Abstract
Objective
Hypercholesterolemia is associated with decreased vascular nitric oxide bioavailability and deletion of endothelial nitric oxide synthase (eNOS) markedly accelerates atherosclerosis development in apolipoprotein E knockout (apoE ko) mice. The current study tests whether atheroprotection provided by a lipid lowering therapy with Ezetimibe depends on eNOS.
Methods/Results
ApoE ko and apoE/eNOS double ko (dko) mice received a high fat diet with or without 0.05% Ezetimibe. Ezetimibe significantly reduced plasma cholesterol concentrations and atherogenic lipoproteins in both genotypes to a similar extent. Moreover, the drug reduced vascular inflammation, as it significantly reduced Vascular Cell Adhesion Molecule-1 (VCAM-1) expression and vascular CD14 expression, a marker for mononuclear cell infiltration, in both genotypes. Neither NOS protein expression nor vascular reactivity of aortic rings were changed in apoE ko mice following Ezetimibe treatment. Significant lesion reduction was seen in Ezetimibe treated male and female apoE ko and apoE/eNOS dko animals (p≤0.05). Interestingly, the drug mediated additional atheroprotection in male apoE ko, compared to male eNOS dko mice, suggesting that lipid lowering does provide additional eNOS dependent atheroprotection in this experimental group.
Conclusion
Lipid lowering with Ezetimibe potently reduces atherosclerosis and vascular inflammation independent of eNOS. Moreover, Ezetimibe did not exert any effects on eNOS protein expression or enzyme activity. However, additional atheroprotection by Ezetimibe was observed in eNOS competent apoE ko mice, suggesting that some of the drug's antiatherosclerotic effects are mediated by the eNOS pathway.
Introduction
Atherosclerosis, the primary cause of coronary artery disease (CAD) and stroke, is a complex inflammatory disease that involves multiple cell types, genes and environmental factors1, 2. Elevated plasma cholesterol levels and low-density-lipoprotein cholesterol (LDL-C) in particular are independent predictors of CAD. In addition, hyperlipidemia reduces the bioavailability of eNOS derived nitric oxide (NO), thereby increasing the risk for vascular disease. Potential reasons are eNOS-substrate (L-arginine) or -cofactor (tetrahydrobiopterin) deficiency, enhanced degradation of NO, or altered membrane signalling3, 4. Lipid lowering therapy with 3-hydroxy-3methyl-glutaryl(HMG)-CoA reductase inhibitors (statins) causes a significant reduction in mortality from CAD in primary and secondary prevention studies and improves endothelial dependent, NO mediated vasorelaxation5-10. Importantly, endothelial dysfunction is a predictor for cardiovascular complications in patients suffering from CAD 11, 12. Nitric oxide was identified to mediate endothelium dependent relaxation of arterial smooth muscle cells and impaired NO bioavailability is of major importance for the development of endothelial dysfunction13-15.
Statins antagonize the enzyme HMG-CoA reductase, which catalyzes the rate limiting step in cholesterol synthesis and consequently lower plasma non-high density lipoproteins. In addition, statins influence cellular pathways potentially protecting from atherosclerosis, which are independent of their lipid lowering properties. These “pleiotropic” effects include increased expression of eNOS through improved mRNA stability, and increased activity of eNOS through induction of phosphorylation via the phosphatoinositol-3 kinase (PI3K)/protein kinase AKT pathway16, 17. However, correction of hyperlipidemia through dietary interventions, treatment with cholestyramine and lipid apharesis also improves endothelial dysfunction18-20. Since lipid lowering and pleiotropic effects of statins can not be easily separated it is difficult to asses the importance of statin mediated eNOS modulation on its atheroprotective properties in vivo18-21.
Compared to statins, Ezetimibe belongs to a new class of cholesterol lowering drugs which is not known to mediate pleiotropic effects. Ezetimibe inhibits cholesterol uptake as well as its reuptake from the enterohepatic cycle in the mucosa of the small intestine, where its direct molecular target was recently identified as the Niemann-Pick-C1-Like1 (NPC1L1) protein22, 23. Results from preclinical and clinical studies have demonstrated that Ezetimibe potently lowers plasma cholesterol and favourably modulates lipoprotein profiles24-26. In a previous study, Ezetimibe prevented atherosclerosis development in apoE ko mice when the drug was given early on with a high fat “western” diet (WD), a low fat diet, or a cholesterol free diet27. In this study inhibition of intestinal cholesterol absorption by Ezetimibe was similar in C57/Bl6 wildtype and apoE ko mice. ApoE ko atherosclerosis closely resembles human disease with regards to lesion distribution and can be substantially accelerated by atherogenic diets. ApoE is an important component of the reverse cholesterol transport pathway and is an essential ligand for the uptake and clearance of atherogenic lipoproteins28. Genetic deletion of apoE in mice, a species normally resistant to atherosclerosis, is associated with severe hypercholesterolemia induced plaque development. Although the pathomechanism differs from common human disease, the apoE ko model has substantially shaped our understanding of the role of apoE in lipid transport and proved to be a valid atherosclerosis model29.
Our current study tests whether the anti-atherosclerotic effects provided by a lipid lowering therapy with Ezetimibe depend on intact eNOS. We compared the effects of Ezetimibe on lesion development and vascular inflammation in apoE ko mice with apoE/eNOS dko mice. Vascular reactivity was assessed in apoE ko and apoE/eNOS dko mice. As previously reported from our laboratory, apoE/eNOS dko mice show a dramatic increase in atherosclerosis development compared to apoE ko controls30, 31. We hypothesise that protection from atherosclerosis by Ezetimibe is directly mediated by the drug's lipid lowering effects. However, even if Ezetimibe does not regulate eNOS expression or enzyme activity, lipid lowering itself could improve local NO bioavailability and therefore some of Ezetimibe's positive effects could be mediated through the eNOS pathway.
Methods
All procedures performed conformed with the policies of the University of Würzburg, the US National Institute of Health (Publication No.85-23, revised 1996) guidelines and an independent governmental committee for care and use of laboratory animals.
Mice
Animals were backcrossed for 10 generations to the C57/Bl6 genetic background. eNOS ko mice, provided by Paul Huang32, and apoE ko (Jackson Laboratories, Bar Harbor, ME33) mice were crossed to generate double heterozygous mice. C57/Bl6 mice were ordered from the Jackson laboratories. Offsprings were crossed and the progenies were genotyped for eNOS by southern blotting and for apoE by polymerase chain reaction using a PCR protocol provided by the Jackson Laboratories. apoE ko and apoE/eNOS dko animals were weaned at 21 days and fed a Western-type diet (42% of total calories from fat; 0.15% cholesterol; Harlan Teklad) for 10 weeks. At this point the diet was switched to a western-type diet containing 0.005% Ezetimibe (EZ; Shering Plough Research Institute, Kenilworth, NJ; diet custom made by Harlan Teklad), or western-diet without Ezetimibe (WD), and maintained for another 8 weeks. Davis et al. calculated the dose of Ezetimibe consumed by apoE ko mice on the above mentioned diet as 5.31-5.93 mg/kg/day. The dose is higher than the one commonly used in humans (10 mg/day) since Ezetimibe is less potent in apoE ko mice compared to other species27.
Lesion assessment
The aorta was dissected and analyzed as previously described31, 34. Animals were anesthetized with pentobarbital (80 μg/kg i.p.), the aorta was perfused with PBS, pH 7.4, dissected from the aortic valve to the iliac bifurcation and fixed in 4% paraformaldehyde. Adventitial tissue was removed and the aorta was opened longitudinally and pinned onto a black wax surface. Serial images were captured with a black and white video camera (COHU) mounted on a stereomicroscope (Leica). Lipid rich intraluminal lesions were stained with Sudan IV. Serial color pictures, captured with a Leica 35mm camera, were used to identify lesions. Image analysis was performed using Image Pro Plus (Version 4.1; Media Cybernetics). The amount of aortic lesion formation in each animal was measured as percent lesion area per total area of the aorta.
Blood pressure measurements
Mice were intubated and ventilated with a rodent respirator (stroke volume 250 μl, rate 90 min-1). The common carotid artery was cannulated and a Millar catheter (1.4F) was advanced into the left ventricle. Blood pressure and heart rate were recorded under light isoflurane anesthesia.
Western blot
The protein expression of eNOS, nNOS and iNOS were analysed in apoE ko and apoE/eNOS dko animals fed with western diet and Ezetimibe. Electrophoresis and immunoblotting were performed using an anti-eNOS, anti-nNOS (BD Biosciences) and anti-iNOS (Santa Cruz Biotechnology, Inc) antibodies. We used a VCAM-1 antibody (R&D systems) to assess the expression of VCAM-1 in the aorta of apoE ko (WD: n=14; EZ: n=10) and apoE/eNOS dko (WD: n=9; EZ: n=4) mice. The same blots were labelled with an anti α-actin antibody (Santa Cruz Biotechnology, Inc). The expression levels of target proteins were analysed densitrometrically using Scan Pack (Biometra, Gottingen, Germany) and the protein signals were normalised to the expression of α-actin.
Real time PCR
First-strand cDNA was synthesized from 2 μg of total RNA using random primers (Fermentas). To determine monocyte recruitment into the vessel wall, mRNA expression of CD14 was quantified in apoE ko (WD:n=17;Ezetimibe:n=4) and apoE/eNOS dko animals (WD:n=12;Ezetimibe:n=5) by real-time PCR (iCycler, Bio-Rad Laboratories, USA). PCR amplification was performed for 40 cycles at primer annealing of 60°C. Target gene expression was normalized to HPRT signal. CD14 sense: 5′-TAC CGA CCA TGG AGC GTG TG-3′ and antisense: 5′-GCC GGT TAC CTC GAG ATT TT-3′. HPRT sense: 5′- GTT GGA TAC AGG CCA GAC TTT GT-3′ and antisense: 5′- CCA CAG GAC TAG AAC ACC TGC-3′. Fluorogenic probes for CD14: 5′-6FAM-TGT TGC TTC TGG TGC ACG CCT CT-TMR; HPRT: 5′-6FAM-CTC GTA TTT GCA GAT TCA ACT TGC GC XT-PH were used (TIB Molbiol, Germany).
Vascular reactivity studies
Studies were performed in apoE ko animals fed either Ezetimibe (female n=12; male n=15) or the western diet alone (female n=11; male n=16) and in apoE/eNOS dko animals on the western diet (male: n=7). The descending thoracic aorta, which commonly displays the lowest plaque burden of the vessel, was dissected and cleaned from connective tissue. Each vessel was cut into 3 mm rings and mounted in an organ bath (FMI, Seeheim, Germany) for isometric force measurements. Following a 30 min equilibration at a resting tension of 1.25 g in oxygenated (95% O2, 5% CO2) Krebs-Henseleit buffer (NaCl 118 mmol/L, KCl 4.7 mmol/L, MgSO4 1.2 mmol/L, CaCl2 1.6 mmol/L, K2HPO4 1.2 mmol/L, NaHCO3 25 mmol/L, Glucose 12 mmol/L; pH 7.4, 37°C) containing diclofenac (1 μmol/L), rings were repeatedly contracted with KCl (with a maximum of 100 mmol/L) until reproducible responses were obtained. A maximal contractile response to phenylephrine (1 μmol/L) was measured. Following, a maximal contractile response to L-NNA was determined as a measure of calcium independent NO formation. Vascular relaxation to cumulative doses of acetylcholine was recorded after preconstriction with phenylephrine to comparable levels. Subsequently, vascular relaxation in response to the endothelium-independent vasodilator 2-(N,N-Diethylamino)-diazenolate-2-oxide (DEA-NONOate, Alexis Biochemicals, San Diego, CA) was determined.
Tissue preparation and histology
The animals were anesthetized with pentobarbital (80μg/kg i.p.) and perfused with 0.9% NaCl using a catheter placed in the left ventricle. For histochemistry and immunohistochemistry tissues of apoE ko on western diet, apoE ko on Ezetimibe, apoE/eNOS dko on western diet and apoE/eNOS dko receiving Ezetimibe were embedded in Tissue-Tek® (Sakura Finetek) and snap-frozen in liquid nitrogen. Serial sections were taken from the aortic root and fixed in acetone prior to staining. Sections were stained with picric acid to assess lesional collagen content. Immunostaining for smooth muscle cells was performed using a monoclonal anti α-actin antibody (Sigma, clone 1A4).
Histomorphometry
Photomicrographs of the aortic valve were taken with a Leitz-camera mounted on a light microscope (Carl-Zeiss, Jena, Germany). Pictures were digitalized and transferred to a PC for planimetry using Image Pro Plus (Version 4.1; Media Cybernetics). All images were analyzed at 100-fold magnification. Areas of positive staining for smooth muscle cells (SMC) and collagen (picric acid staining) were measured in multiple plaques per animal and results were expressed as % positive staining plaque area.
Measurement of vascular NO production by electron paramagnetic spin trapping
Vascular production of NO was measured in aortic rings in an organ bath, in 37°C Krebs-Hepes Buffer (KHB), by using colloid iron (II) diethyldithiocarbamate (Fe(DETC)2) as a spin-trap and Electron Spin Resonance(ESR) detection using an e-scan ESR spectrometer (Bruker BioSpin GmbH, Germany). The method was adapted for detection of baseline NO production in apoE ko mice35. The NO produced by the vessel rings was trapped with Fe-(DETC)2 over a period of 1 hour. The protein content of the samples was quantified using a BCA protein assay kit (Pierce, IL, USA).
Statistical analysis
Two way ANOVA was used for repeated measurements, followed by Scheffe's F-test (Stat View 4.51, Abacus Concepts, Inc., Berkley, CA). A probability value of p≤0.05 was considered significant. Student's t test was used for unpaired data.
Experimental results
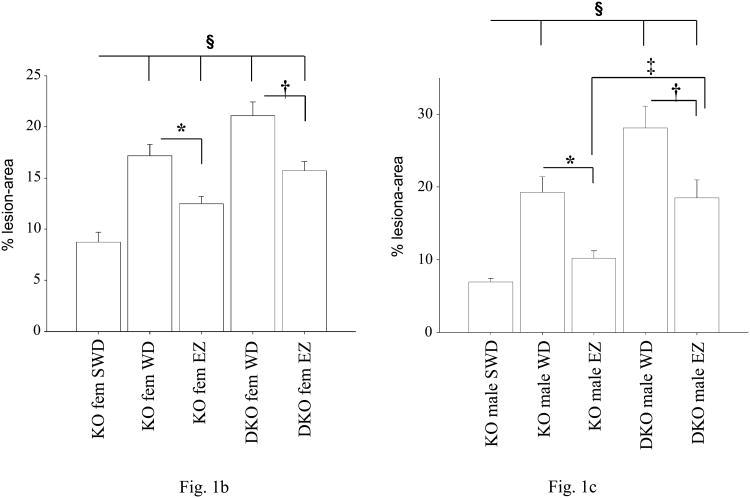
The total area of the aorta and bodyweight of the animals did not differ between mice of the same gender, when compared to different genotypes and feeding regiments (data not shown). Ezetimibe feeding had no effect on mean arterial blood pressure when compared to animals of the same genotype (Table). As previously reported, mean arterial pressure of apoE/eNOS dko animals was elevated compared to apoE ko mice, and Ezetimibe did not change this result30. FPLC analysis of lipoprotein profiles of both genotypes showed a significant but equal reduction in VLDL, IDL and LDL cholesterol-levels and VLDL triglyceride-levels (see data supplement), following Ezetimibe treatment. Parallel to the changes of the lipoprotein profiles, plasma-cholesterol levels were significantly reduced in all experimental groups following Ezetimibe feeding (see data supplement). Following 18 weeks of high fat diet, % lesion area in the “en face” prepared aorta of apoE/eNOS dko mice was significantly increased in females (apoE ko: 17.19±1.09, n=10, vs. apoE/eNOS dko: 21.1±1.34, n=12, p=0.036) and males (apoE ko: 19.24±2.18 SE, n=7, vs. apoE/eNOS dko: 28.1±3.0 SE, n=7, p=0.01), compared to apoE ko animals of the same gender (Figure 1a-c). Ezetimibe reduced plaque progression in female apoE ko animals by 27% (apoE ko WD: 17.19±1.1 SE, n=10, vs. apoE ko Ezetimibe: 12.50±0.7 SE, n=10, p=0.002) and female apoE/eNOS dko mice by 26% (apoE/eNOS dko WD: 21.1±1.34 SE, n=12, vs. apoE/eNOS dko Ezetimibe: 15.7±0.9 SE, n=12, p=0.003), compared to western diet fed animals of the same genotype (Figure 1b). In male apoE ko animals Ezetimibe feeding reduced lesion progression by 47% (apoE ko WD: 19.24±2.2 SE, n=7, vs. apoE ko Ezetimibe: 10.2±1.0 SE, n=14, p=0.0004) and 34% in the group of apoE/eNOS dko animals (apoE/eNOS dko WD: 28.1±3.0 SE, n=7, vs. apoE/eNOS dko Ezetimibe: 18.5±2.5 SE, n=10, p=0.03; Figure 1c).
Blood pressure, heart rate and histomorphometry data.
| BP (mmHg) | n | HR (1/min) | n | % SMC | n | % PA | n | |
|---|---|---|---|---|---|---|---|---|
| apoE ko WD | 85.86±2.04 | 8 | 490±25 | 8 | 2.4±1.1 | 5 | 51±3.4 | 7 |
| apoE ko EZ | 80.67±6.52 | 5 | 522±21 | 5 | 4.2±0.9 | 8 | 48±4.4 | 10 |
| apoE/eNOSdko WD | 101.75±3.64* | 8 | 474±20 | 8 | 3.3±1.4 | 7 | 56±3.3 | 5 |
| apoE/eNOS dko EZ | 100.57±4.43** | 10 | 429±11 | 10 | 5.5±3.4 | 6 | 49±3.2 | 6 |
The mean arterial pressure (BP) in apoE/eNOS dko animals was significantly increased compared to apoE ko animals within each diet group (* apoE ko WD vs. apoE/eNOS dko WD, p=0.002; ** apoE ko EZ vs. apoE/eNOS dko EZ, p=0.024). Ezetimibe had no effect on blood pressure when animals of the same genotype but different diet group were compared. Heart rate (HR) was unchanged between animals of different genotype or diet group. (n=number of animals). Histomorphometry for α smooth muscle actin positive smooth muscle cells (SMC) and collagen content (picric acid positive plaque area (PA)) in aortic root lesions of apoE ko and apoE/eNOS dko animals. Ezetimibe did not change the relative smooth muscle cell or collagen content of the plaque (n=number of plaques).
Figure 1.
(1a) Sudan IV-stained, longitudinally opened aortas from male apoE ko and apoE/eNOS dko animals fed a “western” diet (WD) or a “western” diet containing Ezetimibe (EZ). Luminal side (facing up) displaying lipid rich (red) atherosclerotic lesions. (1b, 1c) Lesion area at 10 weeks of WD, marking the beginning of the EZ treatment is displayed (short western diet, designated SWD). Ezetimibe significantly reduced lesion progression. Mean lesion area expressed as % lesion area of the total aortic area in female (fem) and male apoE ko (KO) and apoE/eNOS dko (DKO) animals on WD, or Ezetimibe (EZ). * apoE ko WD vs. apoE ko EZ, † apoE/eNOS dko WD vs. apoE/eNOS dko EZ; ‡ apoE ko EZ vs. apoE/eNOS dko EZ; § apoE ko (SWD) vs. all (p≤0.05).
However, Ezetimibe fed apoE/eNOS dko mice of both genders developed significantly larger lesion areas when compared to their gender matched, Ezetimibe fed apoE ko controls (female apoE ko: 12.50±0.7 SE, n=10, vs. apoE/eNOS dko: 15.72±0.88, n=12, p=0.02; and male apoE ko: 10.2±1.0 SE, n=14, vs. apoE/eNOS dko: 18.51±2.5, n=10, p=0.003; Figure 1b, 1c). Reduction of plaque progression following Ezetimibe treatment was greater in male apoE ko mice (47% reduction) compared to apoE/eNOS dko (34% reduction) mice (apoE ko Ezetimibe: 10.21±1.0 SE, n=14, vs. apoE/eNOS dko Ezetimibe: 18.51±2.5 SE, n=10, p=0.002), while there was no difference between female animals of those two groups (Figure 1c). Interestingly, lesions in these male apoE ko mice did not progress, compared to the plaque burden of apoE ko mice at the beginning of the Ezetimibe treatment (10 weeks, Figure 1c).
Ezetimibe treatment reduced VCAM-1 protein expression in apoE ko and apoE/eNOS dko mice significantly. (Figure 2a). In addition, Ezetimibe treatment significantly reduced vascular CD14 expression, a marker for mononuclear cell recruitment to the vessel wall, in both genotypes (Figure 2b).
Figure 2.
Western blot for VCAM-1 in total aortic protein lysates of western diet (WD) or Ezetimibe (EZ) treated mice (2a). Real time PCR of CD14 mRNA expression (2b). *Significant difference (p≤0.05). Ezetimibe reduced mean VCAM-1 and CD14 expression levels in apoE ko and apoE/eNOS dko mice.
Immunohistochemical staining for smooth muscle cells (α-smooth muscle actin positive area) and histochemistry for collagen content (picric acid positive area) showed a similar plaque composition between genotypes and diet groups (Table).
Western blot analysis showed equal amounts of eNOS protein in vessels of apoE ko mice, regardless of the treatment group and gender of the animals (Figure 3a). In addition, iNOS and nNOS protein expression levels were not influenced by Ezetimibe treatment in both genotypes (data not shown). To determine if Ezetimibe influences NOS function we compared NO production of aortas from wildtype mice treated with or without Ezetimibe. Using Electron Spin Resonance spectroscopy (ESR), a highly specific and sensitive method for the direct detection of NO, no difference in NO production was observed between the groups (Figure 3b). Vascular reactivity studies documented similar potassium chloride induced vasoconstriction in all experimental groups (Figure 4a). ApoE/eNOS dko mice showed an increased maximum contractile response to phenylephrin (p≤0.05), while the contractile response of all other groups studied revealed no difference (Figure 4b). Administration of cumulative doses of acetylcholine caused equally normal endothelium-dependent vasorelaxation in apoE ko mice regardless of Ezetimibe feeding (Figure 4c). In contrast, apoE/eNOS dko animals showed absence of endothelium dependent relaxation (p≤0.01; Figure 4c). Incubation with the radical scavenger tiron did not change acetylcholine-mediated endothelium-dependent vasorelaxation (Figure 4d). Incubation of the vascular rings with cumulative doses of the NO donor DEA-NONOate revealed similar endothelium-independent vasorelaxation in all groups studied (Figure 4e). The EC50 values did not differ between genotypes and feeding groups (Figure 4f). Moreover, we observed no difference in the L-NNA mediated contractile response between the apoE ko animals fed with western diet or Ezetimibe (Figure 4g).
Figure 3.
(3a) Western blot of total aortic lysates from female apoE ko animals fed with “western” diet (WD) or a “western” diet containing Ezetimibe (EZ). Equal loading was confirmed with an anti α-actin antibody (43 kDa). Densitometric analysis of blots showed no difference in eNOS expression between the diet groups. (3b) Vascular measurements of NO production using Fe-(DETC)2 spintrap. Above: Representative Electron Spin Resonance (ESR) spectrum of the NO-Fe-(DETC)2 from C57/Bl6 mice fed with WD (black line) or EZ (grey line). The arrows indicate the typical three peak ESR signal for NO-Fe-(DETC)2. Ezetimibe did not alter basal NO production in C57/Bl6 animals (Below).
Figure 4.
Potassium chloride- (4a) and phenylephrine-induced (4b) vasoconstriction. Acetylcholine-mediated, endothelium-dependent relaxation in the absence (4c), or presence (4d) of the superoxide scavenger Tiron. DEA-NONOate-mediated endothelium-independent (4e) vasorelaxation. ED50 max relaxation (4f) and effect of NOS inhibitor L-NNA on vascular function (4g). ApoE ko (KO), apoE/eNOS dko (DKO), Ezetimibe (EZ), “western” diet (WD).
Discussion
The present study revealed three major findings: First, Ezetimibe significantly reduced atherosclerosis in apoE ko and apoE/eNOS dko mice, suggesting that the drug's anti-atherosclerotic effects are largely eNOS independent. Second, Ezetimibe did not influence NOS enzyme activity or protein expression. Third, although the drug mediates strong eNOS independent atheroprotection, its anti-atherosclerotic effects were significantly stronger in male eNOS competent apoE ko, compared to apoE/eNOS dko mice, suggesting that some of Ezetimibe's effects are mediated through the eNOS pathway.
In healthy vessels NO production by eNOS protects from vascular damage by inhibiting smooth muscle cell proliferation, platelet and leukocyte activation and adhesion, oxidative stress and the degranulation of pro inflammatory vesicles36-40. In the past, we and others have crossed eNOS ko and apoE ko animals to investigate the effects of eNOS deficiency on atherosclerosis development 31, 41. Results from these studies and the data presented here document that eNOS deficiency accelerates plaque formation, confirming the importance of endothelial NO production for atheroprotection31.
The current study revealed that Ezetimibe potently slowed lesion progression despite the complete lack of endothelial, NO-dependent vasorelaxation in apoE/eNOS dko animals. Endothelial function in apoE ko control mice in both diet groups was not impaired at the time of the study, allowing us to test the drug's effects in the presence and in the absence of functional eNOS between both genotypes. FPLC analysis of plasma samples revealed that Ezetimibe was equally effective in reducing cholesterol content of lipoproteins and plasma total cholesterol in all experimental groups. Western blot analysis of eNOS expression in apoE ko mice showed that Ezetimibe treatment had no effect on eNOS protein expression in the aorta of female or male animals. Additionally, nNOS and iNOS protein expression did not change following Ezetimibe treatment in apoE ko or apoE/eNOS dko animals. Moreover, NO production of wildtype aortas was not affected by Ezetimibe, suggesting that the drug does not directly modulate NOS activity. In contrast, statins increase vascular NO production in wildtype mice by influencing eNOS function42. Invasive blood pressure measurements showed that Ezetimibe treatment did not change mean arterial pressure within animals of the same genotype, when comparing the different diet groups. Blood pressure was significantly elevated in apoE/eNOS dko compared to apoE ko mice but normalization of mean arterial pressure with hydralazine did not prevent acceleration of atherosclerosis in apoE/eNOS dko animals in a previous study from our lab30; we therefore did not address this question in the present study.
Interestingly, Ezetimibe normalized vascular inflammation as it potently reduced vascular VCAM-1 protein expression in apoE ko and apoE/eNOS dkos mice. While Statin mediated reduction of VCAM-1 expression is considered to be dependent on the eNOS pathway43, this is the first report showing that Ezetimibe is capable of reducing vascular VCAM-1 expression independent of eNOS function. This effect may be related to the reduction of plasma LDL cholesterol in our study, as Verna et al. report that LDL can directly induce endothelial adhesion molecule expression by activation of the AP-1 regulatory pathway44. Consistent with the Ezetimibe mediated reduction of endothelial activation, mononuclear cell infiltration of the aorta was significantly reduced in apoE ko mice and apoE/eNOS dko mice.
Ezetimibe treatment reduced lesion formation equally in female apoE ko and apoE/eNOS dko animals. In contrast, Ezetimibe treated male eNOS competent apoE ko mice showed a significantly greater reduction in lesion size, compared to apoE/eNOS dko animals. Since lesion area of female apoE ko was smaller than in males, Ezetimibe's eNOS dependent atheroprotection may only affect advanced atherosclerosis and in this respect may affect female animals at a later time point. However, gender differences in the anti atherosclerotic effects of Ezetimibe can not be ruled out presently.
Vascular reactivity studies of aortic rings confirmed similar endothelial dependent vasorelaxation in western diet and Ezetimibe treated apoE ko mice. One possible explanation for the additional eNOS dependent atheroprotection in male apoE ko mice is that Ezetimibe improved local NO bioavailability in the plaque, which generally can not be assessed by vasoreactivity studies. Certainly, giving the various potential anti-atherosclerotic effects of NO mentioned above, a local improvement in NO bioavailability may decrease plaque progression. Interestingly, Ezetimibe improved endothelial function when given in combination with Atorvastatin as compared to Atorvastatin monotherapy in patients with a metabolic syndrome, suggesting that a combination therapy of both drugs mediates additive effects45.
In summary, the atheroprotection by the lipid lowering therapy with Ezetimibe does not require eNOS, as we observed markedly reduced vascular inflammation and plaque formation in apoE/eNOS dko mice. Our findings suggest that atheroprotection by this lipid lowering therapy does not depend on an initial improvement in eNOS mediated endothelial function. Ezetimibe did not influence eNOS gene expression or enzyme activity. However, in male apoE ko mice Ezetimibe exerted additional eNOS dependent atheroprotection which may be mediated by a local improvement of NO bioavailability as a result of lipid lowering, rather than direct effects of the drug on eNOS expression or function.
Supplementary Material
Acknowledgments
This work was supported by a grant from MSD and essex pharma GmbH to P.K. and J.B. and from the Deutsche-Forschungsgemeinschaft (KU-1206(1) and 1206(2) and the IZKF-Würzburg to P.K., the National Institute of Neurological Disorders and Stroke (NINDS, NS33335) and the National Heart, Lung, and Blood Institute (NHLBI, HL57818) to P.L.H. P.L.H. is an established investigator of the American Heart Association. Ezetimibe was supplied from Schering-Plough Research Institute. We thank Bruker BioSpin GmbH, Karlsruhe, Germany for the use of their ESR facility and Ms. G. Riehl, Mrs. A. Ganscher and Ms. M. Leutke for excellent technical assistance.
References
- 1.Lusis AJ, Fogelman AM, Fonarow GC. Genetic basis of atherosclerosis: part I: new genes and pathways. Circulation. 2004;110:1868–1873. doi: 10.1161/01.CIR.0000143041.58692.CC. [DOI] [PubMed] [Google Scholar]
- 2.Lusis AJ, Fogelman AM, Fonarow GC. Genetic basis of atherosclerosis: part II: clinical implications. Circulation. 2004;110:2066–2071. doi: 10.1161/01.CIR.0000143098.98869.F8. [DOI] [PubMed] [Google Scholar]
- 3.Ohara Y, Peterson TE, Harrison DG. Hypercholesterolemia increases endothelial superoxide anion production. J Clin Invest. 1993;91:2546–2551. doi: 10.1172/JCI116491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Xia Y, Tsai AL, Berka V, Zweier JL. Superoxide generation from endothelial nitric-oxide synthase. A Ca2+/calmodulin-dependent and tetrahydrobiopterin regulatory process. J Biol Chem. 1998;273:25804–25808. doi: 10.1074/jbc.273.40.25804. [DOI] [PubMed] [Google Scholar]
- 5.Randomised trial of cholesterol lowering in 4444 patients with coronary heart disease: the Scandinavian Simvastatin Survival Study (4S) Lancet. 1994;344:1383–1389. [PubMed] [Google Scholar]
- 6.MRC/BHF Heart Protection Study of cholesterol lowering with simvastatin in 20,536 high-risk individuals: a randomised placebo-controlled trial. Lancet. 2002;360:7–22. doi: 10.1016/S0140-6736(02)09327-3. [DOI] [PubMed] [Google Scholar]
- 7.Sever PS, Dahlof B, Poulter NR, Wedel H, Beevers G, Caulfield M, Collins R, Kjeldsen SE, Kristinsson A, McInnes GT, Mehlsen J, Nieminen M, O'Brien E, Ostergren J. Prevention of coronary and stroke events with atorvastatin in hypertensive patients who have average or lower-than-average cholesterol concentrations, in the Anglo-Scandinavian Cardiac Outcomes Trial--Lipid Lowering Arm (ASCOT-LLA): a multicentre randomised controlled trial. Lancet. 2003;361:1149–1158. doi: 10.1016/S0140-6736(03)12948-0. [DOI] [PubMed] [Google Scholar]
- 8.Shepherd J, Cobbe SM, Ford I, Isles CG, Lorimer AR, MacFarlane PW, McKillop JH, Packard CJ. Prevention of coronary heart disease with pravastatin in men with hypercholesterolemia. West of Scotland Coronary Prevention Study Group. N Engl J Med. 1995;333:1301–1307. doi: 10.1056/NEJM199511163332001. [DOI] [PubMed] [Google Scholar]
- 9.Anderson TJ, Meredith IT, Yeung AC, Frei B, Selwyn AP, Ganz P. The effect of cholesterol-lowering and antioxidant therapy on endothelium-dependent coronary vasomotion. N Engl J Med. 1995;332:488–493. doi: 10.1056/NEJM199502233320802. [DOI] [PubMed] [Google Scholar]
- 10.Treasure CB, Klein JL, Weintraub WS, Talley JD, Stillabower ME, Kosinski AS, Zhang J, Boccuzzi SJ, Cedarholm JC, Alexander RW. Beneficial effects of cholesterol-lowering therapy on the coronary endothelium in patients with coronary artery disease. N Engl J Med. 1995;332:481–487. doi: 10.1056/NEJM199502233320801. [DOI] [PubMed] [Google Scholar]
- 11.Schachinger V, Britten MB, Zeiher AM. Prognostic impact of coronary vasodilator dysfunction on adverse long-term outcome of coronary heart disease. Circulation. 2000;101:1899–1906. doi: 10.1161/01.cir.101.16.1899. [DOI] [PubMed] [Google Scholar]
- 12.Suwaidi JA, Hamasaki S, Higano ST, Nishimura RA, Holmes DR, Jr, Lerman A. Long-term follow-up of patients with mild coronary artery disease and endothelial dysfunction. Circulation. 2000;101:948–954. doi: 10.1161/01.cir.101.9.948. [DOI] [PubMed] [Google Scholar]
- 13.Arnold WP, Mittal CK, Katsuki S, Murad F. Nitric oxide activates guanylate cyclase and increases guanosine 3′:5′-cyclic monophosphate levels in various tissue preparations. Proc Natl Acad Sci U S A. 1977;74:3203–3207. doi: 10.1073/pnas.74.8.3203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Furchgott RF, Zawadzki JV. The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature. 1980;288:373–376. doi: 10.1038/288373a0. [DOI] [PubMed] [Google Scholar]
- 15.Ignarro LJ, Byrns RE, Buga GM, Wood KS, Chaudhuri G. Pharmacological evidence that endothelium-derived relaxing factor is nitric oxide: use of pyrogallol and superoxide dismutase to study endothelium-dependent and nitric oxide-elicited vascular smooth muscle relaxation. J Pharmacol Exp Ther. 1988;244:181–189. [PubMed] [Google Scholar]
- 16.Kureishi Y, Luo Z, Shiojima I, Bialik A, Fulton D, Lefer DJ, Sessa WC, Walsh K. The HMG-CoA reductase inhibitor simvastatin activates the protein kinase Akt and promotes angiogenesis in normocholesterolemic animals. Nat Med. 2000;6:1004–1010. doi: 10.1038/79510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Laufs U, La Fata V, Plutzky J, Liao JK. Upregulation of endothelial nitric oxide synthase by HMG CoA reductase inhibitors. Circulation. 1998;97:1129–1135. doi: 10.1161/01.cir.97.12.1129. [DOI] [PubMed] [Google Scholar]
- 18.Leung WH, Lau CP, Wong CK. Beneficial effect of cholesterol-lowering therapy on coronary endothelium-dependent relaxation in hypercholesterolaemic patients. Lancet. 1993;341:1496–1500. doi: 10.1016/0140-6736(93)90634-s. [DOI] [PubMed] [Google Scholar]
- 19.Ohara Y, Peterson TE, Sayegh HS, Subramanian RR, Wilcox JN, Harrison DG. Dietary correction of hypercholesterolemia in the rabbit normalizes endothelial superoxide anion production. Circulation. 1995;92:898–903. doi: 10.1161/01.cir.92.4.898. [DOI] [PubMed] [Google Scholar]
- 20.Tamai O, Matsuoka H, Itabe H, Wada Y, Kohno K, Imaizumi T. Single LDL apheresis improves endothelium-dependent vasodilatation in hypercholesterolemic humans. Circulation. 1997;95:76–82. doi: 10.1161/01.cir.95.1.76. [DOI] [PubMed] [Google Scholar]
- 21.Halcox JP, Deanfield JE. Beyond the laboratory: clinical implications for statin pleiotropy. Circulation. 2004;109:II42–48. doi: 10.1161/01.CIR.0000129500.29229.92. [DOI] [PubMed] [Google Scholar]
- 22.Van Heek M, France CF, Compton DS, McLeod RL, Yumibe NP, Alton KB, Sybertz EJ, Davis HR., Jr In vivo metabolism-based discovery of a potent cholesterol absorption inhibitor, SCH58235, in the rat and rhesus monkey through the identification of the active metabolites of SCH48461. J Pharmacol Exp Ther. 1997;283:157–163. [PubMed] [Google Scholar]
- 23.Garcia-Calvo M, Lisnock J, Bull HG, Hawes BE, Burnett DA, Braun MP, Crona JH, Davis H, Jr, Dean DC, Detmers PA, Graziano MP, Hughes M, Macintyre DE, Ogawa A, O'Neill KA, Iyer SP, Shevell DE, Smith MM, Tang YS, Makarewicz AM, Ujjainwalla F, Altmann SW, Chapman KT, Thornberry NA. The target of ezetimibe is Niemann-Pick C1-Like 1 (NPC1L1) Proc Natl Acad Sci U S A. 2005;102:8132–8137. doi: 10.1073/pnas.0500269102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rosenblum SB, Huynh T, Afonso A, Davis HR, Jr, Yumibe N, Clader JW, Burnett DA. Discovery of 1-(4-fluorophenyl)-(3R)-[3-(4-fluorophenyl)-(3S)-hydroxypropyl]-(4S)-(4 -hydroxyphenyl)-2-azetidinone (SCH 58235): a designed, potent, orally active inhibitor of cholesterol absorption. J Med Chem. 1998;41:973–980. doi: 10.1021/jm970701f. [DOI] [PubMed] [Google Scholar]
- 25.van Heek M, Compton DS, Davis HR. The cholesterol absorption inhibitor, ezetimibe, decreases diet-induced hypercholesterolemia in monkeys. Eur J Pharmacol. 2001;415:79–84. doi: 10.1016/s0014-2999(01)00825-1. [DOI] [PubMed] [Google Scholar]
- 26.van Heek M, Farley C, Compton DS, Hoos L, Alton KB, Sybertz EJ, Davis HR., Jr Comparison of the activity and disposition of the novel cholesterol absorption inhibitor, SCH58235, and its glucuronide, SCH60663. Br J Pharmacol. 2000;129:1748–1754. doi: 10.1038/sj.bjp.0703235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Davis HR, Jr, Compton DS, Hoos L, Tetzloff G. Ezetimibe, a potent cholesterol absorption inhibitor, inhibits the development of atherosclerosis in ApoE knockout mice. Arterioscler Thromb Vasc Biol. 2001;21:2032–2038. doi: 10.1161/hq1201.100260. [DOI] [PubMed] [Google Scholar]
- 28.Mahley RW, Huang Y, Weisgraber KH. Putting cholesterol in its place: apoE and reverse cholesterol transport. J Clin Invest. 2006;116:1226–1229. doi: 10.1172/JCI28632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Breslow JL. Mouse models of atherosclerosis. Science. 1996;272:685–688. doi: 10.1126/science.272.5262.685. [DOI] [PubMed] [Google Scholar]
- 30.Chen J, Kuhlencordt PJ, Astern J, Gyurko R, Huang PL. Hypertension does not account for the accelerated atherosclerosis and development of aneurysms in male apolipoprotein e/endothelial nitric oxide synthase double knockout mice. Circulation. 2001;104:2391–2394. doi: 10.1161/hc4501.099729. [DOI] [PubMed] [Google Scholar]
- 31.Kuhlencordt PJ, Gyurko R, Han F, Scherrer-Crosbie M, Aretz TH, Hajjar R, Picard MH, Huang PL. Accelerated atherosclerosis, aortic aneurysm formation, and ischemic heart disease in apolipoprotein E/endothelial nitric oxide synthase double-knockout mice. Circulation. 2001;104:448–454. doi: 10.1161/hc2901.091399. [DOI] [PubMed] [Google Scholar]
- 32.Huang PL, Huang Z, Mashimo H, Bloch KD, Moskowitz MA, Bevan JA, Fishman MC. Hypertension in mice lacking the gene for endothelial nitric oxide synthase. Nature. 1995;377:239–242. doi: 10.1038/377239a0. [DOI] [PubMed] [Google Scholar]
- 33.Zhang SH, Reddick RL, Piedrahita JA, Maeda N. Spontaneous hypercholesterolemia and arterial lesions in mice lacking apolipoprotein E. Science. 1992;258:468–471. doi: 10.1126/science.1411543. [DOI] [PubMed] [Google Scholar]
- 34.Kuhlencordt PJ, Chen J, Han F, Astern J, Huang PL. Genetic deficiency of inducible nitric oxide synthase reduces atherosclerosis and lowers plasma lipid peroxides in apolipoprotein E-knockout mice. Circulation. 2001;103:3099–3104. doi: 10.1161/01.cir.103.25.3099. [DOI] [PubMed] [Google Scholar]
- 35.Kleschyov AL, Munzel T. Advanced spin trapping of vascular nitric oxide using colloid iron diethyldithiocarbamate. Methods in enzymology. 2002;359:42–51. doi: 10.1016/s0076-6879(02)59170-9. [DOI] [PubMed] [Google Scholar]
- 36.Bath PM. The effect of nitric oxide-donating vasodilators on monocyte chemotaxis and intracellular cGMP concentrations in vitro. Eur J Clin Pharmacol. 1993;45:53–58. doi: 10.1007/BF00315350. [DOI] [PubMed] [Google Scholar]
- 37.Goss SP, Hogg N, Kalyanaraman B. The effect of nitric oxide release rates on the oxidation of human low density lipoprotein. J Biol Chem. 1997;272:21647–21653. doi: 10.1074/jbc.272.34.21647. [DOI] [PubMed] [Google Scholar]
- 38.Matsushita K, Morrell CN, Cambien B, Yang SX, Yamakuchi M, Bao C, Hara MR, Quick RA, Cao W, O'Rourke B, Lowenstein JM, Pevsner J, Wagner DD, Lowenstein CJ. Nitric oxide regulates exocytosis by S-nitrosylation of N-ethylmaleimide-sensitive factor. Cell. 2003;115:139–150. doi: 10.1016/s0092-8674(03)00803-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mooradian DL, Hutsell TC, Keefer LK. Nitric oxide (NO) donor molecules: effect of NO release rate on vascular smooth muscle cell proliferation in vitro. J Cardiovasc Pharmacol. 1995;25:674–678. [PubMed] [Google Scholar]
- 40.Radomski MW, Palmer RM, Moncada S. Modulation of platelet aggregation by an L-arginine-nitric oxide pathway. Trends Pharmacol Sci. 1991;12:87–88. doi: 10.1016/0165-6147(91)90510-y. [DOI] [PubMed] [Google Scholar]
- 41.Knowles JW, Reddick RL, Jennette JC, Shesely EG, Smithies O, Maeda N. Enhanced atherosclerosis and kidney dysfunction in eNOS(-/-)Apoe(-/-) mice are ameliorated by enalapril treatment. J Clin Invest. 2000;105:451–458. doi: 10.1172/JCI8376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Scalia R, Gooszen ME, Jones SP, Hoffmeyer M, Rimmer DM, 3rd, Trocha SD, Huang PL, Smith MB, Lefer AM, Lefer DJ. Simvastatin exerts both anti-inflammatory and cardioprotective effects in apolipoprotein E-deficient mice. Circulation. 2001;103:2598–2603. doi: 10.1161/01.cir.103.21.2598. [DOI] [PubMed] [Google Scholar]
- 43.Tsunekawa T, Hayashi T, Kano H, Sumi D, Matsui-Hirai H, Thakur NK, Egashira K, Iguchi A. Cerivastatin, a hydroxymethylglutaryl coenzyme a reductase inhibitor, improves endothelial function in elderly diabetic patients within 3 days. Circulation. 2001;104:376–379. doi: 10.1161/hc2901.094094. [DOI] [PubMed] [Google Scholar]
- 44.Verna L, Ganda C, Stemerman MB. In vivo low-density lipoprotein exposure induces intercellular adhesion molecule-1 and vascular cell adhesion molecule-1 correlated with activator protein-1 expression. Arterioscler Thromb Vasc Biol. 2006;26:1344–1349. doi: 10.1161/01.ATV.0000222152.83069.3f. [DOI] [PubMed] [Google Scholar]
- 45.Bulut D, Hanefeld C, Bulut-Streich N, Graf C, Mugge A, Spiecker M. Endothelial function in the forearm circulation of patients with the metabolic syndrome--effect of different lipid-lowering regimens. Cardiology. 2005;104:176–180. doi: 10.1159/000088105. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.