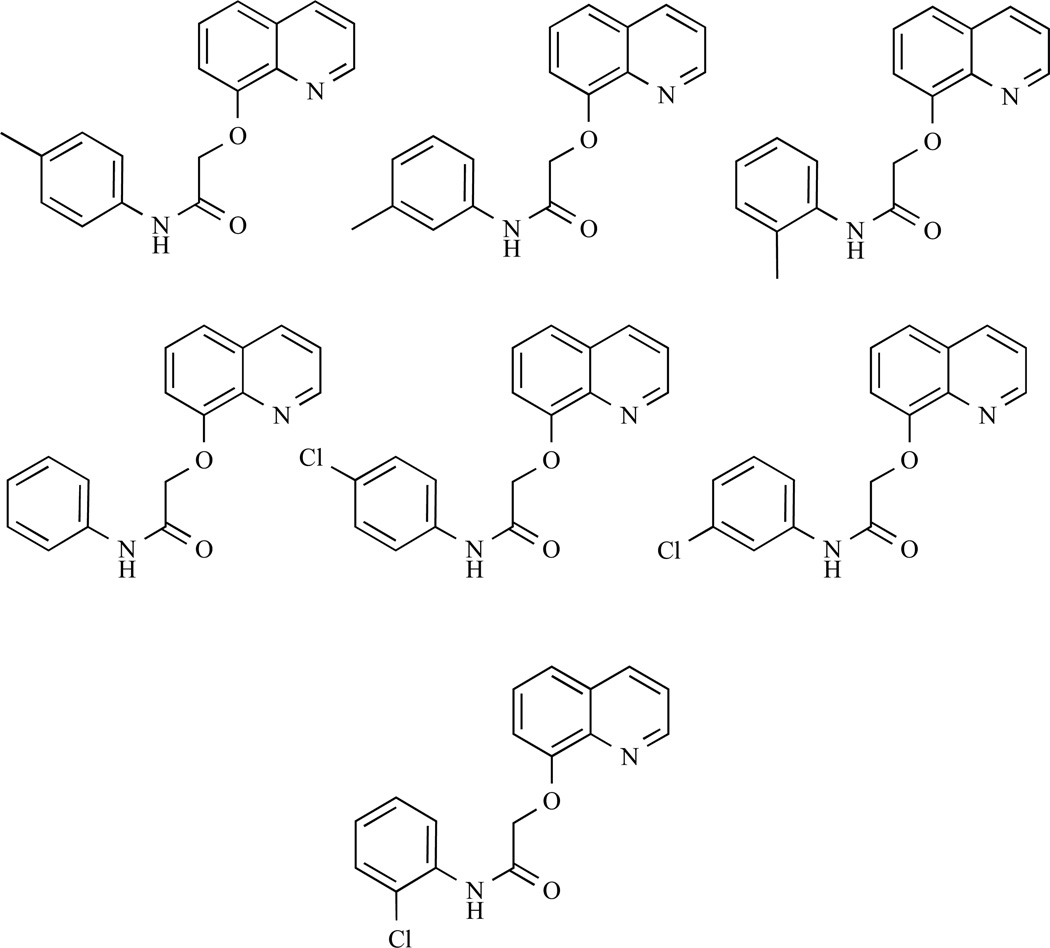
In another project, we have reported synthesis of a novel 64-member library of 8-hydroxylquinoline analogues using a parallel synthesis platform with their copper complexes. We evaluated their biological activities in human breast cancer cells [112], Fig. (6) and found that the polarity of the substituents used for synthesis of these 8-hydroxylquinoline analogues is important for their ability to bind copper and form complexes with anti-proliferative, proteasome-inhibitory, and apoptosis-inducing activities. When the hydroxyl group of 8-hydroxylquinoline was substituted with N-phenylacetamide, the potency of its copper mixture dramatically decreased. When an electron-withdrawing chlorine was attached to the phenyl ring on the N-phenylacetamide substituents, their copper mixtures were not active. However, when an electron-donating methyl group was attached to the phenyl ring on the N-phenylacetamide substituent, the activities of 8-hydroxylquinoline were largely intact. From these observations, we concluded that an electron-donating group on the phenyl ring of the N-phenylacetamide substituent is required for the anti-proliferative activity.
Fig. 6.
Copper mixtures with 8-hydroxylquinoline analogues.
We also applied a parallel synthesis technique to synthesize a PDTC library. Their copper-dependent proteasome-inhibitory and apoptosis-inducing activities [113] were reported. In this work, we modified substitutions on the pyrrolidine ring and studied their structure-activity relationships, Fig. (7). We found that the size and polarity of the ring within PDTC analogues are important for them to complex with copper. After the pyrrolidine ring was substituted with the more polar and larger groups, inhibition activity of the proliferation of breast cancer cells was decreased. We also showed that pyrrolidine substitution with piperidine had almost no effect on the proteasome-inhibitory and cell death-inducing activities. In conclusion, the nontoxic copper-binding DTCs can spontaneously bind tumor cellular copper and form a proteasome inhibitor and an apoptosis inducer. Our study strongly suggest that cancer cells and tissues, which contain elevated copper and are more dependent on proteasome activity for their survival, should be much more sensitive to such a treatment.
Fig. 7.
Structures of the PDTC analogues.
A library of 1:1 Schiff base copper complexes has been reported that these complexes have dose-dependent, antiproliferative, and proapoptotic activity in PC-3 and LNCaP prostate cancer cells [105]. In this library, FPA-137, Table 3, was the most potent proteasome inhibitor. The IC50 of PC-3 and LNCaP cells (4 and 3.2 µM, respectively) is lower than clioquinol and pyrrolidine dithiocarbamate.
5.3. Potential Computational Approach
In silico docking study provides an effective method to screen compounds virtually and design more potent proteasome inhibitors. Using crystal structures of proteasome subunits, molecular models can be built for investigating copper or other metal complexes. In one such model, proteasome binding and inhibition of chymotrypsin-like domain by triphenyl-tin (TPT) was studied [122]. The docking results indicate that TPT has a major docking mode which was repeated for 47 of 100 runs (47% probability). The distances from the Sn atom to nearby amino acids support the hypothesis of coordination between amino acids and the metal center. The molecular modeling in combination with a combinatorial library approach will provide more powerful tools to search for novel anti-cancer proteasome inhibitors.
CONCLUSION
Cancer tissues have a high level of copper that participate in cellular processes such as angiogenesis to promote cancer development. Organic compounds that bind copper provide useful tools to convert cancer-promoting copper to cancer-fighting agents. These copper complexes can serve as anti-angiogenesis agents or or ROS generators to inhibit tumor growth. These agents can also target cellular proteins such as the proteasome to inhibit cancer cell proliferation. Taken together, medicinal chemistry, computation modeling, and combinatorial chemistry can provide powerful tools to explore chemical space and structure-activity relationships in order to optimize and develop more potent and specific anti-cancer copper ligands.
Fig. 8.
A computer model of the chymotrypsin-like site in the cellular proteasome and the docking of triphenyl tin in the active site. Reproduced with permission from Environmental Health Perspectives.
ACKNOWLEDGEMENTS
This work was supported by Shandong University, National Cancer Institute (P30CA027165 to BY), the American Lebanese Syrian Associated Charities (ALSAC) and St. Jude Children’s Research Hospital to BY, and research funds from the Karmanos Cancer Institute of Wayne State University (to QPD), the Department of Defense Breast Cancer Research Program (W81XWH-04-1-0688, DAMD17-03-1-0175, to QPD) and the National Cancer Institute (1R01CA120009, 1R21CA139386-01, to QPD).
REFERENCES
- 1.Gregoriadis GC, Apostolidis NS, Romanos AN, Paradellis TP. A comparative study of trace elements in normal and cancerous colorectal tissues. Cancer. 1983;52:508–519. doi: 10.1002/1097-0142(19830801)52:3<508::aid-cncr2820520322>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 2.O'Dell BL. Biochemistry of copper. Med. Clin. North. Am. 1976;60:687–703. doi: 10.1016/s0025-7125(16)31853-3. [DOI] [PubMed] [Google Scholar]
- 3.Tapiero H, Townsend DM, Tew KD. Trace elements in human physiology and pathology. Copper. Biomed. Pharmacother. 2003;57:386–398. doi: 10.1016/s0753-3322(03)00012-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Weiss KC, Linder MC. Copper transport in rats involving a new plasma protein. Amer. J. Physiol-Endocrinol Met. 1985;249:77–88. doi: 10.1152/ajpendo.1985.249.1.E77. [DOI] [PubMed] [Google Scholar]
- 5.Raju KS, Alessandri G, Ziche M, Gullino PM. Ceruloplasmin, copper ions, and angiogenesis. J. Nat. Cancer. Inst. 1982;69:1183–1188. [PubMed] [Google Scholar]
- 6.Pena MMO, Lee J, Thiele DJ. A Delicate Balance: Homeostatic Control of Copper Uptake and Distribution 1. J. Nutr. 1999;129:1251–1260. doi: 10.1093/jn/129.7.1251. [DOI] [PubMed] [Google Scholar]
- 7.Crichton RR, Ward RJ. Metal-based neurodegeneration: from molecular mechanisms to therapeutic strategies. John Wiley and sons, Incorporated; 2006. [Google Scholar]
- 8.Tanzi RE, Petrukhin K, Chernov I, Pellequer JL, Wasco W, Ross B, Romano DM, Parano E, Pavone L, Brzustowicz LM. The Wilson disease gene is a copper transporting ATPase with homology to the Menkes disease gene. Nat. Genet. 1993;5:344–350. doi: 10.1038/ng1293-344. [DOI] [PubMed] [Google Scholar]
- 9.Brewer GJ, Yuzbasiyan-Gurkan V. Wilson disease. Medicine. 1992;71:139–164. doi: 10.1097/00005792-199205000-00004. [DOI] [PubMed] [Google Scholar]
- 10.Bull PC, Thomas GR, Rommens JM, Forbes JR, Cox DW. The Wilson disease gene is a putative copper transporting P-type ATPase similar to the Menkes gene. Nat. Genet. 1993;5:327–337. doi: 10.1038/ng1293-327. [DOI] [PubMed] [Google Scholar]
- 11.Goka TJ, Stevenson RE, Hefferan PM, Howell RR. Menkes disease: a biochemical abnormality in cultured human fibroblasts. Proc. Nat. Acad. Sci. 1976;73:604–606. doi: 10.1073/pnas.73.2.604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brewer GJ. Anticopper therapy against cancer and diseases of inflammation and fibrosis. Drug Discov. Today. 2005;10:1103–1109. doi: 10.1016/S1359-6446(05)03541-5. [DOI] [PubMed] [Google Scholar]
- 13.Goodman VL, Brewer GJ, Merajver SD. Copper deficiency as an anti-cancer strategy. Endocr-Related Cancer. 2004;11:255–263. doi: 10.1677/erc.0.0110255. [DOI] [PubMed] [Google Scholar]
- 14.Lowndes SA, Harris AL. The role of copper in tumour angiogenesis. J. Mammary Gland Biol. Meoplasi. 2005;10:299–310. doi: 10.1007/s10911-006-9003-7. [DOI] [PubMed] [Google Scholar]
- 15.Apelgot S, Coppey J, Fromentin A, Guille E, Poupon MF, Roussel A. Altered distribution of copper (64Cu) in tumor-bearing mice and rats. Anticancer Res. 1986;6:159–164. [PubMed] [Google Scholar]
- 16.Coates RJ, Weiss NS, Daling JR, Rettmer RL, Warnick GR. Cancer risk in relation to serum copper levels. Cancer Res. 1989;49:4353–4356. [PubMed] [Google Scholar]
- 17.Gupta SK, Shukla VK, Vaidya MP, Roy SK, Gupta S. Serum trace elements and Cu/Zn ratio in breast cancer patients. J. Surg. Oncol. 1991;46:178–181. doi: 10.1002/jso.2930460311. [DOI] [PubMed] [Google Scholar]
- 18.Campbell CH, Brown R, Linder MC. Circulating ceruloplasmin is an important source of copper for normal and malignant animal cells. Biochim Biophys Acta. 1981;678:27–38. doi: 10.1016/0304-4165(81)90044-1. [DOI] [PubMed] [Google Scholar]
- 19.Wirth PL, Linder MC. Distribution of copper among components of human serum. J Natl Cancer Inst. 1985;75:277–284. [PubMed] [Google Scholar]
- 20.Linder MC. Biochemistry of copper. Plenum Pub Corp; 1991. [Google Scholar]
- 21.Folkman J. Tumor angiogenesis: therapeutic implications. N Engl J Med. 1971;285:1182–1186. doi: 10.1056/NEJM197111182852108. [DOI] [PubMed] [Google Scholar]
- 22.Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- 23.Bairey D, Blickstein D, Shaklai M. [Tumor angiogenesis--prognostic and therapeutic implications] Harefuah. 1997;132:117–120. [PubMed] [Google Scholar]
- 24.Pluda JM. Tumor-associated angiogenesis: mechanisms, clinical implications, and therapeutic strategies. Semin Oncol. 1997;24:203–218. [PubMed] [Google Scholar]
- 25.Anna Nasulewicza AM. Adam Opolskia Role of copper in tumour angiogenesis-clinical implications. J. Trace. Elem. Med. Biol. 2004;18:1–8. doi: 10.1016/j.jtemb.2004.02.004. [DOI] [PubMed] [Google Scholar]
- 26.Carmeliet P JR. Angiogenesis in cancer and other diseases. Nature. 2000;407:249–257. doi: 10.1038/35025220. [DOI] [PubMed] [Google Scholar]
- 27.McAuslan BR, Reilly W. Endothelial cell phagokinesis in response to specific metal ions. Exp. Cell Res. 1980;130:147–157. doi: 10.1016/0014-4827(80)90051-8. [DOI] [PubMed] [Google Scholar]
- 28.Hu G. Copper stimulates proliferation of human endothelial cells under culture. J. Cell Biochem. 1998;69:326–335. doi: 10.1002/(sici)1097-4644(19980601)69:3<326::aid-jcb10>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 29.Manders P, Beex L, Tjan-Heijnen VCG, Geurts-Moespot J, Van Tienoven TH, Foekens JA, Sweep CGJ. The prognostic value of vascular endothelial growth factor in 574 node-negative breast cancer patients who did not receive adjuvant systemic therapy. Brit. J. Cancer. 2002;87:772–778. doi: 10.1038/sj.bjc.6600555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Berns EMJJ, Klijn JGM, Look MP, Grebenchtchikov N, Vossen R, Peters H, Geurts-Moespot A, Portengen H, van Staveren IL, Meijer-van Gelder ME. Combined Vascular Endothelial Growth Factor and TP53 Status Predicts Poor Response to Tamoxifen Therapy in Estrogen Receptor-positive Advanced Breast Cancer 1. Clin. Cancer Res. 2003;9:1253–1258. [PubMed] [Google Scholar]
- 31.Manders P, Beex LVAM, Tjan-Heijnen VCG, Span PN, Sweep CFGJ. Vascular endothelial growth factor is associated with the efficacy of endocrine therapy in patients with advanced breast carcinoma. Cancer. 2003;98:2125–2132. doi: 10.1002/cncr.11764. [DOI] [PubMed] [Google Scholar]
- 32.Miller KD, Chap LI, Holmes FA, Cobleigh MA, Marcom PK, Fehrenbacher L, Dickler M, Overmoyer BA, Reimann JD, Sing AP. Randomized phase III trial of capecitabine compared with bevacizumab plus capecitabine in patients with previously treated metastatic breast cancer. J. Clin. Oncol. 2005;23:792–799. doi: 10.1200/JCO.2005.05.098. [DOI] [PubMed] [Google Scholar]
- 33.Hurwitz H, Fehrenbacher L, Novotny W, Cartwright T, Hainsworth J, Heim W, Berlin J, Baron A, Griffing S, Holmgren E. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N. Engl. J. Med. 2004;350:2335–2342. doi: 10.1056/NEJMoa032691. [DOI] [PubMed] [Google Scholar]
- 34.Yang JC, Haworth L, Sherry RM, Hwu P, Schwartzentruber DJ, Topalian SL, Steinberg SM, Chen HX, Rosenberg SA. A randomized trial of bevacizumab, an anti-vascular endothelial growth factor antibody, for metastatic renal cancer. N. Engl. J. Med. 2003;349:427–434. doi: 10.1056/NEJMoa021491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Harper J, Moses MA. Molecular regulation of tumor angiogenesis: mechanisms and therapeutic implications. EXS. 2006:223–268. doi: 10.1007/3-7643-7378-4_10. [DOI] [PubMed] [Google Scholar]
- 36.Papetti M, Herman IM. Mechanisms of normal and tumor-derived angiogenesis. Amer. J. Physiol-Cell Physiol. 2002;282:947–970. doi: 10.1152/ajpcell.00389.2001. [DOI] [PubMed] [Google Scholar]
- 37.De Palma M, Murdoch C, Venneri MA, Naldini L, Lewis CE. Tie2-expressing monocytes: regulation of tumor angiogenesis and therapeutic implications. Trends Immunol. 2007;28:519–524. doi: 10.1016/j.it.2007.09.004. [DOI] [PubMed] [Google Scholar]
- 38.Malonne H, Langer I, Kiss R, Atassi G. Mechanisms of tumor angiogenesis and therapeutic implications: angiogenesis inhibitors. Clin Exp Metastasis. 1999;17:1–14. doi: 10.1023/a:1026443925807. [DOI] [PubMed] [Google Scholar]
- 39.Rockwell S, Knisely JP. Hypoxia and angiogenesis in experimental tumor models: therapeutic implications. EXS. 1997;79:335–360. doi: 10.1007/978-3-0348-9006-9_14. [DOI] [PubMed] [Google Scholar]
- 40.Parke A, Bhattacherjee P, Palmer RM, Lazarus NR. Characterization and quantification of copper sulfate-induced vascularization of the rabbit cornea. Amer. J. Pathol. 1988;130:173–178. [PMC free article] [PubMed] [Google Scholar]
- 41.Brem SS, Zagzag D, Tsanaclis AM, Gately S, Elkouby MP, Brien SE. Inhibition of angiogenesis and tumor growth in the brain. Suppression of endothelial cell turnover by penicillamine and the depletion of copper, an angiogenic cofactor. Amer. J. Pathol. 1990;137:1121–1142. [PMC free article] [PubMed] [Google Scholar]
- 42.Pan Q, Kleer CG, van Golen KL, Irani J, Bottema KM, Bias C, De Carvalho M, Mesri EA, Robins DM, Dick RD. Copper Deficiency Induced by Tetrathiomolybdate Suppresses Tumor Growth and Angiogenesis 1. Cancer Research. 2002;62:4854–4859. [PubMed] [Google Scholar]
- 43.Soncin F, Guitton JD, Cartwright T, Badet J. Interaction of human angiogenin with copper modulates angiogenin binding to endothelial cells. Biocem. Biophys. Res. Commun. 1997;236:604–610. doi: 10.1006/bbrc.1997.7018. [DOI] [PubMed] [Google Scholar]
- 44.Brewer GJ, Dick R, Ullenbruch MR, Jin H, Phan SH. Inhibition of key cytokines by tetrathiomolybdate in the bleomycin model of pulmonary fibrosis. J. Inorg. Biochem. 2004;98:2160–2167. doi: 10.1016/j.jinorgbio.2004.10.006. [DOI] [PubMed] [Google Scholar]
- 45.Satake HSK, Aoki T, Otsuka M, Sugiura Y, Yamamoto T, Inoue J. Cupric Ion Blocks NFκB Activation Through Inhibiting The Signal-Induced Phosphorylation of IκB. Biochem. Biophys. Res. Commun. 1995;216:568–573. doi: 10.1006/bbrc.1995.2660. [DOI] [PubMed] [Google Scholar]
- 46.Maziere C AM, Djavaheri-Mergny M, Packer L, Maziere JC. Oxidized low density lipoproreininduces activation of the transcription factor NFκB in fibroblasts, endothelial and smooth muscle cells. Biochem. Mol. Biol. Int. 1996;39:1201–1207. doi: 10.1080/15216549600201392. [DOI] [PubMed] [Google Scholar]
- 47.Brewer GJ, Dick RD, Johnson V, Wang Y, Yuzbasiyan-Gurkan V, Kluin K, Fink JK, Aisen A. Treatment of Wilson's disease with ammonium tetrathiomolybdate. I Initial therapy in 17 neurologically affected patients. Arch. Neurol. 1994;51:545–554. doi: 10.1001/archneur.1994.00540180023009. [DOI] [PubMed] [Google Scholar]
- 48.Brewer GJ. Wilson’s disease. Curr. Treat Options Neurol. 2000;2:193–203. doi: 10.1007/s11940-000-0002-5. [DOI] [PubMed] [Google Scholar]
- 49.Clarke P. Developmental cell death: morphological diversity and multiple mechanisms. Anat. Embryol. 1990;181:195–213. doi: 10.1007/BF00174615. [DOI] [PubMed] [Google Scholar]
- 50.Gupte A, Mumper RJ. Elevated copper and oxidative stress in cancer cells as a target for cancer treatment. Cancer Treat Rev. 2009;35:32–46. doi: 10.1016/j.ctrv.2008.07.004. [DOI] [PubMed] [Google Scholar]
- 51.Camphausen K, Sproull M, Tantama S, Sankineni S, Scott T, Ménard C, Coleman CN, Brechbiel MW. Evaluation of copper chelation agents as anti-angiogenic therapy. Bioorgan. Med. Chem. 2003;11:4287–4293. doi: 10.1016/s0968-0896(03)00401-2. [DOI] [PubMed] [Google Scholar]
- 52.Yoshida D, Ikeda Y, Nakazawa S. Copper chelation inhibits tumor angiogenesis in the experimental 9L gliosarcoma model. Neurosurgery. 1995;37:287–293. doi: 10.1227/00006123-199508000-00014. [DOI] [PubMed] [Google Scholar]
- 53.Matsubara T, Saura R, Hirohata K, Ziff M. Inhibition of Human Endothelial Cell Proliferation in vitro and Neovascularization In vivo by D-Penicillarnine. J. Clin. Invest. 1989;83:158–167. doi: 10.1172/JCI113853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Brem S, Grossman SA, Carson KA, New P, Phuphanich S, Alavi JB, Mikkelsen T, Fisher JD. Phase 2 trial of copper depletion and penicillamine as antiangiogenesis therapy of glioblastoma. Neuro-Oncology. 2005;7:246–249. doi: 10.1215/S1152851704000869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Brewer GJ. Copper lowering therapy with tetrathiomolybdate produces antiangiogenic, anticancer, antifibrotic, and antiinflammatory effects. Semin. Integr. Med. 2003;1:181–190. [Google Scholar]
- 56.Brewer GJ. Copper-lowering therapy with tetrathiomolybdate for cancer and diseases of fibrosis and inflammation The University of Michigan has recently licensed the antiangiogenic uses of TM to Attenuon LLC, and Dr. Brewer has equity in Attenuon LLC. J. Trace Elem. Exp. Med. 2003;16:191–199. [Google Scholar]
- 57.Brewer GJ, Dick RD, Grover DK, LeClaire V, Tseng M, Wicha M, Pienta K, Redman BG, Jahan T, Sondak VK. Treatment of Metastatic Cancer with Tetrathiomolybdate, an Anti-copper, Antiangiogenic Agent: Phase I Study 1. Clin. Cancer Res. 2000;6:1–10. [PubMed] [Google Scholar]
- 58.Pan Q, Bao LW, Kleer CG, Brewer GJ, Merajver SD. Antiangiogenic Tetrathiomolybdate Enhance the Efficacy of Doxorubicin against Breast Carcinoma 1. Mol. Cancer Ther. 2003;2:617–622. [PubMed] [Google Scholar]
- 59.Khan MK, Miller MW, Taylor J, Gill NK, Dick RD, Van K. Radiotherapy and antiangiogenic TM in lung cancer. Neoplasia. 2002;4:164–170. doi: 10.1038/sj.neo.7900218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Pass HI, Brewer GJ, Dick R, Carbone M, Merajver S. A phase II trial of tetrathiomolybdate after surgery for malignant mesothelioma: final results. Ann. Thorac. Surg. 2008;86:383–390. doi: 10.1016/j.athoracsur.2008.03.016. [DOI] [PubMed] [Google Scholar]
- 61.Botha CJ, Swan GE, Minnaar PP. Pharmacokinetics of ammonium tetrathiomolybdate following intravenous administration in sheep. J. S. Afr. Vet. Assoc. 1995;66:6–10. [PubMed] [Google Scholar]
- 62.Kotsaki-Kovatsi VP, Koehler-Samouilidis G, Kovatsis A, Rozos G. Fluctuation of zinc, copper, magnesium and calcium concentrations in guinea pig tissues after administration of Captopril(SQ 14225) J. Trace Elem. Med. Biol. 1997;11:32–36. doi: 10.1016/S0946-672X(97)80007-7. [DOI] [PubMed] [Google Scholar]
- 63.Richer C, Giroux B, Plouin PF, Maarek B, Giudicelli JF. Captopril: pharmacokinetics, antihypertensive and biological effects in hypertensive patients. B r. J. Clin. Pharmacol. 1984;17:243–250. doi: 10.1111/j.1365-2125.1984.tb02338.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Moriguchi M, Nakajima T, Kimura H, Watanabe T, Takashima H, Mitsumoto Y, Katagishi T, Okanoue T, Kagawa K. The copper chelator trientine has an antiangiogenic effect against hepatocellular carcinoma, possibly through inhibition of interleukin-8 production. Int. J. Cancer. 2002;102:445–452. doi: 10.1002/ijc.10740. [DOI] [PubMed] [Google Scholar]
- 65.Yoshii J, Yoshiji H, Kuriyama S, Ikenaka Y, Noguchi R, Okuda H, Tsujinoue H, Nakatani T, Kishida H, Nakae D. The copper-chelating agent, trientine, suppresses tumor development and angiogenesis in the murine hepatocellular carcinoma cells. Int. J. Cancer. 2001;94:768–773. doi: 10.1002/ijc.1537. [DOI] [PubMed] [Google Scholar]
- 66.Yoshiji H, Kuriyama S, Yoshii J, Ikenaka Y, Noguchi R, Yanase K, Namisaki T, Yamazaki M, Tsujinoue H, Imazu H. The copper-chelating agent, trientine, attenuates liver enzymealtered preneoplastic lesions in rats by angiogenesis suppression. Oncol. Rep. 2003;10:1369–1373. [PubMed] [Google Scholar]
- 67.Tanabe R. Disposition behavior and absorption mechanism of trientine, an orphan drug for Wilson's disease. Hokkaido Igaku Zasshi. 1996;71:217–228. [PubMed] [Google Scholar]
- 68.Bondiolotti G, Sala M, Pollera C, Gervasoni M, Puricelli M, Ponti W, Bareggi SR. Pharmacokinetics and distribution of clioquinol in golden hamsters. J. Pharm. Pharmacol. 2007;59:387–393. doi: 10.1211/jpp.59.3.0008. [DOI] [PubMed] [Google Scholar]
- 69.Ding WQ, Liu B, Vaught JL, Yamauchi H, Lind SE. Anti-cancer activity of the antibiotic clioquinol. Cancer Res. 2005;65:3389–3395. doi: 10.1158/0008-5472.CAN-04-3577. [DOI] [PubMed] [Google Scholar]
- 70.Halliwell B, Aruoma O. DNA damage by oxygen-derived species. Its mechanism and measurement in mammalian systems. FEBS letters. 1991;281:9–19. doi: 10.1016/0014-5793(91)80347-6. [DOI] [PubMed] [Google Scholar]
- 71.Pecheur ML, Bourdon E, Paly E, Farout L, Friguet B, London J. Oxidized SOD1 alters proteasome activities in vitro and in the cortex of SOD1 overexpressing mice. FEBS letters. 2005;579:3613–3618. doi: 10.1016/j.febslet.2005.05.048. [DOI] [PubMed] [Google Scholar]
- 72.Valko M, Leibfritz D, Moncol J, Cronin M, Mazur M, Telser J. Free radicals and antioxidants in normal physiological functions and human disease. Int. J. Biochem. Cell Biol. 2007;39:44–84. doi: 10.1016/j.biocel.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 73.Beretta S, Sala G, Mattavelli L, Ceresa C, Casciati A, Ferri A, Carrì MT, Ferrarese C. Mitochondrial dysfunction due to mutant copper/zinc superoxide dismutase associated with amyotrophic lateral sclerosis is reversed by N-acetylcysteine. Neurobiol. Disease. 2003;13:213–221. doi: 10.1016/s0969-9961(03)00043-3. [DOI] [PubMed] [Google Scholar]
- 74.Tardito S, Marchio L. Copper compounds in anticancer strategies. Curr Med Chem. 2009;16:1325–1348. doi: 10.2174/092986709787846532. [DOI] [PubMed] [Google Scholar]
- 75.Pelicano H, Carney D, Huang P. ROS stress in cancer cells and therapeutic implications. Drug Resist. Update. 2004;7:97–110. doi: 10.1016/j.drup.2004.01.004. [DOI] [PubMed] [Google Scholar]
- 76.Chaiswing L, Bourdeau-Heller J, Zhong W, Oberley T. Characterization of redox state of two human prostate carcinoma cell lines with different degrees of aggressiveness. Free Radical Biol. Med. 2007;43:202–215. doi: 10.1016/j.freeradbiomed.2007.03.031. [DOI] [PubMed] [Google Scholar]
- 77.Kong Q, Beel J, Lillehei K. A threshold concept for cancer therapy. Medical hypotheses. 2000;55:29–35. doi: 10.1054/mehy.1999.0982. [DOI] [PubMed] [Google Scholar]
- 78.McCarty M, Barroso-Aranda J, Contreras F. A two-phase strategy for treatment of oxidant-dependent cancers. Medical hypotheses. 2007;69:489–496. doi: 10.1016/j.mehy.2006.12.065. [DOI] [PubMed] [Google Scholar]
- 79.Kong Q, Lillehei K. Antioxidant inhibitors for cancer therapy. Med. Hypotheses. 1998;51:405–409. doi: 10.1016/s0306-9877(98)90036-6. [DOI] [PubMed] [Google Scholar]
- 80.Fang J, Nakamura H, Iyer A. Tumor-targeted induction of oxystress for cancer therapy. J. Drug Targeting. 2007;15:475–486. doi: 10.1080/10611860701498286. [DOI] [PubMed] [Google Scholar]
- 81.Starkebaum G, Root R. D-Penicillamine: analysis of the mechanism of copper-catalyzed hydrogen peroxide generation. J. Immunol. 1985;134:3371–3378. [PubMed] [Google Scholar]
- 82.Matsubara T, Saura R, Hirohata K, Ziff M. Inhibition of human endothelial cell proliferation in vitro and neovascularization in vivo by D-penicillamine. J. Clin. Invest. 1989;83:158–167. doi: 10.1172/JCI113853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Carvallo-Chaigneau F, Trejo-Solís C, Gómez-Ruiz C, Rodríguez-Aguilera E, Macías-Rosales L, Cortés-Barberena E, Cedillo-Peláez C, Gracia-Mora I, Ruiz-Azuara L, Madrid-Marina V. Casiopeina III-ia induces apoptosis in HCT-15 cells in vitro through caspase-dependent mechanisms and has antitumor effect in vivo . BioMetals. 2008;21:17–28. doi: 10.1007/s10534-007-9089-4. [DOI] [PubMed] [Google Scholar]
- 84.Trejo-Sol s C, Palencia G, Zúniga S, Rodr g-Ropon A, Osorio-Rico L, Luvia ST, Gracia-Mora I, Marquez-Rosado L, Sánchez A, Moreno-Garc á ME. Cas IIgly induces apoptosis in glioma C6 cells in vitro and in vivo through caspase-dependent and caspase-independent mechanisms. Neoplasia. 2005;7:563–574. doi: 10.1593/neo.04607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Liu MC, Lin TS, Cory JG, Cory AH, Sartorelli AC. Synthesis and biological activity of 3-and 5-amino derivatives of pyridine-2-carboxaldehyde thiosemicarbazone. J. Med. Chem. 1996;39:2586–2593. doi: 10.1021/jm9600454. [DOI] [PubMed] [Google Scholar]
- 86.Cory JG, Cory AH, Rappa G, Lorico A, Liu MC, Lin TS, Sartorelli AC. Inhibitors of ribonucleotide reductase. Comparative effects of amino-and hydroxy-substituted pyridine-2-carboxaldehyde thiosemicarbazones. Biochem Pharmacol. 1994;48:335–344. doi: 10.1016/0006-2952(94)90105-8. [DOI] [PubMed] [Google Scholar]
- 87.Agrawal S, Singh NK, Aggarwal RC, Sodhi A, Tandon P. Synthesis, structure, and antitumor activity of N-salicyloyl-N'-(2-furylthiocarbonyl) hydrazine and its copper (II) complex. J. Med. Chem. 1986;29:199–202. doi: 10.1021/jm00152a006. [DOI] [PubMed] [Google Scholar]
- 88.García-Tojal J, García-Orad A, Serra JL, Pizarro JL, Lezama L, Arriortua MI, Rojo T. Synthesis and spectroscopic properties of copper (II) complexes derived from thiophene-2-carbaldehyde thiosemicarbazone. Structure and biological activity of [Cu (C6H6N3S2) 2] J. Inorg. Biochem. 1999;75:45–54. doi: 10.1016/S0162-0134(99)00031-8. [DOI] [PubMed] [Google Scholar]
- 89.Sartorelli AC, Moore EC, Zedeck MS, Agrawal KC. Inhibition of ribonucleoside diphosphate reductase by 1-formylisoquinoline thiosemicarbazone and related compounds. Biochem. 1970;9:4492–4498. doi: 10.1021/bi00825a005. [DOI] [PubMed] [Google Scholar]
- 90.Thelander L, Graslund A. Mechanism of inhibition of mammalian ribonucleotide reductase by the iron chelate of 1-formylisoquinoline thiosemicarbazone. Destruction of the tyrosine free radical of the enzyme in an oxygen-requiring reaction. J. Biol. Chem. 1983;258:4063–4066. [PubMed] [Google Scholar]
- 91.Bymes RW, Mohan M, Antholine WE, Xu RX, Petering DH. Oxidative stress induced by a copper thiosemicarbazones complex. Biochem. 1990;29:7046–7053. doi: 10.1021/bi00482a014. [DOI] [PubMed] [Google Scholar]
- 92.Zhang H, Thomas R, Oupicky D, Peng F. Synthesis and characterization of new copper thiosemicarbazone complexes with an ONNS quadridentate system: cell growth inhibition, S-phase cell cycle arrest and proapoptotic activities on cisplatin-resistant neuroblastoma cells. J. Biol. Inorg. Chem. 2008;13:47–55. doi: 10.1007/s00775-007-0299-6. [DOI] [PubMed] [Google Scholar]
- 93.Marzano C, Gandin V, Pellei M, Colavito D, Papini G, Lobbia GG, Del Giudice E, Porchia M, Tisato F, Santini C. In vitro Antitumor Activity of the Water Soluble Copper (I) Complexes Bearing the Tris (hydroxymethyl) phosphine Ligand. J. Med. Chem. 2008;51:798–808. doi: 10.1021/jm701146c. [DOI] [PubMed] [Google Scholar]
- 94.Eytan E, Ganoth D, Armon T, Hershko A. ATP-dependent incorporation of 20S protease into the 26S complex that degrades proteins conjugated to ubiquitin. Proc. Nat. Acad. Sci. 1989;86:7751–7755. doi: 10.1073/pnas.86.20.7751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Hough R, Pratt G, Rechsteiner M. Purification of two high molecular weight proteases from rabbit reticulocyte lysate. J. Biol. Chem. 1987;262:8303–8313. [PubMed] [Google Scholar]
- 96.Voges D, Zwickl P, Baumeister W. The 26S proteasome: a molecular machine designed for controlled proteolysis. Annu. Rev. Biochem. 1999;68:1015–1068. doi: 10.1146/annurev.biochem.68.1.1015. [DOI] [PubMed] [Google Scholar]
- 97.Ciechanover A, Orian A, Schwartz AL. Ubiquitin-mediated proteolysis: biological regulation via destruction. Bioessays. 2000;22:442–451. doi: 10.1002/(SICI)1521-1878(200005)22:5<442::AID-BIES6>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 98.Cvek B, Milacic V, Taraba J, Dou Q. Ni (II), Cu (II), and Zn (II) Diethyldithiocarbamate Complexes Show Various Activities Against the Proteasome in Breast Cancer Cells. J. Med. Chem. 2008;51:6256–6258. doi: 10.1021/jm8007807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Catley L, Tai YT, Chauhan D, Anderson KC. Perspectives for combination therapy to overcome drug-resistant multiple myeloma. Drug Resist. Update. 2005;8:205–218. doi: 10.1016/j.drup.2005.06.001. [DOI] [PubMed] [Google Scholar]
- 100.Dou QP, Goldfarb RH. Bortezomib (millennium pharmaceuticals) IDrugs. 2002;5:828–834. [PubMed] [Google Scholar]
- 101.Goy A, Younes A, McLaughlin P, Pro B, Romaguera JE, Hagemeister F, Fayad L, Dang NH, Samaniego F, Wang M. Phase II study of proteasome inhibitor bortezomib in relapsed or refractory B-cell non-Hodgkin's lymphoma. J. Clin. Oncol. 2005;23:667–675. doi: 10.1200/JCO.2005.03.108. [DOI] [PubMed] [Google Scholar]
- 102.Richardson PG, Sonneveld P, Schuster MW, Irwin D, Stadtmauer EA, Facon T, Harousseau JL, Ben-Yehuda D, Lonial S, Goldschmidt H. Bortezomib or high-dose dexamethasone for relapsed multiple myeloma. N. Engl. J. Med. 2005;352:2487–2498. doi: 10.1056/NEJMoa043445. [DOI] [PubMed] [Google Scholar]
- 103.An B, Goldfarb RH, Siman R, Dou QP. Novel dipeptidyl proteasome inhibitors overcome Bcl-2 protective function and selectively accumulate the cyclin-dependent kinase inhibitor p27 and induce apoptosis in transformed, but not normal, human fibroblasts. Cell Death Differentiation. 1998;5:1062–1075. doi: 10.1038/sj.cdd.4400436. [DOI] [PubMed] [Google Scholar]
- 104.Soligo D, Servida F, Delia D, Fontanella E, Lamorte G, Caneva L, Fumiatti R, Lambertenghi Deliliers G. The apoptogenic response of human myeloid leukaemia cell lines and of normal and malignant haematopoietic progenitor cells to the proteasome inhibitor PSI. Brit. J. Haematol. 2001;113:126–135. doi: 10.1046/j.1365-2141.2001.02683.x. [DOI] [PubMed] [Google Scholar]
- 105.Chen D, Cui QC, Yang H, Barrea RA, Sarkar FH, Sheng S, Yan B, Reddy GP, Dou QP. Clioquinol, a therapeutic agent for Alzheimer's disease, has proteasome-inhibitory, androgen receptor-suppressing, apoptosis-inducing, and antitumor activities in human prostate cancer cells and xenografts. Cancer Res. 2007;67:1636–1644. doi: 10.1158/0008-5472.CAN-06-3546. [DOI] [PubMed] [Google Scholar]
- 106.Zhai S, Yang L, Cui CQ, Sun Y, Dou QP, Yan B. Tumor cellular proteasome inhibition and growth suppression by 8-hydroxylquinoline requires their capabilities to bind copper and transport copper into cells. J. Biol. Inorg. Chem. 2009 doi: 10.1007/s00775-009-0594-5. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Chen D, Cui QC, Yang H, Dou QP. Disulfiram, a clinically used anti-alcoholism drug and copper-binding agent, induces apoptotic cell death in breast cancer cultures and xenografts via inhibition of the proteasome activity. Cancer Res. 2006;66:10425–10433. doi: 10.1158/0008-5472.CAN-06-2126. [DOI] [PubMed] [Google Scholar]
- 108.Nobel CSI, Kimland M, Lind B, Orrenius S, Slater AFG. Dithiocarbamates induce apoptosis in thymocytes by raising the intracellular level of redox-active copper. J. Biol. Chem. 1995;270:26202–26208. doi: 10.1074/jbc.270.44.26202. [DOI] [PubMed] [Google Scholar]
- 109.Erl W, Weber C, Hansson GK. Pyrrolidine dithiocarbamate-induced apoptosis depends on cell type, density, and the presence of Cu2+ and Zn2+ Amer. J. Physiol-Cell Physiol. 2000;278:1116–1125. doi: 10.1152/ajpcell.2000.278.6.C1116. [DOI] [PubMed] [Google Scholar]
- 110.Chen SH, Liu SH, Liang YC, Lin JK, Lin-Shiau SY. Death signaling pathway induced by pyrrolidine dithiocarbamate-Cu2+ complex in the cultured rat cortical astrocytes. Glia. 2000;31:249–261. doi: 10.1002/1098-1136(200009)31:3<249::aid-glia60>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 111.Schreck R, Meier B, Mannel DN, Droge W, Baeuerle PA. Dithiocarbamates as potent inhibitors of nuclear factor kappa B activation in intact cells. J. Exp. Med. 1992;175:1181–1194. doi: 10.1084/jem.175.5.1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Milacic V, Jiao P, Zhang B, Yan B, Dou QP. Novel 8-hydroxylquinoline analogs induce copper-dependent ptoteasome inhibition and cell death in human breast cancer cells. Int. J. Oncol. 2009 doi: 10.3892/ijo_00000467. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Yu Z, Wang F, Milacic V, Li X, Cui QC, Zhang B, Yan B, Dou QP. Evaluation of copper-dependent proteasome-inhibitory and apoptosis-inducing activities of novel pyrrolidine dithiocarbamate analogues. Int. J. Mol. Med. 2007;20:919–925. [PubMed] [Google Scholar]
- 114.Daniel KG, Gupta P, Harbach RH, Guida WC, Dou QP. Organic copper complexes as a new class of proteasome inhibitors and apoptosis inducers in human cancer cells. Biochem. Pharmacol. 2004;67:1139–1151. doi: 10.1016/j.bcp.2003.10.031. [DOI] [PubMed] [Google Scholar]
- 115.Daniel K, Orlu S, Cui Q, Miller F, Dou QP. Clioquinol and pyrrolidine dithiocarbamate complex with copper to form proteasome inhibitors and apoptosis inducers in human breast cancer cells. Breast Cancer Res. 2005;7:897–908. doi: 10.1186/bcr1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Adsule S, Barve V, Ahmed F, Dou QP, Padhye S, Sarkar FH. Novel Schiff base copper complexes of quinoline-2 carboxaldehyde as proteasome inhibitors in human prostate cancer cells. J. Med. Chem. 2006;49:7242–7246. doi: 10.1021/jm060712l. [DOI] [PubMed] [Google Scholar]
- 117.Milacic V, Chen D, Giovagnini L, Diez A, Fregona D, Dou QP. Pyrrolidine dithiocarbamate-zinc (II) and-copper (II) complexes induce apoptosis in tumor cells by inhibiting the proteasomal activity. Toxicol. Appl. Pharmacol. 2008;231:24–33. doi: 10.1016/j.taap.2008.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Chen QY, Huang J, Guo WJ, Gao J. Synthesis, characterization, DNA interaction and cytotoxic activities of copper complexes with ethyl 2-[bis (2-pyridylmethyl) amino] propionate. Spectrochim. Acta. Pt. A- Mol. Bio. 2009;72:648–653. doi: 10.1016/j.saa.2008.11.006. [DOI] [PubMed] [Google Scholar]
- 119.Zhang H, Liu CS, Bu XH, Yang M. Synthesis, crystal structure, cytotoxic activity and DNA-binding properties of the copper (II) and zinc (II) complexes with 1-[3-(2-pyridyl) pyrazol-1-ylmethyl] naphthalene. J. Inorg. Biochem. 2005;99:1119–1125. doi: 10.1016/j.jinorgbio.2005.02.005. [DOI] [PubMed] [Google Scholar]
- 120.Shrivastav A, Singh NK, Tripathi P, George T, Dimmock JR, Sharma RK. Copper (II) and manganese (III) complexes of N′-[(2-hydroxy phenyl) carbonothioyl] pyridine-2-carbohydrazide: novel therapeutic agents for cancer. Biochimie. 2006;88:1209–1216. doi: 10.1016/j.biochi.2006.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Ambike V, Adsule S, Ahmed F, Wang Z, Afrasiabi Z, Sinn E, Sarkar F, Padhye S. Copper conjugates of nimesulide Schiff bases targeting VEGF, COX and Bcl-2 in pancreatic cancer cells. J. Inorg. Biochem. 2007;101:1517–1524. doi: 10.1016/j.jinorgbio.2007.06.028. [DOI] [PubMed] [Google Scholar]
- 122.Shi G, Chen D, Zhai G, Chen MS, Cui QC, Zhou Q, He B, Dou QP, Jiang G. The proteasome is a molecular target of environmental toxic organotins. Environ. Health Perspect. 2009;117:379–386. doi: 10.1289/ehp.11865. [DOI] [PMC free article] [PubMed] [Google Scholar]