Abstract
Baumycins are coproduced with clinically important anticancer secondary metabolites daunorubicin and doxorubicin, which are glycosylated anthracyclines isolated from Streptomyces peucetius. The distinguishing feature of baumycins is the presence of an unusual acetal moiety appended to daunosamine, which is hydrolyzed during acidic extraction of daunorubicin from fermentation broth. The structure of the baumycin acetal suggests that it is likely derived from an unknown C-3” methyl deoxysugar cleaved between the C-3” and C-4” positions. This is supported by analysis of the baumycin/daunorubicin biosynthetic gene cluster (dox), which also encodes putative proteins consistent with production of an anthracycline dissacharide containing a branched sugar. Notably, the dnmZ gene in the dox gene cluster possesses high translated sequence similarity to nitrososynthases, which are flavin-dependent amine monooxygenases involved in the four electron oxidation of aminosugars to nitrososugars. Herein we demonstrate that DnmZ is an amino sugar nitrososynthase initiating the conversion of thymidine-5’-diphosphate-l-epi-vancosamine to a ring opened product via a previously uncharacterized retro oxime-aldol reaction.
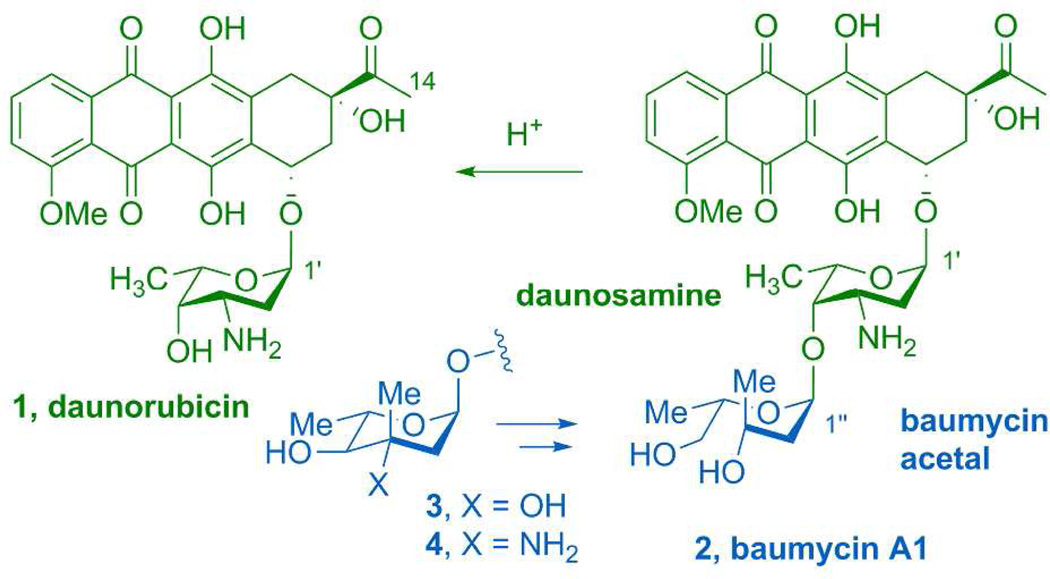
Daunorubicin (1) and its C-14 hydroxylated congener doxorubicin (adriamycin) are archetypal anthracycline natural products that were first entered into clinical practice in 1967 and remain important agents for the treatment of a wide variety of cancers.1,2 Perhaps less widely appreciated is that these anthracyclines are actually chemical degradation products of a group of more elaborated secondary metabolites, the baumycins (2), which possess distinct, potent, and selective antibiotic and antiproliferative properties (Figure 1).3,4 The distinguishing feature of baumycins is an unusual acetal moiety of unknown biosynthetic origin linked at the C-4’ oxygen of daunosamine. Notably, the baumycin acetal is removed during acidic extraction of the fermentation broth of the producing organism Streptomyces peucetius.5 The substitution pattern of this six-carbon baumycin acetal suggests that it likely originates from a deoxy sugar progenitor via oxidative cleavage of a vicinal diol of the hexose ring. However, the nature of the baumycin sugar precursor and the pathway for its transformation into the acid-labile baumycin acetal has never been addressed.
Figure 1.
The baumycin family of natural products includes baumycin A1 (2) which forms daunorubicin (1) upon acid hydrolysis of the acetal moiety (blue in 2). The structure of the acetal moiety and the dox gene cluster suggests that the baumycin acetal is derived from oxidative cleavage of a C3-methyl sugar such as 3 or 4.
As a result of the clinical importance of doxorubicin, dissecting its biosynthesis in S. peucetius has attracted significant attention over the past decades. The identification of the dox biosynthetic gene cluster by the Hutchinson group along with targeted gene disruption experiments demonstrate that the anthracycline core is the product of a type II polyketide synthase and various oxidative and methylating tailoring enzymes.5,6 A glycosyltranferase encoded by dnmS appends 3-amino-2,3,6-trideoxysugar daunosamine to the anthracycline aglycone. An additional glycosyltransferase encoded by dnrH has been linked by gene inactivation experiments to the formation of acid sensitive baumycins.7
We have recently characterized orf36 from the everninomicin gene cluster in Micromonospora carbonacea var. africana, which encodes a nitrososynthase catalyzing the oxidation of an amino sugar to its nitroso congener via two consecutive flavin-dependent monooxygenation reactions.8 Analogous enzymes are found in the biosynthetic pathways of rubradirin9 and kijanimicin10 generating structurally similar nitrososugars. These nitrososugars then serve as the precursors for nitrosugar moieties in natural products of which they are constituents. Biochemical and X-ray structural studies demonstrate that these nitrososynthases can utilize FADH2 or FMNH2 and act on anomeric thymidine diphosphate (TDP) functionalized aminosugar precursors.11,12 Surprising to us during these endeavors was the finding of a putative gene in dox gene cluster, dnmZ, with high translated sequence similarity to that of orf36 (59/70% identity/similarity). This observation was puzzling since daunorubicin and doxorubicin do not possess a nitro(so)sugar. However, analysis of the dox gene cluster reveals a number of additional genes possibly involved in the biosynthesis of a C-3-methylated TDP-deoxysugar.
This information prompted us to hypothesize that the putative glycosyltransferase DnrH attaches an unidentified C3-methylated deoxysugar (e.g., 3 or 4 where X = OH or NH2, respectively) to daunorubicin (1) and that DnmZ is responsible for the oxidative cleavage reaction converting the putative sugar to the acetal appendage in baumycin A1 (2).13 We initially envisioned that a likely substrate of DnmZ is TDP-l-mycarose (Supplementary Figure S3a), which could be oxidized at the 3-position to form a hydroperoxide intermediate, in analogy with the formation of the N-hydroxylamine intermediate from TDP-l-epi-vancosamine (4) in the first step of nitrososynthase-catalyzed reaction. The resulting 3’-hydroperoxide 4’-hydroxy compound could then undergo C-C bond cleavage via a Criegee-type pathway14 resulting in a dicarbonyl progenitor of the baumycin acetal.
To test this proposal, the genes encoding the DnmZ and DnrH proteins were cloned, overexpressed with N-terminal hexahistidine tags in E. coli, and the resulting proteins were purified by nickel affinity chromatography. TDP-l-mycarose (3) was prepared in vitro using reconstituted enzymes from the tylosin pathway from Streptomyces fradiae.15 On incubation of TDP-l-mycarose (3) with DnmZ under typical nitrososynthase reaction conditions, no new products were observed. Furthermore, we also assayed TDP-l-mycarose as a substrate for the putative glycosyltransferase DnrH and found no evidence of daunorubicin or doxorubicin glycosylation.
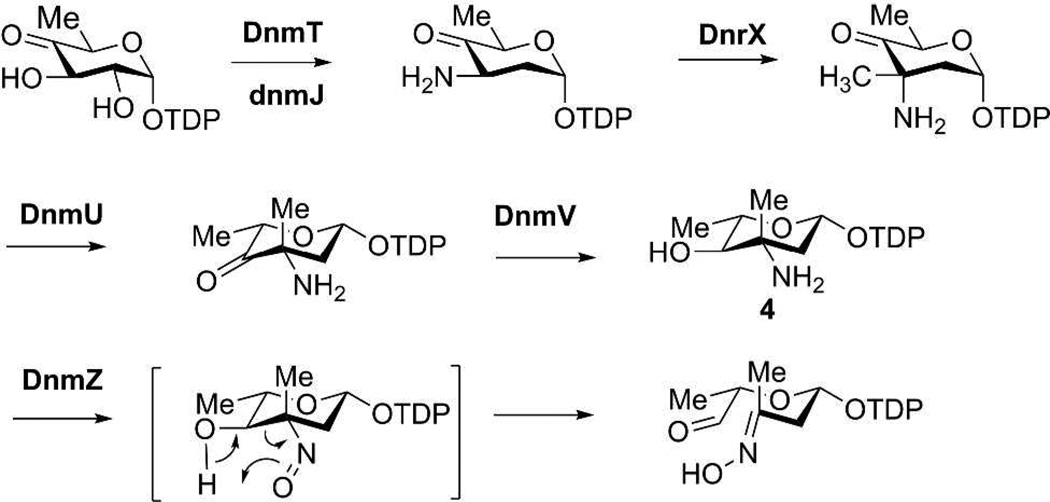
Subsequently, we considered the possibility that the enzymes encoded by the additional sugar biosynthetic genes in the dox gene cluster are responsible for producing TDP-l-epi-vancosamine itself. Indeed, close inspection of these genes revealed them to be fully consistent with the previously described biosynthesis of TDP-l-epi-vancosamine in the chloroeremomicin pathway.16 Specifically, dnrX encodes a putative protein with high sequence similarity (77%) to a C3-methyltransferase, dnmU to a C-5 epimerase (81%), and dnmV to a C-4-ketoreductase (65%) in the epi-vancosmaine pathway (Figure 2). Since the proposed C-C bond cleavage of the retro oxime-aldol reaction occurs at the site of oxidation catalyzed by the nitrososynthase homolog, DnmZ, the final product of its reaction with TDP-l-epi-vancosamine was expected to be an aldehyde-oxime species (Figure 2).
Figure 2.
Proposed role for DnmZ nitrososynthase in baumycin acetal biosynthetic pathway.
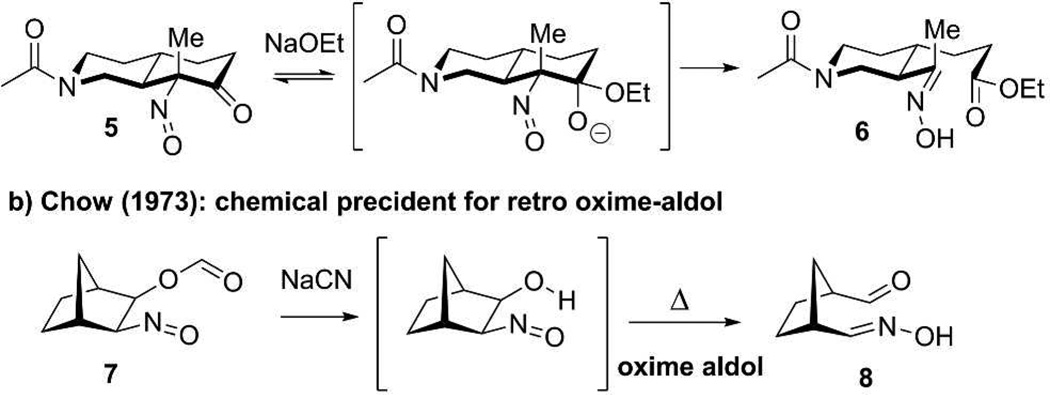
Exploration of the chemical precedent for this ring cleavage transformation reveals two notable entries (Figure 3). Firstly, the work of R.B Woodward in his total synthesis of quinine from 7-hydroxyisoquinoline reveals that a nitroso α–hemiacetal anion of 5 is primed to rapidly cleave to 6 via a retro oxime-Claisen type reaction.17 Of close relevance to this work, Chow and coworkers reported that mild nucleophillic hydrolysis of 2-nitroso-3-formyloxybicyclo[2.2.1]-heptane (7) results in a vicinal hydroxy nitrosospecies that fragments to form the aldehyde-oxime functionalized cyclopentane 8.18 These chemical precedents suggest a similar fragmentation may affect the key cleavage in the formation of baumycin acetal.
Figure 3.
Chemical precedents for nitroso triggered C-C bond fragmentation.
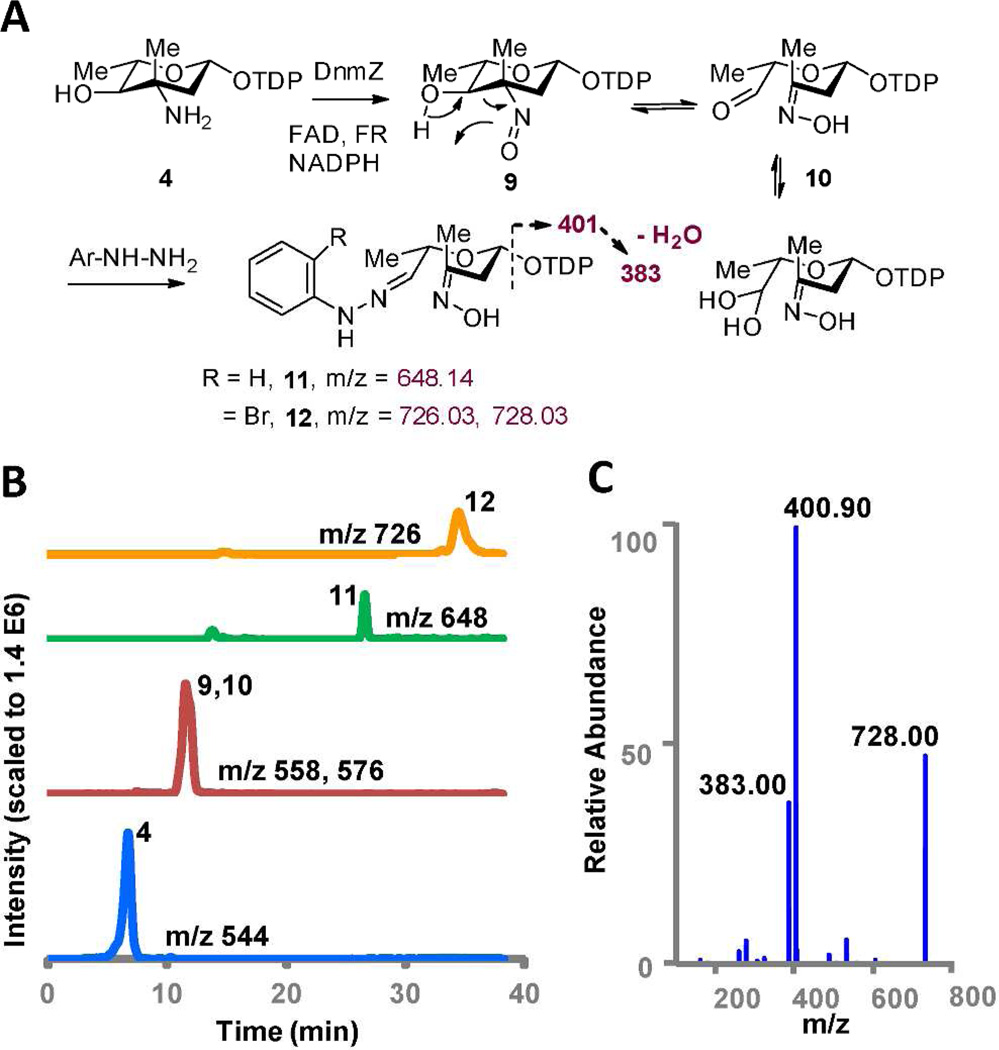
To verify the function of DnmZ, TDP-l-epi-vancosamine (4) was generated from TDP-d-glucose using a six-enzyme multistep sequence, as previously described.16 On incubation of TDP-l-epi-vancosamine with DnmZ, FAD, NADPH, and flavin reductase, we observed the predicted nitroso product of TDP-l-epi-vancosamine (m/z = 558) and an additional chromatographically distinct compound (m/z = 576) corresponding to a hydrated species (Figure 4). The rapid turnover of TDP-l-epi-vancosamine suggested this compound, or a closely related analog, is the substrate for DnmZ.
Figure 4.
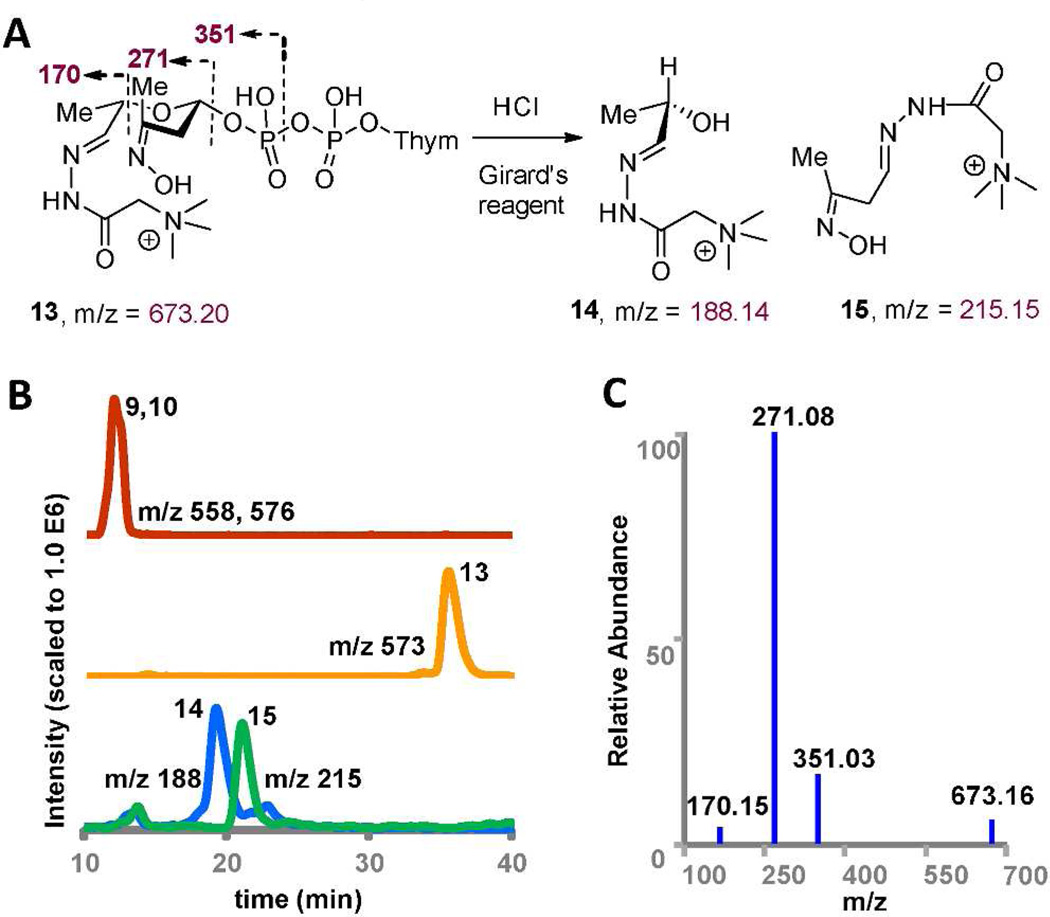
A. Reaction of TDP-L-epi-vancosamine 4 with DnmZ, FAD, Vibrio flavin reductase (FR) and NADPH and subsequent hydrazone derivatization of the aldehyde-oxime product. B. Extracted ion chromatograms of reaction with TDP-L-epi-vancosamine (blue) with DnmZ (red) and separate reactions with added phenyl- and 2-bromophenylhydrazine (green, orange). C. MS/MS spectra of hydrazone adduct 12 (selected precursor ion m/z = 728.03).
These data are consistent with the expectation that the resulting vicinal hydroxy nitrososugar (9) would undergo cleavage via a retro oxime-aldol reaction to 10 in accordance with chemical precedent. Unfortunately, the resulting putative aldehyde-oxime species 10 was found to be highly unstable under reversed phase and ion exchange chromatographic conditions and thus could not be isolated for direct structural characterization. To provide evidence supporting the structure of the aldehyde-oxime 10, we therefore treated 10 in situ with different hydrazine reagents including phenylhydrazine, bromophenylhydrazine, and (carboxymethyl)-trimethyl-ammonium chloride hydrazide (Girard’s reagent) at the end of the DnmZ reaction.19 All hydrazines reacted smoothly with aldehyde 10 to generate the expected hydrazones as confirmed by accurate mass determination (Figure 4, Supplementary Figure S15). MS/MS analysis of the bromophenyl hydrazone species 12 confirmed the fragmentation of the TDP group as observed in negative-ion mode.
The Girard’s hydrazide adduct 13 facilitated tandem mass analysis in positive-ion mode and fragmentation data was fully consistent with the proposed structure. Finally, we subjected the Girard’s hydrazide adduct to acid catalyzed cleavage of the acetals to generate two aldehyde functional fragments, which we captured in situ as the di-hydrazides (14) and 15. As expected, we observed the two derivatized acetal fragments 14 and 15 whose structures are confirmed by accurate mass determination and MS/MS analysis (Figure 5 and Supplementary Figure S15). These results strongly suggest that DnmZ is a flavin-dependent monooxygenase generating a vicinal hydroxy nitroso species which undergoes a subsequent catalyzed or uncatalyzed retro oxime aldol cleavage reaction.
Figure 5.
A. Acid catalyzed degradation of aldehyde-oxime acetal phosphate Girard’s adduct 13 derived from 10. B. HPLC/MS extracted ion chromatograms for DnmZ reaction (red) with Girard reagent (orange) and the HCl degradation products (blue and green). C. MS/MS of hydrazide 13 (selected precursor ion m/z = 673).
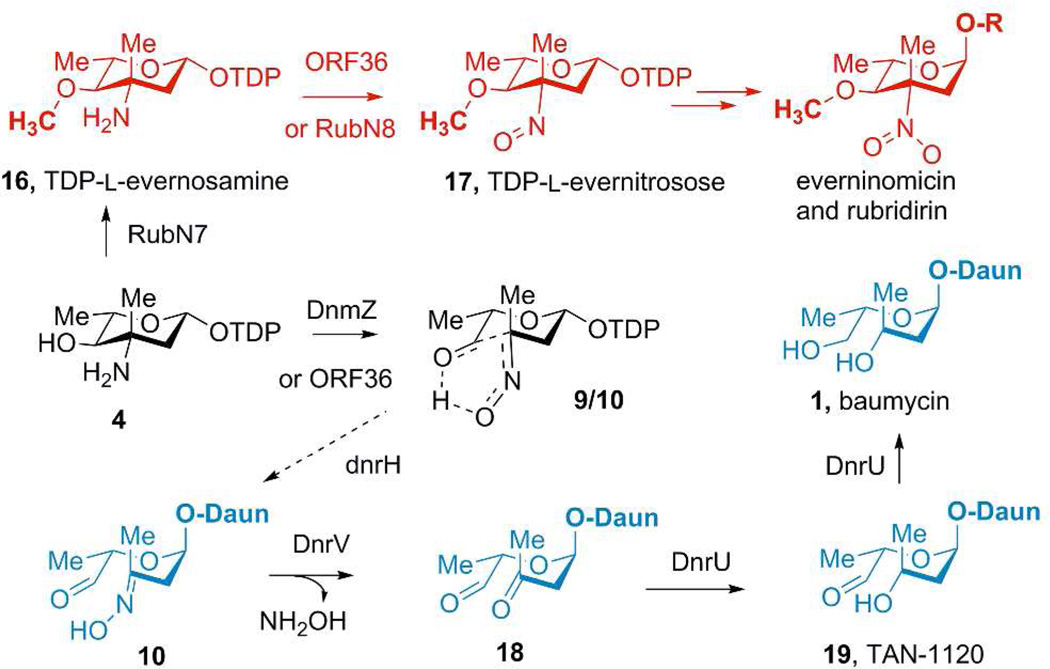
The apparent oxime-aldol reaction, observed subsequent to DnmZ catalyzed nitrososynthase reaction described herein, offers some insights into the biosynthesis of the orthoester antibiotic everninomicin, polyketide rubradirin and the baumycins.9,10,20 In our previous studies of nitrosugar formation in the everninomicin/rubradirin pathways, the nitrososynthase activity of ORF36/RubN8 was established using TDP-l-epi-vancosamine (4) as substrate, which is a biosynthetic precursor to the expected substrate TDP-l-evernosamine (16). We observed the oxime-aldehyde hydrated species (10) (m/z = 576) but were unable to account for its structure in the context of nitrosugar formation.12 The evidence presented herein for the equilibrium between the nitroso and aldehyde-oxime rearrangement products 9/10 helps clarify this unresolved question and demonstrates that oxidation of 4 results in an equilibrium mixture of 9/10 for the nitrososynthase in everninomicin biosynthesis. The new DnmZ data suggests that the true substrate of ORF36 is presumably TDP-l-evernosamine (16), which differs from TDPl-epi-vancosamine (4) by 4-O- methylation (Figure 6), and therefore would be protected from this retro oxime-aldol cleavage reaction.21
Figure 6.
Importance of nitrosylation of TDP-L-epi-vancosamine (4) to everninomicin/rubradirin and baumycin biosynthesis. Red route shows the late stages of evernitrosose formation in which methylation blocks C-C bond cleavage. R designates the everninomicin or rubradirin aglycones. Blue route is a possible pathway for the biosynthesis of baumycin (1) via the known metabolite TAN-1120, which exists in solution as the hemiaminal of (19) with daunosamine.
To test this prediction, we next cloned, overexpressed and purified RubN7, the putative methyltransferase for 4-O-methylation of TDP-l-epi-vancosamine in the rubradirin pathway.9 Upon incubation of TDP-l-epi-vancosamine (4) with SAM and RubN7, we observed the formation of TDP-l-evernosamine (16). On further incubation with ORF36/DnmZ, the formation of TDP-l-evernitrosose (17) was observed and turnover with DnmZ reactions with TDP-l-evernitrosose were slower than with ORF36 (see Supplementary materials S9). These results demonstrate that methylation state of the 4-hydroxyl group of TDP-l-epi-vancosamine determines the fate of this oxidation reaction towards a nitrosugar or the baumycin acetal pathway.
While our results show that the precursor for the acetal in the baumycin pathway is l-epi-vancosamine, questions remain as to the reaction sequence transforming the aldehyde-oxime 10 to the fully reduced baumycin acetal. While highly speculative at this point, it is possible to envision a biosynthetic pathway for baumycin biosynthesis branching from the daunorubicin pathway (Figure 6). Formally, the oxime in 10 must be hydrolyzed to the ketone and both carbonyls in 18 must be reduced to their corresponding alcohols. Of note, the potent angiostatic secondary metabolite TAN-1120 (19) from S. triangulates is a previously isolated member of the baumycin family in which the aldehyde functionality is not reduced. Instead, it is observed as a hemiaminal with the adjacent daunosomine 4’-amine. The existence of TAN-1120 suggests that it may be a biosynthetic intermediate and glycosylation precedes final reduction in the baumycin pathway.3 As revealed by the dox gene cluster, the hydrolysis may be catalyzed by DnrV, a putative hydrolase related to the bleomycin resistance determinant, and DnrU, a putative aldehyde dehydrogenase, may be responsible for one or more of the requisite carbonyl reduction reactions.
Also remaining to be determined is the timing of glycosylation of the progenitor for the baumycin acetal. Incubation of daunorubicin and TDP-l-epi-vancosamine with the putative glycosyltransferase DnrH in the presence and absence of nitrososynthase DnmZ did not yield any new products. This could be due to the anthracycline substrate of DnrH being an upstream precursor of daunorubicin (e.g. rhodomycin-D), or that the donor acetal is one of the more reduced acetal diphosphates.
In summary, this study demonstrates the unexpected role of the nitrososynthase class of flavin monooxygenase in a previously uninvestigated pathway for deoxysugar C3-C4 bond cleaving biochemistry. The enzyme-catalyzed oxidation of TDP-l-epi-vancosamine to its vicinal hydroxy nitrosocongener triggers C-C bond cleavage. The overall rearrangement of the hydroxy nitroso sugar to the aldehyde-oxime product is formally that of a retro oxime-aldol transformation. Future studies will ascertain if the cleavage reaction is enzymatically catalyzed or spontaneous. In either case, from a mechanistic standpoint the cleavage bears a resemblance to class I aldolase enzymes, which activate the retro aldol cleavage via formation of a lysine iminium ion at the 2-keto position to open fructose between C3 and C4.22 In the retro oxime-aldol mechanism, the polarized nitroso functional group is the surrogate for the iminium group of an aldolase. To the best of our knowledge, this study provides the first evidence of a deoxysugar C-C cleavage initiating enzyme and expands our understanding into the late steps of baumycin biosynthesis, which is now functionally linked by this study to the biosynthesis of daunorubicin and doxorubicin in S. peucetius. From a broader perspective, it is conceivable that oxidation of aminosugars by reactive oxygen species could result in the generation of transient vicinal hydroxy nitrososugars. Based on the results presented here, this provides a potential mechanism of oxidative deglycosylation of amino sugar functional oligosaccharides via oxidative burst metabolism.
Supplementary Material
ACKNOWLEDGMENT
This work was supported by the Vanderbilt Institute of Chemical Biology, Office of Naval Research Grant N00014-09-1-012 to B.O.B., National Institutes of Health Grants GM092218 to B.O.B. and GM035906 to H.-w.L., and the Welch Foundation Grant F-1511 to H.-w.L
Footnotes
ASSOCIATED CONTENT
Supporting Information
Supporting Information is available pertaining to enzymological methods and spectral characterization. This material is available free of charge via the Internet at http://pubs.acs.org.
Notes
The authors declare no competing financial interests.
REFERENCES
- 1.Minotti G, Menna P, Salvatorelli E, Cairo G, Gianni L. Pharm. Rev. 2004;56:185. doi: 10.1124/pr.56.2.6. [DOI] [PubMed] [Google Scholar]
- 2.Piekarski M, Jelinska A. Mini Rev. Med. Chem. 2013;13:627. doi: 10.2174/1389557511313050001. [DOI] [PubMed] [Google Scholar]
- 3.Nozaki Y, Hida T, Iinuma S, Ishii T, Sudo K, Muroi M, Kanamaru T. J. Antibiot. 1993;46:569. doi: 10.7164/antibiotics.46.569. [DOI] [PubMed] [Google Scholar]
- 4.Takahashi Y, Naganawa H, Takeuchi T, Umezawa H, Komiyana T, Oki T, Iui T. J. Antibiot. 1977;30:622. doi: 10.7164/antibiotics.30.622. [DOI] [PubMed] [Google Scholar]
- 5.Lomovskaya N, Otten SL, Doi-Katayama Y, Fonstein L, Liu XC, Takatsu T, Inventi-Solari A, Filippini S, Torti F, Colombo AL, Hutchinson CR. J. Bacteriol. 1999;181:305. doi: 10.1128/jb.181.1.305-318.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hutchinson CR. Chem. Rev. 1997;97:2525. doi: 10.1021/cr960022x. [DOI] [PubMed] [Google Scholar]
- 7.Scotti C, Hutchinson CR. J. Bacteriol. 1996;178:7316. doi: 10.1128/jb.178.24.7316-7321.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hu Y, Al-Mestarihi A, Grimes CL, Kahne D, Bachmann BO. J. Am. Chem. Soc. 2008;130:15756. doi: 10.1021/ja8051415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim CG, Lamichhane J, Song KI, Nguyen VD, Kim DH, Jeong TS, Kang SH, Kim KW, Maharjan J, Hong YS, Kang JS, Yoo JC, Lee JJ, Oh TJ, Liou K, Sohng JK. Arch. Microbiol. 2008;189:463. doi: 10.1007/s00203-007-0337-3. [DOI] [PubMed] [Google Scholar]
- 10.Zhang H, White-Phillip JA, Melancon CE, 3rd, Kwon HJ, Yu WL, Liu H-w. J. Am. Chem. Soc. 2007;129:14670. doi: 10.1021/ja0744854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bruender NA, Thoden JB, Holden HM. Biochemistry. 2010;49:3517. doi: 10.1021/bi100318v. [DOI] [PubMed] [Google Scholar]
- 12.Vey JL, Al-Mestarihi A, Hu Y, Funk MA, Bachmann BO, Iverson TM. Biochemistry. 2010;49:9306. doi: 10.1021/bi101336u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Takahashi H, Liu Y-n, Chen H, Liu H-w. J. Am. Chem. Soc. 2005;127:9340. doi: 10.1021/ja051409x. [DOI] [PubMed] [Google Scholar]
- 14.Criegee R. Angew. Chem. Int. Edit. 1975;14:745. [Google Scholar]
- 15.Takahashi H, Liu YN, Liu H-w. J. Am. Chem. Soc. 2006;128:1432. doi: 10.1021/ja0562144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen H, Thomas MG, Hubbard BK, Losey HC, Walsh CT, Burkart MD. Proc. Natl. Acad. Sci. U. S. A. 2000;97:11942. doi: 10.1073/pnas.210395097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Woodward RB, Doering WE. J. Am. Chem. Soc. 1944;66:849. [Google Scholar]
- 18.Chow YL, Chen SC, Mojelsky T. J. Chem. Comm. 1973:827. [Google Scholar]
- 19.Johnson DW. Rapid. Commun. Mass. Spectrom. 2007;21:2926. doi: 10.1002/rcm.3175. [DOI] [PubMed] [Google Scholar]
- 20.Weinstein MJ, Luedemann GM, Oden EM, Wagman GH. Antimicrob. Agents. Chemother. 1964;10:24. [PubMed] [Google Scholar]
- 21.Ganguly AK, Saksena AK. J. Antibiot. 1975;28:707. [PubMed] [Google Scholar]
- 22.Fushinobu S, Nishimasu H, Hattori D, Song HJ, Wakagi T. Nature. 2011;478:538. doi: 10.1038/nature10457. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.