Abstract
The effects of methylphenidate (MPH), atomoxetine (ATMX), and/or physical exercise (EX) on orienting behavior and social interaction were examined in Spontaneously Hypertensive Rats (SHR), a commonly used animal model of (ADHD). During the orienting procedure, rats received repeated presentations of a non-reinforced visual stimulus. As observed previously, orienting behavior (rearing up on the hind legs) habituated across trials in normo-active control rats (Wistars) but not in SHRs, suggesting that SHRs have difficulty ignoring irrelevant behavioral stimuli. Treatment with MPH (0.125 mg/kg), ATMX (0.125 mg/kg), or EX (3 weeks of access to a running wheel), alone or in combination, reduced rearing behavior in SHRs to the level observed in the Wistar control group. Similarly, drug treatment and/or EX reduced the number of social interactions exhibited by SHRs, while having no effects on locomotor activity. Importantly, EX was just as effective as MPH or ATMX in reducing orienting behavior and social interaction. In contrast to the SHRs, neither MPH nor ATMX affected orienting or social behavior in Wistar rats. Together, these findings support the growing literature that EX may be useful as an adjunctive or replacement therapy in ADHD.
Keywords: Attention, Attention-Deficit/Hyperactivity Disorder, rat, methylphenidate, atomoxetine, locomotor activity
INTRODUCTION
Attention deficit/hyperactivity disorder (ADHD) affects 3–7% of children (American Psychiatric Association, 2000; Nair et al., 2006) and is characterized by varying degrees of inattention, hyperactivity, and impulsivity. The standard of care for ADHD continues to be treatment with psychostimulants such as methylphenidate (MPH, Ritalin™) that potentiate dopaminergic and noradrenergic neurotransmission (Kuczenski & Segal, 2001). Other treatments include atomoxetine (ATMX, Strattera™), which selectively affects noradrenergic reuptake (Bymaster et al., 2002; Michelson et al., 2001; Spencer et al., 2001). However, not all individuals respond to drug therapy (Biederman, Spencer, & Wilens, 2004) and for those that do, the benefits typically decrease over time or are only present during active intervention (Jensen et al., 2007). Moreover, concern about abuse potential and long-term health effects have stimulated research on possible adjunctive or alternate therapies (Halperin & Healey, 2001; Scherer et al., 2010; Swanson et al., 2007; Volkow & Insel, 2003).
Given the breadth of behavioral and cognitive symptoms exhibited by persons with ADHD, perhaps it is not surprising that a single drug therapy would be ineffective for some persons or symptoms. Indeed, various subtypes of ADHD have been identified (inattentive, hyperactive, and combined subtypes; Milich, Balentin, & Lyman, 2001) and although the symptoms associated with ADHD are thought to primarily arise from core deficits in behavioral inhibition and delay aversion (Barkley, 1997; Nigg, 2001; Sonuga-Barke, Taylor, Sembi, & Smith, 1992), the range of dysfunction is varied and includes impulsivity, inattention, and excessive-social behavior (Nigg et al., 2005; Pelham, Fabiano, & Massetti, 2005; Sergeant, 2005; Whalen & Henker, 1992). Thus, different drugs may regulate some behaviors associated with ADHD while leaving others unaffected. Yet surprisingly little is known about how different pharmaco-therapies, and their underlying mechanisms of action, relate to specific behavioral phenotypes (Oades et al., 2005).
One goal of the present study was to determine whether MPH and ATMX affect orienting behavior and social behavior in Spontaneously Hypertensive Rats, a commonly used rodent model of ADHD (Davids, Zhang, Tarazi, & Baldessarini, 2003; Sagvolden, 2000; Sagvolden, Russell, Aase, Johansen, & Farshbaf, 2005). SHRs exhibit some of the principal behavioral symptoms and cognitive impairments typically associated with ADHD (Hopkins, Sharma, Evans, & Bucci, 2009; Kantak et al., 2008; Robinson, Hopkins, & Bucci, 2011; Russell, 2007; Sagvolden et al., 2005) as well as dysregulated dopaminergic and noradrenergic neurotransmission (Heal, Smith, Kulkarni, & Rowley, 2008; Russell, 2000; 2002; Solanto & Conners, 1982) that may underlie ADHD in some individuals (Arnsten, 2006; Biederman, 2005). Although other studies have examined the effects of MPH on attention in SHRs, most prior research has focused specifically on the ability to maintain attention to behaviorally-relevant cues (e.g., Thanos et al., 2010). In contrast, there has been little research on the effects of MPH on the ability to ignore extraneous cues. This component of attentional function can be assessed by observing orienting responses to repeated presentations of a non-reinforced visual stimulus. Orienting is defined as rearing up on the hind legs towards the stimulus (Holland, 1977; 1984) and is an often-used measure of attentional processing (Gallagher, Graham, & Holland, 1990; Kaye & Pearce, 1984; Lang, Simons, & Balaban, 1997). In normal rats, rearing behavior rapidly decreases (habituates) when the cue is not followed by reinforcement, which has been suggested to reflect an adaptive decrease in attention to a behaviorally-irrelevant stimulus (Gallagher et al., 1990; Holland, 1997; Kaye & Pearce, 1984). We have shown previously that SHRs exhibit hyper-orienting behavior with little or no habituation compared to normo-active controls strains (Hopkins et al., 2009; Robinson et al., 2011) indicating that they are more prone to respond to distracting, irrelevant stimuli.
Similarly, few studies have focused on impairments in social behavior that are typically associated with ADHD (Pelham et al., 2005; Whalen & Henker, 1992) but notoriously resistant to current interventions (Swanson et al., 2001). Social interaction was assessed in the current study using a procedure adapted from File and colleagues (File, 1980; File & Seth, 2003) and used previously to demonstrate that SHRs exhibit hyper-social behavior. Indeed, compared to normo-active control rats, SHRs initiate more interactions with an unfamiliar rat (Hopkins et al. 2009).
A second goal of this study was to compare the effects of pharmaco-therapies to those resulting from physical exercise. Recent studies have shown that voluntary wheel running can normalize social behavior and attentional function in SHRs (Hopkins et al., 2009; Robinson et al., 2011), and exercise has become increasingly studied for its beneficial effects on mental health and wellness in both children and adults (Carek, Laibstain, & Carek, 2011; Sibley & Etneir, 2003; Strong et al., 2011). Importantly in the context of ADHD, exercise increases dopamine and norepinephrine levels (Hattori, Naoi, & Nishino, 1994; Pagliari & Peyrin, 1995; Winter et al., 2007) and a recent study has reported beneficial effects of an exercise intervention in children with ADHD (Chang, Liu, Yu, & Lee, 2012). Likewise, there has been increased research interest in identifying the neural and behavioral effects of exercise using animal models of ADHD (Hopkins et al., 2011, Naylor, Persson, Eriksoon, Jonsdottir, & Thorlin, 2005; Robinson et al., 2011). Thus, we compared the effects of physical exercise alone or in combination with drug treatment on orienting and social behavior.
Notably, orienting behavior and social interaction in SHRs has been found to be higher in females compared to males (Hopkins et al., 2009; Robinson et al., 2011); consistent with a growing evidence that females diagnosed with ADHD may be more cognitively impaired than males (Gershon, 2002; Nigg, Blaskey, Huang-Pollock, & Rappley, 2002; Spencer, Biederman, & Mick, 2007). In addition, we were specifically interested in testing young adult rats since ADHD is now thought to continue into adulthood for a majority of individuals (Mao et al., 2011) yet there has been little research on adults with ADHD. Importantly, while the hyperactive symptoms of ADHD tend to remit with age, the cognitive impairments do not (Mao et al., 2011). Hence, the present study tested the effects of MPH, ATMX, and exercise in young adult female SHRs compared to untreated young adult female Wistar rats (normo-active control strain).
MATERIALS AND METHODS
Subjects
Experiment 1
Seventy-four female SHRs and 8 female Wistar rats (7–8 weeks old) were obtained from Harlan Laboratories (Indianapolis, IN). Rats were group housed (2–4 rats/cage) and maintained on a 14/10 h light-dark cycle throughout the experiment. For 7 days, rats were allowed to acclimate to the vivarium before behavioral testing began. The SHRs were then divided into six groups (see Table 1 for sample sizes): saline-treated control (SHR:CTL), methylphenidate (SHR:MPH), atomoxetine (SHR:ATMX), exercise (SHR:EX), methylphenidate + exercise (SHR:MPH+EX), and atomoxetine + exercise (SHR:ATMX+EX). The 8 Wistar rats were treated with saline as a normo-active control group (WIS:CTL).
Table 1.
Experimental groups and sample sizes for Experiment 1
| Group | Sample size (orienting task) |
Sample size (social interaction) |
|---|---|---|
| WIS:CTL | 8 | 8 |
| SHR:CTL | 16 | 8 |
| SHR:EX | 11 | 11 |
| SHR:MPH | 12 | 11 |
| SHR:MPH+EX | 12 | 12 |
| SHR:ATMX | 12 | 11 |
| SHR:ATMX+EX | 11 | 11 |
Experiment 2
Based on the findings from Experiment 1, a follow-up experiment was conducted with 32 female Wistar rats (7–8 weeks old, Harlan Laboratories). The rats were divided into three groups: saline-treated control (WIS:CTL, n=12), methylphenidate (WIS:MPH, n=8), and atomoxetine (WIS:ATMX, n=12). The rats were treated identically to the rats in Experiment 1 and all procedures were conducted in accordance with Association for Assessment and Accreditation of Laboratory Animal Care guidelines and the Dartmouth College Institutional Animal Care and Use Committee.
Apparatus
Running wheel
Rats in the EX groups had access to a stainless steel running wheel inside the home cage (34.5cm diameter, 1.3mm rods placed 0.9cm apart; Philips Respironics, Bend, OR) or a wheel that was accessible through an opening in the side of the cage (35.6cm diameter, 4.8mm rods placed 1.6cm apart; Med Associates, St. Albans, VT). An automatic counter mounted on the side of the apparatus monitored wheel rotations.
Orienting and conditioning tasks
The orienting and conditioning procedures were carried out in standard operant chambers (24cm × 30.5cm × 29cm; Med Associates, Inc.) connected to a computer and enclosed in a sound-attenuating chamber (62cm × 56cm × 56cm) equipped with an exhaust fan to provide airflow and background noise (~68dB). The chambers consisted of aluminum front and back walls with clear acrylic side walls and ceiling, and a grid floor. A dimly illuminated food cup was recessed in the center of one wall at a height of 5cm. The stimulus light was a 2.8-W bulb located on the center of the chamber wall opposite the food cup, 1 cm from the ceiling. A red house light (2.8W) was located on the ceiling of the sound-attenuating chambers to provide background lighting. Three pairs of photobeam sensors were mounted in the chamber and used to detect rearing behavior. The sensors were placed 15cm above the grid floor and were evenly spaced along the wall so that a rearing response produced anywhere in the chamber would be detected by one of the sensors. A photobeam was also located across front of the food cup.
Social interaction
The social interaction procedure was conducted in a white plastic tub measuring 119.4cm × 59.7cm × 59.7cm. In the center of the tub was a clear plexiglass cylinder (27.9cm long × 7.6cm diameter) containing an unfamiliar rat of the same strain (‘target rat’). There were five holes on each side of the cylinder (1.9cm diameter), two holes on top (1.9cm diameter), and one hole on either end (3.2cm and 1.9cm). A camera was mounted directly above the center of the tub and was used to videotape the session.
Drug Treatment
Methylphenidate (MPH) and atomoxetine (ATMX; Sigma-Aldrich, St. Louis, MO) were dissolved in sterile water and injected into the intraperitoneal cavity in a volume of 1.0ml/kg. MPH (0.125mg/kg) or ATMX (0.125mg/kg) was injected 10 or 30 min before the start of a behavioral task, respectively. These doses are based on prior studies conducted to identify a dose of each drug that did not affect locomotor behavior, which would have confounded interpretation of the orienting and social behavior. Specifically, we found that doses of 0.25, 0.5, and 1.0mg/kg of MPH increased activity levels and doses of 0.25, 0.5, 1.0, and 2.0 mg/kg of atomoxetine reduced general activity (Eggleston, Robinson, & Bucci, 2011). Rats in the WIS:CTL, SHR:CTL, and SHR:EX groups were injected with saline either 10 min or 30 min before behavioral testing began.
Behavioral procedure
Exercise
Rats in the exercise groups were provided with 24 hr access to a running wheel for 3 weeks prior to the start of the behavioral procedures and continued to have access to the wheels throughout the entire study. The door to the wheel was blocked off 2 hrs prior to behavioral training in order to minimize the potential for exercise-related fatigue. The number of wheel rotations was recorded daily.
Unconditioned orienting procedure
During a single 32-min session, rats received 12 non-reinforced presentations of the stimulus light (10-sec duration) during which the red house light was extinguished and the stimulus light flashed on/off at a frequency of 1Hz. The average inter-stimulus interval was 2.75min.
Conditioning procedure
A subset of rats in the WIS:CTL, SHR:CTL, and SHR:MPH groups (n=6/group) in Experiment 1 also underwent appetitive conditioning after the unconditioned orienting session to determine if the observed effects of MPH on orienting behavior in SHRs translated to effects on conditioned food cup behavior. Prior to the unconditioned orienting session, these rats were weighed for 3 days to establish baseline body weight, which was gradually reduced over a 7-day period and maintained at 85% of the free-feeding weight. The subsequent 8 daily conditioning sessions consisted of 6 trials during which the stimulus light was presented for 10 sec as described above and followed immediately by the delivery of two 45-mg food pellets (BioServ, Inc, Frenchtown, NJ). Note that there were no differences in unconditioned orienting behavior between rats that had been food-restricted and those that were not food restricted (p > 0.1).
Social interaction procedure
Following completion of the conditioning procedure, 65 of the SHRs and 8 of the Wistar rats used in Experiment 1 were tested in the social interaction procedure. Eight of the remaining 9 SHRs were not tested because they were for another study not reported here. In addition, one SHR was used as the target rat for the social interaction task; see Table 1 for group sample sizes in Experiment 1. In Experiment 2, all of the rats in each group were tested in the social interaction procedure. Rats were allowed free access to food and water for 7 days before being exposed to the social interaction apparatus. At the beginning of the session, each rat was placed in the corner of the tub and was allowed to explore the tub for 10 min. After the session was over all surfaces were cleaned with disinfectant (Quatricide™).
Behavioral measures and data analysis
Wheel running
Since a group of rats shared each wheel it was not possible to know the exact distance run by each individual rat. However, previous studies have shown that group-housed rodents spend approximately equal amounts of time on the wheel when it is freely accessible (54). Thus, we divided the number of wheel rotations recorded each day by the number of rats in the cage. The average daily distance run by an individual rat was then calculated by multiplying the number of rotations by the circumference of the wheel (1.12m for the Med Associates wheel and 1.08m for the Philips Respironics wheels) to convert to meters.
Unconditioned orienting behavior
During each presentation of the visual stimulus, the number of breaks in the photobeams mounted on the walls of the chamber were monitored by a computer and used to measure orienting behavior. Orienting was defined as rearing on the hind legs with both forepaws off the ground (Holland, 1977). The number of breaks in the three photobeams used to detect rearing behavior was summed for each trial, because previous studies indicate that it is unlikely that a rearing response will simultaneously break more than one photobeam (Keene & Bucci, 2007). The number of beam breaks was then summed across blocks of 4 trials in order to assess habituation of the unconditioned orienting response. A repeated measures analysis of variance (ANOVA) was used to assess differences in the number of rears using Block as the within-subjects variable and Group (WIS:CTL, SHR:CTL, SHR:EX, SHR:MPH, SHR:MPH+EX, SHR:ATMX, and SHR:ATMX+EX in Experiment 1; and WIS:CTL, WIS:MPH, and WIS:ATMX in Experiment 2) as the between-subjects variable. P-values were corrected with the Huynh-Feldt procedure and significant differences were subsequently analyzed using Fisher’s PLSD test.
Conditioned food cup behavior
During the conditioning sessions, a computer monitored breaks in the photobeam located across the entry to the food cup. The number of beam breaks during presentation of the light was used as the primary measure of conditioned responding. The number of breaks was averaged across the trials in each session and group differences were analyzed using a repeated measures ANOVA with Group as the between-subjects variable and Session as the within-subject variable.
Social interaction
The primary measure of social interaction was the number of times the experimental rat approached and sniffed inside one of the holes in the cylinder that contained the target rat. In addition, walking around the cylinder with continuous sniffing was counted as an interaction. Exploration of other parts of the cylinder (i.e., areas without holes) was not counted as an interaction. Interactions initiated by the target rat when they poked their noses out of the front hole were not scored unless the experimental rat reciprocated the interaction. The number of social interactions was analyzed using a one-way ANOVA with Group as the between-subject variable. Significant differences were subsequently analyzed using Fisher’s PLSD test.
Locomotor activity
The recording of the social interaction session was also scored to assess the levels of general locomotor activity in each group of rats. Locomotor activity was measured to assess whether any differences that emerged in orienting behavior or social interaction might be due to exercise-induced fatigue or to effects of MPH or ATMX on activity. To measure locomotion, two lines were drawn perpendicular to the long side of the tub on the video screen, thus dividing the tub into three equal areas. A line crossing was counted when all four paws crossed over onto the other side of the line. The number of line crossings was analyzed using a one-way ANOVA with Group as the between-subject variable and significant differences were subsequently analyzed using Fisher’s PLSD test. All statistical analyses were conducted using an alpha level of 0.05.
RESULTS
EXPERIMENT 1
Exercise
The average daily distance run by each rat in the exercise groups was 4.9 km for the SHR:EX group, 5.4 km for the SHR:MPH + EX group, and 5.1 km for the SHR:ATMX+EX group.
Unconditioned orienting behavior
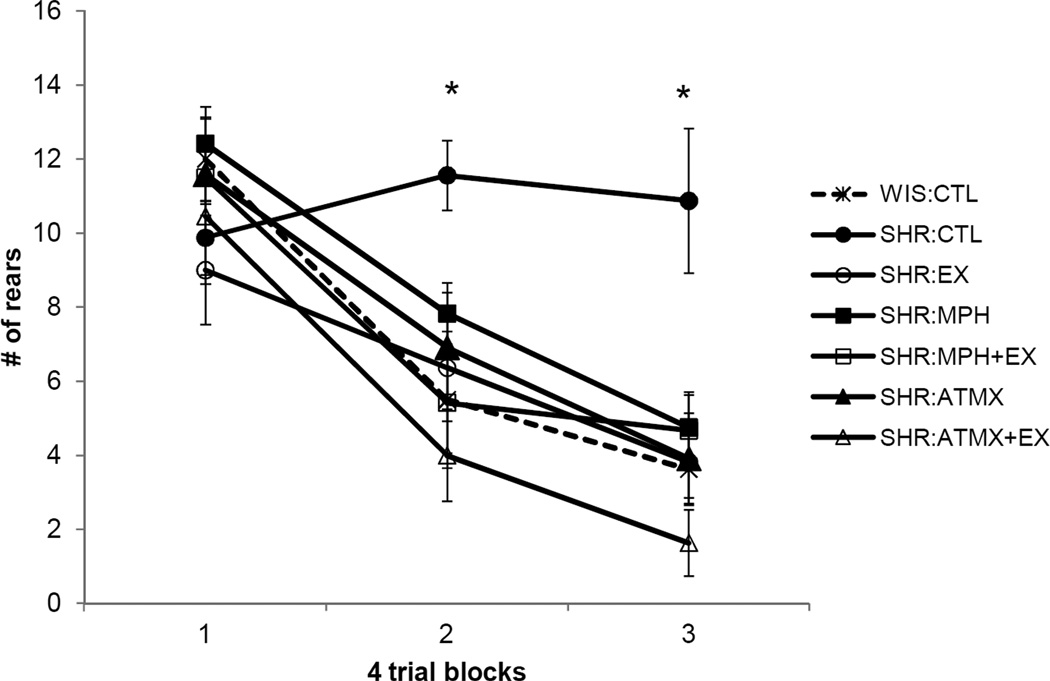
The amount of rearing behavior exhibited by rats in each group during non-reinforced presentations of the stimulus light is illustrated in Figure 1. A repeated measures ANOVA revealed a significant main effect of Block [F (2,150) = 59.9, p < 0.001] as well as a significant main effect of Group [F (6,75) = 3.6, p < 0.005] and a significant Block × Group interaction [F (2,150) = 3.7, p < 0.001]. Post hoc analysis of the main effect of Group revealed that the SHR: CTL group exhibited rearing more than all other groups (ps < 0.01), with the exception of the SHR:MPH group, which approached statistical significance (p = 0.06). In addition, there was a significant difference between the SHR:MPH and SHR:ATMX+EX groups (p = 0 .04).
Figure 1.
Unconditioned orienting (rearing behavior) observed during repeated presentation of a non-reinforced visual stimulus in Experiment 1. The number of breaks in the photobeams used to detect rearing behavior is shown on the y-axis. Blocks of trials (first 4, middle 4, and last 4 trials) are shown on the x-axis. All groups exhibited habituation of the orienting response except the SHR:CTL group (*p<0.05). Data are means ± standard error. Abbreviations: WIS = Wistar; SHR = Spontaneously Hypertensive; CTL = control (no exercise and only treated with saline); EX = 3 weeks access to a running wheel; MPH = methylphenidate (0.125mg/kg); ATMX = atomoxotine (0.125mg/kg).
The Block × Group interaction was subsequently decomposed using repeated measures ANOVAs that compared rearing behavior across blocks in each group. As expected, rearing behavior did not habituate in the SHR:CTL group (p > 0.5); in contrast, all other groups exhibited a significant decrease in rearing over blocks (ps < 0.03). In addition, one-way ANOVAs were carried out to compare differences between the groups during each block. During Block 1 there was no significant difference between groups [F (6,81) = 0.9, p > 0.4], indicating that the amount of rearing behavior was initially comparable in all groups. However, there were significant group differences in the amount of rearing during Block 2 [F (6,81) = 4.3, p < 0.001] with post-hoc tests revealing that SHR:CTL rats reared more than any other group (ps < 0.03). Also, rats in the SHR:ATMX+EX group reared less than rats in the SHR:MPH group (p < 0.04); there were no other group differences during Block 2. During Block 3, there was also a significant group difference in the amount of orienting behavior [F (6,81) = 5.6, p < 0.001] with post-hoc tests revealing a significant difference between SHR:CTL rats and all other groups (ps < 0.002).
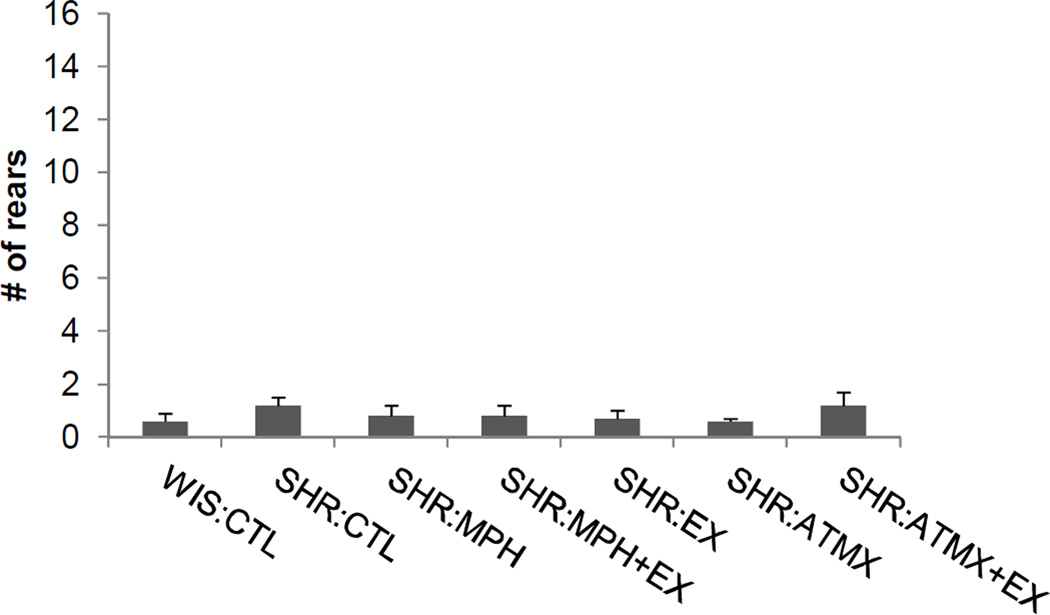
Two other analyses were conducted to test for potential confounding effects of drug treatment or exercise on rearing. An analysis of the amount of rearing exhibited during the 5-sec period before the first presentation of the visual stimulus did not reveal any group differences [F (6,81) = 0.6, p > 0.7], indicating that all groups exhibited similar baseline levels of rearing behavior. Indeed, the amount rearing exhibited prior to the first trial was uniformly low, as shown in Figure 2. In addition, a one-way ANOVA did not reveal any group differences in rearing during the first trial [F (6,81) = 1.3, p > 0.2], indicating that rats in all groups displayed similar amounts of orienting behavior to the initial presentation of the light.
Figure 2.
Rearing behavior prior to onset of the visual stimulus in Experiment 1. The amount of rearing behavior observed during the preCS period (i.e., 5 sec before the visual stimulus was presented) was uniformly low across each treatment group. Data are mean ± SEM (abbreviations as defined in Figure 1).
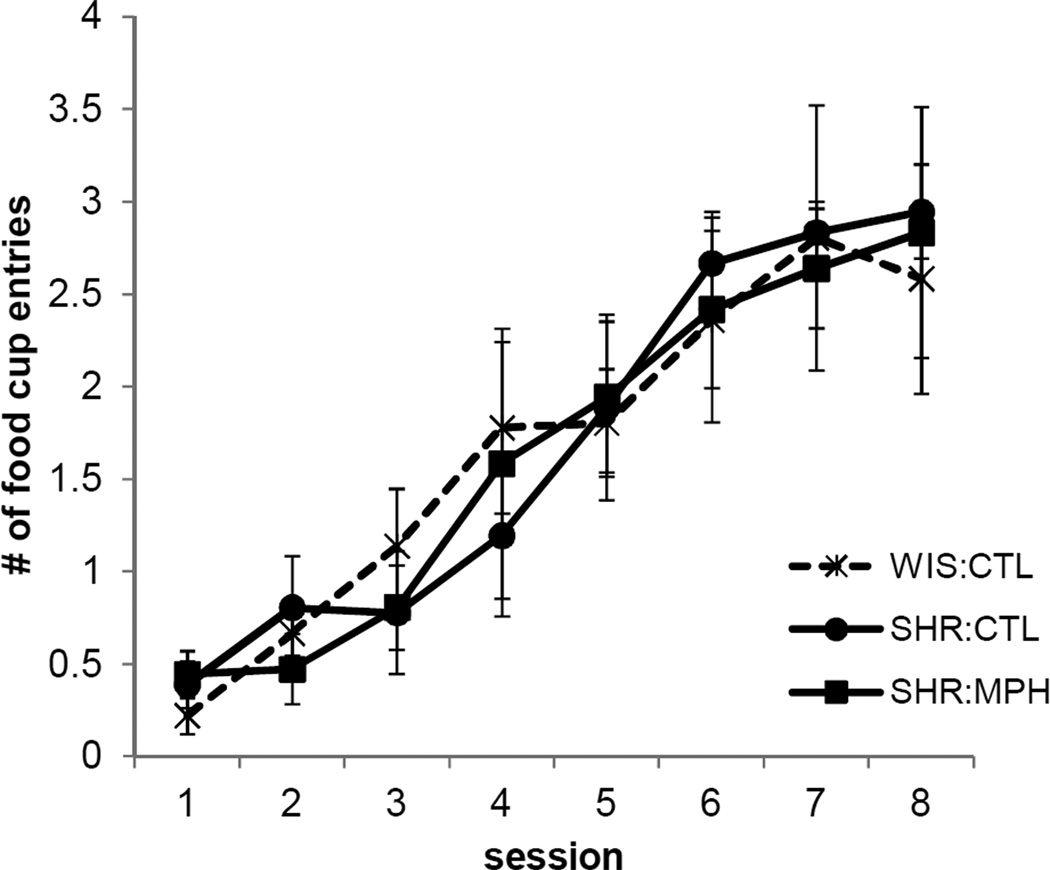
Conditioned food cup behavior
When the light was later paired with food for a subset of WIS:CTL, SHR:CTL, and SHR:MPH rats, all groups exhibited increased food cup behavior over the course of training, as shown in Figure 3 (main effect of Session [F (7,9) = 13.0, p < 0.001]). There were no Group differences [F (2,15) = 0.009, p > 0.9] and no Group × Session interaction [F (14,18) = 0.5, p > 0.8], indicating that learning about the light-food contingency was not affected by MPH or strain.
Figure 3.
Conditioned food cup behavior during the 8 sessions in which the light was paired with a food reward in Experiment 1. MPH did not affect conditioning tot the light. The number of beam breaks into the food cup during the last 5 sec of the presentation of the light is shown on the y-axis. Data are mean ± SEM (abbreviations as defined in Figure 1).
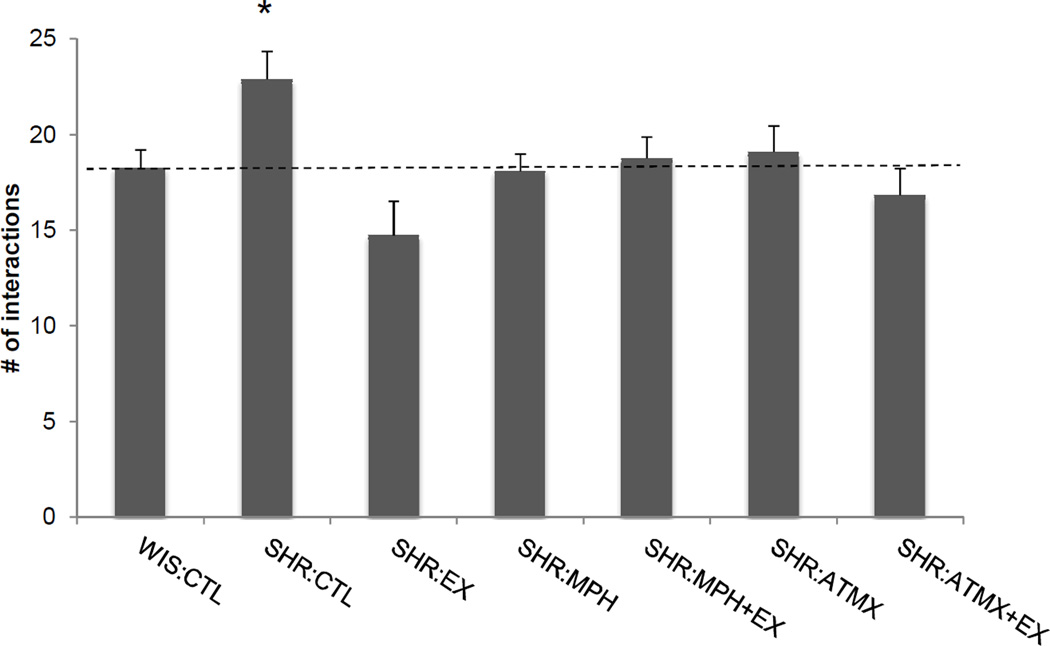
Social behavior
The data from one rat was not included in the analysis because of a technical difficulty with the video recording. Final samples sizes are indicated in Table 1. Figure 4 illustrates the number of social interactions made during the 10 min free-exploration session. A one-way ANOVA revealed a significant difference between groups in the number of interactions made with the target rat [F (6,65) = 3.1, p < 0.01]. Post-hoc tests revealed a significant difference between the SHR:CTL group and all other groups (ps < 0.04) except the SHR:ATMX group, which approached statistical significance (p = 0.06).
Figure 4.
The average number of social interactions made by each group in Experiment 1. The number of social interactions reflects the number of contacts made by an experimental rat with holes in the cylinder containing the target rat during a 10-min session. The number of social interactions was significantly higher in the SHR:CTL group (*p<0.05) compared to all other groups (except the SHR:ATMX group, which approached statistical significance). Data are means ± standard error (abbreviations as defined in Figure 1). Dashed line indicates the number of interactions exhibited by the WIS:CTL group
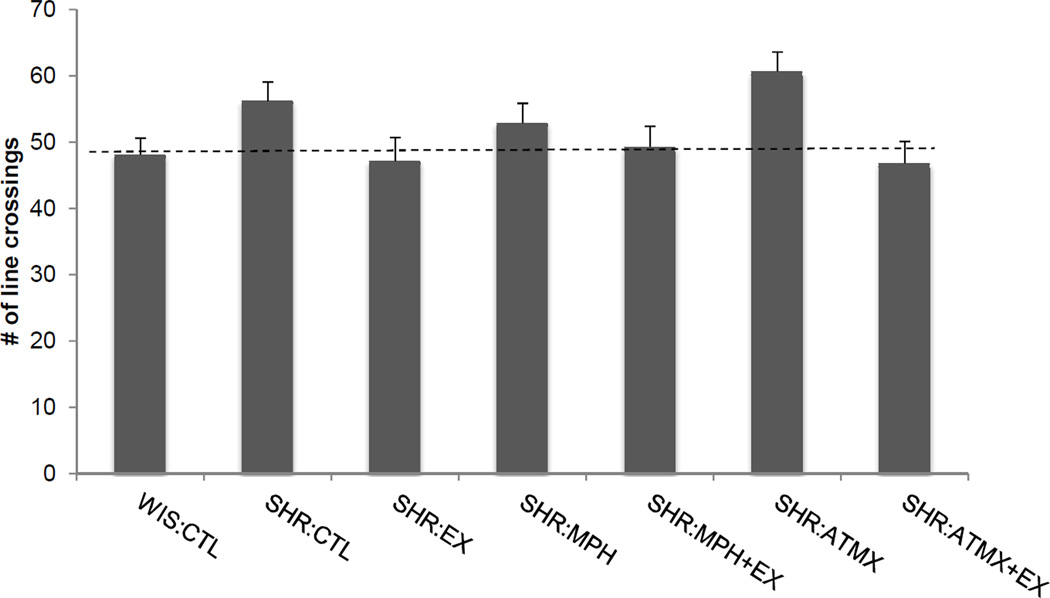
Locomotor behavior
Locomotor activity during the social interaction task is shown in Figure 5. A one-way ANOVA revealed a significant difference between groups in the number of line crossings made during the 10 min social interaction session [F (6,65) = 2.8, p < 0.02]. Post-hoc comparisons only revealed that the difference between the SHR:CTL group and the SHR:ATMX+EX group was marginally significant (p=0.048).
Figure 5.
Locomotor activity measured during the social interaction task in Experiment 1. Data reflect the number of times rats in each group crossed a line that separated the arena into thirds. There were no differences between any of the SHR groups and the WIS:CTL group. Data are means ± SEM (abbreviations as defined in Figure 1). Dashed line indicates the number of line crossing in the WIS:CTL group.
EXPERIMENT 2
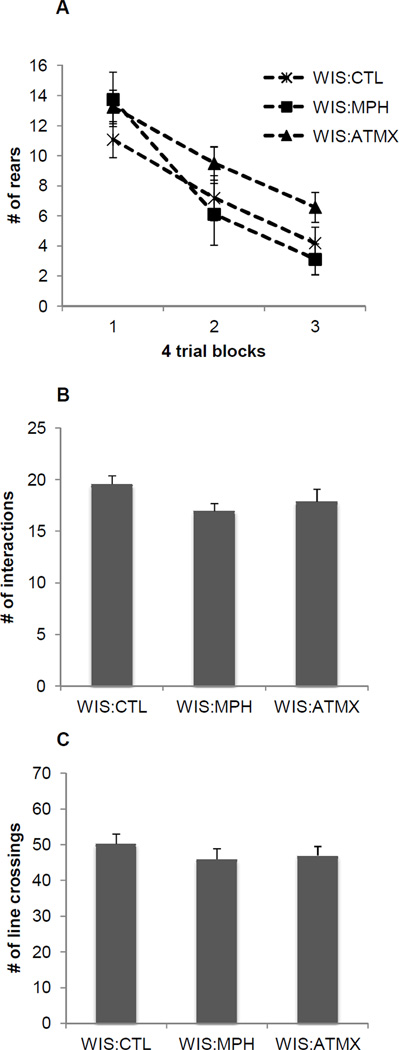
The results for the Wistar rats are illustrated in Figure 6. In the unconditioned orienting task (Figure 6A), all Wistar rats habituated to the visual stimulus as indicated by a main effect of Block [F (2,58) = 44.2, p < 0.001]. There was no main effect of Group [F (2,29) = 1.7, p > 0.18] or a significant Block × Group interaction [F (4,58) = 1.3, p > 0.29], indicating MPH and ATMX did not affect orienting behavior in Wistar rats. In addition, neither MPH nor ATMX affected social interaction (Figure 6B, [F (2,31) = 1.6, p > 0.2]) or locomotor activity (Figure 6C, [F (2,31) = 0.6, p > 0.5]) compared to Wistar control rats.
Figure 6.
Unconditioned orienting behavior (A), social interaction (B), and locomotor activity (C) observed in Experiment 2. Neither MPH nor ATMX affected orienting behavior, social interaction, or locomotor behavior in Wistar rats. Data are means ± SEM
DISCUSSION
Experiment 1 tested and compared the effects of drug treatment, exercise, or a combination of exercise and drug treatment on orienting behavior and social interaction in SHRs, a commonly used animal model of ADHD. One goal was to determine whether MPH and ATMX, which have differential effects on dopamine and norepinephrine reuptake, have different effects on orienting behavior and on social behavior in female SHRs. A second goal of the study was to determine if physical exercise would be as effective as MPH or ATMX in reducing distractibility and hyper-social behavior.
Consistent with its hyper-responsive phenotype (Sagvolden et al., 2005) and our prior findings (Hopkins et al., 2009; Robinson et al., 2011), SHRs that did not exercise or receive MPH or ATMX (SHR:CTL) failed to exhibit habituation of the orienting response during repeated presentations of a non-reinforced visual stimulus, suggesting that SHRs have difficulty ignoring (or learning to ignore) distracting, irrelevant stimuli. In contrast, SHRs that were treated with MPH or ATMX exhibited a robust decrease in orienting behavior across trials, indicating that blocking the reuptake of dopamine and/or norepinephrine may reduce distractibility in SHRs. Similarly, SHRs that had access to a running wheel for three weeks also exhibited habituation of the orienting response. Notably, the orienting behavior of SHRs treated with MPH, ATMX, or EX was indistinguishable from normo-active rats (WIS:CTL), and exercise alone was as efficacious as drug treatment in reducing orienting behavior.
The finding that exercise was at least as effective as MPH or ATMX in reducing orienting behavior suggests that physical exercise might be a useful adjunctive or replacement therapy for attentional dysfunction in persons with ADHD, consistent with a recent report that acute physical exercise enhanced inhibitory function in children with ADHD (Chang et al., 2012). Similar to our findings, a recent study (Kim et al., 2011) found that exercise was as effective as MPH in reducing hyperactivity and improving spatial learning in SHRs. In addition they found that MPH and treadmill exercise both increased expression of tyrosine hydroxylase in the striatum and brain derived neurotrophic factor (BDNF) in the hippocampus, which were decreased in SHRs when compared to controls. Indeed, there is currently great interest in using exercise as a replacement or adjunctive therapies for psychostimulants (Halperin & Healey, 2011) given mounting concerns about long-term health effects and limitations in the efficacy of drug therapies (Biederman et al., 2004; Scherer et al., 2010; Swanson et al., 2007; Volkow & Insel, 2003). In addition, exercise has the potential to produce longer lasting effects on behavior, unlike drug therapies, in which the effects are usually only present during active treatment (Jensen et al., 2007). For instance, recent studies have shown that exercise (particularly during adolescence) results in cognitive improvements that last long after exercise is terminated (Hopkins et al., 2011; Gomes da Silva et al., 2012).
Moreover, we found that combining exercise and drug treatment was no more effective than either intervention alone; suggesting that exercise, MPH, and ATMX may act through similar neurobiological mechanisms to affect orienting behavior. For instance, both MPH and ATMX reduce the reuptake of norepinephrine (Kuczenski & Segal, 2001; Michelson et al., 2001; Spencer et al., 2001), and physical exercise has likewise been shown to increase norepinephrine levels (Hattori, Naoi, & Nishino, 1994; Winter et al., 2007). However, future studies are needed to determine if shorter durations of exercise in combination with lower drug doses could produce additive effects in reducing excessive orienting behavior in SHRs in the event that the current findings reflect a floor effect.
In the social interaction task, SHR:CTL rats initiated more social interactions than WIS:CTL rats, consistent with prior studies using another normo-active control strain (Hopkins et al., 2009) and supporting the notion that SHRs exhibit hyper-social behavior as reported in persons with ADHD (Pelman et al., 2005; Whalen & Henker, 1992). Three weeks of wheel running or treatment with MPH reduced the number of social interactions compared to the SHR:CTL group. Combining MPH and exercise did not reduce the number of social interactions any further than MPH or exercise alone. The effect of ATMX alone on social interaction approached statistical significance, while ATMX treatment significantly reduced social interactions when it was combined with exercise. As was the case with orienting behavior, drug treatment or wheel running reduced the number of social interactions to the level exhibited by the normo-active control group (WIS:CTL) and exercise was just as effective as MPH or ATMX in reducing social interactions. Although hyper-social behavior is not considered a core deficit in ADHD, it is often associated with the disorder (Pelman et al., 2005; Whalen & Henker, 1992) and notoriously resistant to interventions (Swanson et al., 2001). Thus, physical exercise may have potential use as a replacement therapy for psychostimulants in treating impairments in social behavior. However, additional research in needed on the nature of social dysfunction in ADHD, which is likely a complex phenomenon with characteristics that are not fully realized in the rat model used here.
It is unlikely that the effects of exercise, MPH, or ATMX on reducing orienting behavior or social behavior could be attributed simply to reductions in overall activity levels. Indeed, locomotor behavior was comparable between all of the SHR groups and the WIS:CTL group. In addition, we purposefully chose to test doses that were lower than those that affected locomotor activity (Eggleston et al., 2011). Likewise, there were no group differences in the amount of orienting behavior exhibited during the first block of presentations of the visual stimulus and no difference in baseline levels of orienting behavior. It is also interesting to note that when the light was later paired with a food reward, SHR:CTL, SHR MPH, and WIS:CTL rats in Experiment 1 learned the light-food contingency comparably. This further indicates that the effects of MPH, ATMX, and EX on orienting behavior and social interaction were not simply due to non-specific effects on behavior. Moreover, the finding that MPH affected unconditioned orienting behavior but not conditioning in SHRs is consistent with prior literature in humans indicating that MPH affects attention but not achievement or learning (Advokat, 2010).
In addition, we found that the effects of MPH and ATMX on orienting behavior and social interaction were specific to SHRs, in that neither drug affected these behaviors in Wistar rats (Experiment 2). This is consistent with evidence that SHRs exhibit hypo-functioning dopamine and norepinephrine systems compared to normo-active control rats (Heal et al., 2008; Russell, 2000, 2002; Solanto & Conners, 1982), suggesting that the doses used in the study had a greater impact on behavior in rats with pre-existing neurochemical dysfunction. Moreover, our findings suggest that low doses of MPH, that do not affect locomotor behavior, can be affective in alleviating cognitive impairments, consistent with prior studies that used doses that had little effect on locomotor behavior (Berridge, Devilbliss, Andrzejewski, Arnsten et al., 2006).
In summary, the present findings are among the first to compare the effects of physical exercise and standard of care pharmacological treatments on ADHD-like behaviors. Physical exercise was at least as effective as a psychostimulant or selective norepinephrine drug in reducing excessive orienting behavior and hyper-social behavior in SHRs, a commonly used animal model of ADHD. Importantly, SHRs that exercised or were treated with MPH or ATMX exhibited ‘normal’ levels of orienting and social behavior in the sense that they were indistinguishable from untreated Wistar control rats. Given the intense interest in developing new therapies for ADHD, these findings have important implications for using physical exercise as an adjunctive or replacement therapy for psychostimulants. In addition, these findings address current gaps in the literature regarding the paucity of studies on ADHD in adults, and the lack of research on female subjects. It should be noted, however, that SHRs do not necessarily model all aspects of ADHD-related behavior and data obtained with SHRs may be more or less relevant to particular subtypes of ADHD (Wickens, Hyland, & Tripp, 2011). Similarly, although SHRs exhibit alterations in catecholaminergic neurotransmission, such abnormalities have not been widely identified in persons with ADHD. Nevertheless, the present findings suggest that exercise may be a useful non-pharmacological intervention to treat at least some of the cognitive and behavioral symptoms of ADHD.
ACKNOWLEDGEMENTS
This research was supported by NIH Grant R01MH082893. The authors thank Dr. Alexandra Potter for comments on previous versions of the manuscript.
REFERENCES
- Advokat C. What are the cognitive effects of stimulant medications? Emphasis on adults with attention-deficit/hyperactivity disorder (ADHD) Neuroscience & Biobehavioral Reviews. 2010;34(8):1256–1266. doi: 10.1016/j.neubiorev.2010.03.006. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 3rd edition. Washington, DC: American Psychiatric Association; 2000. [Google Scholar]
- Arnsten AF. Stimulants: Therapeutic actions in ADHD. Neuropsychopharmacology. 2006;31(11):2376–2383. doi: 10.1038/sj.npp.1301164. [DOI] [PubMed] [Google Scholar]
- Barkley RA. Attention-deficit/hyperactivity disorder, self-regulation, and time: toward a more comprehensive theory. Journal of Developmental & Behavioral Pediatrics. 1997;18(4):271–279. [PubMed] [Google Scholar]
- Berridge CW, Devilbiss DM, Andrzejewski ME, Arnsten AF, Kelley AE, Schmeichel B, et al. Methylphenidate preferentially increases catecholamine neurotransmission within the prefrontal cortex at low doses that enhance cognitive function. Biological Psychiatry. 2006;60(10):1111–1120. doi: 10.1016/j.biopsych.2006.04.022. [DOI] [PubMed] [Google Scholar]
- Biederman J. Attention-deficit/hyperactivity disorder: a selective overview. Biological Psychiatry. 2005;57(11):1215–1220. doi: 10.1016/j.biopsych.2004.10.020. [DOI] [PubMed] [Google Scholar]
- Biederman J, Spencer T, Wilens T. Evidence-based pharmacotherapy for attention-deficit hyperactivity disorder. The International Journal of Neuropsychopharmacology. 2004;7(1):77–97. doi: 10.1017/S1461145703003973. [DOI] [PubMed] [Google Scholar]
- Bymaster FP, Katner JS, Nelson DL, Hemrick-Luecke SK, Threlkeld PG, Heiligenstein JH, et al. Atomoxetine increases extracellular levels of norepinephrine and dopamine in prefrontal cortex of rat: a potential mechanism for efficacy in attention deficit/hyperactivity disorder. Neuropsychopharmacology. 2002;27(5):699–711. doi: 10.1016/S0893-133X(02)00346-9. [DOI] [PubMed] [Google Scholar]
- Carek PJ, Laibstain SE, Carek SM. Exercise for the treatment of depression and anxiety. The International Journal of Psychiatry in Medicine. 2011;41(1):15–28. doi: 10.2190/PM.41.1.c. [DOI] [PubMed] [Google Scholar]
- Chang YK, Liu S, Yu HH, Lee YH. Effect of acute exercise on executive function in children with attention deficit hyperactivity disorder. Archives of Clinical Neuropsychology. 2012;27(2):225–237. doi: 10.1093/arclin/acr094. [DOI] [PubMed] [Google Scholar]
- Davids E, Zhang K, Tarazi FI, Baldessarini RJ. Animal models of attention-deficit hyperactivity disorder. Brain Research. Brain Research Reviews. 2003;42(1):1–21. doi: 10.1016/s0165-0173(02)00274-6. [DOI] [PubMed] [Google Scholar]
- Eggleston RL, Robinson AM, Bucci DJ. Society for Neuroscience Abstracts. Washington, D.C.: 2011. A comparison of the effects of physical exercise and pharmaco-therapies on attentional dysfunction and hyper-social behavior in a rat model of ADHD. [Google Scholar]
- File SE. The use of social interaction as a method for detecting anxiolytic activity of chlordiazepoxide-like drugs. Journal of Neuroscience Methods. 1980;2(3):219–238. doi: 10.1016/0165-0270(80)90012-6. [DOI] [PubMed] [Google Scholar]
- File SE, Seth P. A review of 25 years of the social interaction test. European Journal of Pharmacology. 2003;463(1–3):35–53. doi: 10.1016/s0014-2999(03)01273-1. [DOI] [PubMed] [Google Scholar]
- Fox JH, Hammack SE, Falls WA. Exercise is associated with reduction in the anxiogenic effect of mCPP on acoustic startle. Behavioral Neuroscience. 2008;122(4):943–948. doi: 10.1037/0735-7044.122.4.943. [DOI] [PubMed] [Google Scholar]
- Gallagher M, Graham PW, Holland PC. The amygdala central nucleus and appetitive Pavlovian conditioning: lesions impair one class of conditioned behavior. The Journal of Neuroscience. 1990;10(6):1906–1911. doi: 10.1523/JNEUROSCI.10-06-01906.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gershon J. A meta-analytic review of gender differences in ADHD. Journal of Attention Disorders. 2002;5:143–154. doi: 10.1177/108705470200500302. [DOI] [PubMed] [Google Scholar]
- Gomes da Silva S, Unsain N, Masco DH, Toscano-Silva M, de Amorim HA, Silva Araújo BH, et al. Early exercise promotes positive hippocampal plasticity and improves spatial memory in the adult life of rats. Hippocampus. 2012;22(2):347–358. doi: 10.1002/hipo.20903. [DOI] [PubMed] [Google Scholar]
- Halperin JM, Healey DM. The influences of environmental enrichment, cognitive enhancement, and physical exercise on brain development: can we alter the developmental trajectory of ADHD? Neuroscience & Biobehavioral Reviews. 2011;35(3):621–634. doi: 10.1016/j.neubiorev.2010.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hattori S, Naoi M, Nishino H. Striatal dopamine turnover during treadmill running in the rat: relation to the speed of running. Brain Research Bulletin. 1994;35(1):41–49. doi: 10.1016/0361-9230(94)90214-3. [DOI] [PubMed] [Google Scholar]
- Heal DJ, Smith SL, Kulkarni RS, Rowley HL. New perspectives from microdialysis studies in freely-moving, spontaneously hypertensive rats on the pharmacology of drugs for the treatment of ADHD. Pharmacology Biochemistry and Behavior. 2008;90(2):184–197. doi: 10.1016/j.pbb.2008.03.016. [DOI] [PubMed] [Google Scholar]
- Holland PC. Conditioned stimulus as a determinant of the form of the Pavlovian conditioned response. Journal of Experimental Psychology: Animal Behavior Processes. 1977;3(1):77–104. doi: 10.1037//0097-7403.3.1.77. [DOI] [PubMed] [Google Scholar]
- Holland PC. Unblocking in Pavlovian appetitive conditioning. Journal of Experimental Psychology: Animal Behavior Processes. 1984;10(4):476–497. [PubMed] [Google Scholar]
- Holland PC. Brain mechanisms for changes in processing of conditioned stimuli in Pavlovian conditioning: Implications for behavior theory. Animal Learning & Behavior. 1997;25:373–399. [Google Scholar]
- Hopkins ME, Sharma M, Evans GC, Bucci DJ. Voluntary physical exercise alters attentional orienting and social behavior in a rat model of attention-deficit/hyperactivity disorder. Behavioral Neuroscience. 2009;123(3):599–606. doi: 10.1037/a0015632. [DOI] [PubMed] [Google Scholar]
- Hopkins ME, Nitecki R, Bucci DJ. Physical exercise during adolescence versus adulthood: Differential effects on object recognition memory and BDNF expression. Neuroscience. 2011;194:84–94. doi: 10.1016/j.neuroscience.2011.07.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen PS, Arnold LE, Swanson JM, Vitiello B, Abikoff HB, Greenhill LL, et al. 3-year follow-up of the NIMH MTA study. Journal of the American Academy of Child and Adolescent Psychiatry. 2007;46(8):989–1002. doi: 10.1097/CHI.0b013e3180686d48. [DOI] [PubMed] [Google Scholar]
- Kantak KM, Singh T, Kerstetter KA, Dembro KA, Mutebi MM, Harvey RC, et al. Advancing the spontaneous hypertensive rat model of attention deficit/hyperactivity disorder. Behavioral Neuroscience. 2008;22(2):340–357. doi: 10.1037/0735-7044.122.2.340. [DOI] [PubMed] [Google Scholar]
- Kaye H, Pearce JM. The strength of the orienting response during Pavlovian conditioning. Journal of Experimental Psychology: Animal Behavior Processes. 1984;10(1):90–109. [PubMed] [Google Scholar]
- Keene CS, Bucci DJ. Automated measure of conditioned orienting behavior in rats. Behavior Research Methods. 2007;39(2):303–308. doi: 10.3758/bf03193161. [DOI] [PubMed] [Google Scholar]
- Kim H, Heo HI, Kim DH, Ko IG, Lee SS, Kim SE, et al. Treadmill exercise and methylphenidate ameliorate symptoms of attention deficit/hyperactivity disorder through enhancing dopamine synthesis and brain-derived neurotrophic factor expression in spontaneous hypertensive rats. Neuroscience Letters. 2011;504(1):35–39. doi: 10.1016/j.neulet.2011.08.052. [DOI] [PubMed] [Google Scholar]
- Kuczenski R, Segal DS. Locomotor effects of acute and repeated threshold doses of amphetamine and methylphenidate: relative roles of dopamine and norepinephrine. Journal of Pharmacology and Experimental Therapeutics. 2001;296(3):876–883. [PubMed] [Google Scholar]
- Lang PJ, Simons RG, Balaban M. Attention and orienting: Sensory and motivational processes. Mahwah, NJ: Erlbaum; 1997. [Google Scholar]
- Mao AR, Babcock T, Brams M. ADHD in adults: current treatment trends with consideration of abuse potential of medications. Journal of Psychiatry Practice. 2011;17(4):241–250. doi: 10.1097/01.pra.0000400261.45290.bd. [DOI] [PubMed] [Google Scholar]
- Michelson D, Faries D, Wernicke J, Kelsey D, Kendrick K, Sallee FR, et al. Atomoxetine in the treatment of children and adolescents with attention-deficit/hyperactivity disorder: a randomized, placebo-controlled, dose-response study. Pediatrics. 2001;108(5):E83. doi: 10.1542/peds.108.5.e83. [DOI] [PubMed] [Google Scholar]
- Milich R, Balentin AC, Lynam DR. ADHD combined type and ADHD predominantly inattentive type are distinct and unrelated disorders. Clinical Psychology: Science And Practice. 2001;8:463–488. [Google Scholar]
- Naylor AS, Persson AI, Eriksson PS, Jonsdottir IH, Thorlin T. Extended voluntary running inhibits exercise-induced adult hippocampal progenitor proliferation in the spontaneously hypertensive rat. Journal of Neurophysiology. 2005;93(5):2406–2414. doi: 10.1152/jn.01085.2004. [DOI] [PubMed] [Google Scholar]
- Nair J, Ehimare U, Beitman BD, Nair SS, Lavin A. Clinical review: evidence-based diagnosis and treatment of ADHD in children. Molecular Medicine. 2006;103(6):617–621. [PubMed] [Google Scholar]
- Nigg JT. Is ADHD a disinhibitory disorder? Psychological Bulletin. 2001;127(5):571–598. doi: 10.1037/0033-2909.127.5.571. [DOI] [PubMed] [Google Scholar]
- Nigg JT, Blaskey LG, Huang-Pollock CL, Rappley MD. Neuropsychological executive functions and DSM–IV ADHD subtypes. Journal of the American Academy of Child and Adolescent Psychiatry. 2002;41:59–66. doi: 10.1097/00004583-200201000-00012. [DOI] [PubMed] [Google Scholar]
- Nigg JT, Stavro G, Ettenhofer M, Hambrick DZ, Miller T, Henderson JM. Executive functions and ADHD in adults: evidence for selective effects on ADHD symptom domains. Journal of Abnormal Psychology. 2005;114(4):706–717. doi: 10.1037/0021-843X.114.3.706. [DOI] [PubMed] [Google Scholar]
- Oades RD, Sadile AG, Sagvolden T, Viggiano D, Zuddas A, Devoto P, et al. The control of responsiveness in ADHD by catecholamines: evidence for dopaminergic, noradrenergic and interactive roles. Developmental Science. 2005;8(2):122–131. doi: 10.1111/j.1467-7687.2005.00399.x. [DOI] [PubMed] [Google Scholar]
- Pagliari R, Peyrin L. Norepinephrine release in the rat frontal cortex under treadmill exercise: a study with microdialysis. Journal of Applied Physiology. 1995;78(6):2121–2130. doi: 10.1152/jappl.1995.78.6.2121. [DOI] [PubMed] [Google Scholar]
- Pelham WE, Jr, Fabiano GA, Massetti GM. Evidence-based assessment of attention deficit hyperactivity disorder in children and adolescents. Journal of Clinical Child & Adolescent Psychology. 2005;34(3):449–476. doi: 10.1207/s15374424jccp3403_5. [DOI] [PubMed] [Google Scholar]
- Robinson AM, Hopkins ME, Bucci DJ. Effects of physical exercise on ADHD-like behavior in male and female adolescent spontaneously hypertensive rats. Developmental Psychobiology. 2011;53(4):383–390. doi: 10.1002/dev.20530. [DOI] [PubMed] [Google Scholar]
- Russell VA. The nucleus accumbens motor-limbic interface of the spontaneously hypertensive rat as studied in vitro by the superfusion slice technique. Neuroscience & Biobehavioral Reviews. 2000;24(1):133–136. doi: 10.1016/s0149-7634(99)00056-1. [DOI] [PubMed] [Google Scholar]
- Russell VA. Hypodopaminergic and hypernoradrenergic activity in prefrontal cortex slices of an animal model for attention-deficit hyperactivity disorder--the spontaneously hypertensive rat. Behavioural Brain Research. 2002;130(1–2):191–196. doi: 10.1016/s0166-4328(01)00425-9. [DOI] [PubMed] [Google Scholar]
- Russell VA. Neurobiology of animal models of attention-deficit hyperactivity disorder. Journal of Neuroscience Methods. 2007;161(2):185–198. doi: 10.1016/j.jneumeth.2006.12.005. [DOI] [PubMed] [Google Scholar]
- Sagvolden T. Behavioral validation of the spontaneously hypertensive rat (SHR) as an animal model of attention-deficit/hyperactivity disorder (AD/HD) Neuroscience & Biobehavioral Reviews. 2000;24(1):31–39. doi: 10.1016/s0149-7634(99)00058-5. [DOI] [PubMed] [Google Scholar]
- Sagvolden T, Russell VA, Aase H, Johansen EB, Farshbaf M. Rodent models of attention-deficit/hyperactivity disorder. Biological Psychiatry. 2005;57(11):1239–1247. doi: 10.1016/j.biopsych.2005.02.002. [DOI] [PubMed] [Google Scholar]
- Scherer EB, da Cunha MJ, Matté C, Schmitz F, Netto CA, Wyse AT. Methylphenidate affects memory, brain-derived neurotrophic factor immunocontent and brain acetylcholinesterase activity in the rat. Neurobiology of Learning and Memory. 2010;94(2):247–253. doi: 10.1016/j.nlm.2010.06.002. [DOI] [PubMed] [Google Scholar]
- Sergeant JA. Modeling attention-deficit/hyperactivity disorder: a critical appraisal of the cognitive-energetic model. Biological Psychiatry. 2005;57:1248–1255. doi: 10.1016/j.biopsych.2004.09.010. [DOI] [PubMed] [Google Scholar]
- Sibley BA, Etnier JL. The relationship between physical activity and cognition in children: A meta-analysis. Pediatric Exercise Science. 2003;15:243–256. [Google Scholar]
- Solanto MV, Conners CK. A dose-response and time-action analysis of autonomic and behavioral effects of methylphenidate in attention deficit disorder with hyperactivity. Psychophysiology. 1982;19(6):658–667. doi: 10.1111/j.1469-8986.1982.tb02519.x. [DOI] [PubMed] [Google Scholar]
- Sonuga-Barke EJ, Taylor E, Sembi S, Smith J. Hyperactivity and delay aversion--I. The effect of delay on choice. The Journal of Child Psychology and Psychiatry. 1992;33(2):387–398. doi: 10.1111/j.1469-7610.1992.tb00874.x. [DOI] [PubMed] [Google Scholar]
- Spencer TJ, Biederman J, Mick E. Attention-deficit/hyperactivity disorder: Diagnosis, lifespan, comorbidities, and neurobiology. Journal of Pediatric Psychology. 2007;3:631–642. doi: 10.1093/jpepsy/jsm005. [DOI] [PubMed] [Google Scholar]
- Spencer T, Biederman J, Heiligenstein J, Wilens T, Faries D, Prince J, et al. An open-label, dose-ranging study of atomoxetine in children with attention deficit hyperactivity disorder. Journal of Child and Adolescent Psychopharmacology. 2001;11(3):251–265. doi: 10.1089/10445460152595577. [DOI] [PubMed] [Google Scholar]
- Strong WB, Malina RM, Blimkie CJ, Daniels SR, Dishman RK, Gutin B, et al. Evidence based physical activity for school-age youth. Journal of Pediatrics. 2011;146(6):732–737. doi: 10.1016/j.jpeds.2005.01.055. [DOI] [PubMed] [Google Scholar]
- Swanson JM, Elliott GR, Greenhill LL, Wigal T, Arnold LE, Vitiello B, et al. Effects of stimulant medication on growth rates across 3 years in the MTA follow-up. Journal of the American Academy of Child and Adolescent Psychiatry. 2007;46(8):1015–1027. doi: 10.1097/chi.0b013e3180686d7e. [DOI] [PubMed] [Google Scholar]
- Swanson JM, Kraemer HC, Hinshaw SP, Arnold LE, Conners CK, Abikoff HB, et al. Clinical relevance of the primary findings of the MTA: success rates based on severity of ADHD and ODD symptoms at the end of treatment. Journal of the American Academy of Child and Adolescent Psychiatry. 2001;40(2):168–179. doi: 10.1097/00004583-200102000-00011. [DOI] [PubMed] [Google Scholar]
- Thanos PK, Ivanov I, Robinson JK, Michaelides M, Wang GJ, Swanson JM, et al. Dissociation between spontaneously hypertensive (SHR) and Wistar-Kyoto (WKY) rats in baseline performance and methylphenidate response on measures of attention, impulsivity and hyperactivity in a Visual Stimulus Position Discrimination Task. Pharmacology, Biochemistry, & Behavior. 2010;94(3):374–379. doi: 10.1016/j.pbb.2009.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Insel TR. What are the long-term effects of methylphenidate treatment? Biological Psychiatry. 2003;54(12):1307–1309. doi: 10.1016/j.biopsych.2003.10.019. [DOI] [PubMed] [Google Scholar]
- Whalen CK, Henker B. The social profile of attention-deficit hyperactivity disorder: Five fundamental facets. Child & Adolescent Psychiatric Clinics of North America. 1992;1:395–410. [Google Scholar]
- Wickens JR, Hyland BI, Tripp G. Animal models to guide clinical drug development in ADHD: lost in translation? British Journal of Pharmacology. 2011;164:1107–1128. doi: 10.1111/j.1476-5381.2011.01412.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winter B, Breitenstein C, Mooren FC, Voelker K, Fobker M, Lechtermann A, et al. High impact running improves learning. Neurobiology of Learning and Memory. 2007;87(4):597–609. doi: 10.1016/j.nlm.2006.11.003. [DOI] [PubMed] [Google Scholar]